Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States
Abstract
1. Introduction
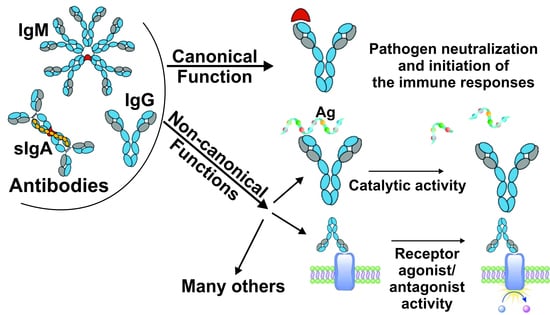
2. Canonical and Non-Canonical Functions of Immunoglobulins
3. Diversity of Abs Types: Antigen-Specific, Natural, Polyreactive, Broadly Neutralizing, Homophilic, Bispecific, Catalytic Abs
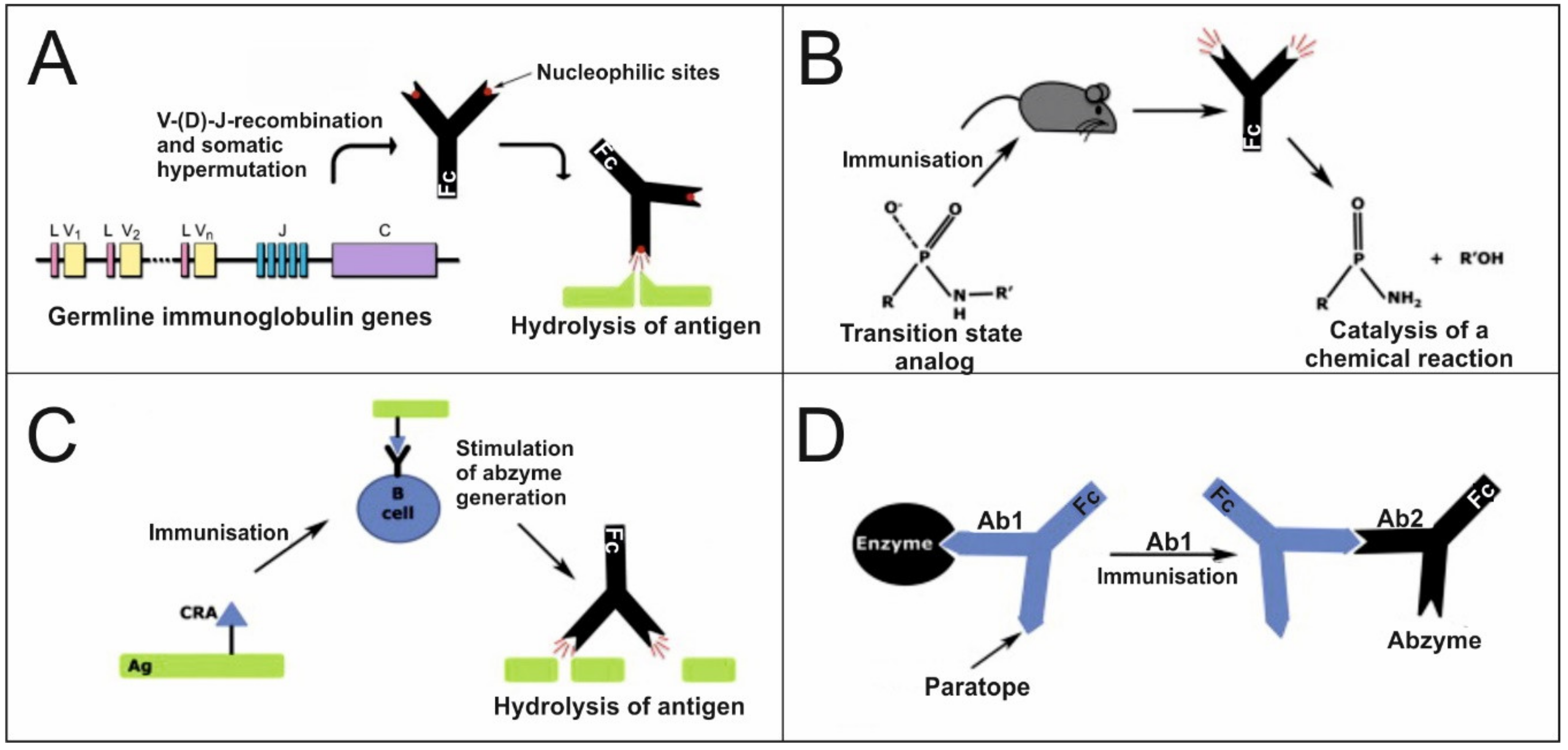
4. Catalytic Immunoglobulins
4.1. Origin and Generation of Catalytic Immunoglobulins
4.2. Catalytic Abs in Normal and Pathological Conditions
4.2.1. Autoimmune and Neurodegenerative Diseases
4.2.2. Inflammatory and Infectious Diseases
4.2.3. Cancer
4.2.4. Alloimmune Diseases
4.2.5. Metabolic Diseases
4.2.6. Psychiatric Disorders
4.2.7. Normal Physiological Conditions
5. The Biological Role of Catalytic Abs and Their Use in Medicine and Biotechnology
6. IVIg-Mediated Effector Functions Are Determined by Canonical and Non-Canonical Functions of Abs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab(s) | Antibody(ies) |
| AIDs | Autoimmune disease(s) |
| BamA | β-barrel assembly machine |
| Igs | Immunoglobulin(s) |
| IVIg | Intravenous immunoglobulins |
| MBP | Myelin basic protein |
| MS | Multiple sclerosis |
| sIgA | Secretory immunoglobulin A |
| SLE | Systemic lupus erythematosus |
| TIR | Toll-interleukin 1 receptor |
| TIRAP | TIR domain-containing adapter protein |
References
- Forthal, D.N. Functions of antibodies. In Antibodies for Infectious Diseases; Crowe, J.E., Jr., Boraschi, D., Rappuoli, R., Eds.; Wiley Online Library: Washington, DC, USA, 2015; pp. 23–48. [Google Scholar]
- Avrameas, S.; Alexopoulos, H.; Moutsopoulos, H.M. Natural Autoantibodies: An Undersugn Hero of the Immune System and Autoimmune Disorders—A Point of View. Front. Immunol. 2018, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.J.; Vanhoorelbeke, K.; Leypoldt, F.; Kaya, Z.; Bieber, K.; McLachlan, S.M.; Komorowski, L.; Luo, J.; Cabral-Marques, O.; Hammers, C.M.; et al. Mechanisms of autoantibody-induced pathology. Front. Immunol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Maddur, M.S.; Lacroix-Desmazes, S.; Dimitrov, J.D.; Kazatchkine, M.D.; Bayry, J.; Kaveri, S.V. Natural antibodies: From first-line defense against pathogens to perpetual immune homeostasis. Clin. Rev. Allergy Immunol. 2020, 58, 213–228. [Google Scholar] [CrossRef]
- Galeotti, C.; Kaveri, S.V.; Bayry, J. IVIG-mediated Effector Functions in Autoimmune and Inflammatory Diseases. Int. Immunol. 2017, 29, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Horns, F.; Vollmers, C.; Dekker, C.L.; Quake, S.R. Signatures of selection in the human antibody repertoire: Selective sweeps, competing subclones, and neutral drift. Proc. Natl. Acad. Sci. USA. 2019, 116, 1261–1266. [Google Scholar] [CrossRef]
- Miho, E.; Yermanos, A.; Weber, C.R.; Berger, C.T.; Reddy, S.T.; Greiff, V. Computational strategies for dissecting the high-dimensional complexity of adaptive immune repertoires. Front. Immunol. 2018, 9, 224. [Google Scholar] [CrossRef]
- Norman, R.A.; Ambrosetti, F.; Bonvin, A.M.; Colwell, L.J.; Kelm, S.; Kumar, S.; Krawczyk, K. Computational approaches to therapeutic antibody design: Established methods and emerging trends. Brief. Bioinform. 2019, bbz095. [Google Scholar] [CrossRef]
- Rees, A.R. Understanding the human antibody repertoire. MAbs. 2020, 12, 1729683. [Google Scholar] [CrossRef]
- Dimitrov, J.D.; Lacroix-Desmazes, S. Noncanonical Functions of Antibodies. Trends Immunol. 2020, 41, 379–393. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- Bournazos, S.; Wang, T.T.; Dahan, R.; Maamary, J.; Ravetch, J.V. Signaling by antibodies: Recent progress. Annu. Rev. Immunol. 2017, 35, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.S.; Baeten, D.L.P.; den Dunnen, J. The inflammatory function of human IgA. Cell. Mol. Life Sci. 2019, 76, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Michaud, E.; Mastrandrea, C.; Rochereau, N.; Paul, S. Human Secretory IgM: An Elusive Player in Mucosal Immunity. Trends Immunol. 2020, 41, 141–156. [Google Scholar] [CrossRef]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The neonatal Fc receptor (FcRn): A misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Oettgen, H.C. Fifty years later: Emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J. Allergy Clin. Immunol. 2016, 137, 1631–1645. [Google Scholar] [CrossRef]
- Luker, A.J.; Lownik, J.C.; Conrad, D.H.; Martin, R.K. A new look at IgE beyond allergies. F1000Res. 2019, 8. [Google Scholar] [CrossRef]
- Gutzeit, C.; Chen, K.; Cerutti, A. The enigmatic function of IgD: Some answers at last. Eur. J. Immunol. 2018, 48, 1101–1113. [Google Scholar] [CrossRef]
- Buneva, V.N.; Nevinsky, G.A. Exceptional Diversity of Catalytic Antibodies in the Blood of Patients with Autoimmune and Viral Diseases. Mol. Biol. (Mosk). 2017, 51, 969–984. [Google Scholar] [CrossRef]
- Fransen, F.; Zagato, E.; Mazzini, E.; Fosso, B.; Manzari, C.; El Aidy, S.; Chiavelli, A.; D’Erchia, A.M.; Sethi, M.K.; Pabst, O.; et al. BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity 2015, 43, 527–540. [Google Scholar] [CrossRef]
- Uchimura, Y.; Fuhrer, T.; Li, H.; Lawson, M.A.; Zimmermann, M.; Yilmaz, B.; Zindel, J.; Ronchi, F.; Sorribas, M.; Hapfelmeier, S.; et al. Antibodies set boundaries limiting microbial metabolite penetration and the resultant mammalian host response. Immunity 2018, 49, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Diard, M.; Sellin, M.E.; Felmy, B.; Wotzka, S.Y.; Toska, A.; Bakkeren, E.; Arnoldini, M.; Bansept, F.; Co, A.D.; et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 2017, 544, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Günther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Riemekasten, G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 648–656. [Google Scholar] [CrossRef]
- Meyer, S.; Woodward, M.; Hertel, C.; Vlaicu, P.; Haque, Y.; Kärner, J.; Macagno, A.; Onuoha, S.C.; Fishman, D.; Peterson, H.; et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell 2016, 166, 582–595. [Google Scholar] [CrossRef]
- Israel, L.; Wang, Y.; Bulek, K.; Della, M.E.; Zhang, Z.; Pedergnana, V.; Chrabieh, M.; Lemmens, N.A.; Sancho-Shimizu, V.; Descatoire, M.; et al. Human adaptive immunity rescues an inborn error of innate immunity. Cell 2017, 168, 789–800. [Google Scholar] [CrossRef]
- Kubagawa, H.; Honjo, K.; Ohkura, N.; Sakaguchi, S.; Radbruch, A.; Melchers, F.; Jani, P.K. Functional roles of the IgM Fc receptor in the immune system. Front. Immunol. 2019, 10, 945. [Google Scholar] [CrossRef]
- Sharp, T.H.; Boyle, A.L.; Diebolder, C.A.; Kros, A.; Koster, A.J.; Gros, P. Insights into IgM-mediated complement activation based on in situ structures of IgM-C1-C4b. Proc. Natl. Acad. Sci. USA. 2019, 116, 11900–11905. [Google Scholar] [CrossRef]
- Noviski, M.; Mueller, J.L.; Satterthwaite, A.; Garrett-Sinha, L.A.; Brombacher, F.; Zikherman, J. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. Elife 2018, 7, e35074. [Google Scholar] [CrossRef]
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; Van Kasteren, P.B. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef]
- Irvine, E.B.; Alter, G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 2020, 30, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Kanyavuz, A.; Marey-Jarossay, A.; Lacroix-Desmazes, S.; Dimitrov, J.D. Breaking the law: Unconventional strategies for antibody diversification. Nat. Rev. Immunol. 2019, 19, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Nakajima, A.; Vogelzang, A.; Maruya, M.; Miyajima, M.; Murata, M.; Son, A.; Kuwahara, T.; Tsuruyama, T.; Yamada, S.; et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 2018, 215, 2019–2034. [Google Scholar] [CrossRef]
- Storek, K.M.; Auerbach, M.R.; Shi, H.; Garcia, N.K.; Sun, D.; Nickerson, N.N.; Vij, R.; Lin, Z.; Chiang, N.; Schneider, K.; et al. Monoclonal antibody targeting the β-barrel assembly machine of Escherichia coli is bactericidal. Proc. Natl. Acad. Sci. USA 2018, 115, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Mayes, P.A.; Hance, K.W.; Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug Discov. 2018, 17, 509–527. [Google Scholar] [CrossRef]
- Tanigaki, K.; Sacharidou, A.; Peng, J.; Chambliss, K.L.; Yuhanna, I.S.; Ghosh, D.; Ahmed, M.; Szalai, A.J.; Vongpatanasin, W.; Mattrey, R.F.; et al. Hyposialylated IgG activates endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance. J. Clin. Invest. 2018, 128, 309–322. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D. B cell responses: Cell interaction dynamics and decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining natural antibodies. Front. Immunol. 2017, 8, 872. [Google Scholar] [CrossRef]
- Smith, F.L.; Baumgarth, N. B-1 cell responses to infections. Cur. Opin. Immunol. 2019, 57, 23–31. [Google Scholar] [CrossRef]
- Savage, H.P.; Kläsener, K.; Smith, F.L.; Luo, Z.; Reth, M.; Baumgarth, N. TLR induces reorganization of the IgM-BCR complex regulating murine B-1 cell responses to infections. Elife 2019, 8, e46997. [Google Scholar] [CrossRef] [PubMed]
- Kreuk, L.S.; Koch, M.A.; Slayden, L.C.; Lind, N.A.; Chu, S.; Savage, H.P.; Kantor, A.B.; Baumgarth, N.; Barton, G.M. B cell receptor and Toll-like receptor signaling coordinate to control distinct B-1 responses to both self and the microbiota. Elife 2019, 8, e47015. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Seagal, J.; Otipoby, K.L.; Lam, K.P.; Ayoub, S.; Zhang, B.; Sander, S.; Chu, V.T.; Rajewsky, K. BCR-dependent lineage plasticity in mature B cells. Science 2019, 363, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. The role of B-1 cells in inflammation. Immunol. Res. 2015, 63, 153–166. [Google Scholar] [CrossRef]
- Panda, S.; Ding, J.L. Natural antibodies bridge innate and adaptive immunity. J. Immunol. 2015, 194, 13–20. [Google Scholar] [CrossRef]
- Gunti, S.; Notkins, A.L. Polyreactive antibodies: Function and quantification. J. Infect. Dis. 2015, 212, S42–S46. [Google Scholar] [CrossRef]
- Liu, M.; Yang, G.; Wiehe, K.; Nicely, N.I.; Vandergrift, N.A.; Rountree, W.; Bonsignori, M.; Alam, S.M.; Gao, J.; Haynes, B.F.; et al. Polyreactivity and autoreactivity among HIV-1 antibodies. J. Virol. 2015, 89, 784–798. [Google Scholar] [CrossRef]
- Bunker, J.J.; Erickson, S.A.; Flynn, T.M.; Henry, C.; Koval, J.C.; Meisel, M.; Jabri, B.; Antonopoulos, D.A.; Wilson, P.C.; Bendelac, A. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 2017, 358, eaan6619. [Google Scholar] [CrossRef]
- Sok, D.; Burton, D.R. Recent progress in broadly neutralizing antibodies to HIV. Nat. Immunol. 2018, 19, 1179–1188. [Google Scholar] [CrossRef]
- Caskey, M.; Klein, F.; Nussenzweig, M.C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 2019, 25, 547–553. [Google Scholar] [CrossRef]
- Dimitrov, J.D. Harnessing the Therapeutic Potential of ‘Rogue’Antibodies. Trends Pharmacol. Sci. 2020, 4, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Imkeller, K.; Scally, S.W.; Bosch, A.; Martí, G.P.; Costa, G.P.; Triller, G.; Murugan, R.; Renna, V.; Jumaa, H.; Kremsner, P.G.; et al. Antihomotypic affinity maturation improves human B cell responses against a repetitive epitope. Science 2018, 360, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, S.E.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G.A. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Devel. Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, S.E.; Lekchnov, E.A.; Prince, V.V.; Buneva, V.N.; Nevinsky, G.A. Half molecular exchange of IgGs in the blood of healthy humans: Chimeric lambda-kappa-immunoglobulins containing HL fragments of antibodies of different subclasses (IgG1–IgG4). Mol. Biosyst. 2016, 12, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Lekchnov, E.A.; Sedykh, S.E.; Dmitrenok, P.S.; Buneva, V.N.; Nevinsky, G.A. Human placenta: Relative content of antibodies of different classes and subclasses (IgG1–IgG4) containing lambda-and kappa-light chains and chimeric lambda-kappa-immunoglobulins. Int. Immunol. 2015, 27, 297–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. Human milk IgGs contain various combinations of different antigen-binding sites resulting in multiple variants of their bispecificity. PLoS ONE 2012, 7, e42942. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. Human milk sIgA molecules contain various combinations of different antigen-binding sites resulting in a multiple binding specificity of antibodies and enzymatic activities of abzymes. PLoS ONE 2012, 7, e48756. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies – abzymes. In Catalytic Antibodies; Keinan, E., Ed.; WILEY-VCH Verlag GmbH&Co: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Mahendra, A.; Sharma, M.; Rao, D.N.; Peyron, I.; Planchais, C.; Dimitrov, J.D.; Kaveri, S.V.; Lacroix-Desmazes, S. Antibody-mediated catalysis: Induction and therapeutic relevance. Autoimmun. Rev. 2013, 12, 648–652. [Google Scholar] [CrossRef]
- Collins, A.M.; Jackson, K.J.L. On being the right size: Antibody repertoire formation in the mouse and human. Immunogenetics 2018, 70, 143–158. [Google Scholar] [CrossRef]
- Paul, S.; Nishiyama, Y.; Planque, S.; Taguchi, H. Theory of proteolytic antibody occurrence. Immunol. Lett. 2006, 103, 8–16. [Google Scholar] [CrossRef]
- Le Minoux, D.; Mahendra, A.; Kaveri, S.; Limnios, N.; Friboulet, A.; Avalle, B.; Boquet, D.; Lacroix-Desmazes, S.; Padiolleau-Lefèvre, S. A novel molecular analysis of genes encoding catalytic antibodies. Mol. Immunol. 2012, 50, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.J.; Jacobs, J.W.; Schultz, P.G. Selective chemical catalysis by an antibody. Science 1986, 234, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, A.; Janda, K.D.; Lerner, R.A. Catalytic antibodies. Science 1986, 234, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, P.; Ashley, J.A.; Lo, C.H.; Janda, K.D.; Lerner, R.A. Reactive immunization. Science 1995, 270, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Planque, S.; Zhou, Y.X.; Taguchi, H.; Bhatia, G.; Karle, S.; Hanson, C.; Nishiyama, Y. Specific HIV gp120-cleaving antibodies induced by a covalently reactive analog of gp120. J. Biol. Chem. 2003, 278, 20429–20435. [Google Scholar] [CrossRef]
- Taguchi, H.; Planque, S.; Sapparapu, G.; Boivin, S.; Hara, M.; Nishiyama, Y.; Paul, S. Exceptional amyloid β peptide hydrolyzing activity of nonphysiological immunoglobulin variable domain scaffolds. J. Biol. Chem. 2008, 283, 36724–36733. [Google Scholar] [CrossRef]
- Stanova, A.K.; Ryabkova, V.A.; Utekhin, S.V.; Shoenfeld, V.J.; Churilov, L.P.; Shoenfeld, Y. Anti-Idiotypic Agonistic Antibodies: Candidates for the Role of Universal Remedy. Antibodies (Basel). 2020, 9, 19. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. Investigations into the development of catalytic activity in anti-acetylcholinesterase idiotypic and anti-idiotypic antibodies. J. Mol. Recognit. 2009, 22, 188–196. [Google Scholar] [CrossRef]
- Lefevre, S.; Debat, H.; Thomas, D.; Friboulet, A.; Avalle, B. A suicide-substrate mechanism for hydrolysis of β-lactams by an anti-idiotypic catalytic antibody. FEBS Lett. 2001, 489, 25–28. [Google Scholar] [CrossRef]
- Ponomarenko, N.A.; Pillet, D.; Paon, M.; Vorobiev, II.; Smirnov, IV.; Adenier, H.; Avalle, B.; Kolesnikov, A.V.; Kozyr, A.V.; Thomas, D.; et al. Anti-idiotypic antibody mimics proteolytic function of parent antigen. Biochemistry 2007, 46, 14598–14609. [Google Scholar] [CrossRef]
- Krasnorutskii, M.A.; Buneva, V.N.; Nevinsky, G.A. Antibodies against DNA hydrolyze DNA and RNA. Biochem. (Mosk). 2008, 73, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Krasnorutskii, M.A.; Buneva, V.N.; Nevinsky, G.A. Antibodies against RNA hydrolyze RNA and DNA. J. Mol. Recognit. 2008, 21, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Durova, O.M.; Vorobiev, I.I.; Smirnov, I.V.; Reshetnyak, A.V.; Telegin, G.B.; Shamborant, O.G.; Orlova, N.A.; Genkin, D.D.; Bacon, A.; Ponomarenko, N.A.; et al. Strategies for induction of catalytic antibodies toward HIV-1 glycoprotein gp120 in autoimmune prone mice. Mol. Immunol. 2009, 47, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Doronin, V.B.; Parkhomenko, T.A.; Korablev, A.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaja, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; et al. Changes in different parameters, lymphocyte proliferation, and hematopoietic progenitor colony formation in EAE mice treated with myelin oligodendrocyte glycoprotein. J. Cell. Mol. Med. 2016, 20, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dickerson, T.J.; Rogers, C.J.; Kaufmann, G.F.; Mee, J.M.; McKenzie, K.M.; Janda, K.D.; Wilson, I.A. Complete reaction cycle of a cocaine catalytic antibody at atomic resolution. Structure 2006, 14, 205–216. [Google Scholar] [CrossRef][Green Version]
- Paul, S.; Li, L.; Kalaga, R.; Wilkins-Stevens, P.; Stevens, F.J.; Solomon, A. Natural catalytic antibodies: Peptide-hydrolyzing activities of Bence Jones proteins and VL fragment. J. Biol. Chem. 1995, 270, 15257–15261. [Google Scholar] [CrossRef]
- Lawrence, D.A.; McCabe, M.J., Jr. Immunomodulation by metals. Int. Immunopharmacol. 2002, 2, 293–302. [Google Scholar] [CrossRef]
- Fournel, S.; Muller, S. Anti-nucleosome antibodies and T-cell response in systemic lupus erythematosus. Ann. Med. Interne 2002, 153, 513–519. [Google Scholar]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in haematopoietic progenitor colony differentiation and proliferation and the production of different abzymes in EAE mice treated with DNA. J. Cell. Mol. Med. 2017, 21, 3795–3809. [Google Scholar] [CrossRef]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sedykh, S.E.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in cell differentiation and proliferation lead to production of abzymes in EAE mice treated with DNA–Histone complexes. J. Cell. Mol. Med. 2018, 22, 5816–5832. [Google Scholar] [CrossRef]
- Aulova, K.S.; Urusov, A.E.; Toporkova, L.B.; Sedykh, S.E.; Shevchenko, Y.A.; Tereshchenko., V.P.; Sennikov, S.V.; Budde, T.; Meuth, S.G.; Popova, N.A.; et al. Production of Abzymes in Th, CBA, and C57BL/6 Mice before and after MOG Treatment: Comparing Changes in Cell Differentiation and Proliferation. Biomolecules 2019, 28, 53. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.J.; Metzler, G.; Wray-Dutra, M.; Jackson, S.W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017, 17, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y. Evolution of catalytic antibody repertoire in autoimmune mice. J. Immunol. Methods. 2002, 269, 213–233. [Google Scholar] [CrossRef]
- Bowen, A.; Wear, M.; Casadevall, A. Antibody-mediated catalysis in infection and immunity. Infect. Immun. 2017, 85, 202–217. [Google Scholar] [CrossRef]
- Uda, T.; Emi, H. Super catalytic antibody and antigenase. J. Biosci. Bioeng. 2004, 97, 143–152. [Google Scholar] [CrossRef]
- Sapparapu, G.; Planque, S.; Mitsuda, Y.; McLean, G.; Nishiyama, Y.; Paul, S. Constant domain-regulated antibody catalysis. J. Biol. Chem. 2012, 287, 36096–36104. [Google Scholar] [CrossRef]
- Shuster, A.M.; Gololobov, G.V.; Kvashuk, O.A.; Bogomolova, A.E.; Smirnov, I.V.; Gabibov, A.G. DNA hydrolyzing autoantibodies. Science 1992, 256, 665–667. [Google Scholar] [CrossRef]
- Andrievskaya, O.A.; Buneva, V.N.; Baranovskii, A.G.; Gal’vita, A.V.; Benzo, E.S.; Naumov, V.A.; Nevinsky, G.A. Catalytic diversity of polyclonal RNA-hydrolyzing IgG antibodies from the sera of patients with systemic lupus erythematosus. Immunol. Lett. 2002, 81, 191–198. [Google Scholar] [CrossRef]
- Andrievskaya, O.A.; Buneva, V.N.; Naumov, V.A.; Nevinsky, G.A. Catalytic heterogenity of polyclonal RNA-hydrolyzing IgM from sera of patients with lupus erythematosus. Med. Sci. Monit. 2000, 6, 460–470. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural Catalytic Antibodies in Norm, Autoimmune, Viral, and bacterial Diseases. ScientificWorldJournal 2010, 10, 1203–1233. [Google Scholar] [CrossRef]
- Bezuglova, A.M.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. IgGs containing light chains of the λ-and κ-type and of all subclasses (IgG1–IgG4) from the sera of patients with systemic lupus erythematosus hydrolyze myelin basic protein. Int. Immunol. 2012, 24, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Magorivska, I.B.; Bilyy, R.O.; Havrylyuk, A.M.; Chop’yak, V.V.; Stoika, R.S.; Kit, Y.Y. Anti-histone H1 IgGs from blood serum of systemic lupus erythematosus patients are capable of hydrolyzing histone H1 and myelin basic protein. J. Mol. Recognit. 2010, 23, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple sites of the cleavage of 17-and 19-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic lupus erythematosus. Peptides 2012, 37, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kulminskaya, A.A.; Saveliev, A.N.; Neustroev, K.N. Human abzymes with amylolytic activity. Trends Glycosci. Glyc. 2004, 16, 17–31. [Google Scholar] [CrossRef][Green Version]
- Tolmacheva, A.S.; Buneva, V.N.; Nevinsky, G.A. Substrate specificity of IgGs with peroxidase and oxidoreductase activities from sera of patients with systemic lupus erythematosus and multiple sclerosis. J. Mol. Recognit. 2019, 32, e2807. [Google Scholar] [CrossRef]
- Baranovskii, A.G.; Ershova, N.A.; Buneva, V.N.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Doronin, B.M.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunol. Lett. 2001, 76, 163–167. [Google Scholar] [CrossRef]
- Parkhomenko, T.A.; Legostaeva, G.A.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. IgGs containing light chains of the λ and κ type and of all subclasses (IgG1-IgG4) from sera of patients with multiple sclerosis hydrolyze DNA. J. Mol. Recognit. 2010, 23, 486–494. [Google Scholar] [CrossRef]
- Parkhomenko, T.A.; Doronin, V.B.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of DNA-hydrolyzing antibodies from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e93001. [Google Scholar] [CrossRef]
- Ponomarenko, N.A.; Durova, O.M.; Vorobiev, I.I.; Belogurov, A.A., Jr.; Kurkova, I.N.; Petrenko, A.G.; Telegin, G.B.; Suchkov, S.V.; Kiselev, S.L.; Lagarkova, M.A.; et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc. Natl. Acad. Sci. USA. 2006, 103, 281–286. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J. Cell. Mol. Med. 2004, 8, 359–368. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Buneva, V.N.; Doronin, B.M.; Tyshkevich, O.B.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by IgM and IgA antibodies from the sera of patients with multiple sclerosis. Med. Sci. Monit. 2005, 11, 266–272. [Google Scholar]
- Saveliev, A.N.; Ivanen, D.R.; Kulminskaya, A.A.; Ershova, N.A.; Kanyshkova, T.G.; Buneva, V.N.; Mogelnitskii, A.S.; Doronin, B.M.; Favorova, O.O.; Nevinsky, G.A.; et al. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol. Lett. 2003, 86, 291–297. [Google Scholar] [CrossRef]
- Baranova, S.V.; Mikheeva, E.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the sera of multiple sclerosis patients efficiently hydrolyze five histones. Biomolecules 2019, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Kostrikina, I.A.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Extreme Diversity of IgGs Against Histones, DNA, and Myelin Basic Protein in the Cerebrospinal Fluid and Blood of Patients with Multiple Sclerosis. Biomolecules 2020, 10, 630. [Google Scholar] [CrossRef]
- Li, L.; Paul, S.; Tyutyulkova, S.; Kazatchkine, M.D.; Kaveri, S. Catalytic activity of anti-thyroglobulin antibodies. J. Immunol. 1995, 154, 3328–3332. [Google Scholar]
- Nevinsky, G.A.; Breusov, A.A.; Baranovskii, A.G.; Prints, A.V.; Kanyshkova, T.G.; Galvita, A.V.; Naumov, V.A.; Buneva, V.N. Effect of different drugs on the level of DNA-hydrolyzing polyclonal IgG antibodies in sera of patients with Hashimoto’s thyroiditis and nontoxic nodal goiter. Med. Sci. Monit. 2001, 7, 201–211. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 1–107. [Google Scholar]
- Kamalanathan, A.S.; Goulvestre, C.; Weill, B.; Vijayalakshmi, M.A. Proteolysis activity of IgM antibodies from rheumatoid arthritis patients’ sera: Evidence of atypical catalytic site. J. Mol. Recognit. 2010, 23, 577–582. [Google Scholar] [CrossRef]
- Wootla, B.; Dasgupta, S.; Dimitrov, J.D.; Bayry, J.; Lévesque, H.; Borg, J.Y.; Borel-Derlon, A.; Rao, D.N.; Friboulet, A.; Kaveri, S.V.; et al. Factor VIII hydrolysis mediated by anti-factor VIII autoantibodies in acquired hemophilia. J. Immunol 2008, 180, 7714–7720. [Google Scholar] [CrossRef]
- Wootla, B.; Christophe, O.D.; Mahendra, A.; Dimitrov, J.D.; Repessé, Y.; Ollivier, V.; Friboulet, A.; Borel-Derlon, A.; Levesque, H.; Borg, J.Y.; et al. Proteolytic antibodies activate factor IX in patients with acquired hemophilia. Blood 2011, 117, 2257–2264. [Google Scholar] [CrossRef]
- Kundzer, A.V.; Volkova, M.V.; Bogdanos, D.P.; Rödiger, S.; Schierack, P.; Generalov, I.; Nevinsky, G.A.; Roggenbuck, D. Deoxyribonuclease activity of polyclonal IgGs: A putative serological marker in patients with spondyloarthritides. Immunol. Res. 2013, 56, 457–464. [Google Scholar] [CrossRef]
- Suchkov, S.V.; Gabibov, A.G. Introduction to medical abzymology: State of the problem and prospects. Vestn. Ross. Akad. Med. Nauk 2005, 10, 44–53. [Google Scholar]
- Taguchi, H.; Planque, S.; Nishiyama, Y.; Symersky, J.; Boivin, S.; Szabo, P.; Friedland, R.P.; Ramsland, P.A.; Edmundson, A.B.; Weksler, M.E.; et al. Autoantibody-catalyzed hydrolysis of amyloid β peptide. J. Biol. Chem. 2008, 283, 4714–4722. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Volle, D.J.; Beach, C.M.; Johnson, D.R.; Powell, M.J.; Massey, R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Desmazes, S.; Bayry, J.; Kaveri, S.V.; Hayon-Sonsino, D.; Thorenoor, N.; Charpentier, J.; Luyt, C.E.; Mira, J.P.; Nagaraja, V.; Kazatchkine, M.D.; et al. High levels of catalytic antibodies correlate with favorable outcome in sepsis. Proc. Natl. Acad. Sci. USA 2005, 102, 4109–4113. [Google Scholar] [CrossRef] [PubMed]
- Barnay-Verdier, S.; Borde, C.; Fattoum, L.; Wootla, B.; Lacroix-Desmazes, S.; Kaveri, S.; Gibot, S.; Maréchal, V. Emergence of antibodies endowed with proteolytic activity against High-mobility group box 1 protein (HMGB1) in patients surviving septic shock. Cell. Immunol. 2020, 347, 104020. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskiĭ, A.G.; Siziakina, L.P.; Buneva, V.N.; Nevinskiĭ, G.A. DNA-hydrolyzing IgG antibodies from the blood of patients with acquired immune deficiency syndrome. Mol. Biol. (Mosk). 2006, 40, 857–864. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Sizyakina, L.P.; Buneva, V.N.; Nevinsky, G.A. Proteolytic activity of IgG antibodies from blood of acquired immunodeficiency syndrome patients. Biochem. (Mosk). 2006, 71, 251–261. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Zakharova, O.D.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing IgM antibodies from sera of HIV-infected patients. Int. Immunol. 2010, 22, 671–680. [Google Scholar] [CrossRef][Green Version]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Rasskazov, V.A.; Calmels, C.; Parissi, V.; Andreola, M.L.; Buneva, V.N.; Zakharova, O.D.; Nevinsky, G.A. Antibodies to HIV integrase catalyze site-specific degradation of their antigen. Int. Immunol. 2011, 23, 601–612. [Google Scholar] [CrossRef][Green Version]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing antibodies from sera of HIV-infected patients. Biochimie 2009, 91, 1081–1086. [Google Scholar] [CrossRef]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care-Pandemic Approaches in the 21st Century; Kasenga, F., Ed.; InTech: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H1 histone from the sera of HIV-infected patients recognize and catalyze site-specific degradation of this histone. J. Mol. Recognit. 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H2a and H2b histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. Mol. Biosyst. 2017, 13, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Dmitrenok, P.S.; Zubkova, A.D.; Ivanisenko, N.V.; Odintsova, E.S.; Buneva, V.N.; Nevinsky, G.A. Antibodies against H3 and H4 histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. J. Mol. Recognit. 2018, 31, e2703. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Dmitrienok, P.S.; Buneva, V.N.; Nevinsky, G.A. Autoantibodies in HIV-infected patients: Cross site-specific hydrolysis of H1 histone and myelin basic protein. Biofactors 2019, 45, 211–222. [Google Scholar] [CrossRef]
- Parkhomenko, T.A.; Buneva, V.N.; Tyshkevich, O.B.; Generalov, I.I.; Doronin, B.M.; Nevinsky, G.A. DNA-hydrolyzing activity of IgG antibodies from the sera of patients with tick-borne encephalitis. Biochimie 2010, 92, 545–554. [Google Scholar] [CrossRef]
- Parkhomenko, T.A.; Odintsova, E.S.; Buneva, V.N.; Kunder, E.V.; Zhyltsov, I.V.; Senkovich, S.A.; Generalov, I.I.; Nevinsky, G.A. DNA-hydrolysing activity of IgG antibodies from the sera of patients with diseases caused by different bacterial infections. J. Cell. Mol. Med. 2009, 13, 2875–2887. [Google Scholar] [CrossRef][Green Version]
- Hifumi, E.; Fujimoto, N.; Arakawa, M.; Saito, E.; Matsumoto, S.; Kobayashi, N.; Uda, T. Biochemical features of a catalytic antibody light chain, 22F6, prepared from human lymphocytes. J. Biol. Chem. 2013, 288, 19558–19568. [Google Scholar] [CrossRef]
- Bilyy, R.; Tomin, A.; Mahorivska, I.; Shalay, O.; Lohinskyy, V.; Stoika, R.; Kit, Y. Antibody-mediated sialidase activity in blood serum of patients with multiple myeloma. J. Mol. Recognit. 2011, 24, 576–584. [Google Scholar] [CrossRef]
- Sinohara, H.; Matsuura, K. Does catalytic activity of Bence-Jones proteins contribute to the pathogenesis of multiple myeloma? Appl. Biochem. Biotechnol. 2000, 83, 85–92. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Dannenbring, R.; Matsuura, K.; Tramontano, A.; Gololobov, G.; Paul, S. Monoclonal antibody light chain with prothrombinase activity. Biochemistry 2000, 39, 6459–6465. [Google Scholar] [CrossRef]
- Kozyr, A.V.; Kolesnikov, A.V.; Aleksandrova, E.S.; Sashchenko, L.P.; Gnuchev, N.V.; Favorov, P.V.; Kotelnikov, M.A.; Iakhnina, E.I.; Astsaturov, I.A.; Prokaeva, T.B.; et al. Novel functional activities of anti-DNA autoantibodies from sera of patients with lymphoproliferative and autoimmune diseases. Appl. Biochem. Biotechnol. 1998, 75, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Desmazes, S.; Bayry, J.; Misra, N.; Horn, M.P.; Villard, S.; Pashov, A.; Stieltjes, N.; d’Oiron, R.; Saint-Remy, J.M.; Hoebeke, J.; et al. The prevalence of proteolytic antibodies against factor VIII in hemophilia A. N. Engl. J. Med. 2002, 346, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Desmazes, S.; Wootla, B.; Dasgupta, S.; Delignat, S.; Bayry, J.; Reinbolt, J.; Hoebeke, J.; Saenko, E.; Kazatchkine, M.D.; Friboulet, A.; et al. Catalytic IgG from patients with hemophilia A inactivate therapeutic factor VIII. J. Immunol. 2006, 177, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Wootla, B.; Nicoletti, A.; Patey, N.; Dimitrov, J.D.; Legendre, C.; Christophe, O.D.; Friboulet, A.; Kaveri, S.V.; Lacroix-Desmazes, S.; Thaunat, O. Hydrolysis of coagulation factors by circulating IgG is associated with a reduced risk for chronic allograft nephropathy in renal transplanted patients. J. Immunol. 2008, 180, 8455–8460. [Google Scholar] [CrossRef]
- Galvita, A.V.; Baranovskii, A.G.; Kuznetsova, I.A.; Vinshu, N.V.; Galenok, V.A.; Buneva, V.N.; Nevinsky, G.A. Features of DNA hydrolysis by antibodies from the blood of patients with diabetes mellitus. Rus. Immunol. J. 2007, 1, 116–131. [Google Scholar]
- Pagetta, A.; Tramentozzi, E.; Corbetti, L.; Frasson, M.; Brunati, A.M.; Finotti, P. Characterization of immune complexes of idiotypic catalytic and anti-idiotypic inhibitory antibodies in plasma of type 1 diabetic subjects. Mol. Immunol. 2007, 44, 2870–2883. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Cuchacovich, M.; Francos, R.; Cuchacovich, S.; Blanco, A.; Sandoval, R.; Gomez, C.F.; Valenzuela, J.A.; Ray, R.; Pizzo, S.V. Catalytic autoantibodies against myelin basic protein (MBP) isolated from serum of autistic children impair in vitro models of synaptic plasticity in rat hippocampus. J. Neuroimmunol. 2015, 287, 1–8. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Smirnova, L.P.; Parkhomenko, T.A.; Dmitrenok, P.S.; Krotenko, N.M.; Fattakhov, N.S.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; et al. DNA-hydrolysing activity of IgG antibodies from the sera of patients with schizophrenia. Open Biol. 2015, 5, 150064. [Google Scholar] [CrossRef]
- Buneva, V.N.; Ermakov, E.A.; Nevinsky, G.A. Immune System Dysregulation and Autoimmunity in Schizophrenia: IgGs from Sera of Patients with Several Catalytic Activities. In Psychotic Disorders–An Update; InTechOpen: London, UK, 2018; pp. 41–101. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Hydrolysi s by catalytic IgGs of microRNA specific for patients with schizophrenia. IUBMB Life 2018, 70, 153–164. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Blood-Derived RNA- and microRNA-Hydrolyzing IgG Antibodies in Schizophrenia Patients. Biochem. (Mosc). 2018, 83, 507–526. [Google Scholar] [CrossRef]
- Parshukova, D.; Smirnova, L.P.; Ermakov, E.A.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Autoimmunity and immune system dysregulation in schizophrenia: IgGs from sera of patients hydrolyze myelin basic protein. J. Mol. Recognit. 2019, 32, e2759. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Smirnova, L.P.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Catalase activity of IgG antibodies from the sera of healthy donors and patients with schizophrenia. PLoS ONE. 2017, 12, e0183867. [Google Scholar] [CrossRef] [PubMed]
- Nevinsky, G.A.; Kanyshkova, T.G.; Semenov, D.V.; Vlassov, A.V.; Gal’vita, A.V.; Buneva, V.N. Secretory immunoglobulin a from health y human mothers’ milk catalyzes nucleic acid hydrolysis. Appl. Biochem. Biotechnol. 2000, 83, 115–130. [Google Scholar] [CrossRef]
- Savel’ev, A.N.; Kanyshkova, T.G.; Kulminskaya, A.A.; Buneva, V.N.; Eneyskaya, E.V.; Filatov, M.V.; Nevinsky, G.A.; Neustroev, K.N. Amylolytic activity of IgG and sIgA immunoglobulins from human milk. Clin. Chim. Acta. 2001, 314, 141–152. [Google Scholar] [CrossRef]
- Semenov, D.V.; Kanyshkova, T.G.; Karotaeva, N.A.; Krasnorutskii, M.A.; Kuznetsova, I.A.; Buneva, V.N.; Nevinsky, G.A. Catalytic nucleotide-hydrolyzing antibodies in milk and serum of clinically healthy human mothers. Med. Sci. Monit. 2004, 10, 23–33. [Google Scholar]
- Buneva, V.N.; Kudryavtseva, A.N.; Galvita, A.V.; Dubrovskaya, V.V.; Khokhlova, O.V.; Kalinina, I.A.; Galenok, V.A.; Nevinsky, G.A. Dynamics of the level of nuclease activity of a woman’s blood antibodies during pregnancy and lactation. Biochem. (Mosc). 2003, 68, 1088–1100. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Buneva, V.N.; Nevinsky, G.A. Casein-hydrolyzing activity of sIgA antibodies from human milk. J. Mol. Recognit. 2005, 18, 413–421. [Google Scholar] [CrossRef]
- Barrera, G.J.; Portillo, R.; Mijares, A.; Rocafull, M.A.; del Castillo, J.R.; Thomas, L.E. Immunoglobulin A with protease activity secreted in human milk activates PAR-2 receptors, of intestinal epithelial cells HT-29, and promotes beta-defensin-2 expression. Immunol. Lett. 2009, 123, 52–59. [Google Scholar] [CrossRef]
- Kit, Y.Ya.; Semenov, D.V.; Nevinsky, G.A. Phosphorylation of Different Human Milk Proteins by Human Catalytic Secretory Immunoglobulin A. Biochem. Mol. Biol. Int. 1996, 39, 521–527. [Google Scholar] [CrossRef]
- Gorbunov, D.V.; Karataeva, N.A.; Buneva, V.N.; Nevinsky, G.A. Lipid kinase activity of antibodies from milk of clinically healthy human mothers. Biochim. Biophys. Acta. 2005, 1735, 153–166. [Google Scholar] [CrossRef]
- Karataeva, N.A.; Gorbunov, D.; Prokudin, I.V.; Buneva, V.N.; Kulminskaya, A.A.; Neustroev, K.N.; Nevinsky, G.A. Human milk antibodies with polysaccharide kinase activity. Immunol. Lett. 2006, 103, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kompaneets, I.Yu.; Ermakov, E.A.; Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. sIgGs from human milk hydrolyze microRNAs. Molecules 2020, 25, 2366. [Google Scholar] [CrossRef] [PubMed]
- Kompaneets, I.Yu.; Ermakov, E.A.; Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. Secretory immunoglobulin A from human milk hydrolyzes microRNA. J. Dairy Sci. 2020, 103, 6782–6797. [Google Scholar] [CrossRef]
- Mitsuda, Y.; Planque, S.; Hara, M.; Kyle, R.; Taguchi, H.; Nishiyama, Y.; Paul, S. Naturally occurring catalytic antibodies: Evidence for preferred development of the catalytic function in IgA class antibodies. Mol. Biotechnol. 2007, 36, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Karle, S.; Planque, S.; Taguchi, H.; Salas, M.; Nishiyama, Y.; Handy, B.; Hunter, R.; Edmundson, A.; Hanson, C. Naturally Occurring Proteolytic Antibodies: Selective immunoglobulin M-catalyzed hydrolysis of HIV gp120. J. Biol. Chem. 2004, 279, 39611–39619. [Google Scholar] [CrossRef] [PubMed]
- Planque, S.; Mitsuda, Y.; Taguchi, H.; Salas, M.; Morris, M.K.; Nishiyama, Y.; Kyle, R.; Okhuysen, P.; Escobar, M.; Hunter, R.; et al. Characterization of gp120 hydrolysis by IgA antibodies from humans without HIV infection. AIDS Res. Hum. Retroviruses 2007, 23, 1541–1554. [Google Scholar] [CrossRef]
- Planque, S.; Nishiyama, Y.; Taguchi, H.; Salas, M.; Hanson, C.; Paul, S. Catalytic antibodies to HIV: Physiological role and potential clinical utility. Autoimmun. Rev. 2008, 7, 473–479. [Google Scholar] [CrossRef]
- Planque, S.A.; Nishiyama, Y.; Hara, M.; Sonoda, S.; Murphy, S.K.; Watanabe, K.; Mitsuda, Y.; Brown, E.L.; Massey, R.J.; Primmer, S.R.; et al. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. J. Biol. Chem. 2014, 289, 13243–13258. [Google Scholar] [CrossRef]
- Brown, E.L.; Nishiyama, Y.; Dunkle, J.W.; Aggarwal, S.; Planque, S.; Watanabe, K.; Csencsits-Smith, K.; Bowden, M.G.; Kaplan, S.L.; Paul, S. Constitutive production of catalytic antibodies to a Staphylococcus aureus virulence factor and effect of infection. J. Biol. Chem. 2012, 287, 9940–9951. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Blinova, E.A.; Ermakov, E.A.; Buneva, V.N.; Vasilenko, N.L.; Nevinsky, G.A. IgG abzymes with peroxidase and oxidoreductase activities from the sera of healthy humans. J. Mol. Recognit. 2015, 28, 565–580. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Ermakov, E.A.; Buneva, V.N.; Nevinsky, G.A. Substrate specificity of healthy human sera IgG antibodies with peroxidase and oxydoreductase activities. R. Soc. Open Sci. 2018, 5, 171097. [Google Scholar] [CrossRef] [PubMed]
- Planque, S.; Bangale, Y.; Song, X.T.; Karle, S.; Taguchi, H.; Poindexter, B.; Bick, R.; Edmundson, A.; Nishiyama, Y.; Paul, S. Ontogeny of Proteolytic Immunity IgM serine proteases. J. Biol. Chem. 2004, 279, 14024–14032. [Google Scholar] [CrossRef] [PubMed]
- Andryushkova, A.A.; Kuznetsova, I.A.; Buneva, V.N.; Toporkova, L.B.; Sakhno, L.V.; Tikhonova, M.A.; Chernykh, E.R.; Orlovskaya, I.A.; Nevinsky, G.A. Formation of different abzymes in autoimmune-prone MRL-lpr/lpr mice is associated with changes in colony formation of haematopoietic progenitors. J. Cell. Mol. Med. 2007, 11, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry—Novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Zank, M.; Klein, R.; Stransky, E.; Batra, A.; Buchkremer, G.; Schott, K. Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res. 2008, 158, 83–86. [Google Scholar] [CrossRef]
- Sugisawa, E.; Takayama, Y.; Takemura, N.; Kondo, T.; Hatakeyama, S.; Kumagai, Y.; Sunagawa, M.; Tominaga, M.; Maruyama, K. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell 2020. In Press. [Google Scholar] [CrossRef]
- Kozyr, A.V.; Sashchenko, L.P.; Kolesnikov, A.V.; Zelenova, N.A.; Khaidukov, S.V.; Ignatova, A.N.; Bobik, T.V.; Gabibov, A.G.; Alekberova, Z.S.; Suchkov, S.V.; et al. Anti-DNA autoantibodies reveal toxicity to tumor cell lines. Immunol. Lett. 2002, 80, 41–47. [Google Scholar] [CrossRef]
- Lee, E.J.; Jang, E.J.; Lee, E.; Yu, J.; Chung, H.Y.; Jang, Y.J. Cell-penetrating autoantibody induces caspase-mediated apoptosis through catalytic hydrolysis of DNA. Bioorg. Med. Chem. 2007, 15, 2016–2023. [Google Scholar] [CrossRef]
- Jang, J.Y.; Jeong, J.G.; Jun, H.R.; Lee, S.C.; Kim, J.S.; Kim, Y.S.; Kwon, M.H. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell. Mol. Life Sci. 2009, 66, 1985–1997. [Google Scholar] [CrossRef]
- Lee, G.; Yu, J.; Cho, S.; Byun, S.J.; Kim, D.H.; Lee, T.K.; Kwon, M.H.; Lee, S. A nucleic-acid hydrolyzing single chain antibody confers resistance to DNA virus infection in hela cells and C57BL/6 mice. PLoS Pathog. 2014, 10, e1004208. [Google Scholar] [CrossRef]
- Weisbart, R.H.; Gera, J.F.; Chan, G.; Hansen, J.E.; Li., E.; Cloninger, C.; Levine, A.J.; Nishimura, R.N. A cell-penetrating bispecific antibody for therapeutic regulation of intracellular targets. Mol. Cancer Ther. 2012, 11, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Belogurov, A., Jr.; Kozyr, A.; Ponomarenko, N.; Gabibov, A. Catalytic antibodies: Balancing between Dr. Jekyll and Mr. Hyde. Bioessays 2009, 31, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to watch in 2020. MAbs 2020, 12, 1703531. [Google Scholar] [CrossRef] [PubMed]
- Sigounas, G.; Harindranath, N.; Donadel, G.; Notkins, A.L. Half-life of polyreactive antibodies. J. Clin. Immunol. 1994, 14, 134–140. [Google Scholar] [CrossRef]
- Padiolleau-Lefèvre, S.; Naya, R.B.; Shahsavarian, M.A.; Friboulet, A.; Avalle, B. Catalytic antibodies and their applications in biotechnology: State of the art. Biotechnol Lett. 2014, 36, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Planque, S.A.; Massey, R.J.; Paul, S. Catalytic antibody (catabody) platform for age-associated amyloid disease: From Heisenberg’s uncertainty principle to the verge of medical interventions. Mech. Ageing Dev. 2020, 185, 111188. [Google Scholar] [CrossRef]
- Hanson, C.V.; Nishiyama, Y.; Paul, S. Catalytic antibodies and their applications. Curr. Opin. Biotechnol. 2005, 16, 631–636. [Google Scholar] [CrossRef]
- Smirnov, I.; Carletti, E.; Kurkova, I.; Nachon, F.; Nicolet, Y.; Mitkevich, V.A.; Débat, H.; Avalle, B.; Belogurov, A.A.Jr.; Kuznetsov, N. Reactibodies generated by kinetic selection couple chemical reactivity with favorable protein dynamics. Proc. Natl. Acad. Sci. USA 2011, 108, 15954–15959. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Golovin, A.V.; Chatziefthimiou, S.D.; Stepanova, A.V.; Peng, Y.; Zolotareva, O.I.; Belogurov, A.A.Jr.; Kurkova, I.N.; Ponomarenko, N.A.; Wilmanns, M. Robotic QM/MM-driven maturation of antibody combining sites. Sci. Adv. 2016, 2, e1501695. [Google Scholar] [CrossRef]
- Bowen, A.; Wear, M.P.; Cordero, R.; Oscarson, S.; Casadevall, A. (2016). A monoclonal antibody to Cryptococcus neoformans glucuronoxylomannan manifests hydrolytic activity for both peptides and polysaccharides. J. Biol. Chem. 2017, 292, 417–434. [Google Scholar] [CrossRef]
- Fukuchi, K.I.; Yang, J.; Kou, J.; Song, M.; Lalonde, R.; Planque, S.A.; Paul, S. Prophylactic and Therapeutic Applications of Catalytic Immunoglobulin Gene Delivery in a Mouse Model of Alzheimer’s Disease. In Gene Therapy in Neurological Disorders; Li, M., Snider, B., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 139–161. [Google Scholar] [CrossRef]
- Lee, W.R.; Jang, J.Y.; Kim, J.S.; Kwon, M.H.; Kim, Y.S. Gene silencing by cell-penetrating, sequence-selective and nucleic-acid hydrolyzing antibodies. Nucleic Acids Res. 2010, 38, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Rader, C. Chemically programmed antibodies. Trends Biotechnol. 2014, 32, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Chaigne, B.; Mouthon, L. Mechanisms of action of intravenous immunoglobulin. Transfus. Apher. Sci. 2017, 56, 45–49. [Google Scholar] [CrossRef]
- Barahona Afonso, A.F.; João, C.M.P. The production processes and biological effects of intravenous immunoglobulin. Biomolecules 2016, 6, 15. [Google Scholar] [CrossRef]
- Tenti, S.; Fabbroni, M.; Mancini, V.; Russo, F.; Galeazzi, M.; Fioravanti, A. Intravenous Immunoglobulins as a new opportunity to treat discoid lupus erythematosus: A case report and review of the literature. Autoimmun. Rev. 2018, 17, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Forbat, E.; Ali, F.R.; Al-Niaimi, F. Intravenous immunoglobulins in dermatology. Part 1: Biological mechanisms and methods of administration. Clin. Exp. Dermatol. 2018, 43, 513–517. [Google Scholar] [CrossRef]
- Graeter, S.; Simon, H.U.; von Gunten, S. Granulocyte death mediated by specific antibodies in intravenous immunoglobulin (IVIG). Pharmacol. Res. 2020, 154, 104168. [Google Scholar] [CrossRef]
| Ig Classes | Canonical Functions of Abs 1 | |
|---|---|---|
| Fab-Dependent | Fc- and Whole Ab-Dependent | |
| IgG-mediated |
|
|
| IgM-mediated |
|
|
| IgA-mediated |
|
|
| IgE-mediated |
|
|
| IgD-mediated |
|
|
| Non-Canonical Functions of Abs 1 | ||
| Ig Classes | Fab-Dependent | Fc- and Whole Ab-Dependent |
| IgG-mediated |
|
|
| IgA-mediated |
|
|
| IgM-mediated |
|
|
| Abs Type | Origin | Affinity | Specificity | Biological Roles |
|---|---|---|---|---|
| Antigen-specific adaptive Abs | B2 cells | High | High |
|
| Natural Abs | B1 cells and marginal zone B cells | Low | Low |
|
| Polyreactive Abs | B1 cells | Low | Moderate | The same functions as natural Abs |
| Broadly neutralizing Abs | B1 cells | Low | Moderate | The same functions as natural Abs |
| Homophilic Abs | B2 cells | High | High | The same functions as antigen-specific Abs |
| Bispecific Abs | B2 cells | High | High | The same functions as antigen-specific Abs |
| Catalytic Abs | Unknown, presumably B1 cells | Low | Moderate |
|
| Disease/Condition | Catalytic Activity of Abs | Substrate | References |
|---|---|---|---|
| Autoimmune and Neurodegenerative Diseases | |||
| Systemic lupus erythematosus | DNA-hydrolyzing | DNA plasmid pUC19 *, d(pA)10 *, d(pA)13 | [89,90,91,92,93,94,95,96,97] |
| RNA-hydrolyzing | p(A)13, p(U)10, poly(А), poly(C), poly(U), сСМР, yeast RNA | ||
| Proteolytic | MBP **, OP17-MBP, OP19-MBP, histone H1** | ||
| Amololytic | Different maltooligosaccharides | ||
| Peroxidase and oxydoreductase | DAB, ATBS, OPD, pHQ and others in the presence and absence of hydrogen peroxide | ||
| Multiple sclerosis(IgG, IgA and IgM from blood serum and CSF) | DNA-hydrolyzing | HeteroODN15, d(pT)10 | [96,97,98,99,100,101,102,103,104,105,106] |
| RNA-hydrolyzing | Poly(А), poly(C), poly(U), сСМР | ||
| Proteolytic | MBP, OP85-101-MBP, H-Pro-Phe-Arg-MCA, histones H1, H2а, H2b, H3, H4 | ||
| Amilolytic | Different maltooligosaccharides | ||
| Peroxidase and oxydoreductase | DAB, ATBS, OPD, pHQ and others in the presence and absence of hydrogen peroxide | ||
| Hashimoto’s thyroiditis | Proteolytic | Pro-Phe-Arg-MCA, thyroglobulin | [107,108,109] |
| DNA-hydrolyzing | DNA plasmid pBR322 | ||
| RNA-hydrolyzing | poly(А), poly(C), poly(U), сСМР, yeast RNA, тРНКPhe, тРНКLys | ||
| Rheumatoid arthritis | Proteolytic | Pro-Phe-Arg-MCA and other MCA-labeled peptides | [110] |
| Systemic scleroderma | DNA-hydrolyzing | DNA plasmid pUC19 | [89] |
| Acquired hemophilia | Proteolytic | Factor-VIII, factor-IX | [111,112] |
| Spondyloarthropathy, polyarthritis | DNA-hydrolyzing | Calf thymus DNA, DNA plasmid pBR322 | [109,113] |
| RNA-hydrolyzing | Poly(А), poly(C), poly(U), сСМР, yeast RNA, тРНКPhe, тРНКLys | ||
| Autoimmune myocarditis | Proteolytic | Cardiomyosin | [114] |
| DNA-hydrolyzing | Plasmid DNA | ||
| Alzheimer’s disease | Proteolytic | β-amyloid, Glu-Ala-Arg- MCA | [115] |
| Inflammatory and Infectious Diseases | |||
| Bronchial asthma | Proteolytic | VIP | [116] |
| Sepsis | Proteolytic | Factor-VIII, factor-IX, Pro-Phe-Arg-MCA, HMGB1 protein | [117,118] |
| HIV-infection (IgG and IgM) | DNA-hydrolyzing | DNA plasmid pBluescript | [119,120,121,122,123,124,125,126,127,128] |
| Proteolytic | β-casein, reverse transcriptase and integrase, HIV **, histones ** H1, H2а, H2b, H3, H4, different peptides | ||
| Hepatitis А, В, С, D | DNA-hydrolyzing | DNA plasmid pBR322 | [92] |
| RNA-hydrolyzing | cCMP, poly(U), poly(A), poly(C), тРНКPhe | ||
| Tick-borne encephalitis | DNA-hydrolyzing | DNA plasmid pBluescript | [129] |
| Streptococcal infection | DNA-hydrolyzing | DNA plasmid pBluescript | [130] |
| Urogenital Chlamydia Associated with Arthritis | DNA-hydrolyzing | DNA plasmid pBluescript | [130] |
| Meningococcal meningitis | DNA-hydrolyzing | DNA plasmid pBluescript | [130] |
| Shigellosis | DNA-hydrolyzing | DNA plasmid pBluescript | [130] |
| Purulent surgical infections caused by Staphylococcus aureus and Staphylococcus epidermidis | DNA-hydrolyzing | DNA plasmid pBluescript | [130] |
| Genitourinary ureaplasmosis associated with reactive arthritis | DNA-hydrolyzing | DNA plasmid pBluescript | [130] |
| Influenza (light chains) | Nuclease | DNA plasmid pBR322, genome RNA from Noda virus | [131] |
| Proteolytic | Peptide-AMC | ||
| Cancer | |||
| Multiple myeloma (light chains) | Sialidase | 2′- (4-methylumbelliferyl) -α-d-N-acetylneuraminic acid | [132,133,134,135] |
| Proteolytic | BApNA, prothrombin | ||
| DNA-hydrolyzing | DNA plasmid pUC19 | ||
| Chronic lymphocytic leukemia | DNA-hydrolyzing | DNA plasmid pUC19 | [135] |
| Mantle cell lymphoma | DNA-hydrolyzing | DNA plasmid pUC19 | [135] |
| Marginal area lymphoma | DNA-hydrolyzing | DNA plasmid pUC19 | [135] |
| Follicular lymphoma | DNA-hydrolyzing | DNA plasmid pUC19 | [135] |
| Waldenstrom macroglobulinemia | Proteolytic | β-amyloid | [135] |
| Alloimmune Diseases | |||
| Hemophilia A (after replacement therapy) | Proteolytic | Factor-VIII | [136,137] |
| Transplant rejection reaction | Proteolytic | Pro-Phe-Arg-MCA, factor-VIII, factor-IX | [138] |
| Metabolic Diseases | |||
| Diabetes | DNA-hydrolyzing | DNA plasmid pBluescript | [139,140] |
| Proteolytic | BApNA, β-casein | ||
| Psychiatric Disorders | |||
| Autism (IgA, IgG, and IgM) | Proteolytic | MBP**, D-Ile-Pro-Arg-pNA, D-Leu-pNA, other | [141] |
| Schizophrenia | DNA-hydrolyzing | DNA plasmid pBluescript | [142,143,144,145,146,147] |
| RNA-hydrolyzing | cСMP, poly(С), poly(А), yeast RNA, microRNA: miR-137, miR-9-5p, miR-219-2-3p, miR-219a-5p | ||
| Proteolytic | MBP, different peptides | ||
| Catalase-like | Hydrogen peroxide | ||
| Normal Physiological Conditions | |||
| Pregnancy and feeding a newborn (sIgA and IgG from milk and blood serum) | DNA-hydrolyzing | DNA plasmid pBR322, Phage λ DNA, тРНКLys, d(pA)10, d(pT)10, d(pC)10 | [59,92,148,149,150,151,152,153,154,155,156,157,158] |
| RNA-hydrolyzing | r(A)10, r(T)10, r(C)10, microRNA: miR-137, miR-219a-5p, miR-219-2-3p, and miR-9-5p | ||
| Amilolytic | Different maltooligosaccharides | ||
| Nucleotide hydrolyzing | ATP, GTP, CTP, dATP, dGTP, dCTP, AMP, etc. | ||
| Proteolytic | β-casein, BSA, activated protease receptor 2 *, BApNA | ||
| Proteinkinase | β-casein in the presence of γ-[32Р]NTP or γ-[32Р]dNTP | ||
| Lipid kinase | lipids in the presence of γ-[32Р]ATP and γ-[32Р]Pi | ||
| Oligo- and polysaccharide kinase | Oligo- and polysaccharides in the presence of γ-[32Р]ATP and γ-[32Р]Pi | ||
| Healthy condition (IgA, IgG, and IgM) | Proteolytic | Pro-Phe-Arg-MCA, Glu-Ala-Arg-AMC, etc.; HIV gp120 protein, transthyretin, extracellular fibrinogen binding protein S. aureus | [110,159,160,161,162,163,164,165,166] |
| Peroxidase and oxydoreductase | DAB, ATBS, OPD, pHQ, and others in the presence and absence of hydrogen peroxide | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermakov, E.A.; Nevinsky, G.A.; Buneva, V.N. Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. Int. J. Mol. Sci. 2020, 21, 5392. https://doi.org/10.3390/ijms21155392
Ermakov EA, Nevinsky GA, Buneva VN. Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. International Journal of Molecular Sciences. 2020; 21(15):5392. https://doi.org/10.3390/ijms21155392
Chicago/Turabian StyleErmakov, Evgeny A., Georgy A. Nevinsky, and Valentina N. Buneva. 2020. "Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States" International Journal of Molecular Sciences 21, no. 15: 5392. https://doi.org/10.3390/ijms21155392
APA StyleErmakov, E. A., Nevinsky, G. A., & Buneva, V. N. (2020). Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. International Journal of Molecular Sciences, 21(15), 5392. https://doi.org/10.3390/ijms21155392