Proteomic Analysis in Seminal Plasma of Fertile Donors and Infertile Patients with Sperm DNA Fragmentation
Abstract
1. Introduction
2. Results
2.1. Sperm DNA Fragmentation
2.2. Analysis of Differentially Expressed Proteins Using 2D-Differential Gel Electrophoresis (2D-DIGE)
2.3. Identification of Differentially Expressed Proteins Using Mass Spectrometry (MS)
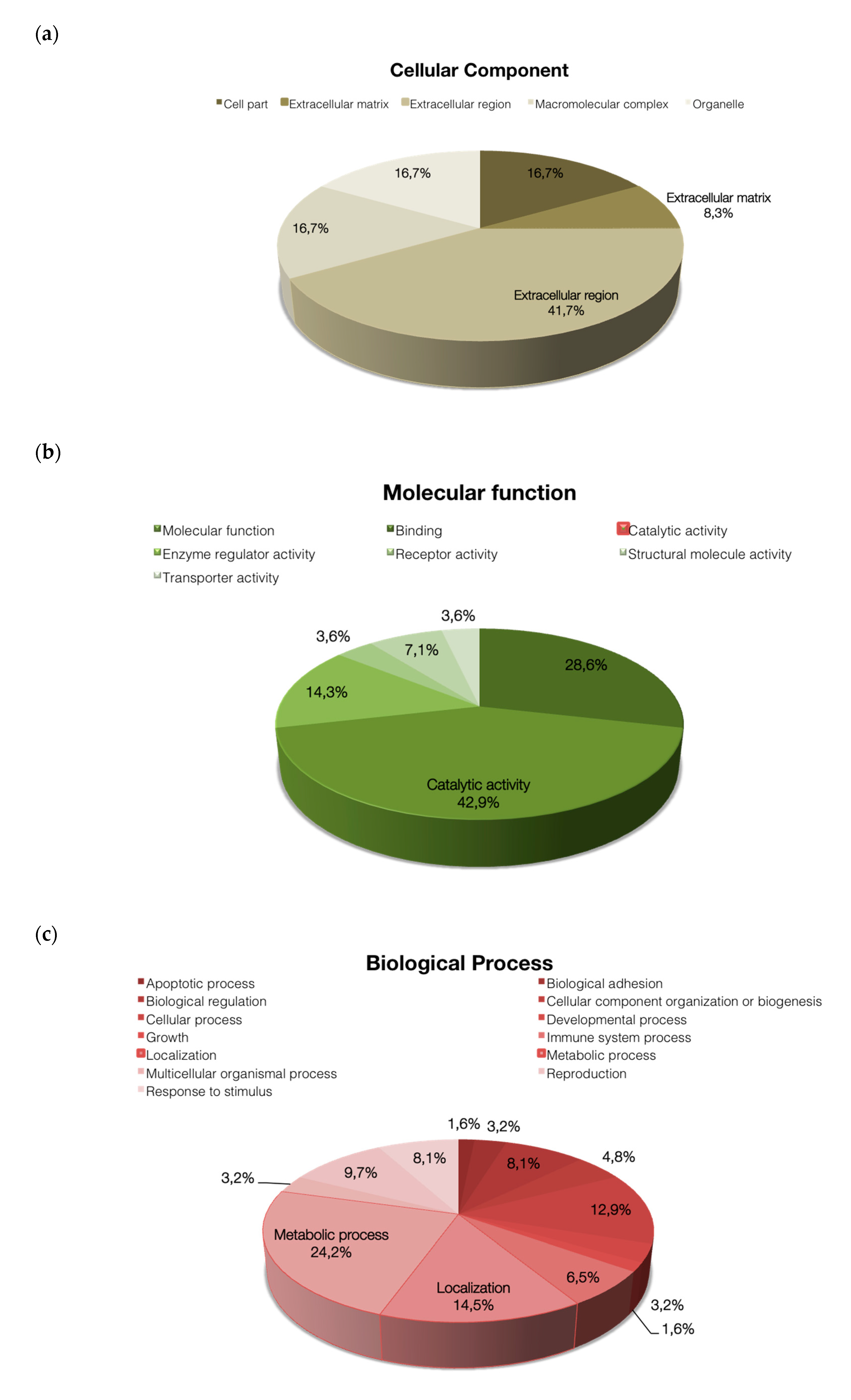
2.4. Functional Classification of Protein and Protein-Protein Interaction Network
3. Discussion
4. Materials and Methods
4.1. Semen Samples and Sperm DNA Fragmentation
4.1.1. Samples and Study Design
- 1.
- Fertile Donors (FD), including samples from six fertile donors who presented normal semen analysis, low DNA degraded sperm (DDS), low SDF by alkaline and neutral Comet assay.
- 2.
- Recurrent Miscarriage (RM), including samples from six recurrent miscarriage patients without female factor, who presented normal semen analysis (sperm count, motility and morphology), low DDS, low SDF by alkaline and high SDF by neutral Comet assay.
- 3.
- Asthenoteratozoospermic infertile patients without varicocele (ATZ), including samples from six ATZ patients, with low DDS, high SDF by alkaline and neutral Comet assay.
- 4.
- Asthenoteratozoospermic infertile patients with varicocele (ATZ-VAR), including samples from six ATZ patients with varicocele, with high values of DDS and SDF for both Comet assays.
4.1.2. Semen Collection, Basic Semen Analysis, and Cryopreservation
4.1.3. DNA Integrity Tests: SCD, Alkaline Comet and Neutral Comet
4.2. Proteomic Analysis of Seminal Plasma
4.2.1. Sample Preparation for 2-D DIGE
4.2.2. Protein Labeling with CyDye DIGE
4.2.3. Two-Dimensional Gel Electrophoresis
4.2.4. Spot Picking and In-Gel Digestion for MS Analysis
4.2.5. Protein Identification
4.2.6. Functional Enrichment Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-DIGE | 2D-Differential Gel Electrophoresis |
| SP | Seminal Plasma |
| SDF | Sperm DNA Fragmentation |
| FD | Fertile Donors |
| ATZ | Asthenoteratozoospermic Group |
| ATZ-VAR | Asthenoteratozoospermic with Varicocele Group |
| RM | Recurrent Miscarriage |
| SCD | Sperm Chromatin Dispersion |
| MS | Mass Spectrometry |
| DDS | Degraded DNA Sperm |
| ART | Assisted Reproductive Technology |
| ICSI | Intra-Cytoplasmic Sperm Injection |
References
- Mosher, W.D.; Pratt, W.F. Fecundity and infertility in the United States: Incidence and trends. Fertil. Steril. 1991, 56, 192–193. [Google Scholar] [CrossRef]
- Sigman, M.; Baazeem, A.; Zini, A. Semen analysis and sperm function assays: What do they mean? Semin. Reprod. Med. 2009, 27, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Baker, H.W. Evaluation and assessment of semen for IVF/ICSI. Asian J. Androl. 2002, 4, 281–285. [Google Scholar]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; Aitken, R.J.; Björndahl, L.; Carrell, D.T.; de Boer, P.; Kvist, U.; Lewis, S.E.M.; Perreault, S.D.; Perry, M.J.; Ramos, L.; et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications—A position report. Hum. Reprod. 2010, 25, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef]
- Ward, W.S. Eight tests for sperm DNA fragmentation and their roles in the clinic. Transl. Androl. Urol. 2017, 6 (Suppl. 4), S468–S470. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Benet, J. Single and double strand sperm DNA damage: Different reproductive effects on male fertility. Genes 2019, 10, 105. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: A guideline. Fertil. Steril. 2013, 99, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ayaz, A.; Samanta, L.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Sabanegh, E. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin. Proteom. 2015, 12, 23. [Google Scholar] [CrossRef]
- Simon, L.; Carrell, D.T. Sperm DNA damage measured by comet assay. Methods Mol. Biol. 2013, 927, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mihalas, B.P.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. Molecular mechanisms responsible for increased vulnerability of the ageing oocyte to oxidative damage. Oxid. Med. Cell. Longev. 2017, 2017, 4015874. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Maheshwari, A.; Mollison, J. Factors associated with failed treatment: An analysis of 121,744 women embarking on their first IVF cycles. PLoS ONE 2013, 8, e82249. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010, 16, 3–13. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Baldi, E. Sperm DNA fragmentation: Mechanisms of origin. Adv. Exp. Med. Biol. 2019, 1166, 75–85. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia 2020, e13706. [Google Scholar] [CrossRef] [PubMed]
- García-Peiró, A.; Martínez-Heredia, J.; Oliver-Bonet, M.; Abad, C.; Amengual, M.J.; Navarro, J.; Jones, C.; Coward, K.; Gosálvez, J.; Benet, J. Protamine 1 to protamine 2 ratio correlates with dynamic aspects of DNA fragmentation in human sperm. Fertil. Steril. 2011, 95. [Google Scholar] [CrossRef]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.C. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef]
- Lettieri, G.; D′Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, R.; Notari, T.; et al. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Gosálvez, J.; Cortés-Gutierez, E.; López-Fernández, C.; Fernández, J.L.; Caballero, P.; Nuñez, R. Sperm deoxyribonucleic acid fragmentation dynamics in fertile donors. Fertil. Steril. 2009, 92. [Google Scholar] [CrossRef] [PubMed]
- Alsaikhan, B.; Alrabeeah, K.; Delouya, G.; Zini, A. Epidemiology of varicocele. Asian J. Androl. 2016, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.M.; Ahmed, H.H.; Kaddah, A.N. A global view of the pathophysiology of varicocele. Andrology 2018, 6, 654–661. [Google Scholar] [CrossRef]
- Vakalopoulos, I.; Kampantais, S.; Lymperi, S.; Grivas, N.; Ioannidis, A.; Mykoniatis, I.; Nikolaou, V.; Dimitriadis, G. Should we expand the indications for varicocele treatment? Transl. Androl. Urol. 2017, 6, 931–942. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Verze, P.; Cai, T.; Lorenzetti, S. The role of the prostate in male fertility, health and disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278. [Google Scholar] [CrossRef]
- Szczykutowicz, J.; Kałuza, A.; Kaźmierowska-Niemczuk, M.; Ferens-Sieczkowska, M. The potential role of seminal plasma in the fertilization outcomes. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.; Moore Dan, H.; Miller, E.G. Proteins of human seminal plasma. J. Biol. Chem. 1942, 144, 667. [Google Scholar]
- Thelen, J.J.; Miernyk, J.A. The proteomic future: Where mass spectrometry should be taking us. Biochem. J. 2012, 444, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Starita-Geribaldi, M.; Poggioli, S.; Zucchini, M.; Garin, J.; Chevallier, D.; Fenichel, P.; Pointis, G. Mapping of seminal plasma proteins by two-dimensional gel electrophoresis in men with normal and impaired spermatogenesis. Mol. Hum. Reprod. 2001, 7, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Yoshida, K.; Nishikawa, H.; Kato, T.; Iwamoto, T. Comparative analysis of interindividual variations in the seminal plasma proteome of fertile men with identification of potential markers for azoospermia in infertile patients. J. Androl. 2007, 28, 858–865. [Google Scholar] [CrossRef]
- Pilch, B.; Mann, M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006, 7, R40. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Zhu, P.; Wang, Y.; Wang, J.; Zhang, W.; Wang, W.; Li, N.; Wang, X.; Zhang, C.; et al. Characterization of seminal plasma proteomic alterations associated with the IVF and rescue-ICSI pregnancy in assisted reproduction. Andrology 2020, 8, 407–420. [Google Scholar] [CrossRef]
- Intasqui, P.; Camargo, M.; Del Giudice, P.T.; Spaine, D.M.; Carvalho, V.M.; Cardozo, K.H.; Zylbersztejn, D.S.; Bertolla, R.P. Sperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasma. BJU Int. 2013, 112, 835–843. [Google Scholar] [CrossRef]
- Intasqui, P.; Camargo, M.; Antoniassi, M.P.; Cedenho, A.P.; Carvalho, V.M.; Cardozo, K.H.M.; Zylbersztejn, D.S.; Bertolla, R.P. Association between the seminal plasma proteome and sperm functional traits. Fertil. Steril. 2016, 105, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Barbăroșie, C.; Ambar, R.; Finelli, R. The impact of single- and double-strand dna breaks in human spermatozoa on assisted reproduction. Int. J. Mol. Sci. 2020, 21, 3882. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; García-Peiró, A.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Alkaline and neutral comet assay profiles of sperm DNA damage in clinical groups. Hum. Reprod. 2012, 27, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Gosálvez, J.; López-Fernández, C.; Núñez-Calonge, R.; Caballero, P.; Agarwal, A.; Fernández, J.L. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int. Urol. Nephrol. 2015, 47, 1471–1477. [Google Scholar] [CrossRef]
- Lacerda, J.I.; Del Giudice, P.T.; da Silva, B.F.; Nichi, M.; Fariello, R.M.; Fraietta, R.; Restelli, A.E.; Blumer, C.G.; Bertolla, R.P.; Cedenho, A.P. Adolescent varicocele: Improved sperm function after varicocelectomy. Fertil. Steril. 2011, 95, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.; Farber, N.; Vij, S. Conventional semen analysis and advanced sperm function tests in diagnosis and management of varicocele. Andrologia 2020, e13629. [Google Scholar] [CrossRef] [PubMed]
- Batruch, I.; Lecker, I.; Kagedan, D.; Smith, C.R.; Mullen, B.J.; Grober, E.; Lo, K.C.; Diamandis, E.P.; Jarvi, K.A. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J. Proteome Res. 2011, 10, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, P.T.; Da Silva, B.F.; Lo Turco, E.G.; Fraietta, R.; Spaine, D.M.; Santos, L.F.A.; Pilau, E.J.; Gozzo, F.C.; Cedenho, A.P.; Bertolla, R.P. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil. Steril. 2013, 100, 667–672. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Du Plessis, S.S.; Gopalan, B.; Willard, B.; Yadav, S.P.; Sabanegh, E. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 85. [Google Scholar] [CrossRef]
- Ahlgren, G.; Rannevik, G.; Lilja, H. Impaired secretory function of the prostate in men with oligo-asthenozoospermia. J. Androl. 1995, 16, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Elzanaty, S.; Richthoff, J.; Malm, J.; Giwercman, A. The impact of epididymal and accessory sex gland function on sperm motility. Hum. Reprod. 2002, 17, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H. Structure, function, and regulation of the enzyme activity of prostate-specific antigen. World J. Urol. 1993, 11, 188–191. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Zhu, P.-Y.; Hu, Y.-A.; Ge, Y.-F. Examination and significance of semenial prostate specific antigen in infertile patients. Zhonghua Nan Ke Xue 2003, 9, 596–598. [Google Scholar] [PubMed]
- Végvári, Á.; Rezeli, M.; Sihlbom, C.; Häkkinen, J.; Carlsohn, E.; Malm, J.; Lilja, H.; Laurell, T.; Marko-Varga, G. Molecular microheterogeneity of prostate specific antigen in seminal fluid by mass spectrometry. Clin. Biochem. 2012, 45, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.B.; de Lamirande, E.; Gagnon, C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol. Reprod. 1999, 61, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Drevet, J.R.; Aitken, R.J. Oxidative damage to sperm DNA: Attack and defense. Adv. Exp. Med. Biol. 2019, 1166, 107–117. [Google Scholar] [CrossRef]
- Tomar, A.K.; Sooch, B.S.; Singh, S.; Yadav, S. Quantification studies in human seminal plasma samples identify prolactin inducible protein as a plausible marker of azoospermia. Biomarkers 2012, 17, 545–551. [Google Scholar] [CrossRef]
- Capková, J.; Elzeinová, F.; Novák, P. Increased expression of secretory actin-binding protein on human spermatozoa is associated with poor semen quality. Hum. Reprod. 2007, 22. [Google Scholar] [CrossRef][Green Version]
- Martínez-Heredia, J.; de Mateo, S.; Vidal-Taboada, J.M.; Ballescà, J.L.; Oliva, R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum. Reprod. 2008, 23, 783–791. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.S.; Ryu, D.-Y.; Kwon, W.-S.; Pang, M.-G. Elevated aminopeptidase n affects sperm motility and early embryo development. PLoS ONE 2017, 12, e0184294. [Google Scholar] [CrossRef]
- Khatun, A.; Kang, K.-H.; Ryu, D.-Y.; Rahman, M.S.; Kwon, W.-S.; Pang, M.-G. Effect of aminopeptidase N on functions and fertility of mouse spermatozoa in vitro. Theriogenology 2018, 118. [Google Scholar] [CrossRef]
- Shui, L.-J.; Meng, Y.; Huang, C.; Qian, Y.; Liu, J.-Y. Aminopeptidase N expression in the endometrium could affect endometrial receptivity. Biochem. Biophys. Res. Commun. 2019, 514, 469–474. [Google Scholar] [CrossRef]
- Intasqui, P.; Antoniassi, M.P.; Camargo, M.; Nichi, M.; Carvalho, V.M.; Cardozo, K.H.; Zylbersztejn, D.S.; Bertolla, R.P. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertil. Steril. 2015, 104. [Google Scholar] [CrossRef]
- Maciel, V.L., Jr.; Tamashiro, L.K.; Bertolla, R.P. Post-translational modifications of seminal proteins and their importance in male fertility potential. Expert Rev. Proteom. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Komander, D. The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 2009, 37, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Zigo, M.; Manaskova-Postlerova, P.; Jonakova, V.; Kerns, K.; Sutovsky, P. Compartmentalization of the proteasome-interacting proteins during sperm capacitation. Sci. Rep. 2019, 9, 12583. [Google Scholar] [CrossRef]
- Sutovsky, P.; Moreno, R.; Ramalho-Santos, J.; Dominko, T.; Thompson, W.E.; Schatten, G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001, 114, 1665–1675. [Google Scholar]
- Muratori, M.; Marchiani, S.; Criscuoli, L.; Fuzzi, B.; Tamburino, L.; Dabizzi, S.; Pucci, C.; Evangelisti, P.; Forti, G.; Noci, I.; et al. Biological meaning of ubiquitination and DNA fragmentation in human spermatozoa. Soc. Reprod. Fertil. Suppl. 2007, 63, 153. [Google Scholar] [PubMed]
- Ribas-Maynou, J.; García-Peiró, A.; Fernandez-Encinas, A.; Amengual, M.J.; Prada, E.; Cortés, P.; Navarro, J.; Benet, J. Double stranded sperm DNA breaks, measured by comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS ONE 2012, 7, e44679. [Google Scholar] [CrossRef] [PubMed]
- Martiáñez, T.; Carrascal, M.; Lamarca, A.; Segura, M.; Durany, N.; Masgrau, R.; Abian, J.; Gella, A. UTP affects the schwannoma cell line proteome through P2Y receptors leading to cytoskeletal reorganisation. Proteomics 2012, 12, 145–156. [Google Scholar] [CrossRef]
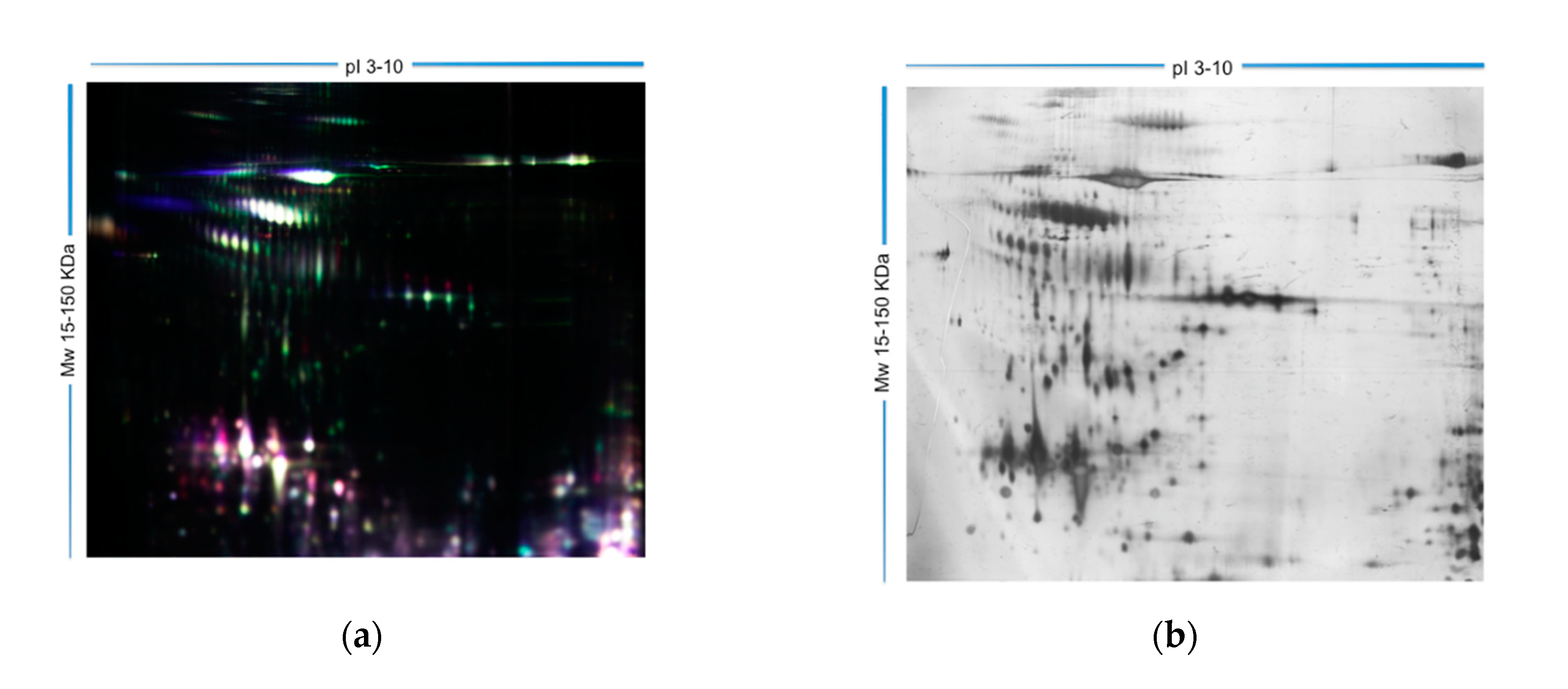

| Group and Pathology | SDF (Alkaline Comet) | SDF (Neutral Comet) | DDS (SCD Test) |
|---|---|---|---|
| Group FD (n = 6) | 23.53 ± 10.46 | 12.36 ± 4.31 | <0.33 |
| Group RM (n = 6) | 36.73 ± 16.42 | 62.74 ± 12.47 | <0.33 |
| Group ATZ (n = 6) | 67.29 ± 12.66 | 74.29 ± 5.91 | <0.33 |
| Group ATZ-VAR (n = 6) | 71.22 ± 5.91 | 86.59 ± 11.83 | >0.33 |
| Group and Pathology | Protein Name | Symbol | Uniprot Accession Number | Fold-Change | ||
|---|---|---|---|---|---|---|
| RM | ATZ | ATZ-VAR | ||||
| Group 1: FD (n = 6) | N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 | DDAH1_HUMAN | O94760 | 2.0 | 2.7 | 2.2 |
| Annexin A3 | ANXA3_HUMAN | P12429 | 0.022 | 0.946 | 0.021 | |
| Clusterin | CLUS_HUMAN | P10909 | 13.1 | 11.7 | 2.0 | |
| Prostaglandin-H2 D-isomerase | PTGDS_HUMAN | P41222 | 2.3 | 2.6 | 2.6 | |
| Semenogelin-1 | SEMG1_HUMAN | P04279 | 2.8 | 1.5 | 2.7 | |
| Beta-2-microglobulin | B2MG_HUMAN | Q91966 | 3.7 | 2.5 | 2.5 | |
| Prostate-specific antigen | KLK3_HUMAN | P07288 | 15.0 | 11.4 | 2.2 | |
| Protein-glutamine gamma-glutamyltransferase 4 | TGM4_HUMAN | P49221 | 3.8 | 4.1 | 4.4 | |
| cDNA FLJ78262, highly similar to Homo sapiens semenogelin II (SEMG2), mRNA | A8K6Z6_HUMAN | A8K6Z6 | 4.6 | 5.0 | 3.9 | |
| cDNA FLJ75803, highly similar to Homo sapiens cysteine-rich secretory protein 1 (CRISP1), transcript variant 1, mRNA | A8K8Y2_HUMAN | A8K8Y2 | 1.9 | 2.5 | 1.8 | |
| Prostatic acid phosphatase | PPAP_HUMAN | P15309 | 2.0 | 2.7 | 2.2 | |
| Ankyrin repeat domain-containing protein SOWAHA | SWAHA_HUMAN | Q2M3V2 | 3.7 | 2.5 | 2.5 | |
| Serine/threonine-protein phosphatase PP1-beta catalytic subunit | PP1B_HUMAN | P62140 | 2.0 | 2.7 | 2.2 | |
| Epididymal secretory protein E3-beta | EP3B_HUMAN | P56851 | 2.2 | 2.2 | 2.6 | |
| Albumin | F6KPG5_HUMAN | F6KPG5 | 4.2 | 2.7 | 4.7 | |
| Apolipoprotein E | Apolipoprotein E (Fragment) | D9ZB55 | 1.9 | 2.5 | 1.8 | |
| cDNA, FLJ92074, highly similar to Homo sapiens progestagen-associated endometrial protein (placental protein 14, pregnancy-associated endometrial alpha-2-globulin, alpha uterine protein) (PAEP), mRNA | B2R4F9_HUMAN | B2R4F9 | 5.4 | 3.6 | 2.9 | |
| RM | ATZ | ATZ-VAR | ||||
| Group 2: normal semen analysis (FD and RM) vs. abnormal semen analysis (ATZ and ATZ-VAR) | Creatine kinase B-type | KCRB_HUMAN | P02787 | 1.3 | 2.3 | 2.2 |
| Gastrisin | PEPC_HUMAN | P20142 | 1.7 | 2.0 | 2.1 | |
| Actin, cytoplasmic 1 | ACTB_HUMAN | P60709 | 1.0 | 1.8 | 1.9 | |
| Fibronectin 1 (FN1) | Q9UQS6_HUMAN | Q9UQS6 | 1.2 | 2.0 | 2.0 | |
| RM | ATZ | FD | ||||
| Group 3: ATZ vs. other groups | Serotransferrin | TRFE_HUMAN | P02787 | 1.3 | 1.1 | 2.5 |
| Prolactin-inducible protein | PIP_HUMAN | P12273 | 1.3 | 1.4 | 2.2 | |
| Beta-actin | F1BXA6_HUMAN | F1BXA6 | 1.3 | 1.4 | 2.2 | |
| Neutrophil defensin 1 | DEF1_HUMAN | P59665 | 1.3 | 1.4 | 2.2 | |
| Cystatin-S | CYTS_HUMAN | P01036 | 2.4 | 2.7 | 4.4 | |
| Alpha-1-antitrypsin | A1AT_HUMAN | P01009 | 1.5 | 1.9 | 2.2 | |
| RM | ATZ | FD | ||||
| Group 4: ATZ-VAR vs. other groups | Aminopeptidase N | AMPN_HUMAN | P15144 | 3.1 | 2.5 | 2.0 |
| Group and Pathology | DDS (SCD Test) | SDF (Alkaline Comet) | SDF (Neutral Comet) |
|---|---|---|---|
| FD (n = 6) | <0.33 | <45% | <50% |
| RM (n = 6) | <0.33 | <45% | >50% |
| ATZ (n = 6) | <0.33 | >45% | >50% |
| ATZ-VAR (n = 6) | >0.33 | >45% | >50% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Encinas, A.; García-Peiró, A.; del Rey, J.; Ribas-Maynou, J.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Proteomic Analysis in Seminal Plasma of Fertile Donors and Infertile Patients with Sperm DNA Fragmentation. Int. J. Mol. Sci. 2020, 21, 5046. https://doi.org/10.3390/ijms21145046
Fernandez-Encinas A, García-Peiró A, del Rey J, Ribas-Maynou J, Abad C, Amengual MJ, Prada E, Navarro J, Benet J. Proteomic Analysis in Seminal Plasma of Fertile Donors and Infertile Patients with Sperm DNA Fragmentation. International Journal of Molecular Sciences. 2020; 21(14):5046. https://doi.org/10.3390/ijms21145046
Chicago/Turabian StyleFernandez-Encinas, Alba, Agustín García-Peiró, Javier del Rey, Jordi Ribas-Maynou, Carlos Abad, Maria José Amengual, Elena Prada, Joaquima Navarro, and Jordi Benet. 2020. "Proteomic Analysis in Seminal Plasma of Fertile Donors and Infertile Patients with Sperm DNA Fragmentation" International Journal of Molecular Sciences 21, no. 14: 5046. https://doi.org/10.3390/ijms21145046
APA StyleFernandez-Encinas, A., García-Peiró, A., del Rey, J., Ribas-Maynou, J., Abad, C., Amengual, M. J., Prada, E., Navarro, J., & Benet, J. (2020). Proteomic Analysis in Seminal Plasma of Fertile Donors and Infertile Patients with Sperm DNA Fragmentation. International Journal of Molecular Sciences, 21(14), 5046. https://doi.org/10.3390/ijms21145046





