Expanding Role of Dopaminergic Inhibition in Hypercapnic Responses of Cultured Rat Carotid Body Cells: Involvement of Type II Glial Cells
Abstract
1. Introduction
2. Results
2.1. Dopamine Attenuates UTP-Evoked Intracellular Ca2+ Responses in Type II Cells
2.2. Reversal of Dopaminergic Inhibition of P2Y2R-Mediated Ca2+ Signalling in Type II Cells by Sulpiride, a D2/3 Receptor Antagonist
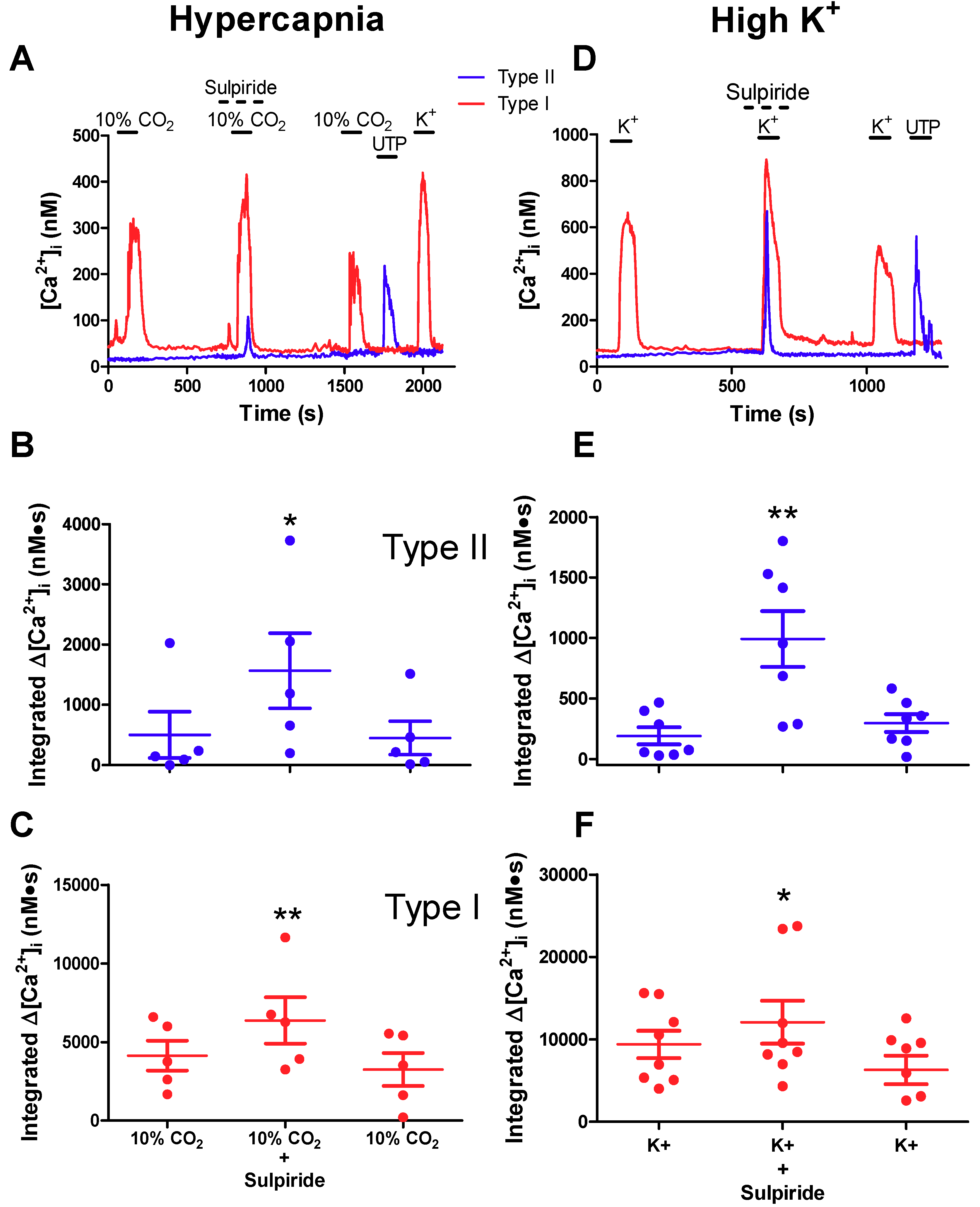
2.3. Effects of Sulpiride on Paracrine Signalling between Type I Chemoreceptor Cells and Type II Glial Cells
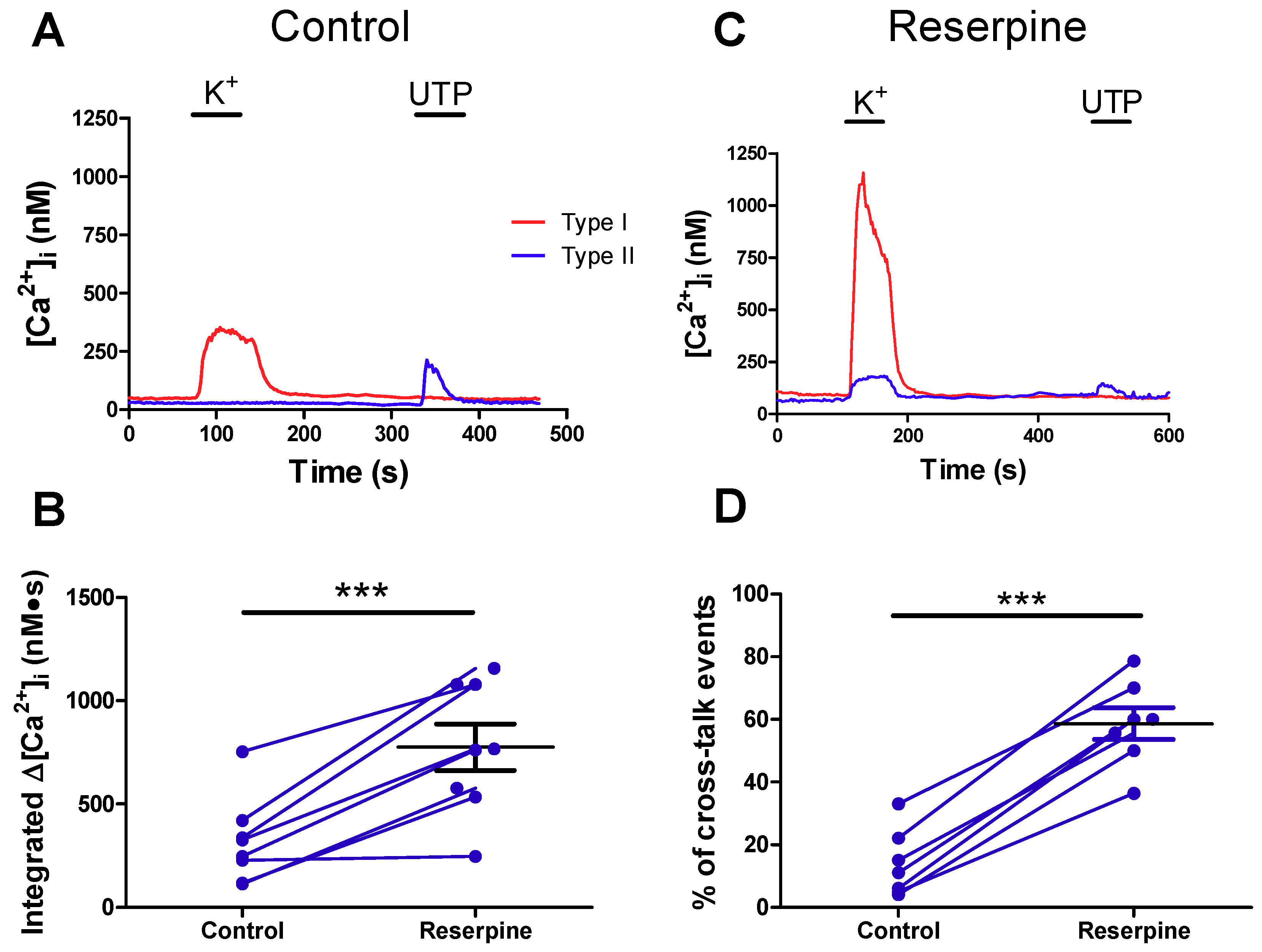
2.4. Pre-Treatment with Reserpine to Deplete Dopamine Stores Facilitates Crosstalk from Type I to Type II Cells
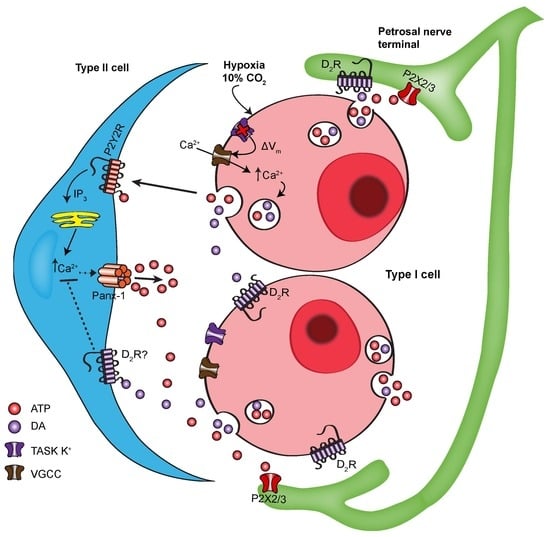
2.5. Role of Dopaminergic Inhibition at the Reconstituted Chemosensory Complex Consisting of Petrosal Neurons, Type I Cells, and Type II Cells In Vitro
2.6. Evidence for Crosstalk between Type II Cells and Petrosal Neurons in Cocultures
3. Discussion
3.1. Dopaminergic Inhibition of P2Y2R-Mediated Calcium Signalling in Type II Cells
3.2. Significance of DA Inhibition and Type II Cell Signalling to the Integrated Carotid Body Sensory Output
3.3. Limitations
4. Materials and Methods
4.1. Ethical Approval
4.2. Carotid Body Cultures and Petrosal Neuron-Carotid Body Cocultures
4.3. Fura-2 Measurements of Intracellular Calcium
4.4. Solutions and Drugs
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CB | Carotid body |
| DA | Dopamine |
| D2/3R | Dopamine receptor type 2/3 |
| Panx-1 | Pannexin-1 |
| PN | petrosal neuron |
| VMAT | Vesicular monoamine transporter |
| VGCC | Voltage-gated calcium channel |
| TASK | Two-pore domain, acid-sensitive K+ |
References
- Gonzalez, C.; Almaraz, L.; Obeso, A.; Rigual, R. Carotid body chemoreceptors: From natural stimuli to sensory discharges. Physiol. Rev. 1994, 74, 829–898. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Prabhakar, N.R. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr. Physiol. 2012, 2, 141–219. [Google Scholar] [PubMed]
- Nurse, C.A.; Piskuric, N.A. Signal processing at mammalian carotid body chemoreceptors. Semin. Cell Dev. Biol. 2013, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Alcayaga, J. Neurotransmission in the carotid body: Transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res. Brain Res. Rev. 2004, 47, 46–53. [Google Scholar] [CrossRef]
- Nurse, C.A. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J. Physiol. 2014, 592, 3419–3426. [Google Scholar] [CrossRef]
- Conde, S.V.; Monteiro, E.C.; Obeso, A.; Gonzalez, C. Adenosine in peripheral chemoreception: New insights into a historically overlooked molecule--invited article. Adv. Exp. Med. Biol. 2009, 648, 145–159. [Google Scholar]
- Conde, S.V.; Monteiro, E.C.; Rigual, R.; Obeso, A.; Gonzalez, C. Hypoxic intensity: A determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J. Appl. Physiol. 2012, 112, 2002–2010. [Google Scholar] [CrossRef]
- Iturriaga, R.; Alcayaga, J.; Gonzalez, C. Neurotransmitters in carotid body function: The case of dopamine—invited article. Adv. Exp. Med. Biol. 2009, 648, 137–143. [Google Scholar]
- Lopez-Barneo, J.; Gonzalez-Rodriguez, P.; Gao, L.; Fernandez-Aguera, M.C.; Pardal, R.; Ortega-Saenz, P. Oxygen sensing by the carotid body: Mechanisms and role in adaptation to hypoxia. Am. J. Physiol. Cell Physiol. 2016, 310, C629–C642. [Google Scholar] [CrossRef]
- Gauda, E.B. Gene expression in peripheral arterial chemoreceptors. Microsc. Res. Tech. 2002, 59, 153–167. [Google Scholar] [CrossRef]
- Bairam, A.; Carroll, J.L. Neurotransmitters in carotid body development. Respir. Physiol. Neurobiol. 2005, 149, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, M.J.; Kahlin, J.; Ebberyd, A.; Schulte, G.; Mkrtchian, S.; Eriksson, L.I. The human carotid body: Expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiologists 2010, 113, 1270–1279. [Google Scholar] [CrossRef]
- Bairam, A.; Frenette, J.; Dauphin, C.; Carroll, J.L.; Khandjian, E.W. Expression of dopamine D1-receptor mRNA in the carotid body of adult rabbits, cats and rats. Neurosci. Res. 1998, 31, 147–154. [Google Scholar] [CrossRef]
- Zhang, M.; Vollmer, C.; Nurse, C.A. Adenosine and dopamine oppositely modulate a hyperpolarization-activated current Ih in chemosensory neurons of the rat carotid body in co-culture. J. Physiol. 2018, 596, 3101–3117. [Google Scholar] [CrossRef] [PubMed]
- Benot, A.R.; Lopez-Barneo, J. Feedback Inhibition of Ca2+ Currents by Dopamine in Glomus Cells of the Carotid Body. Eur. J. Neurosci. 1990, 2, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.L.; Boyle, K.M.; Wasicko, M.J.; Sterni, L.M. Dopamine D2 receptor modulation of carotid body type 1 cell intracellular calcium in developing rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L910–L916. [Google Scholar] [CrossRef]
- Conde, S.V.; Gonzalez, C.; Batuca, J.R.; Monteiro, E.C.; Obeso, A. An antagonistic interaction between A2B adenosine and D2 dopamine receptors modulates the function of rat carotid body chemoreceptor cells. J. Neurochem. 2008, 107, 1369–1381. [Google Scholar] [CrossRef]
- Xu, J.; Tse, F.W.; Tse, A. ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J. Physiol. 2003, 549, 739–747. [Google Scholar] [CrossRef]
- Tse, A.; Yan, L.; Lee, A.K.; Tse, F.W. Autocrine and paracrine actions of ATP in rat carotid body. Can. J. Physiol. Pharmacol. 2012, 90, 705–711. [Google Scholar] [CrossRef]
- Zhang, M.; Piskuric, N.A.; Vollmer, C.; Nurse, C.A. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: Potential role in amplifying the neurotransmitter ATP. J. Physiol. 2012, 590, 4335–4350. [Google Scholar] [CrossRef]
- Murali, S.; Zhang, M.; Nurse, C.A. Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. J. Physiol. 2014, 592, 4747–4762. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Nurse, C.A. Purinergic signalling mediates bidirectional crosstalk between chemoreceptor type I and glial-like type II cells of the rat carotid body. J. Physiol. 2016, 594, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Nurse, C.A.; Leonard, E.M.; Salman, S. Role of glial-like type II cells as paracrine modulators of carotid body chemoreception. Physiol. Genom. 2018, 50, 255–262. [Google Scholar] [CrossRef]
- Prabhakar, N.R. O2 sensing at the mammalian carotid body: Why multiple O2 sensors and multiple transmitters? Exp. Physiol. 2006, 91, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nurse, C.A. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp. Physiol. 2010, 95, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Kasparov, S. Astrocytes as brain interoceptors. Exp. Physiol. 2011, 96, 411–416. [Google Scholar] [CrossRef]
- Piskuric, N.A.; Nurse, C.A. Effects of chemostimuli on Ca2+i responses of rat aortic body type I cells and endogenous local neurons: Comparison with carotid body cells. J. Physiol. 2012, 590, 2121–2135. [Google Scholar] [CrossRef]
- Conde, S.V.; Ribeiro, M.J.; Obeso, A.; Rigual, R.; Monteiro, E.C.; Gonzalez, C. Chronic caffeine intake in adult rat inhibits carotid body sensitization produced by chronic sustained hypoxia but maintains intact chemoreflex output. Mol. Pharmacol. 2012, 82, 1056–1065. [Google Scholar] [CrossRef]
- Leonard, E.M.; Salman, S.; Nurse, C.A. Sensory processing and integration at the carotid body tripartite synapse: Neurotransmitter functions and effects of chronic hypoxia. Front. Physiol. 2018, 9, 225. [Google Scholar] [CrossRef]
- Buttigieg, J.; Nurse, C.A. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem. Biophys. Res. Commun. 2004, 322, 82–87. [Google Scholar] [CrossRef]
- Donnelly, D.F. Does catecholamine secretion mediate the hypoxia-induced increase in nerve activity? Biol. Neurosignals 1995, 4, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Mandela, P.; Chandley, M.; Xu, Y.Y.; Zhu, M.Y.; Ordway, G.A. Reserpine-induced reduction in norepinephrine transporter function requires catecholamine storage vesicles. Neurochem. Int. 2010, 56, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhong, H.; Vollmer, C.; Nurse, C.A. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J. Physiol. 2000, 525 Pt 1, 143–158. [Google Scholar] [CrossRef]
- Zhang, M.; Nurse, C.A. CO2/pH chemosensory signaling in co-cultures of rat carotid body receptors and petrosal neurons: Role of ATP and ACh. J. Neurophysiol. 2004, 92, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Zhang, M.; Nurse, C.A. Evidence that 5-HT stimulates intracellular Ca2+ signalling and activates pannexin-1 currents in type II cells of the rat carotid body. J. Physiol. 2017, 595, 4261–4277. [Google Scholar] [CrossRef]
- Prasad, M.; Fearon, I.M.; Zhang, M.; Laing, M.; Vollmer, C.; Nurse, C.A. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: Role in chemosensory signalling. J. Physiol. 2001, 537, 667–677. [Google Scholar] [CrossRef]
- Rong, W.; Gourine, A.V.; Cockayne, D.A.; Xiang, Z.; Ford, A.P.; Spyer, K.M.; Burnstock, G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J. Neurosci. 2003, 23, 11315–11321. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Herman, J.K.; O’Halloran, K.D.; Keith, I.M.; Bisgard, G.E. Pharmacological and immunochemical evidence of the dopamine D3 receptor in the goat carotid body. Adv. Exp. Med. Biol. 2001, 499, 49–53. [Google Scholar]
- Wakai, J.; Takayama, A.; Yokoyama, T.; Nakamuta, N.; Kusakabe, T.; Yamamoto, Y. Immunohistochemical localization of dopamine D2 receptor in the rat carotid body. Acta Histochem. 2015, 117, 784–789. [Google Scholar] [CrossRef]
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef]
- Swapna, I.; Bondy, B.; Morikawa, H. Differential dopamine regulation of Ca2+ signaling and its timing dependence in the nucleus accumbens. Cell. Rep. 2016, 15, 563–573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salman, S.; Vollmer, C.; McClelland, G.B.; Nurse, C.A. Characterization of ectonucleotidase expression in the rat carotid body: Regulation by chronic hypoxia. Am. J. Physiol. Cell Physiol. 2017, 313, C274–C284. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.P.; Ray, C.J.; Pearson, S.A.; Coney, A.M.; Kumar, P. Ecto-5’-nucleotidase (CD73) regulates peripheral chemoreceptor activity and cardiorespiratory responses to hypoxia. J. Physiol. 2018, 596, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhang, M.; Nurse, C.A. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J. Physiol. 1997, 503 Pt 3, 599–612. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonard, E.M.; Nurse, C.A. Expanding Role of Dopaminergic Inhibition in Hypercapnic Responses of Cultured Rat Carotid Body Cells: Involvement of Type II Glial Cells. Int. J. Mol. Sci. 2020, 21, 5434. https://doi.org/10.3390/ijms21155434
Leonard EM, Nurse CA. Expanding Role of Dopaminergic Inhibition in Hypercapnic Responses of Cultured Rat Carotid Body Cells: Involvement of Type II Glial Cells. International Journal of Molecular Sciences. 2020; 21(15):5434. https://doi.org/10.3390/ijms21155434
Chicago/Turabian StyleLeonard, Erin M., and Colin A. Nurse. 2020. "Expanding Role of Dopaminergic Inhibition in Hypercapnic Responses of Cultured Rat Carotid Body Cells: Involvement of Type II Glial Cells" International Journal of Molecular Sciences 21, no. 15: 5434. https://doi.org/10.3390/ijms21155434
APA StyleLeonard, E. M., & Nurse, C. A. (2020). Expanding Role of Dopaminergic Inhibition in Hypercapnic Responses of Cultured Rat Carotid Body Cells: Involvement of Type II Glial Cells. International Journal of Molecular Sciences, 21(15), 5434. https://doi.org/10.3390/ijms21155434