Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization
Abstract
1. Introduction
2. Results
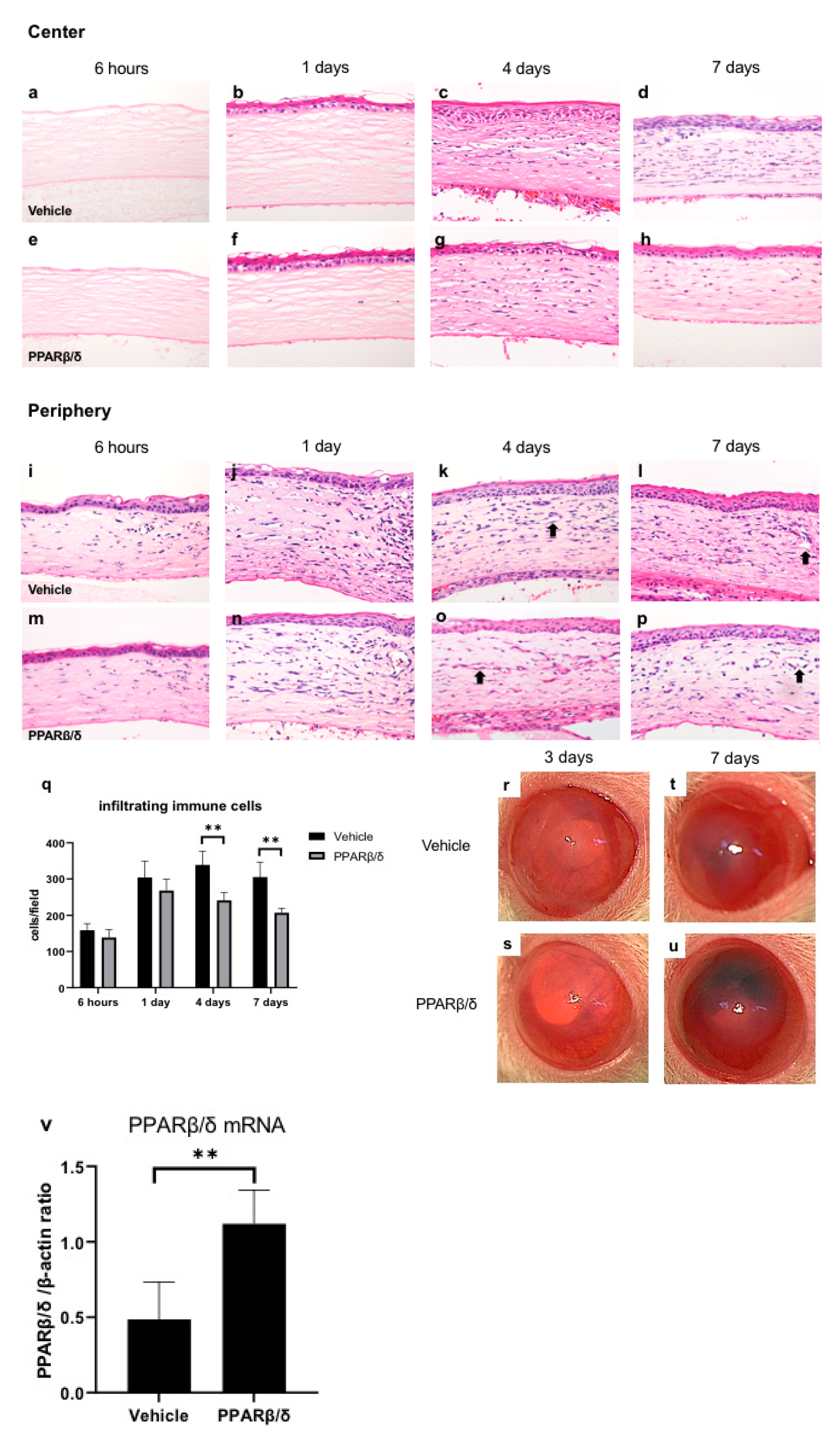
2.1. Wound Healing Process and Expression of PPARβ/δ
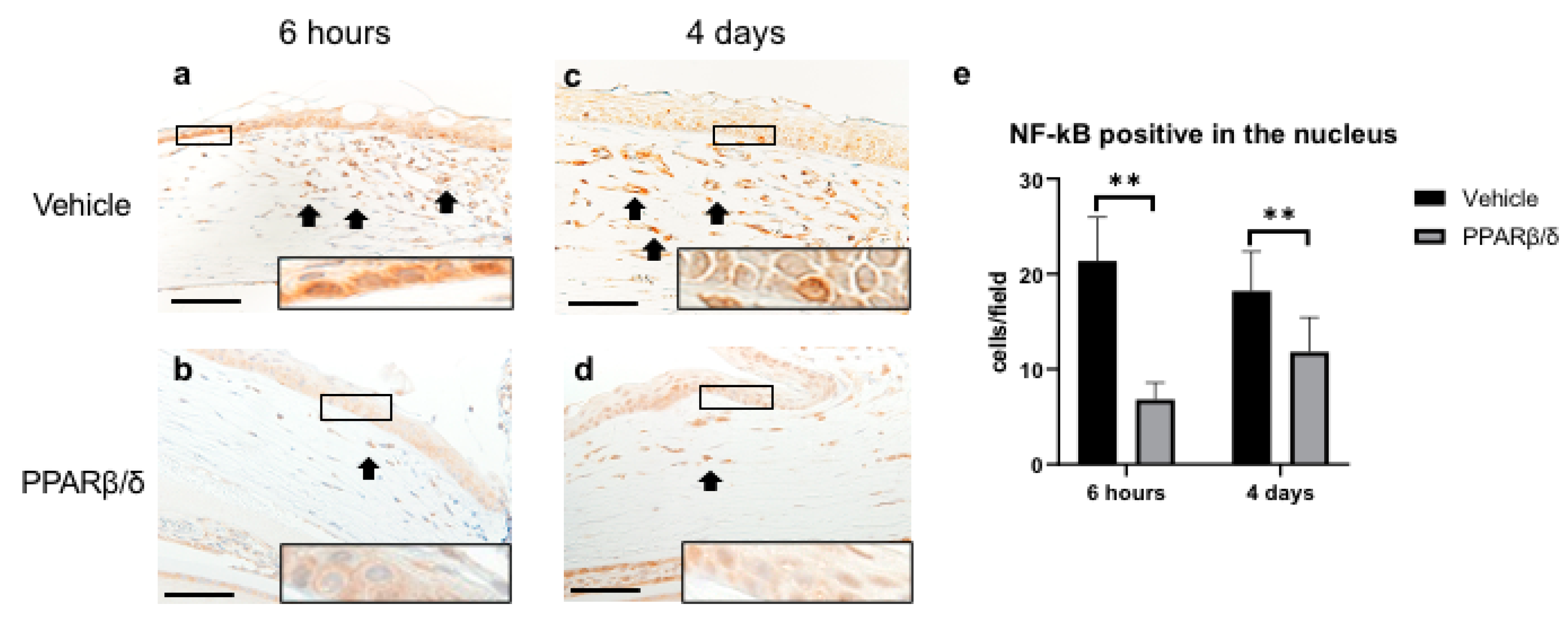
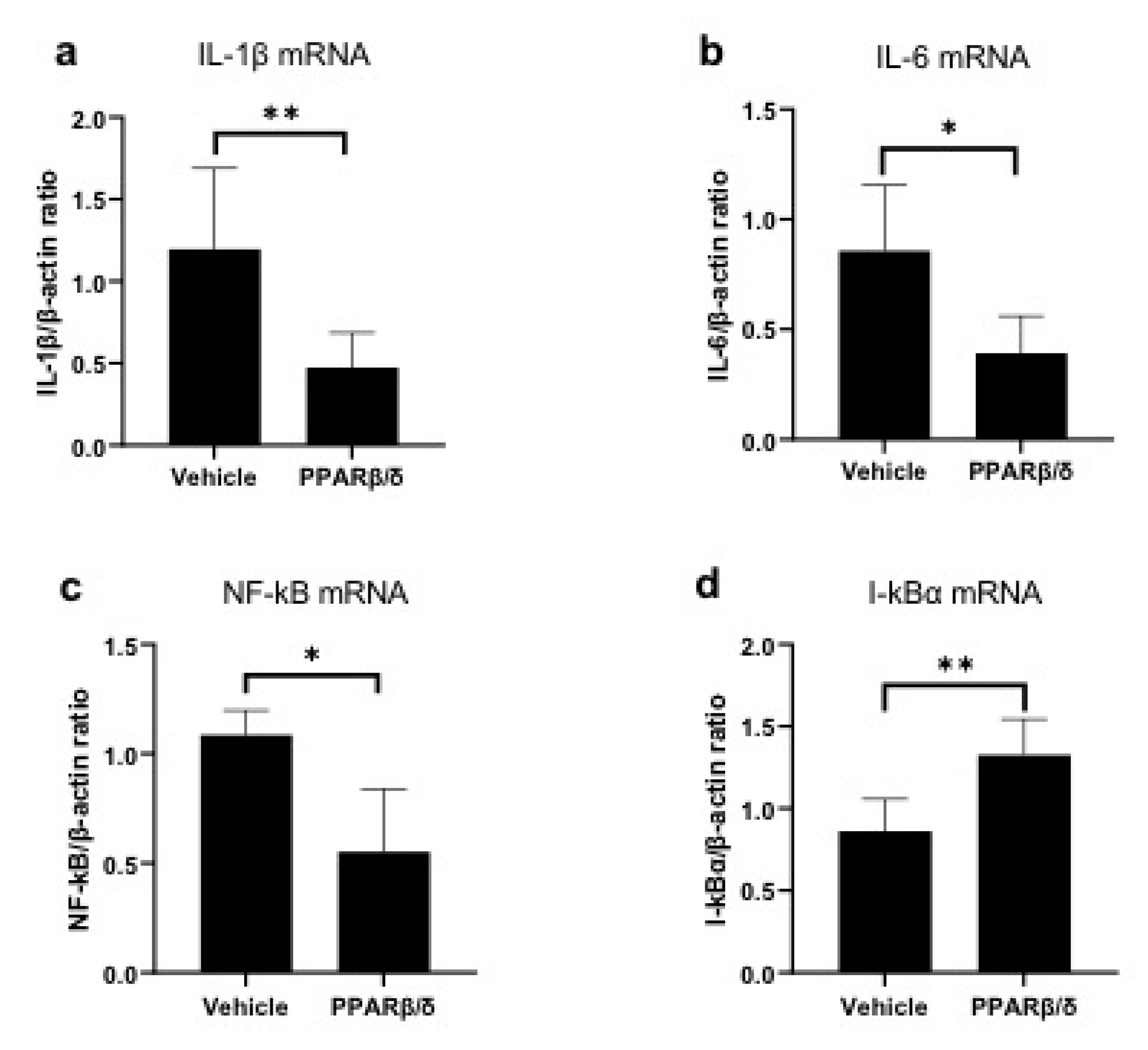
2.2. Anti-Inflammatory Effect of PPARβ/δ
2.3. Involvement of PPARβ/δ in Macrophage Polarization
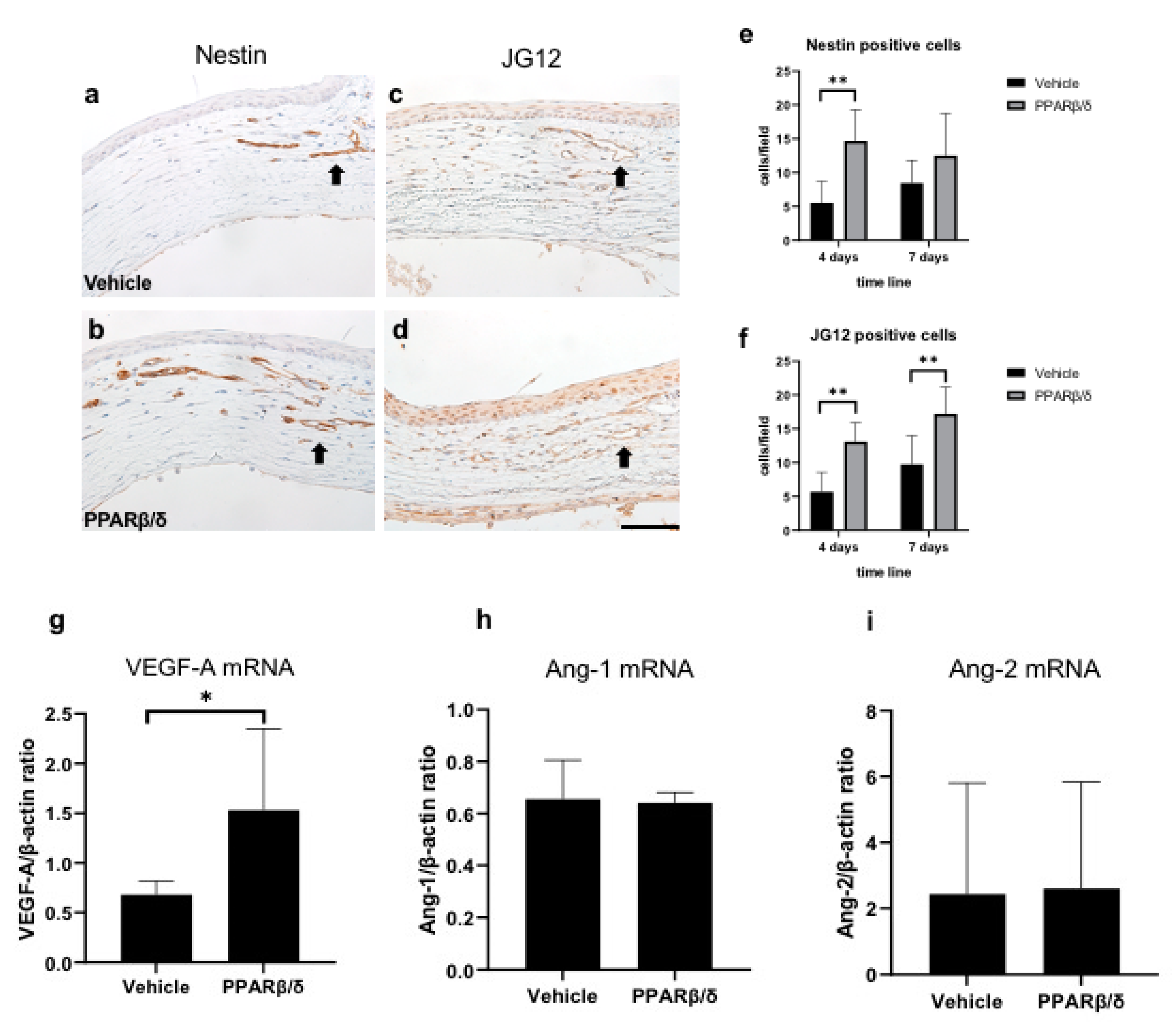
2.4. Promotion of Neovascularization
2.5. Effects of the PPARβ/δ Ligand on the Normal Cornea
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Experimental Procedures
4.3. Histological and Immunohistochemical Analyses
4.4. Real-Time RT-PCR
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PPAR | Peroxisome proliferator-activated receptor |
| RT-PCR | Reverse transcription polymerase chain reaction |
| VEGF | Vascular endothelial growth factor |
| HE | Hematoxylin-eosin |
| EST | Naphthol AS-D chloroacetate esterase |
| iNOS | Inducible nitric oxide synthase |
| TNF-α | Tumor necrosis factor alpha |
| Arg-1 | Arginase 1 |
| CD206 | Mannose receptor |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear factor-kappa B |
| I-κBα | kappa light polypeptide gene enhancer in the B-cell inhibitor, alpha |
| ED-1 | CD68 antibody |
| ED-2 | CD163 antibody |
| JG12 | Aminopeptidase P |
| Ang-1 | Angiopoietin-1 |
| Ang-2 | Angiopoietin-2 |
References
- Berger, J.P.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, G.; Sahebkar, A.; Maffioli, P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J. Cell. Physiol. 2017, 233, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [PubMed]
- Barbier, O.; Torra, I.P.; Duguay, Y.; Blanquart, C.; Fruchart, J.-C.; Glineur, C.; Staels, B. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arter. Thromb. Vasc. Boil. 2002, 22, 717–726. [Google Scholar] [CrossRef]
- Takada, I.; Makishima, M. Peroxisome proliferator-activated receptor agonists and antagonists: a patent review (2014-present). Expert Opin. Ther. Patents 2019, 30, 1–13. [Google Scholar] [CrossRef]
- Tan, N.S.; Vázquez-Carrera, M.; Montagner, A.; Sng, M.K.; Guillou, H.; Wahli, W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Prog. Lipid Res. 2016, 64, 98–122. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: the ‘future’ in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef]
- Becker, J.; Delayre-Orthez, C.; Frossard, N.; Pons, F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam. Clin. Pharmacol. 2006, 20, 429–447. [Google Scholar] [CrossRef]
- Nakano, Y.; Uchiyama, M.; Arima, T.; Nagasaka, S.; Igarashi, T.; Shimizu, A.; Takahashi, H. PPARα Agonist Suppresses Inflammation after Corneal Alkali Burn by Suppressing Proinflammatory Cytokines, MCP-1, and Nuclear Translocation of NF-κB. Molecules 2018, 24, 114. [Google Scholar] [CrossRef]
- Arima, T.; Uchiyama, M.; Nakano, Y.; Nagasaka, S.; Kang, D.; Shimizu, A.; Takahashi, H. Peroxisome proliferator-activated receptor alpha agonist suppresses neovascularization by reducing both vascular endothelial growth factor and angiopoietin-2 in corneal alkali burn. Sci. Rep. 2017, 7, 17763. [Google Scholar] [CrossRef]
- Uchiyama, M.; Shimizu, A.; Masuda, Y.; Nagasaka, S.; Fukuda, Y.; Takahashi, H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013, 19, 2135–2150. [Google Scholar]
- Choi, H.; Phillips, C.; Oh, J.Y.; Stock, E.M.; Kim, D.-K.; Won, J.-K.; Fulcher, S. Comprehensive Modeling of Corneal Alkali Injury in the Rat Eye. Curr. Eye Res. 2017, 42, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ma, M.; Li, Y.; Su, T.; Zhou, X.-Z.; Ye, L.; Yuan, Q.; Zhu, P.; Min, Y.; Shi, W.; et al. The role of pirfenidone in alkali burn rat cornea. Int. Immunopharmacol. 2018, 64, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yazu, H.; Kozuki, N.; Dogru, M.; Shibasaki, A.; Fujishima, H. The Effect of Long-Term Use of an Eyewash Solution on the Ocular Surface Mucin Layer. Int. J. Mol. Sci. 2019, 20, 5078. [Google Scholar] [CrossRef]
- Oliver, W.R.; Shenk, J.L.; Snaith, M.R.; Russell, C.S.; Plunket, K.D.; Bodkin, N.L.; Lewis, M.C.; Winegar, D.A.; Sznaidman, M.L.; Lambert, M.H.; et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA 2001, 98, 5306–5311. [Google Scholar] [PubMed]
- Barish, G.D.; Narkar, V.A.; Evans, R.M. PPAR delta: A dagger in the heart of the metabolic syndrome. J. Clin. Invest. 2006, 116, 590–597. [Google Scholar] [PubMed]
- Dressel, U.; Allen, T.L.; Pippal, J.B.; Rohde, P.R.; Lau, P.; Muscat, G. The Peroxisome Proliferator-Activated Receptor β/δ Agonist, GW501516, Regulates the Expression of Genes Involved in Lipid Catabolism and Energy Uncoupling in Skeletal Muscle Cells. Mol. Endocrinol. 2003, 17, 2477–2493. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin. Pharmacother. 2014, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Heuvel, J.P.V.; Vandenheuvel, J. Modulation of PPAR activity via phosphorylation. Biochim. et Biophys. Acta (BBA)-Mol. Cell Boil. Lipids 2007, 1771, 952–960. [Google Scholar] [CrossRef]
- Defaux, A.; Zurich, M.G.; Braissant, O.; Honegger, P.; Monnet-Tschudi, F. Effects of the PPAR-beta agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination. J. Neuroinflamm. 2009, 6, 15. [Google Scholar]
- Yin, Y.; Russell, R.G.; Dettin, L.E.; Bai, R.; Wei, Z.L.; Kozikowski, A.P.; Kopelovich, L.; Kopleovich, L.; Glazer, R.I. Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005, 65, 3950–3957. [Google Scholar] [PubMed]
- Lee, C.-H.; Chawla, A.; Urbiztondo, N.; Liao, D.; Boisvert, W.A.; Evans, R. Transcriptional Repression of Atherogenic Inflammation: Modulation by PPAR. Science 2003, 302, 453–457. [Google Scholar] [CrossRef]
- Takata, Y.; Liu, J.; Yin, F.; Collins, A.R.; Lyon, C.J.; Lee, C.H.; Atkins, A.R.; Downes, M.; Barish, G.D.; Evans, R.M.; et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 4277–4282. [Google Scholar] [PubMed]
- Bishop-Bailey, D.; Bystrom, J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol. Ther. 2009, 124, 141–150. [Google Scholar] [PubMed]
- Coll, T.; Barroso, E.; Alvarez-Guardia, D.; Serrano, L.; Salvadó, L.; Merlos, M.; Palomer, X.; Vázquez-Carrera, M. The Role of Peroxisome Proliferator-Activated Receptor beta/delta on the Inflammatory Basis of Metabolic Disease. PPAR Res. 2010, 2010. [Google Scholar] [CrossRef]
- Rival, Y.; Benéteau, N.; Taillandier, T.; Pezet, M.; Dupont-Passelaigue, E.; Patoiseau, J.F.; Junquéro, D.; Colpaert, F.C.; Delhon, A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur. J. Pharmacol. 2002, 435, 143–151. [Google Scholar]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Niederberger, E.; Geisslinger, G. Proteomics and NF-κB: an update. Expert Rev. Proteom. 2013, 10, 189–204. [Google Scholar] [CrossRef]
- Brancato, S.K.; Albina, J.E. Wound Macrophages as Key Regulators of Repair. Am. J. Pathol. 2011, 178, 19–25. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Red Eagle, A.; Vats, D.; Morel, C.R.; Goforth, M.H.; Subramanian, V.; Mukundan, L.; Ferrante, A.W.; Chawla, A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008, 7, 496–507. [Google Scholar] [PubMed]
- Kang, K.; Reilly, S.M.; Karabacak, V.; Gangl, M.R.; Fitzgerald, K.; Hatano, B.; Lee, C.H. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008, 7, 485–495. [Google Scholar] [PubMed]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARβ/δ Induces Endothelial Cell Proliferation and Angiogenesis. Arter. Thromb. Vasc. Boil. 2007, 27, 63–69. [Google Scholar] [CrossRef]
- Fauconnet, S.; Lascombe, I.; Chabannes, E.; Adessi, G.-L.; Desvergne, B.; Wahli, W.; Bittard, H. Differential Regulation of Vascular Endothelial Growth Factor Expression by Peroxisome Proliferator-activated Receptors in Bladder Cancer Cells. J. Boil. Chem. 2002, 277, 23534–23543. [Google Scholar] [CrossRef]
- Stephen, R.L.; Gustafsson, M.C.U.; Jarvis, M.; Tatoud, R.; Marshall, B.R.; Knight, D.; Ehrenborg, E.; Harris, A.L.; Wolf, C.R.; A Palmer, C.N. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004, 64, 3162–3170. [Google Scholar] [PubMed]
- Gu, Y.; Li, X.; He, T.; Jiang, Z.; Hao, P.; Tang, X. The Antifibrosis Effects of Peroxisome Proliferator-Activated Receptor δ on Rat Corneal Wound Healing after Excimer Laser Keratectomy. PPAR Res. 2014, 2014, 464935. [Google Scholar]
- Mokrý, J.; Nĕmecek, S. Immunohistochemical detection of intermediate filament nestin. Acta Med. (Hradec Kral. Czech Repub. ) 1998, 41, 73–80. [Google Scholar]
- Matsui, K.; Nagy-Bojarsky, K.; Laakkonen, P.; Krieger, S.; Mechtler, K.; Uchida, S.; Geleff, S.; Kang, D.-H.; Johnson, R.J.; Kerjaschki, D. Lymphatic Microvessels in the Rat Remnant Kidney Model of Renal Fibrosis: Aminopeptidase P and Podoplanin Are Discriminatory Markers for Endothelial Cells of Blood and Lymphatic Vessels. J. Am. Soc. Nephrol. 2003, 14, 1981–1989. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar]




| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| β-actin | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC |
| iNOS | TCACCTTCGAGGGCAGCCGA | CAGACGCCATGGTGCAGGGG |
| TNF-α | AAATGGGCTCCCTCTCATCAGTTC | TCTGCTTGGTGGTTTGCTACGAC |
| Arg-1 | ATTCACCCCGGCTACGGGCA | AGGAGCAGCGTTGGCCTGGT |
| CD206 | GACGGACGAGGAGTTCATTATAC | GTTGGAGAGATAGGCACAGAAG |
| IL-1β | TACCTATGTCTTGCCCGTGGAG | ATCATCCCACGAGTCACAGAGG |
| IL-6 | GTCAACTCCATCTGCCCTTCAG | GGCAGTGGCTGTCAACAACAT |
| NF-κB | TGGACGATCTGTTTCCCCTC | TCGCACTTGTAACGGAAACG |
| I-κBα | TGACCATGGAAGTGATTGGTCAG | GATCACAGCCAAGTGGAGTGGA |
| VEGF-A | GCAGCGACAAGGCAGACTAT | GCAACCTCTCCAAACCGTTG |
| PPARβ/δ | GCCGCCCTACAACGAGATCA | CCACCAGCAGTCCGTCTTTGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int. J. Mol. Sci. 2020, 21, 5296. https://doi.org/10.3390/ijms21155296
Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. International Journal of Molecular Sciences. 2020; 21(15):5296. https://doi.org/10.3390/ijms21155296
Chicago/Turabian StyleTobita, Yutaro, Takeshi Arima, Yuji Nakano, Masaaki Uchiyama, Akira Shimizu, and Hiroshi Takahashi. 2020. "Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization" International Journal of Molecular Sciences 21, no. 15: 5296. https://doi.org/10.3390/ijms21155296
APA StyleTobita, Y., Arima, T., Nakano, Y., Uchiyama, M., Shimizu, A., & Takahashi, H. (2020). Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. International Journal of Molecular Sciences, 21(15), 5296. https://doi.org/10.3390/ijms21155296





