Surfactin as a Green Agent Controlling the Growth of Porous Calcite Microstructures
Abstract
1. Introduction
2. Results and Discussion
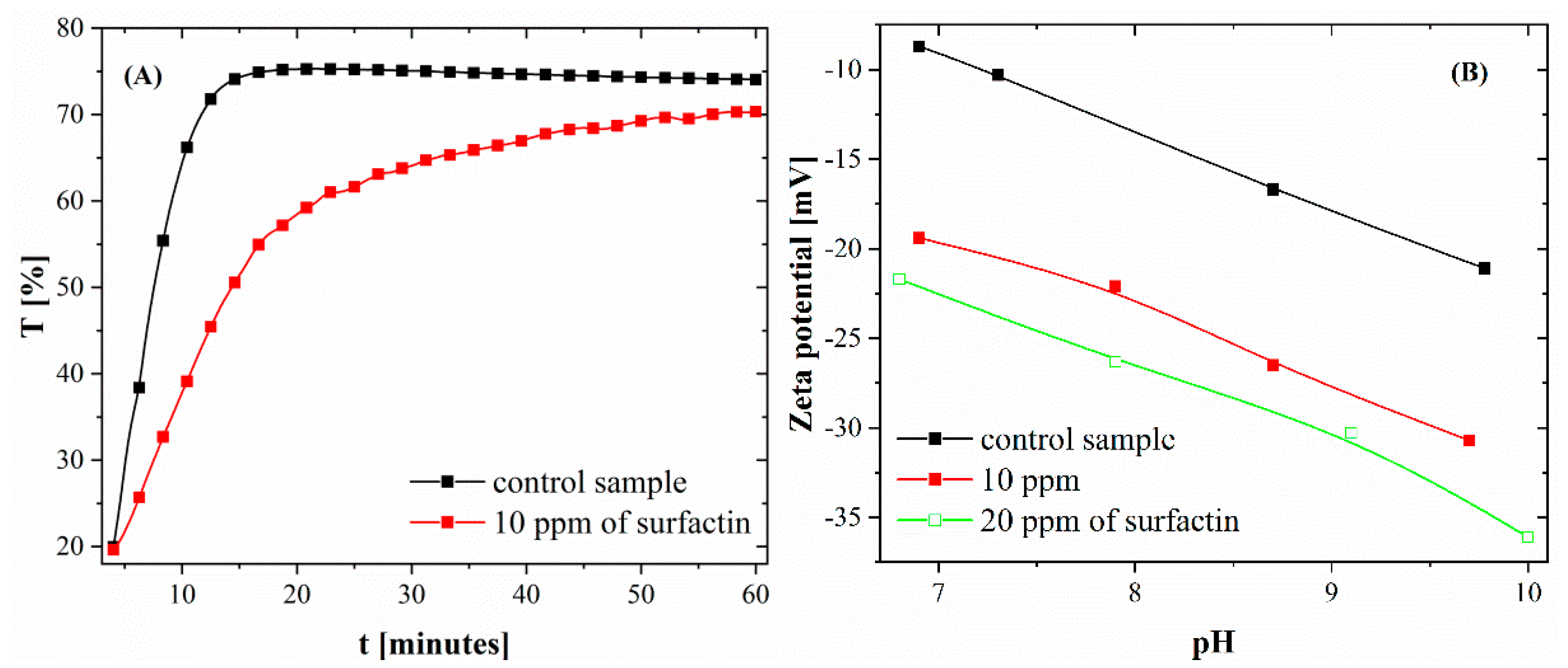
2.1. Particle Size Distribution and Zeta Potential of Calcium Carbonate after 24 H of Ageing
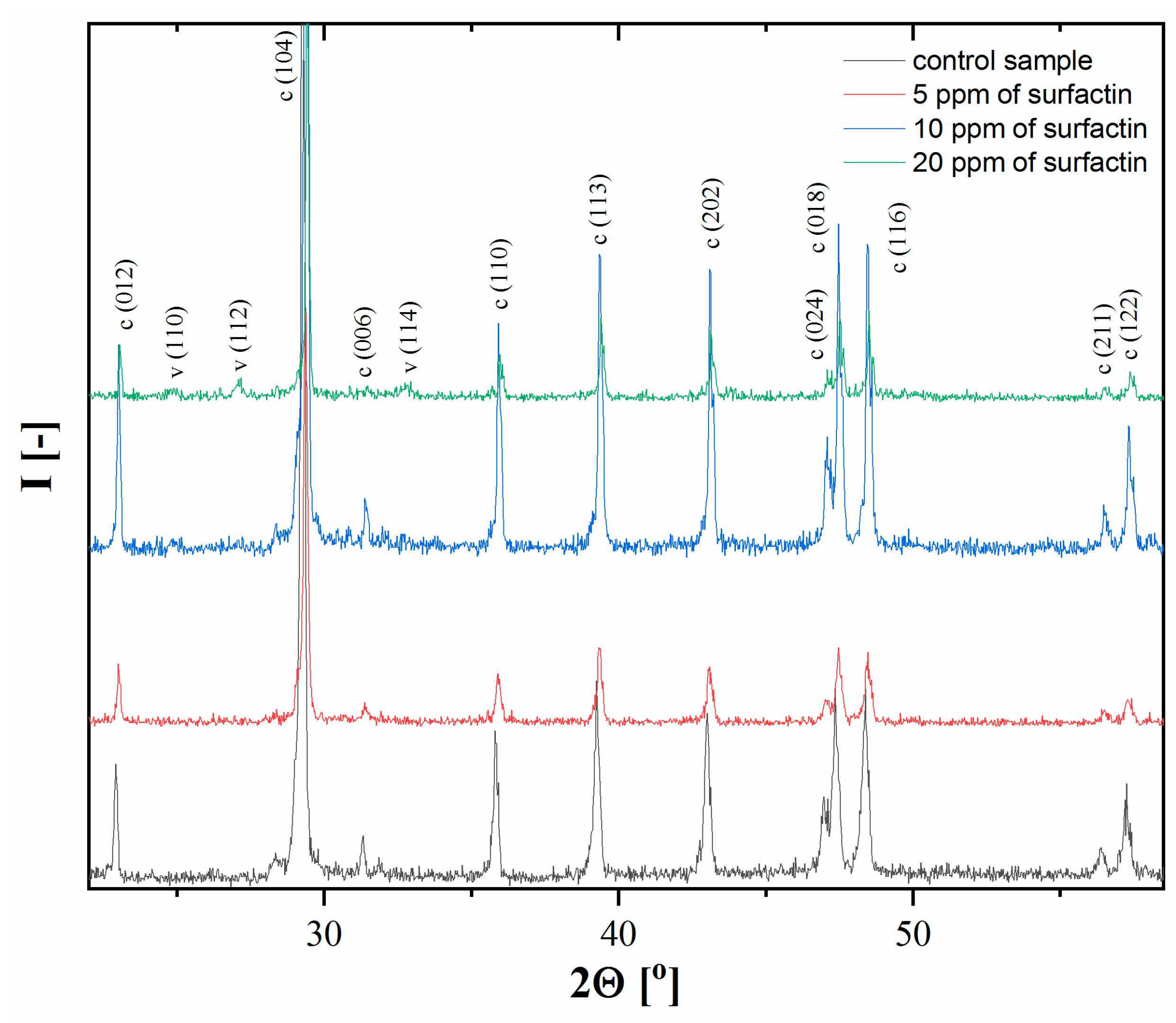
2.2. XRD Analysis of Calcium Carbonate after 24 H of Ageing
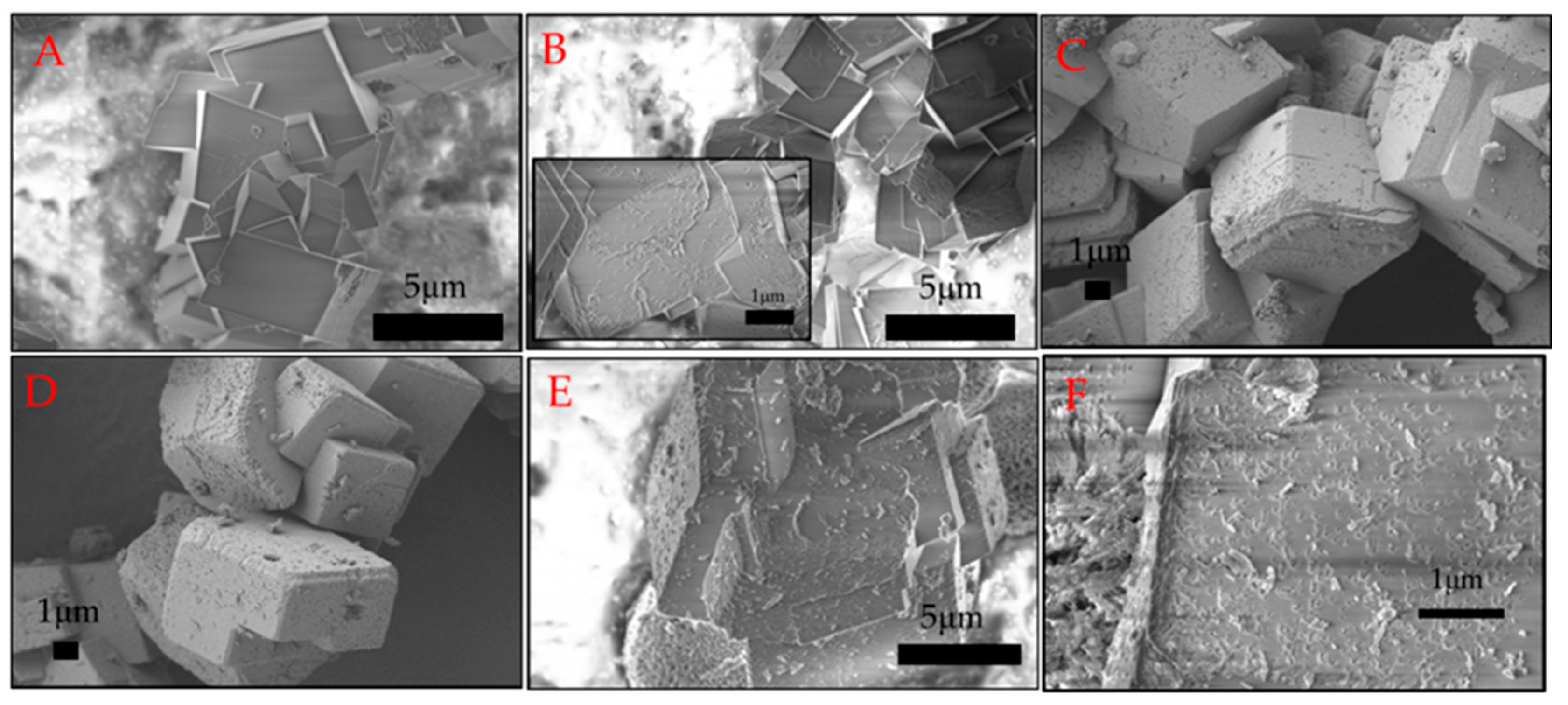
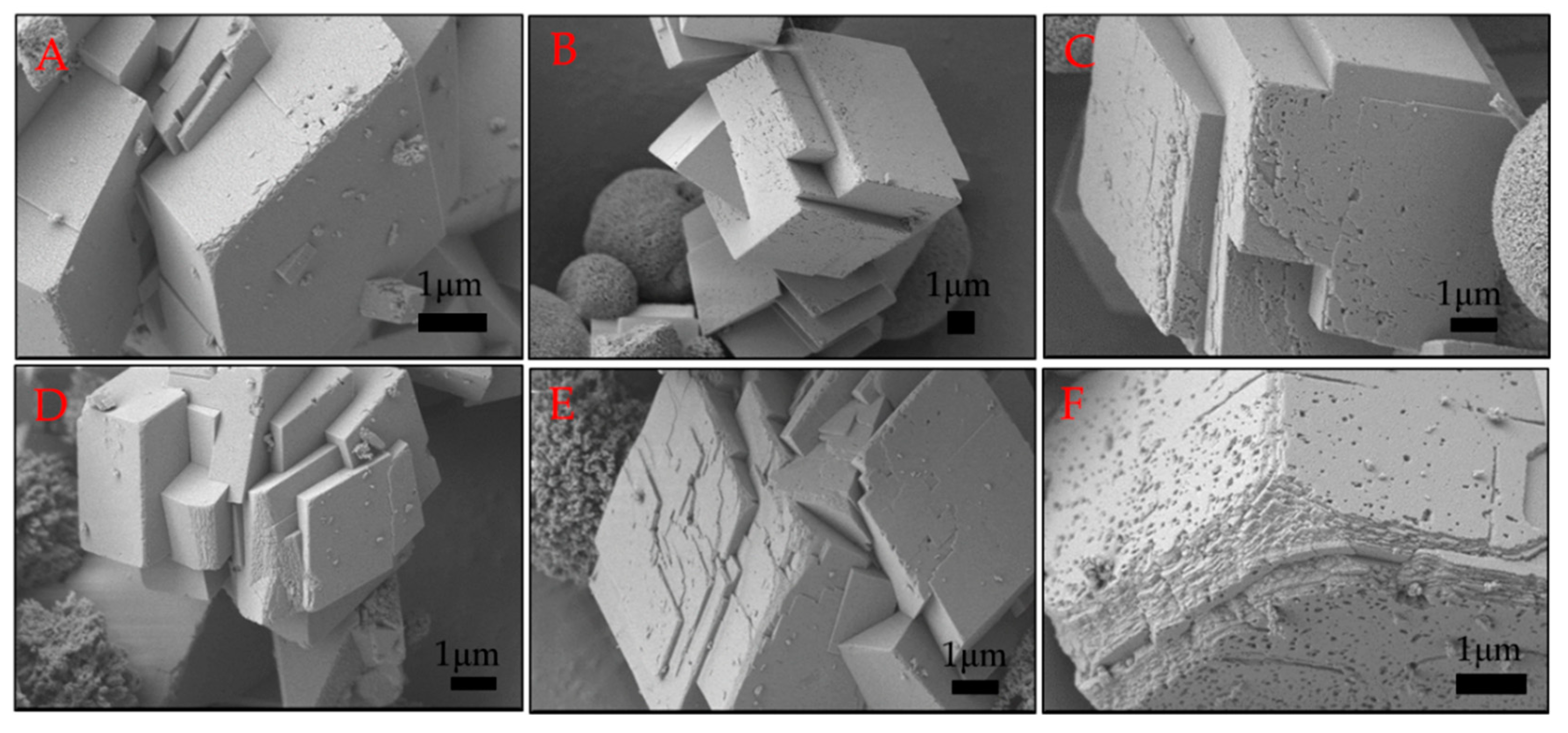
2.3. Morphology of Calcium Carbonate Crystals after 24 H of Ageing
2.4. BET Analysis of Calcium Carbonate after 24 H of Ageing
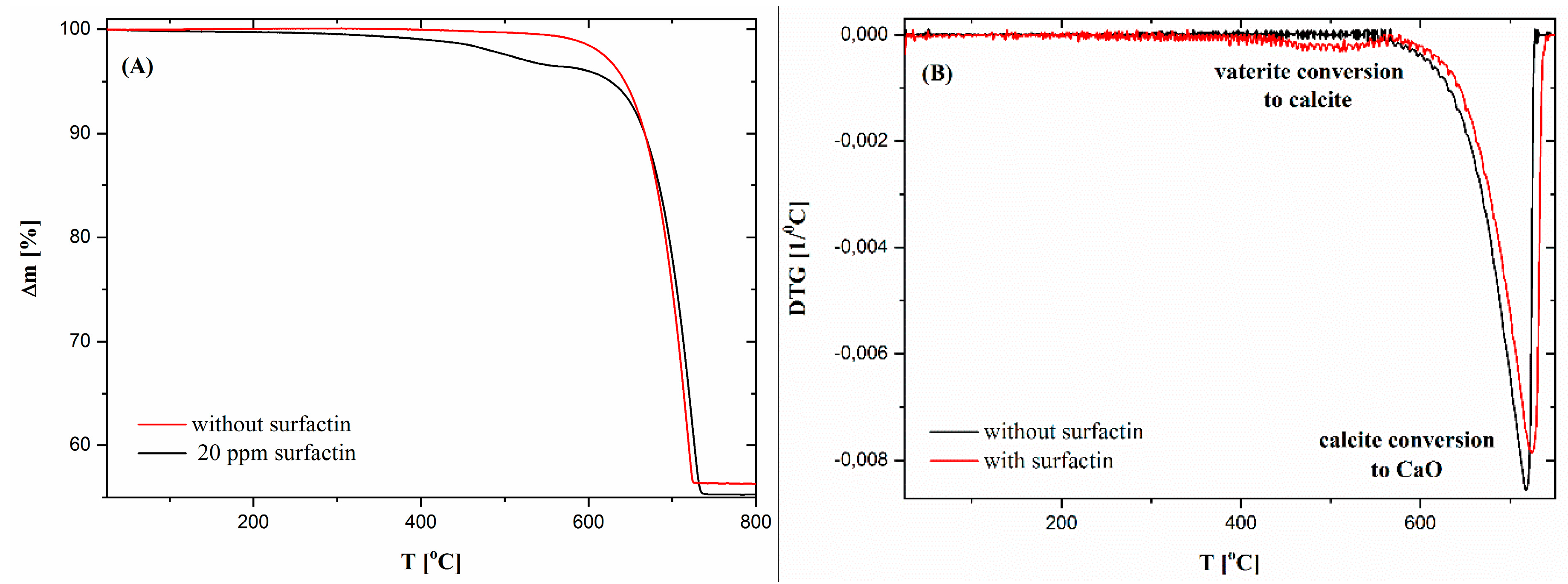
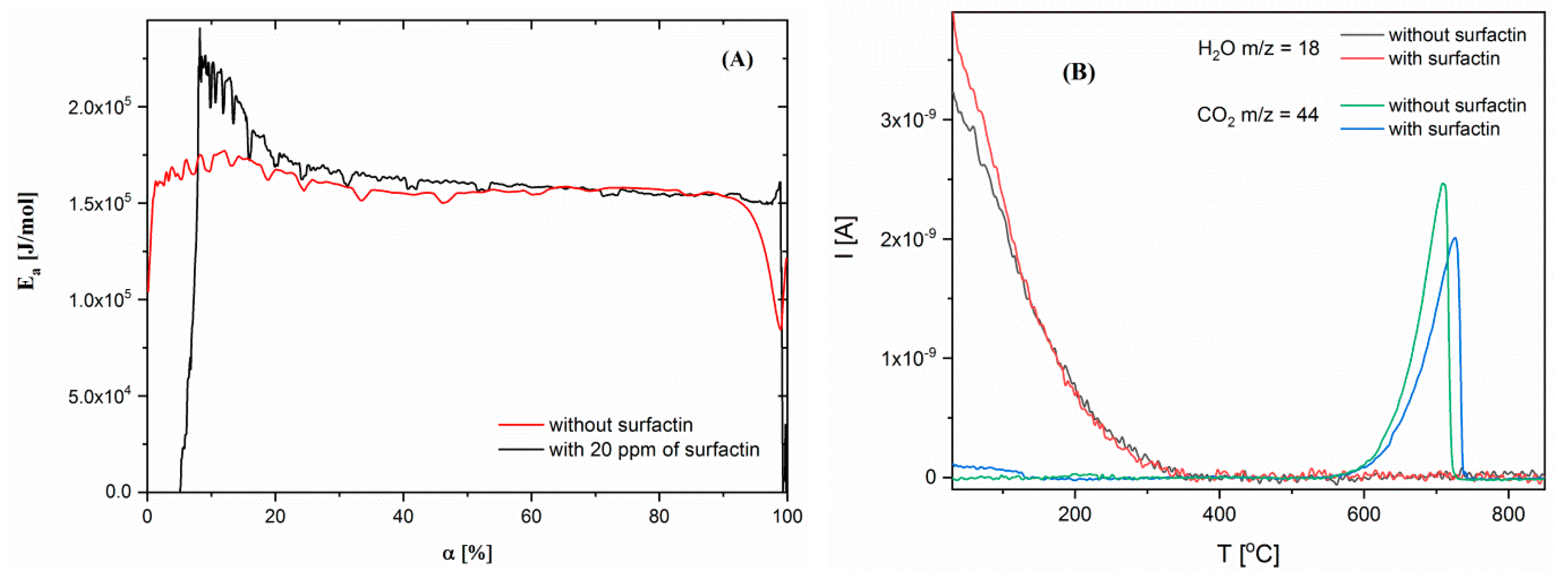
2.5. Thermogravimetric Characteristics of Calcium Carbonate Structures
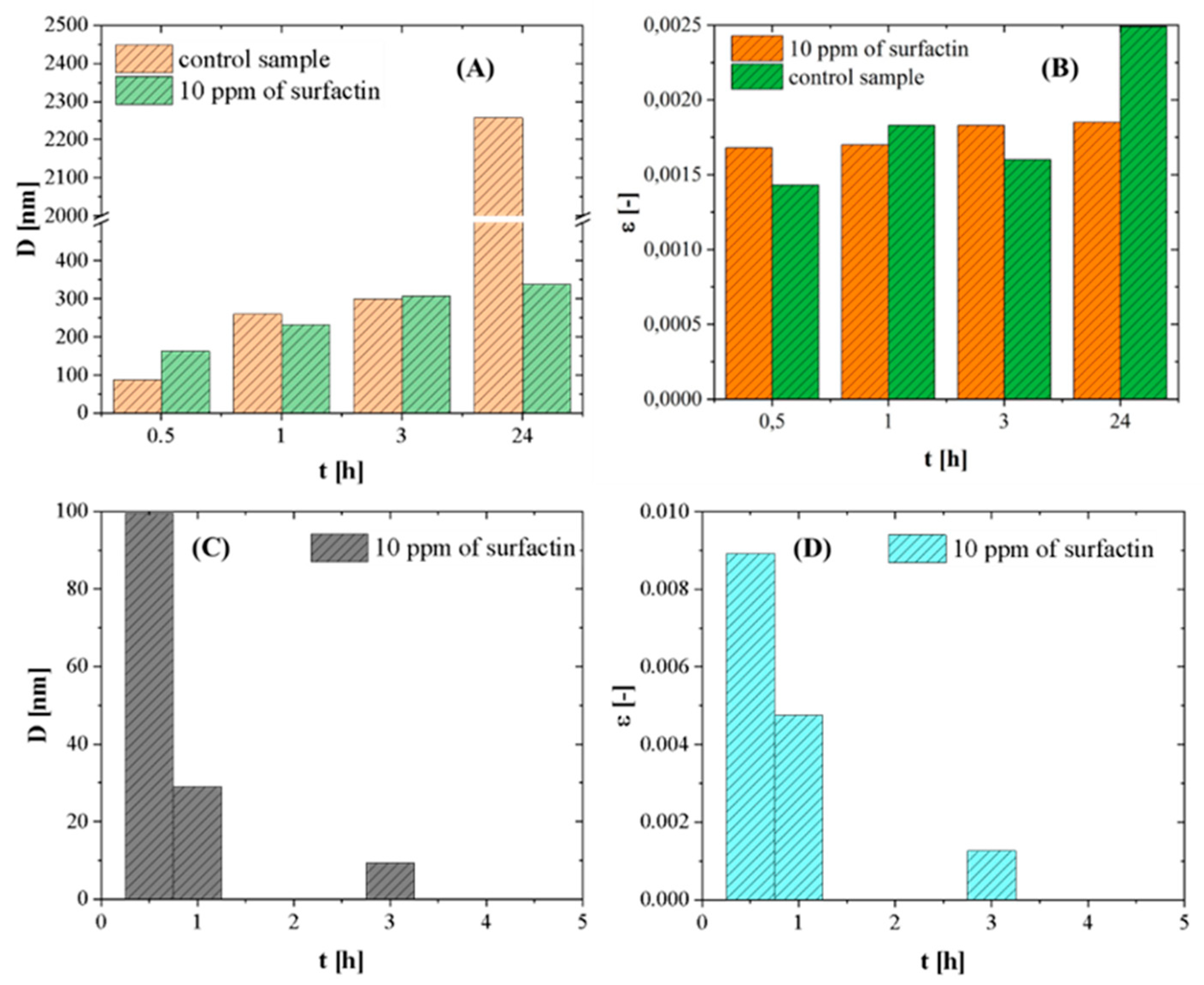
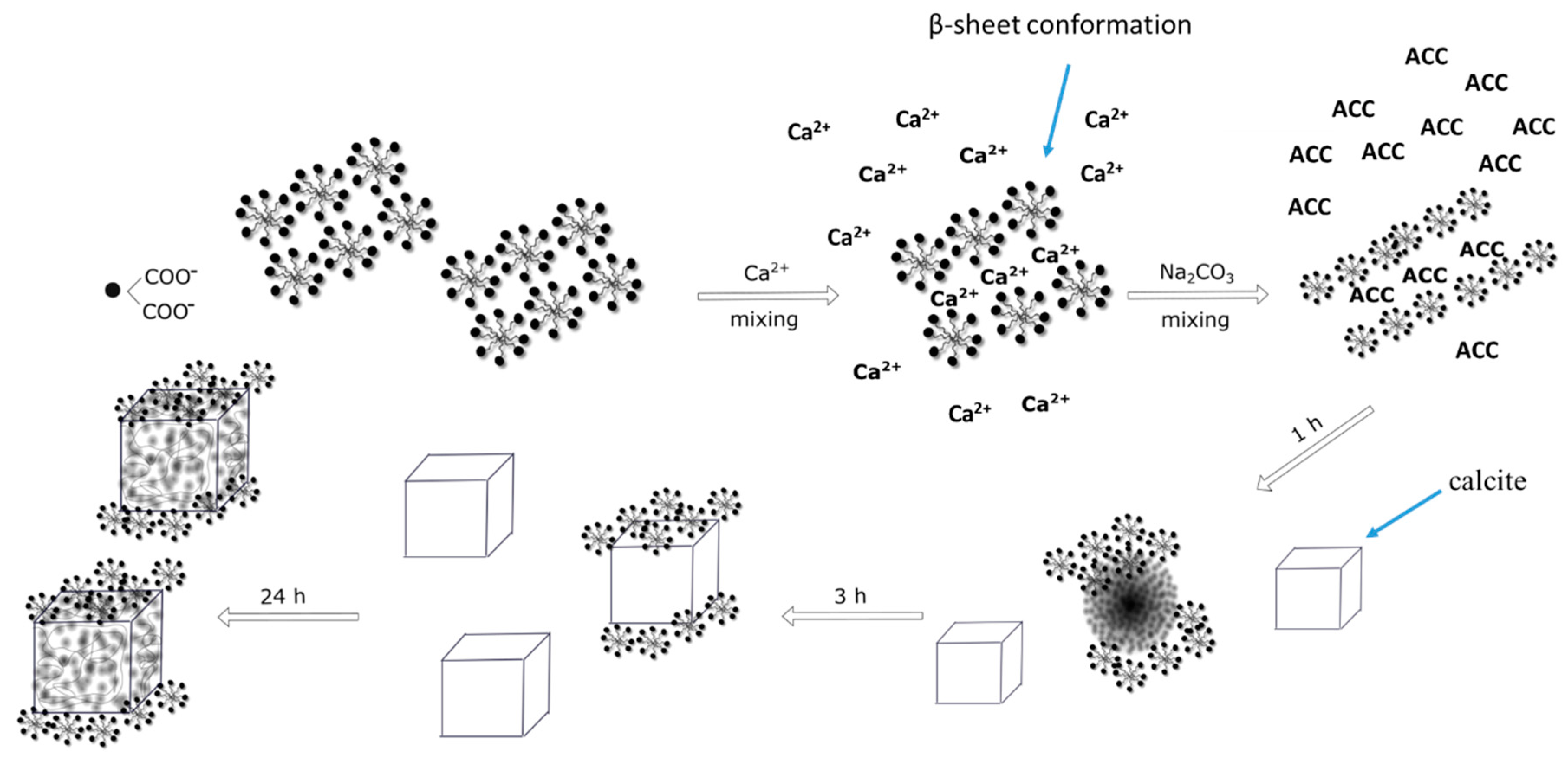
2.6. Effect of Ageing Time on Crystal Growth
- (a)
- in solution during the nucleation stage (formation of Ca-surfactin complexes),
- (b)
- on the surface of formed CaCO3 crystallites.
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, A.W.; Ma, Y.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415–449. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their application. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Xiang, Y.; Han, J.; Zhang, G.; Zhan, F.; Cai, D.; Wu, Z. Efficient synthesis of starch-regulated porous calcium carbonate microspheres as a carrier for slow-release herbicide. ACS Sustain. Chem. Eng. 2018, 6, 3649–3658. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef]
- Trochimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Tymin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 1–36. [Google Scholar]
- Svenskaya, Y.; Parakhonskiy, B.; Haase, A.; Atkin, V.; Lukyanets, E.; Gorin, D.; Antolini, R. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys. Chem. 2013, 182, 11–15. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Liang, T.; Deng, Y.; Qi, X.; Jiang, H.; Wu, Y.; Gao, H. Hierarchical porous calcium carbonate microspheres as drug delivery vector. Proc. Nat. Sci. Mater. 2017, 27, 674–677. [Google Scholar] [CrossRef]
- Zhou, G.T.; Guan, Y.B.; Yao, Q.Z.; Fu, S.Q. Biomimetic mineralization of prismatic calcite mesocrystals: Relevance to biomineralization. Chem. Geol. 2010, 279, 63–72. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 1997, 77, 689–702. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press Inc.: New York, NY, USA, 2001. [Google Scholar]
- Njegic-Džakula, B.; Reggi, M.; Falini, G.; Weber, I.; Brecevic, L.; Kralj, D. The influence of a protein fragment extracted from abalone shell green layer on the precipitation of calcium carbonate polymorphs in aqueous media. Croat. Chem. Acta 2013, 86, 39–47. [Google Scholar] [CrossRef]
- Pastero, L.; Aquilano, D. Calcium carbonate polymorphs growing in the presence of sericin: A new composite mimicking the hierarchic structure of nacre. Crystals 2018, 8, 263. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Okoye, P.U.; Ariffin, K.S.; Hussin, H.B.; Baharun, N. Continuous synthesis of precipitated calcium carbonate using a tubular reactor with the aid of aloe vera (Aloe barbadensis Miller) extract as a green morphological modifier. J. Clean. Prod. 2017, 150, 104–111. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants-a new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 22, 81–90. [Google Scholar] [CrossRef]
- Płaza, G.A.; Chojniak, J.; Banat, I.B. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int. J. Mol. Sci. 2014, 15, 13720–13737. [Google Scholar] [CrossRef] [PubMed]
- Bastrzyk, A.; Fiedot-Toboła, M.; Polowczyk, I.; Legawiec, K.; Płaza, G. Effect of a lipopeptide biosurfactant on the precipitation of calcium carbonate. Colloids Surf. B Biointerfaces 2019, 174, 145–152. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Terpiłowski, K.; Chibowski, S.; Urban, T.; Zarko, V.I.; Gun’ko, V.M. Investigation of stabilization and destabilization possibilities of water alumina suspension in polyelectrolyte presence. Int. J. Min. Proc. 2014, 132, 34–42. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef]
- Dang, H.; Xu, Z.; Chen, Z.; Wu, W.; Feng, J.; Sun, Y.; Jin, F.; Li, J.; Ge, F. A facile and controllable method to in situ synthesize stable hydrophobic vaterite particles. Crys. Res. Technol. 2019, 54, 1–7. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, Y.; Ma, Y.; Zhou, Y.; Nie, F.; Liu, X.; Pei, C. Egg-white-mediated crystallization of calcium carbonate. J. Cryst. Growth 2012, 361, 217–224. [Google Scholar] [CrossRef]
- Szcześ, A.; Sternik, D. Properties of calcium carbonate precipitated in the presence of DPPC liposomes modified with phospholipase A2. J. Therm. Anal. Calorim. 2016, 123, 2357–2365. [Google Scholar] [CrossRef]
- Pokroy, B.; Fitch, A.; Zolotoyabko, E. The microstructure of biogenic calcite: A view by high-resolution synchrotron powder diffraction. Adv. Mater. 2006, 18, 2363–2368. [Google Scholar] [CrossRef]
- Borukhin, S.; Bloch, L.; Radlauer, T.; Hill, A.H.; Fitch, A.N.; Porkroy, B. Screening the incorporation of amino acids into an inorganic crystalline host: The case of calcite. Adv. Funct. Mater. 2012, 22, 4216–4224. [Google Scholar] [CrossRef]
- Różycka, M.; Coronado, I.; Brach, K.; Olesiak-Bańska, J.; Samoć, M.; Zarębski, M.; Dobrucki, J.; Ptak, M.; Weber, E.; Polishchuck, I.; et al. Lattice shrinkage by incorporation of recombinant Starmarker-like protein within bioinspired calcium carbonate crystals. Chem. Eur. J. 2019, 25, 12740–12750. [Google Scholar] [CrossRef] [PubMed]
- Štainer, L.; Kontrec, J.; Njegic- Džakula, B.; Maltar-Strmečki, N.; Plodinec, M.; Lyons, D.M.; Kralj, D. The effect of different amino acids on spontaneous precipitation of calcium carbonate polymorphs. J. Cryst. Growth 2018, 486, 71–81. [Google Scholar] [CrossRef]
- Polowczyk, I.; Bastrzyk, A.; Fiedot, M. Protein-mediated precipitation of calcium carbonate. Materials 2016, 99, 944. [Google Scholar] [CrossRef]
- Zhang, A.; Xie, H.; Liu, N.; Chen, B.L.; Ping, H.; Fu, Z.Y.; Su, B.L. Crystallization of calcium carbonate under the influences of casein and magnesium ions. RSC Adv. 2016, 6, 110362–110366. [Google Scholar] [CrossRef]
- Wang, X.; Kong, R.; Pan, X.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Role of ovalbumin in the stabilization of metastable vaterite in calcium carbonate mineralization. J. Phys. Chem. B 2009, 113, 8975–8982. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.-H. Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 289–303. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Semsarilar, M.; Carloni, J.D.; Rae Cho, K.; Kulak, A.N.; Polishchuk, I.; Hendley, C.T., IV; Smeets, P.J.M.; Fielding, L.A.; Pokroy, B.; et al. Structure and properties of nanocomposites formed by the occlusion of block copolymer worms and vesicles within calcite crystals. Adv. Funct. Mater. 2016, 26, 1382–1392. [Google Scholar] [CrossRef]
- Juhasz-Bortuzzo, J.A.; Myszka, B.; Silva, R.; Boccaccini, A.R. Sonosynthesis of vaterite-type calcium carbonate. Cryst. Growth Des. 2017, 17, 2351–2356. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikanadan, E.; Jayachandran, M. Enhanced polymer induced precipitation of polymorphous in calcium carbonate: Calcite aragonite vaterite phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Bakar Zakaria, Z.A. Synthesis and characterisation of calcium carbonate aragonite nanocrystals from Cockle Shell Powder (Anadara granosa). J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Vyazovkin, S. Model free kinetics. J. Therm. Anal. Calorim. 2006, 83, 45–61. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators. B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Fiedot, M.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Teterycz, H. Chlorine gas sensor to work in high humidity atmosphere. In Proceedings of the 40th International Spring Seminar on Electronics Technology (ISSE), Sofia, Bulgaria, 10–14 May 2017; pp. 1–4. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Akdogan, E.K.; Leonard, M.R.; Safari, A. Size effects in ferroelectric ceramics. In Handbook of Low and High Dielectric Constant Materials and Their Application; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 2, pp. 60–112. [Google Scholar]
- Kim, Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Sand, K.K.; Benning, L.G. Chapter 5. ACC and vaterite as intermediates in the solution-based crystallization of CaCO3. In New Perspectives on Mineral Nucleation and Growth: From Solution Precursors to Solid Material; Van Driessche, A.E.S., Kellermeier, M., Benning, L.G., Gebauer, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 93–111. [Google Scholar]
- Dhali, D.; Coutte, F.; Arias, A.A.; Auger, S.; Bidnenko, V.; Chataigné, G.; Lalk, M.; Neihren, J.; De Sousa, J.; Versari, C.; et al. Genetic engineering of the branched fatty acid metabolic pathway of Bacillus subtilis for the overproduction of surfactin C14 isoform. Biotechnol. J. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Nogueira Felix, A.K.; Martins, J.J.L.; Lima Almeida, J.G.; Giro, M.E.A.; Cavalcante, K.F.; Maciel Melo, V.M.; Loiola Pessoa, O.D.; Valderez Ponte Rocha, M.; Rocha Barros Gonҫalves, L.; Saraiva de Santiago Aguiar, R. Purification and characterization of a biosurfactant produced by Bacillus subtilis in cashew apple juice and its application in the remediation of oil-contaminated soil. Colloids Surf. B Biointerfaces 2019, 175, 256–263. [Google Scholar] [CrossRef]
- Ishigami, Y.; Osman, M.; Nakahara, H.; Sano, Y.; Ishiguro, R.; Matsumoto, M. Significance of β-sheet formation for micellization and surface adsorption of surfactin. Colloids Surf. B Biointerfaces 1995, 4, 341–348. [Google Scholar] [CrossRef]
- Shao, C.; Liu, L.; Gang, H.; Yang, S.; Mu, B. Structural diversity of the microbial surfactin derivatives from selective esterification approach. Int. J. Mol. Sci. 2015, 16, 1855–1872. [Google Scholar] [CrossRef]
- Li, Y.; Zou, A.H.; Ye, R.Q.; Mu, B.Z. Counterion-induced changes to the micellization of surfactin-C-16 aqueous solution. J. Phys. Chem. B 2009, 113, 15272–15277. [Google Scholar] [CrossRef] [PubMed]
- Dhanarajan, G.; Patra, P.; Rangarajan, V.; Samosundaran, P.; Sen, R. Modeling and analysis of micellar and microbubble dynamics to derive new insight in molecular interactions impacting the packing behavior of a green surfactant for potential engineering application. ACS Sustain. Chem. Eng. 2018, 6, 4046–4055. [Google Scholar] [CrossRef]
- Heerklotz, H.; Seeling, J. Detergent-like action of antibiotic peptide surfactin on lipid membranes. Biophys. J. 2001, 81, 1547–1554. [Google Scholar] [CrossRef]
- Carrillo, C.; Cejas, S.; Huber, A.; González, N.S.; Algranati, I.D. Lack of arginine decarboxylase in Trypanosoma cruzi epimastigotes. J. Eukaryot. Microbiol. 2003, 50, 312–316. [Google Scholar] [CrossRef]
- Wu, Z.G.; Wang, J.; Guo, Y.; Jia, Y.R. Exploring the influence of reaction parameters on the preparation of calcium carbonate by spontaneous precipitation. Cryst. Res. Technol. 2018, 53, 1–4. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Leen Penn, R.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthethic, biogenic, and geologic environments. Science 2015, 349, 498–507. [Google Scholar] [CrossRef]
- Jauregi, P.; Coutte, F.; Catiau, L.; Lecouturier, D.; Jacques, P. Micelles size characterization of lipopeptides produced by B. subtilis and their recovery by the two-step ultrafiltration process. Sep. Purif. Technol. 2013, 104, 175–182. [Google Scholar] [CrossRef]
- Shih, S.J.; Lin, Y.C.; Posma Panjaitan, L.V.; Rahayu, D.; Sari, M. The correlation of surfactant concentration on the properties of mesoporous bioactive glass. Materials 2016, 99, 58. [Google Scholar] [CrossRef]
| Surfactin Concentration [ppm] | d10 [µm] | d50 [µm] | d90 [µm] |
|---|---|---|---|
| 0 | 12.5 | 24.4 | 44.6 |
| 5 | 8.4 | 15.4 | 26.0 |
| 10 | 6.3 | 12.4 | 21.6 |
| 20 | 5.0 | 10.6 | 19.6 |
| Surfactin Concentration [ppm] | Calcite | Vaterite | ||
|---|---|---|---|---|
| Content [%] | Structural Parameters | Content [%] | Structural Parameters | |
| 0 | 100 | a = b = 4.9900(2) Å | - | - |
| c = 17.0550(7) Å | ||||
| α = β = 90°, γ = 120° | ||||
| V = 367.776 (Å)3 | ||||
| 5 | 100 | a = b = 4.9923(3) Å | - | - |
| c = 17.0537(14) Å | ||||
| α = β = 90°, γ = 120° | ||||
| V = 368.087 (Å)3 | ||||
| 10 | 98.6 | a = b = 4.9927(1) Å | 1.4 | a = b = 4.1268(4) Å |
| c = 17.0608(6) Å | c = 8.4791(20) Å | |||
| α = β = 90°, γ = 120° | α = β = 90°, γ = 120° | |||
| V = 368.299 (Å)3 | V = 125.057 (Å)3 | |||
| 20 | 85.1 | a = b = 4.9926(2) Å | 14.9 | a = b = 4.1299(5) Å |
| c = 17.0619(9) Å | c = 8.4699(25) Å | |||
| α = β = 90°, γ = 120° | α = β = 90°, γ = 120° | |||
| V = 368.308 (Å)3 | V = 125.109 (Å)3 | |||
| Surfactin Concentration [ppm] | SSA [m2g−1] | Average Pores Volume [cm3g−1] | Average Pores Diameter [nm] |
|---|---|---|---|
| 0 | 0.18 ± 0.05 | - | - |
| 10 | 1.67 ± 0.1 | 0.00513 ± 0.0002 | 8.7 ± 1.0 |
| 20 | 4.87 ± 0.09 | 0.0123 ± 0.001 | 11.7 ± 1.2 |
| Surfactin Concentration [ppm] | ∆m1 (25–400 °C) [%] | ∆m2 (400–566 °C) [%] | T1 [°C] | T2 [°C] | ∆mtotal [%] |
|---|---|---|---|---|---|
| 0 | 0.71 | - | 717 | 43.69 | |
| 20 | 0.95 | 0.95 | 510 | 724 | 44.71 |
| Time [hours] | Calcite [%] | Vaterite [%] |
|---|---|---|
| 0.5 | 59.6 | 40.5 |
| 1 | 67.8 | 32.2 |
| 3 | 90.5 | 9.5 |
| 24 | 98.6 | 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastrzyk, A.; Fiedot-Toboła, M.; Maniak, H.; Polowczyk, I.; Płaza, G. Surfactin as a Green Agent Controlling the Growth of Porous Calcite Microstructures. Int. J. Mol. Sci. 2020, 21, 5526. https://doi.org/10.3390/ijms21155526
Bastrzyk A, Fiedot-Toboła M, Maniak H, Polowczyk I, Płaza G. Surfactin as a Green Agent Controlling the Growth of Porous Calcite Microstructures. International Journal of Molecular Sciences. 2020; 21(15):5526. https://doi.org/10.3390/ijms21155526
Chicago/Turabian StyleBastrzyk, Anna, Marta Fiedot-Toboła, Halina Maniak, Izabela Polowczyk, and Grażyna Płaza. 2020. "Surfactin as a Green Agent Controlling the Growth of Porous Calcite Microstructures" International Journal of Molecular Sciences 21, no. 15: 5526. https://doi.org/10.3390/ijms21155526
APA StyleBastrzyk, A., Fiedot-Toboła, M., Maniak, H., Polowczyk, I., & Płaza, G. (2020). Surfactin as a Green Agent Controlling the Growth of Porous Calcite Microstructures. International Journal of Molecular Sciences, 21(15), 5526. https://doi.org/10.3390/ijms21155526






