Peroxidan Plays a Tumor-Promoting Role in Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
2.1. PXDN Expression in OSCC Specimens
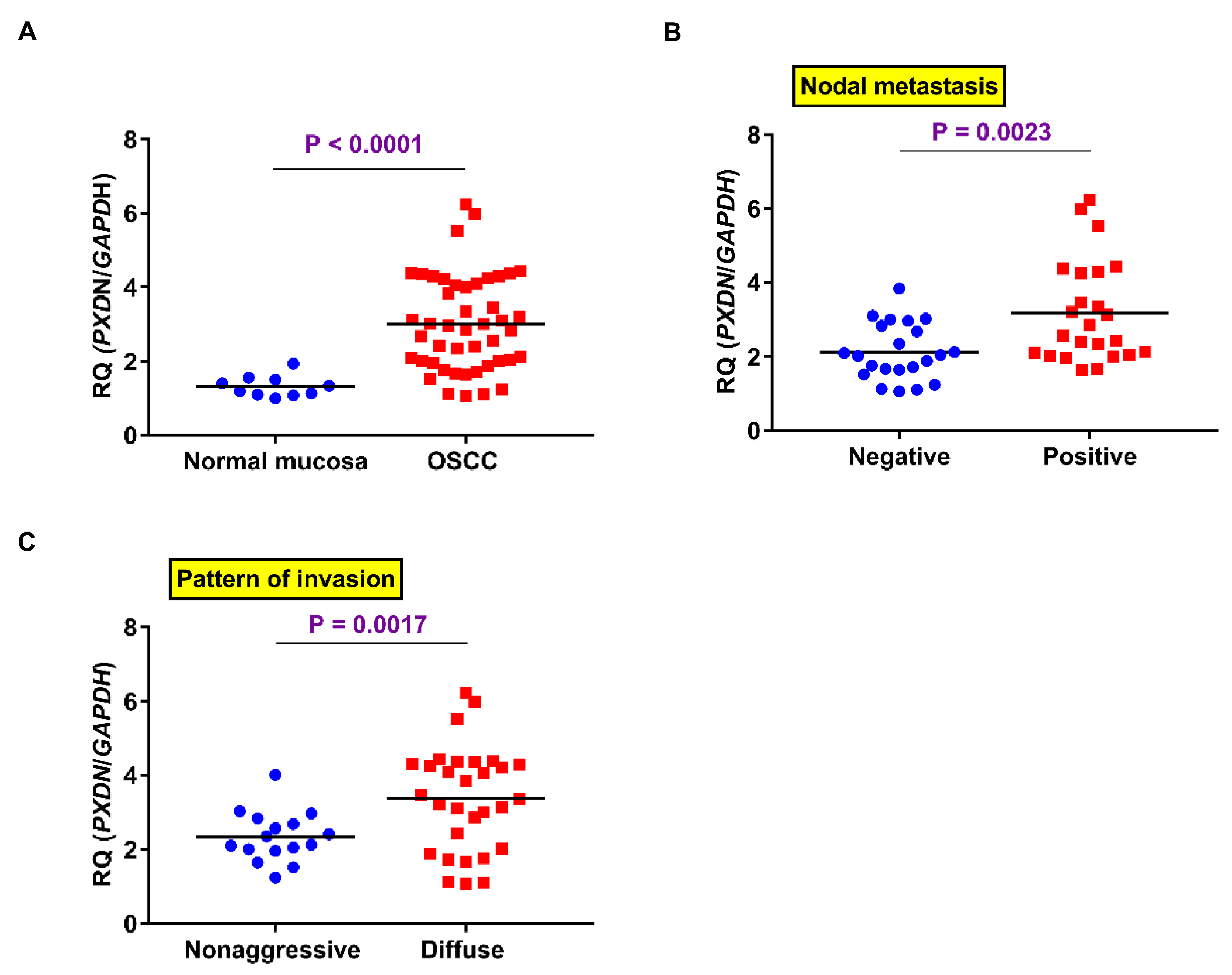
2.2. PXDN mRNA Expression in OSCC Specimens
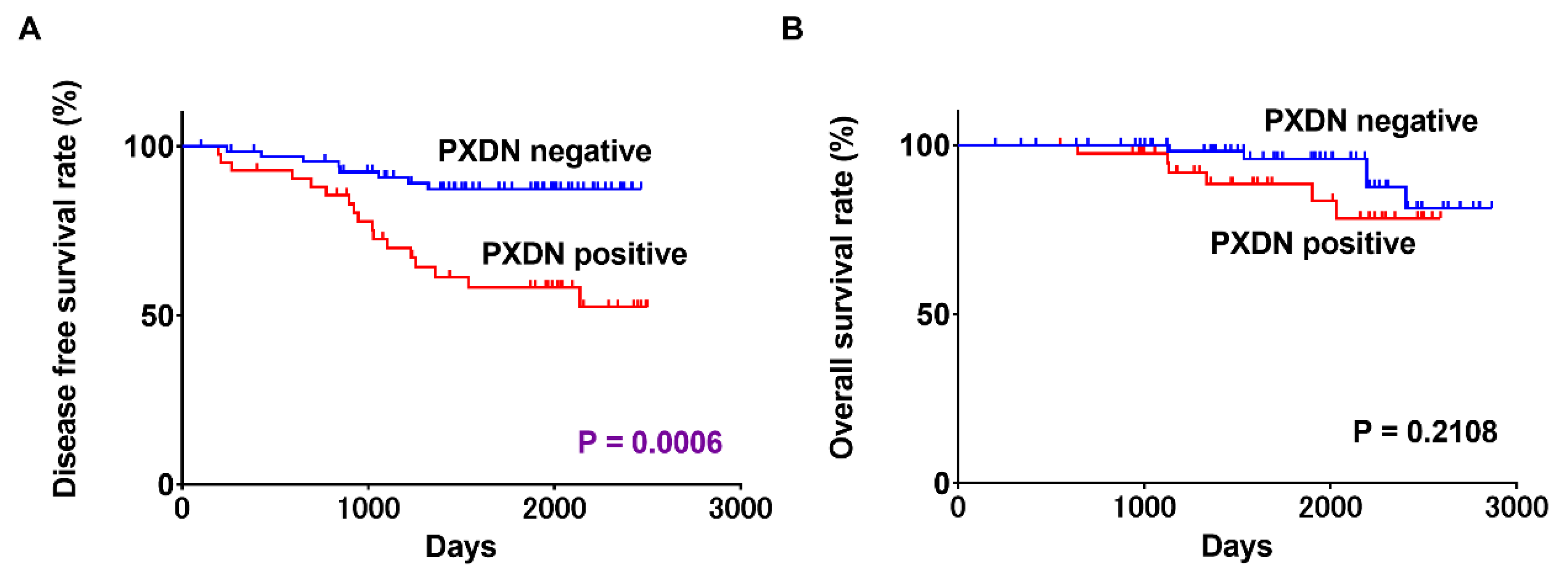
2.3. Associations between PXDN Expression and Prognosis in OSCC
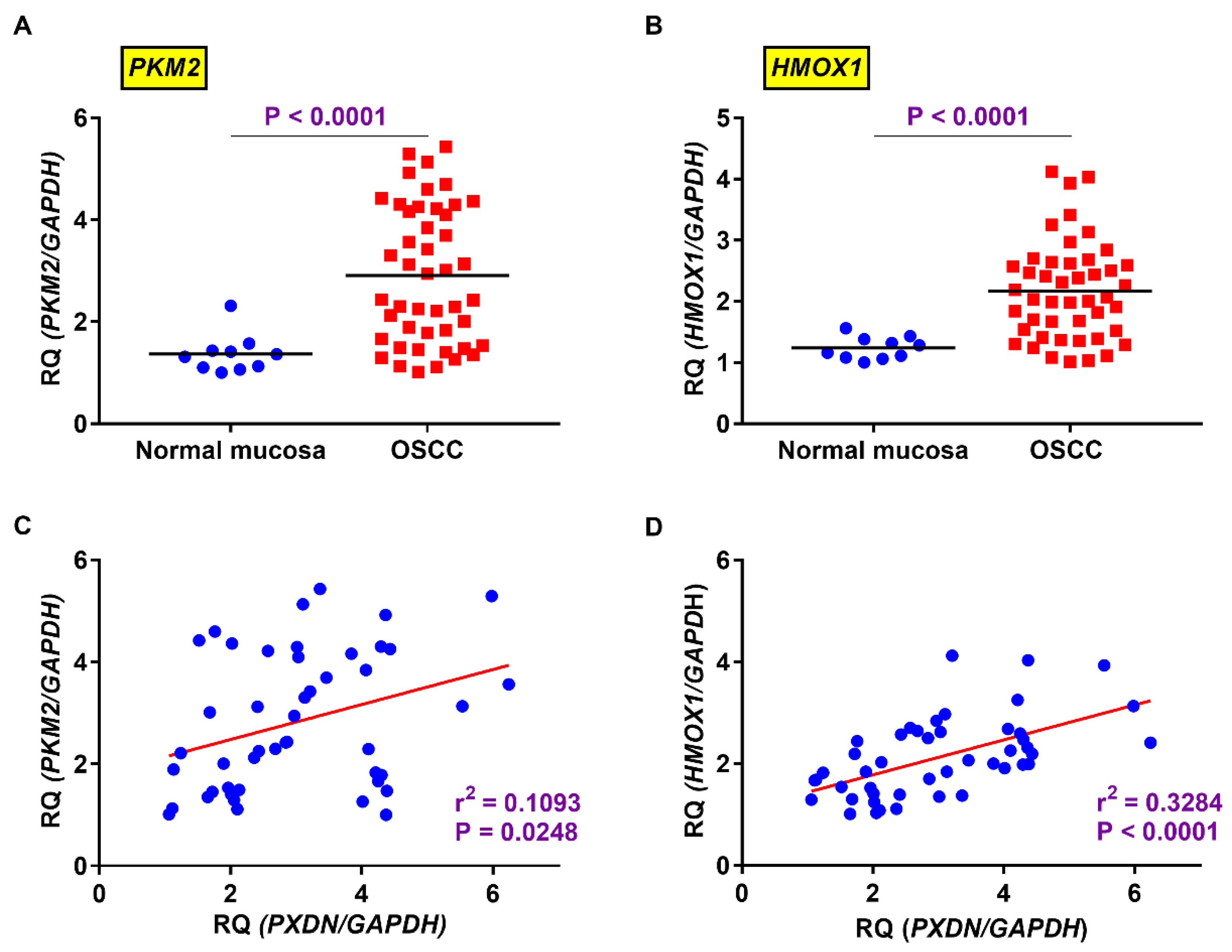
2.4. Relevance of Expression Levels of PXDN and HMOX1 or PKM2 in OSCC
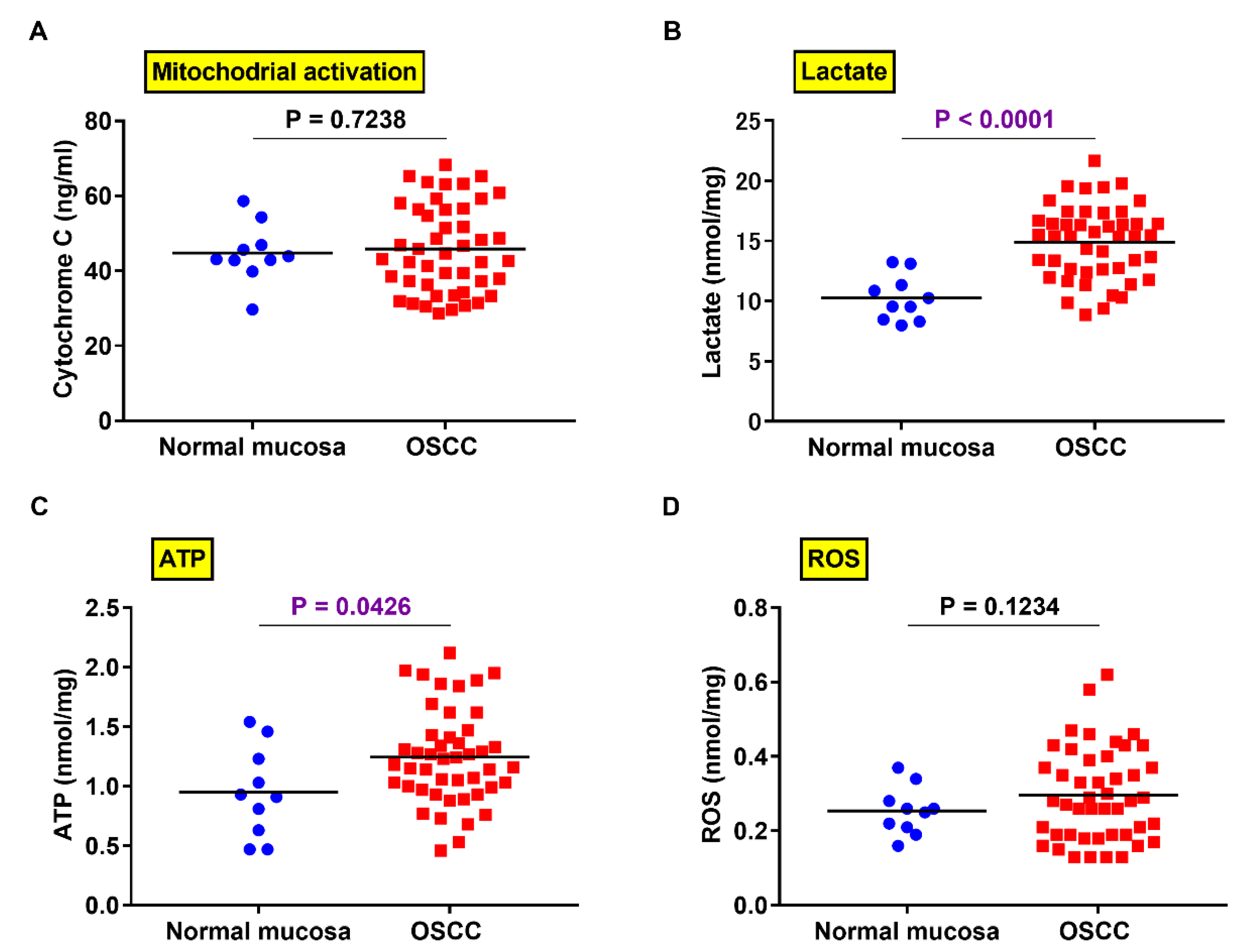
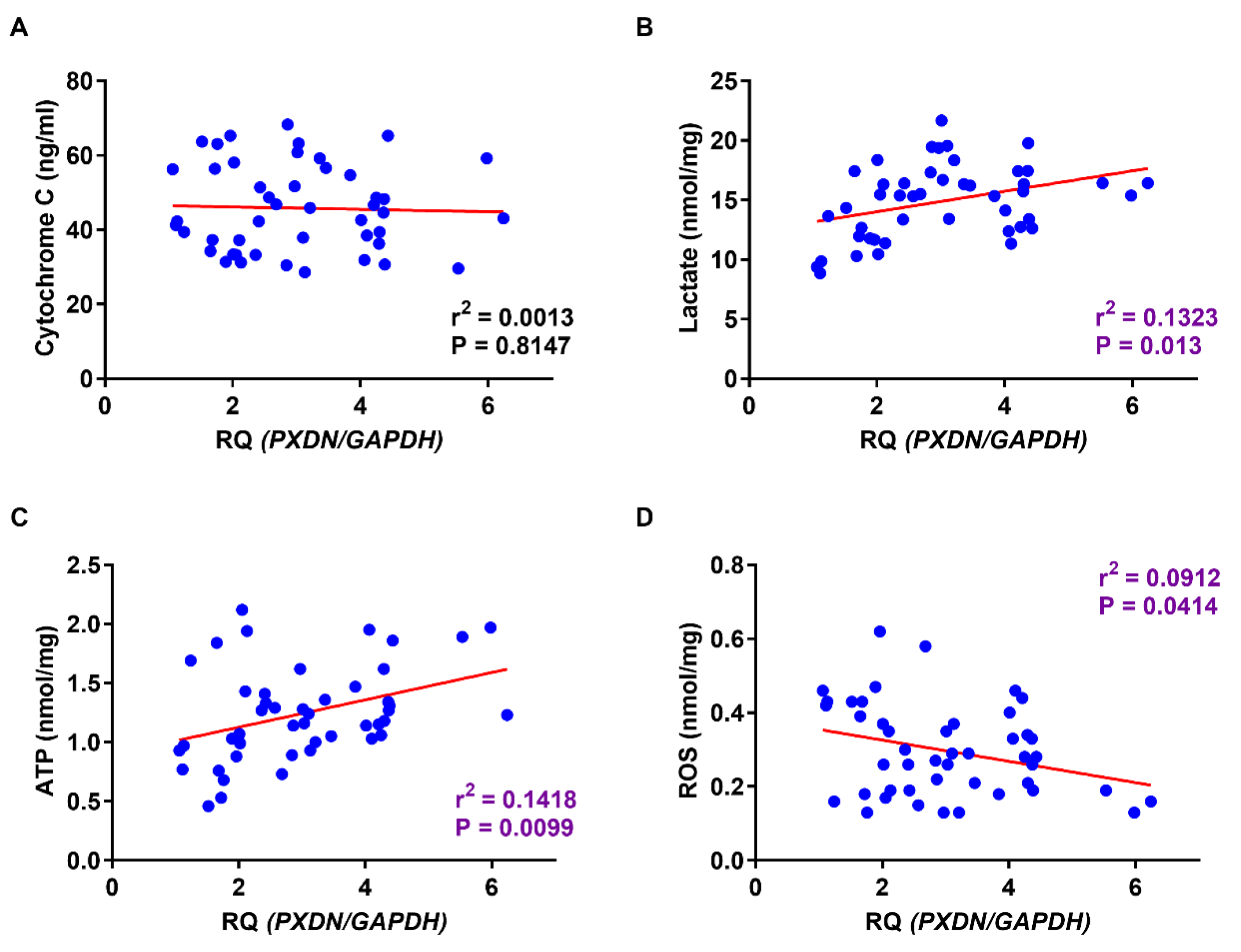
2.5. Comparison of PXDN Expression and Production of Lactate, ATP, and ROS in OSCC and Normal Mucosa
2.6. Relationship between PXDN Expression and Production of Lactate, ATP, and ROS in OSCC Specimens
3. Discussion
4. Materials and Methods
4.1. Surgical Specimens
4.2. Immunohistochemistry
4.3. Evaluation of Immunohistochemistry
4.4. Quantitative Gene Expression Analysis
4.5. ELISA and Measurement of ATP and ROS Production
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DFS | Disease-free survival |
| ELISA | Enzyme-linked immunosorbent assay |
| HO-1 | Heme oxigenase-1 |
| OSCC | Oral squamous cell carcinoma |
| PK | Pyruvate kinase |
| POI | Pattern of invasion |
| PXDN | Peroxidasin |
| ROS | Reactive oxygen species |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Shimomura, H.; Sasahira, T.; Nakashima, C.; Kurihara-Shimomura, M.; Kirita, T. Non-SMC condensin I complex subunit H (NCAPH) is associated with lymphangiogenesis and drug resistance in oral squamous cell carcinoma. J. Clin. Med. 2019, 9, 72. [Google Scholar] [CrossRef]
- Sasahira, T.; Kurihara, M.; Nishiguchi, Y.; Fujiwara, R.; Kirita, T.; Kuniyasu, H. NEDD 4 binding protein 2-like 1 promotes cancer cell invasion in oral squamous cell carcinoma. Virchows Arch. 2016, 469, 163–172. [Google Scholar] [CrossRef]
- Péterfi, Z.; Tóth, Z.E.; Kovács, H.A.; Lázár, E.; Sum, A.; Donkó, A.; Sirokmány, G.; Shah, A.M.; Geiszt, M. Peroxidasin-like protein: A novel peroxidase homologue in the human heart. Cardiovasc. Res. 2014, 101, 393–399. [Google Scholar] [CrossRef]
- Dougan, J.; Hawsawi, O.; Burton, L.J.; Edwards, G.; Jones, K.; Zou, J.; Nagappan, P.; Wang, G.; Zhang, Q.; Danaher, A.; et al. Proteomics-Metabolomics combined approach identifies peroxidasin as a protector against metabolic and oxidative stress in prostate cancer. Int. J. Mol. Sci. 2019, 20, 3046. [Google Scholar] [CrossRef]
- Liu, Y.; Carson-Walter, E.B.; Cooper, A.; Winans, B.N.; Johnson, M.D.; Walter, K.A. Vascular gene expression patterns are conserved in primary and metastatic brain tumors. J. Neuro-Oncol. 2010, 99, 13–24. [Google Scholar] [CrossRef]
- Jayachandran, A.; Prithviraj, P.; Lo, P.H.; Walkiewicz, M.; Anaka, M.; Woods, B.L.; Tan, B.; Behren, A.; Cebon, J.; McKeown, S.J. Identifying and targeting determinants of melanoma cellular invasion. Oncotarget 2016, 7, 41186–41202. [Google Scholar] [CrossRef]
- Cai, X.; Yang, X.; Jin, C.; Li, L.; Cui, Q.; Guo, Y.; Dong, Y.; Yang, X.; Guo, L.; Zhang, M. Identification and verification of differentially expressed microRNAs and their target genes for the diagnosis of esophageal cancer. Oncol. Lett. 2018, 16, 3642–3650. [Google Scholar] [CrossRef]
- Di, Y.; Chen, D.; Yu, W.; Yan, L. Bladder cancer stage-associated hub genes revealed by WGCNA co-expression network analysis. Hereditas 2019, 156, 7. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Liang, L. High expression of PXDN is associated with poor prognosis and promotes proliferation, invasion as well as migration in ovarian cancer. Ann. Diagn. Pathol. 2018, 34, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.; Jais, A.; Jeitler, M.; Haider, S.; Husa, J.; Lindroos, J.; Knöfler, M.; Mayerhofer, M.; Pehamberger, H.; Wagner, O.; et al. Transcriptome analysis of human cancer reveals a functional role of heme oxygenase-1 in tumor cell adhesion. Mol. Cancer 2010, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Lebelo, M.T.; Joubert, A.M.; Visagie, M.H. Warburg effect and its role in tumourigenesis. Arch. Pharm. Res. 2019, 42, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016, 17, 1721–1730. [Google Scholar] [CrossRef]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Kurihara-Shimomura, M.; Sasahira, T.; Nakashima, C.; Kuniyasu, H.; Shimomura, H.; Kirita, T. The multifarious functions of pyruvate kinase M2 in oral cancer cells. Int. J. Mol. Sci. 2018, 19, 2907. [Google Scholar] [CrossRef]
- Li, Y.; Bai, S.; Carroll, W.; Dayan, D.; Dort, J.C.; Heller, K.; Jour, G.; Lau, H.; Penner, C.; Prystowsky, M.; et al. Validation of the risk model: High-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013, 7, 211–223. [Google Scholar] [CrossRef]
- Heerema, M.G.; Melchers, L.J.; Roodenburg, J.L.; Schuuring, E.; de Bock, G.H.; van der Vegt, B. Reproducibility and prognostic value of pattern of invasion scoring in low-stage oral squamous cell carcinoma. Histopathology 2016, 68, 388–397. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bansal, V.; Malik, V.; Bhagat, R.; Punia, R.S.; Handa, U.; Gupta, A.; Dass, A. Tumor budding and worse pattern of invasion can predict nodal metastasis in oral cancers and associated with poor survival in early-stage tumors. Ear Nose Throat J. 2019, 98, E112–E119. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.; Ishibashi, K.; Katoh, H.; Kitamoto, S.; Shirai, T.; Tanaka, S.; Kajita, M.; Ishikawa, S.; Yamauchi, H.; Yako, Y.; et al. Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat. Cell Biol. 2017, 19, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim. Biophys. Acta 2010, 1797, 1225–1230. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Mahmoud, A.A.; El Sawy, S.A.; Abdelaal, E.A.; Fouad, A.M.; Yousif, R.S.; Hashim, M.S.; Hemdan, S.B.; Kadry, Z.M.; Abdelmoaty, M.A.; et al. Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med. Hypotheses 2013, 81, 866–870. [Google Scholar] [CrossRef]
- Mandai, M.; Amano, Y.; Yamaguchi, K.; Matsumura, N.; Baba, T.; Konishi, I. Ovarian clear cell carcinoma meets metabolism; HNF-1β confers survival benefits through the Warburg effect and ROS reduction. Oncotarget 2015, 6, 30704–30714. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007, 12, 230–238. [Google Scholar] [CrossRef]
- Harris, I.S.; Brugge, J.S. Cancer: The enemy of my enemy is my friend. Nature 2015, 527, 170–171. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Chandel, N.S.; Tuveson, D.A. The promise and perils of antioxidants for cancer patients. New Engl. J. Med. 2014, 371, 177–178. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Sasahira, T.; Kirita, T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 2413. [Google Scholar] [CrossRef]
- Barnett, P.; Arnold, R.S.; Mezencev, R.; Chung, L.W.; Zayzafoon, M.; Odero-Marah, V. Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells. Biochem. Biophys. Res. Commun. 2011, 404, 34–39. [Google Scholar] [CrossRef]
- Sitole, B.N.; Mavri-Damelin, D. Peroxidasin is regulated by the epithelial-mesenchymal transition master transcription factor Snai1. Gene 2018, 646, 195–202. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, J.L. MicroRNA and heme oxygenase-1 in allergic disease. Int. Immunopharmacol. 2020, 80, 106132. [Google Scholar] [CrossRef]

| Parameters | PXDN Expression | p Value * | ||
|---|---|---|---|---|
| Negative (%) | Positive (%) | |||
| Gender | Male | 40 (65.6) | 21 (34.4) | |
| Female | 29 (58) | 21 (42) | 0.4376 | |
| Age | <65 | 26 (65) | 14 (35) | |
| >65 | 43 (60.6) | 28 (39.4) | 0.6877 | |
| Site | Tongue | 29(59.2) | 20 (40.8) | |
| Other | 40 (64.5) | 22 (35.3) | 0.6938 | |
| Histological differentiation | Well | 34 (58.6) | 24 (41.4) | |
| Moderately, Poorly | 35 (66) | 18 (34) | 0.4405 | |
| T classification | T1–T2 | 40 (58.8) | 28 (41.2) | |
| T3–T4 | 29 (67.4) | 14 (32.6) | 0.4242 | |
| Clinical stage | I–II | 40 (58.8) | 28 (41.2) | |
| III–IV | 29 (67.4) | 14 (32.6) | 0.4242 | |
| Nodal metastasis | Negative | 53 (71.6) | 21 (28.4) | |
| Positive | 16 (43.2) | 21 (56.8) | 0.0065 | |
| POI | Nonaggressive pattern | 31 (75.6) | 10 (24.4) | |
| Diffuse invasion pattern | 38 (54.3) | 32 (45.7) | 0.0277 | |
| Parameters | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Gender | M | 1.00 | |||||
| F | 1.1734 | 0.5367–2.5634 | 0.6851 | ||||
| Age | <65 | 1.00 | |||||
| >65 | 1.1734 | 0.8296–3.9450 | 0.1341 | ||||
| Site | Tongue 29 (59.2) | 1.00 | |||||
| Other | 1.0041 | 0.4589–2.2311 | 0.9917 | ||||
| Histology | Well | 1.00 | |||||
| Mod, Por | 1.8282 | 0.8324–4.2970 | 0.1345 | ||||
| T factor | T1–T2 | 1.00 | |||||
| T3–T4 | 1.1916 | 0.5330–2.5817 | 0.6608 | ||||
| Clinical stage | I–II | 1.00 | |||||
| III–IV | 1.8837 | 0.8571–4.4289 | 0.1163 | ||||
| Nodal Metastasis | Negative | 1.00 | 1.00 | ||||
| Positive | 3.8307 | 1.7584–8.7592 | 0.0007 | 2.5907 | 1.0710–6.7677 | 0.0342 | |
| POI | Nonaggressive pattern | 1.00 | 1.00 | ||||
| Diffuse invasion pattern | 2.6793 | 1.0898–8.0350 | 0.030 | 1.4048 | 0.4892–4.6331 | 0.5393 | |
| PXDN expression | Negative | 1.00 | 1.00 | ||||
| Positive | 3.4853 | 1.5873–8.1899 | 0.0018 | 2.5971 | 1.1499–6.2578 | 0.0214 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara-Shimomura, M.; Sasahira, T.; Shimomura, H.; Kirita, T. Peroxidan Plays a Tumor-Promoting Role in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 5416. https://doi.org/10.3390/ijms21155416
Kurihara-Shimomura M, Sasahira T, Shimomura H, Kirita T. Peroxidan Plays a Tumor-Promoting Role in Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2020; 21(15):5416. https://doi.org/10.3390/ijms21155416
Chicago/Turabian StyleKurihara-Shimomura, Miyako, Tomonori Sasahira, Hiroyuki Shimomura, and Tadaaki Kirita. 2020. "Peroxidan Plays a Tumor-Promoting Role in Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 21, no. 15: 5416. https://doi.org/10.3390/ijms21155416
APA StyleKurihara-Shimomura, M., Sasahira, T., Shimomura, H., & Kirita, T. (2020). Peroxidan Plays a Tumor-Promoting Role in Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 21(15), 5416. https://doi.org/10.3390/ijms21155416





