The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis
Abstract
1. Introduction
2. Results
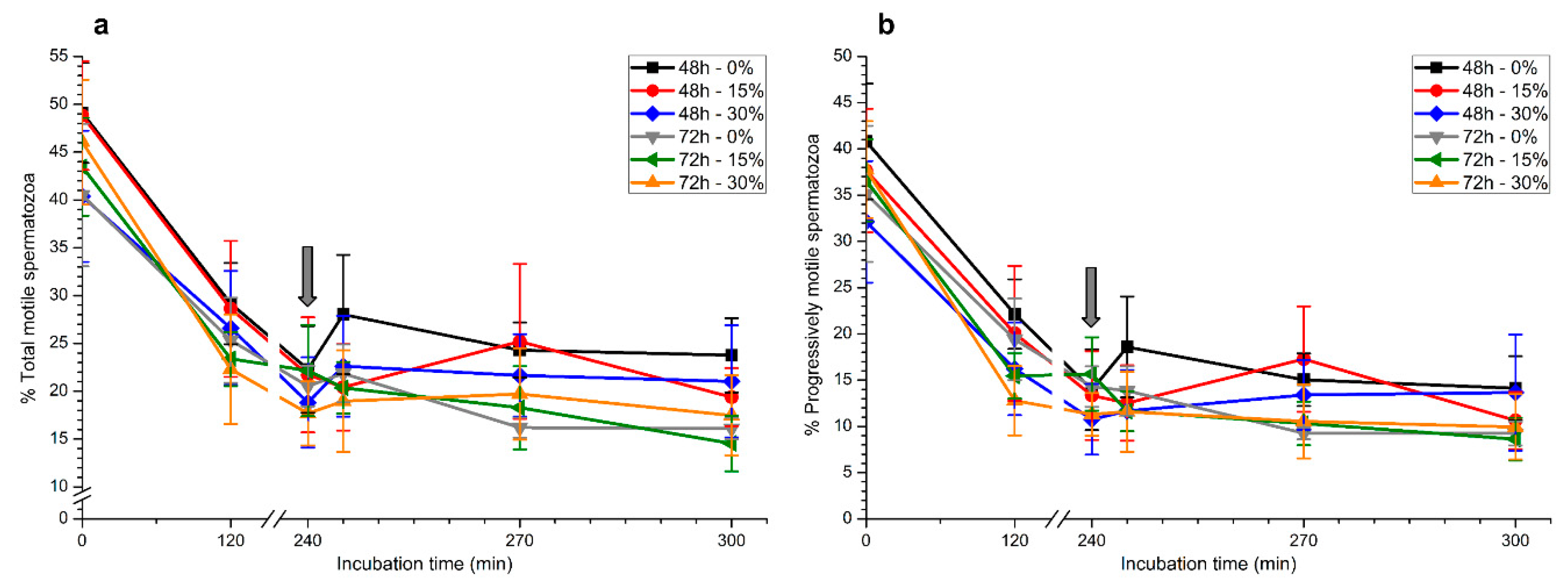
2.1. Sperm Motility
2.1.1. Total and Progressive Sperm Motility
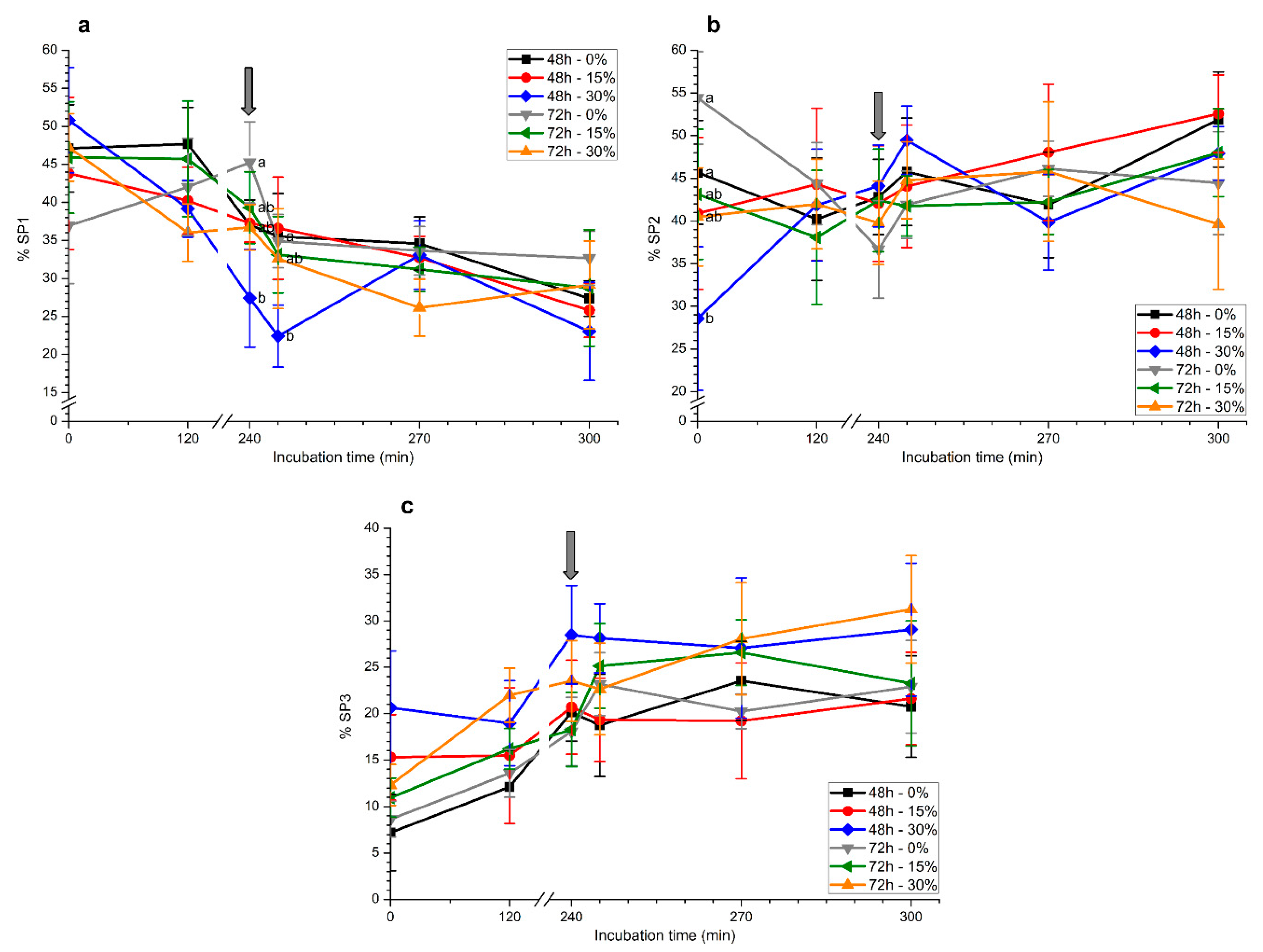
2.1.2. Motile Sperm Subpopulations
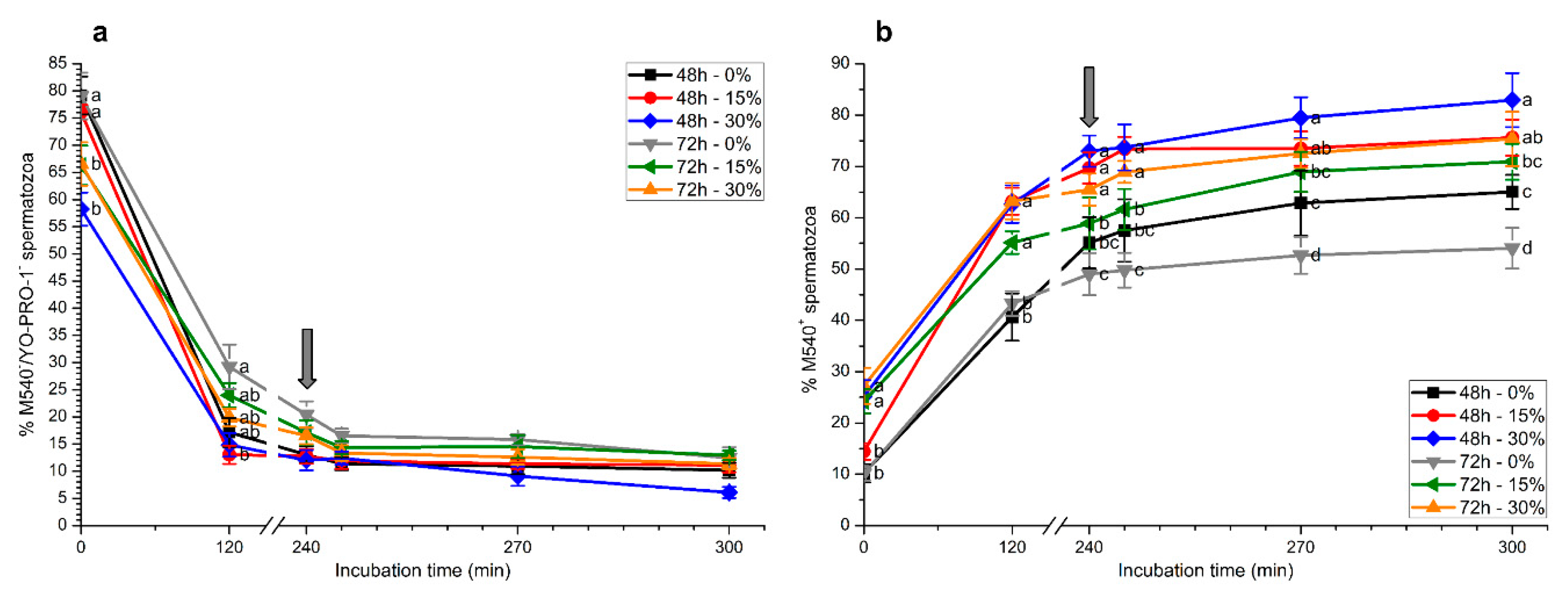
2.2. Plasma Membrane and Acrosome Integrity
2.3. Membrane Lipid Disorder
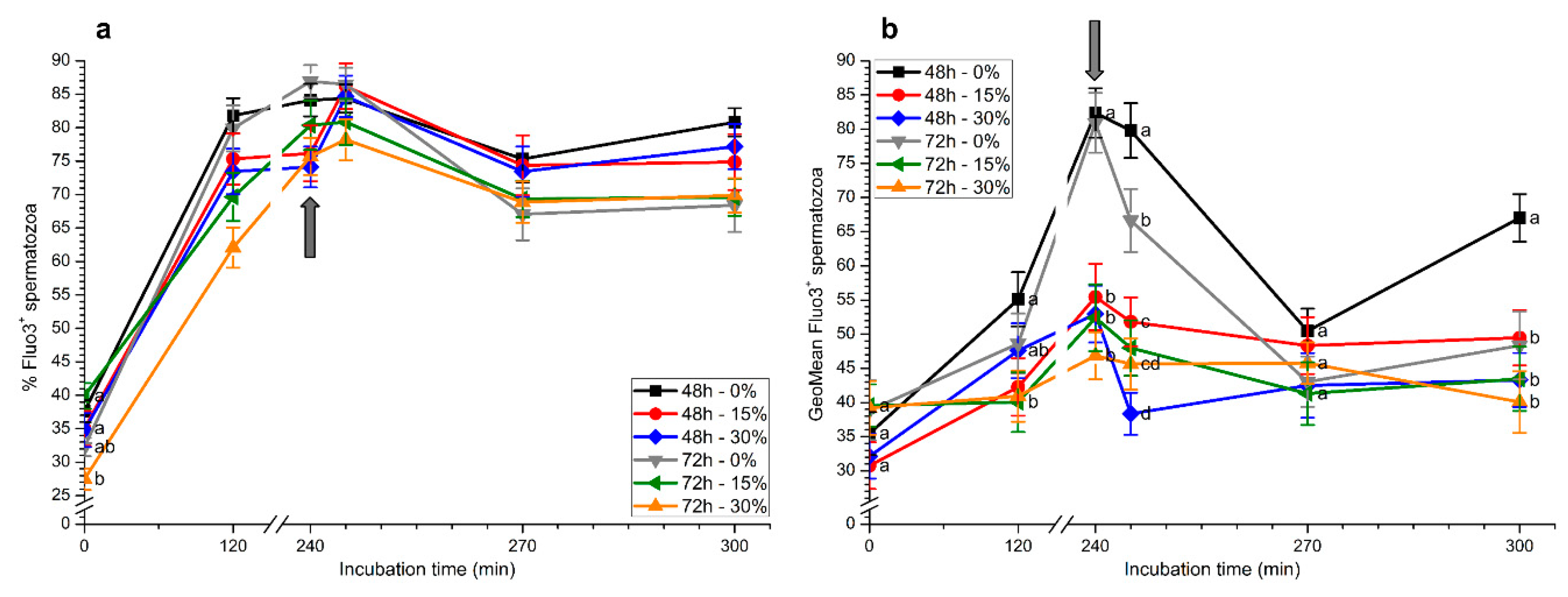
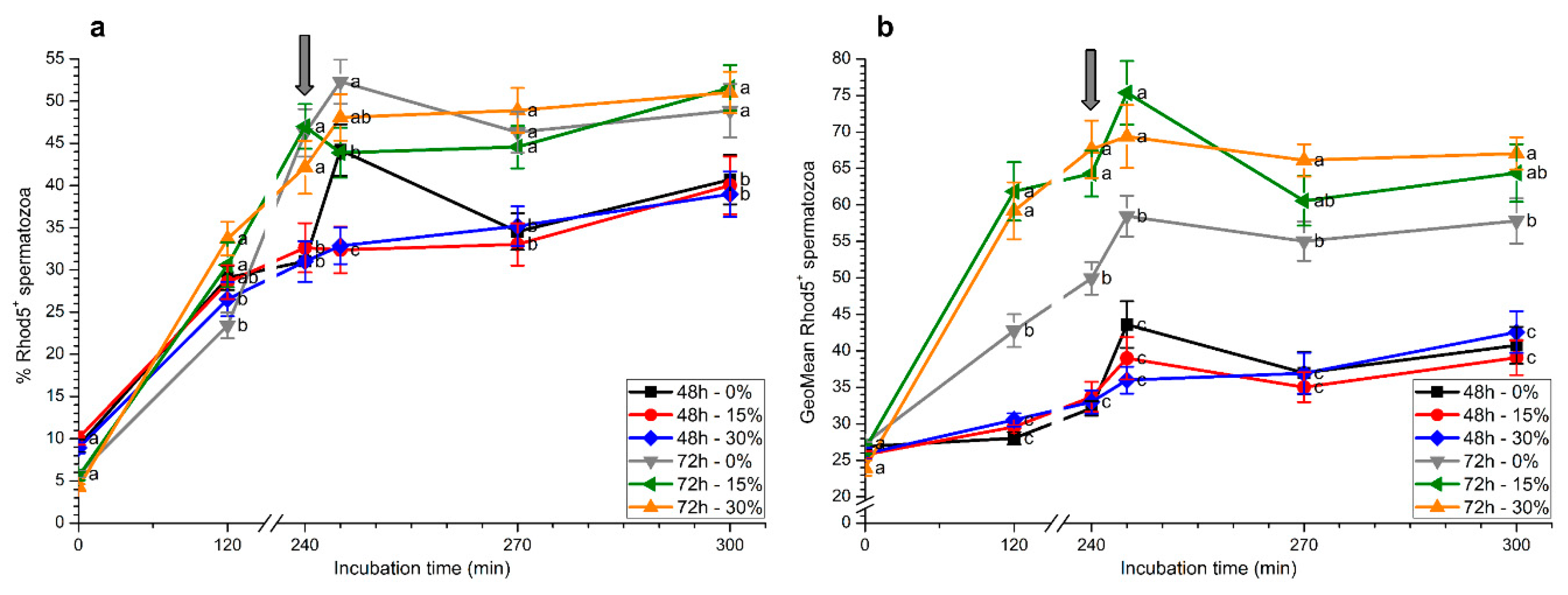
2.4. Intracellular Calcium Levels
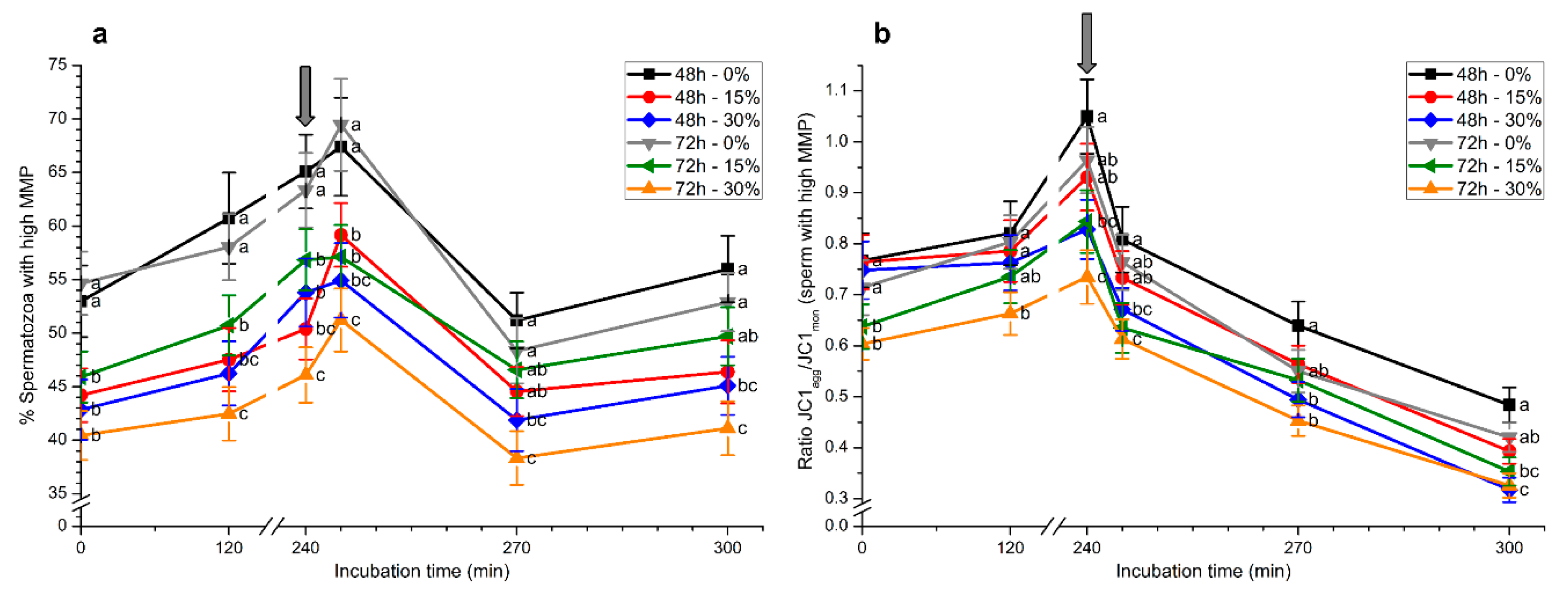
2.5. Mitochondrial Membrane Potential
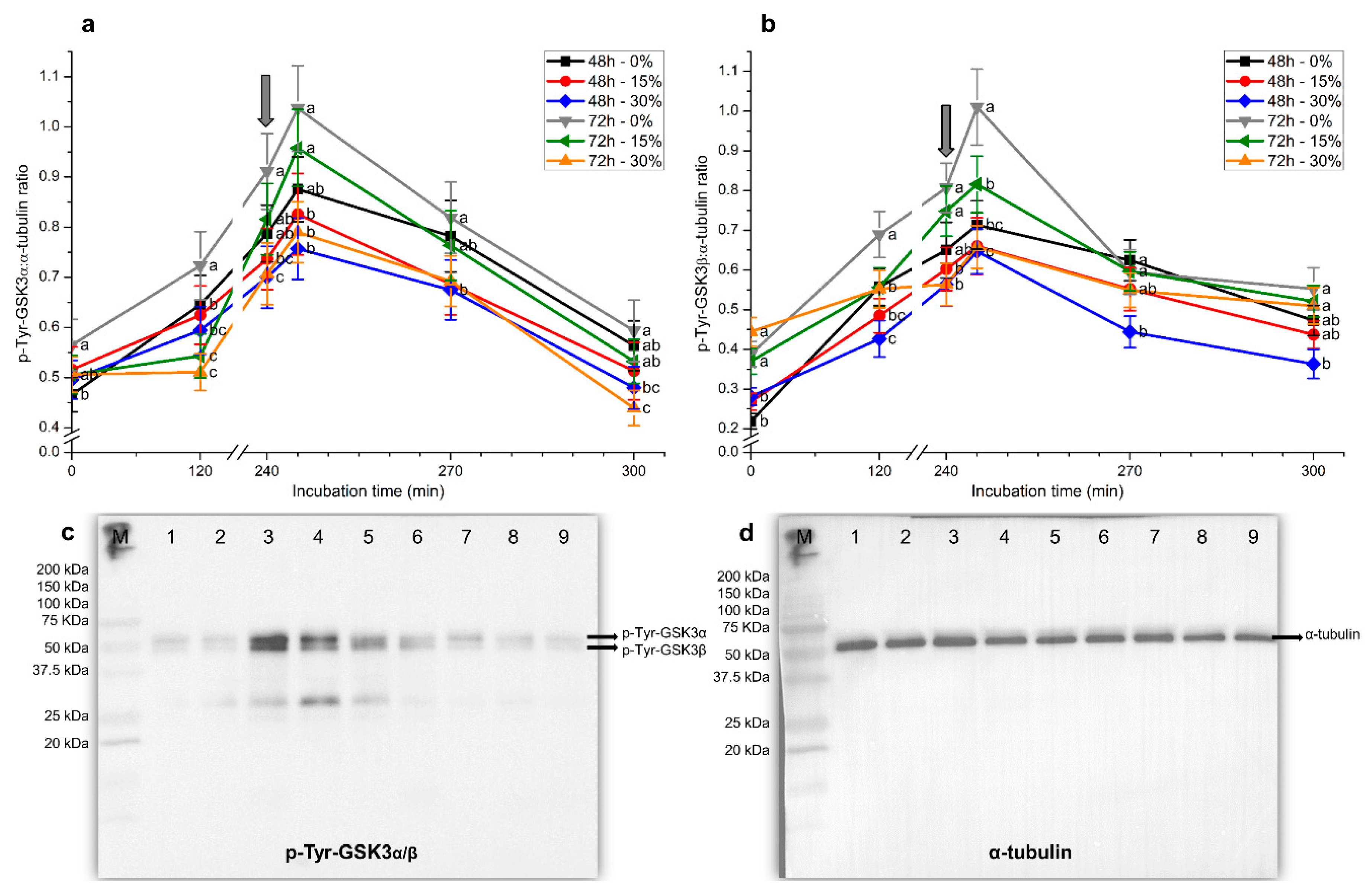
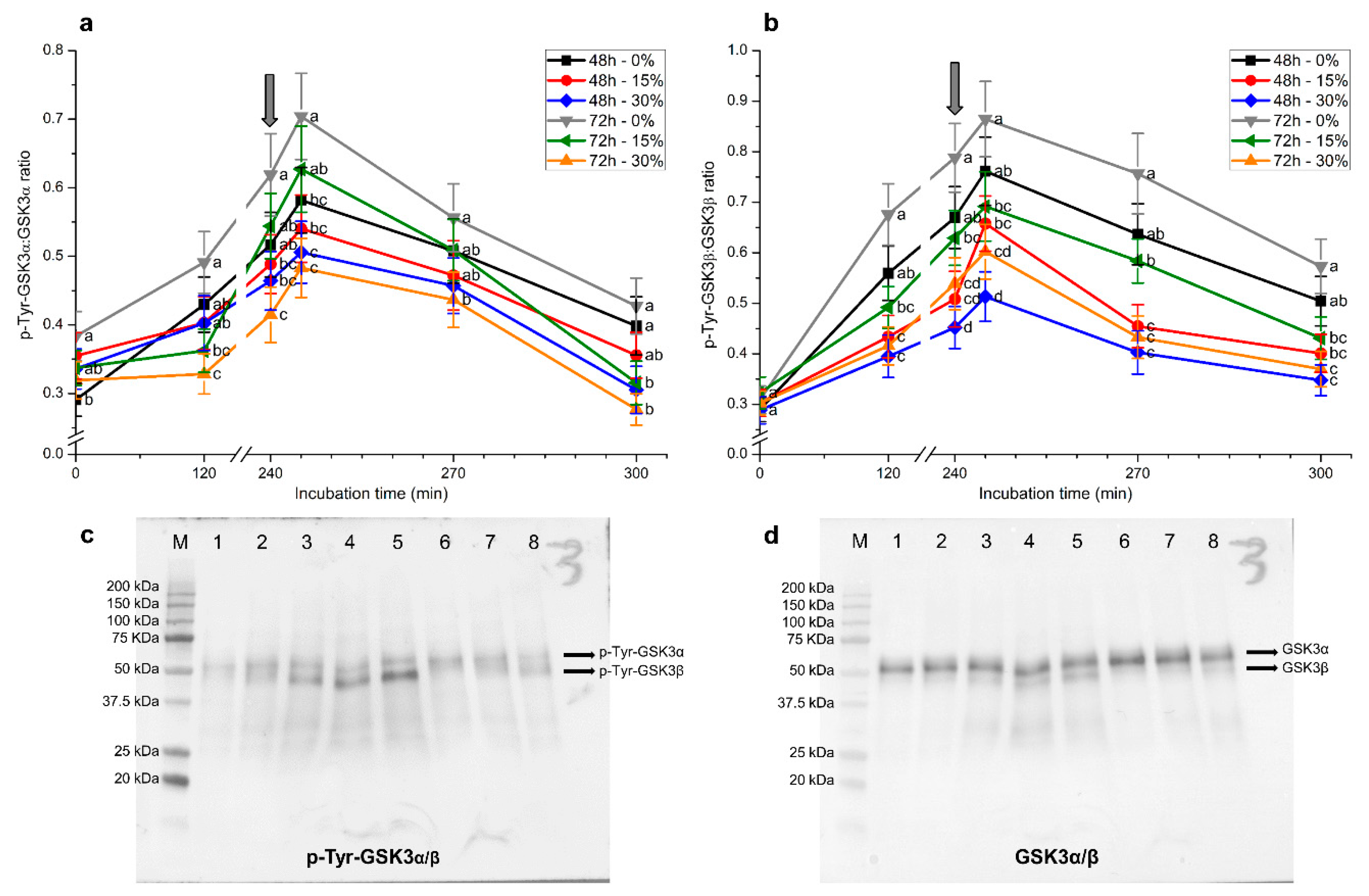
2.6. Tyrosine Phosphorylation Levels of GSK3α/β
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Semen Samples
4.3. Treatments and Semen Storage Procedures
4.4. In Vitro Capacitation and Progesterone-Induced Acrosomal Exocytosis
4.5. Computer-Assisted Sperm Analysis
4.6. Flow Cytometry
4.6.1. Acrosome Integrity
4.6.2. Membrane Lipid Disorder
4.6.3. Intracellular Calcium Levels
4.6.4. Mitochondrial Membrane Potential
4.7. Tyrosine Phosphorylation Levels of GSK3α/β
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALH | amplitude of lateral head displacement |
| BCF | beat-cross frequency |
| BSA | bovine serum albumin |
| CASA | computer-assisted sperm analysis |
| CM | capacitating medium |
| DANCE | forward movement of the head |
| EV | electronic volume |
| FSC | forward scatter |
| GSK3a | glycogen synthase kinase-3 isoform α |
| GSK3ß | glycogen synthase kinase-3 isoform β |
| ISAS | integrated sperm analysis system |
| LIN | linearity |
| M540 | merocyanine-540 |
| MAD | mean angular displacement |
| PCA | principal component analysis |
| PI | propidium iodide |
| PMOT | progressive motility |
| SEM | standard error of the mean |
| SP | motile subpopulation |
| SRF | sperm-rich fraction |
| SSC | side scatter |
| STR | straightness |
| TMOT | total motility |
| VAP | average path velocity |
| VCL | curvilinear velocity |
| VSL | straight linear velocity |
| WOB | wobble coefficient |
References
- Juyena, N.S.; Stelletta, C. Seminal plasma: An essential attribute to spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Padilla, L.; Parrilla, I.; Álvarez-Barrientos, A.; Pérez-Patiño, C.; Peña, F.J.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J. Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci. Rep. 2019, 9, 11584. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Ljunggren, S.A.; Karlsson, H.; Rodriguez-Martinez, H. Exosomes in specific fractions of the boar ejaculate contain CD44: A marker for epididymosomes? Theriogenology 2019, 140, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832–58847. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Latifi, Z.; Kusama, K.; Nakamura, K.; Shimada, M.; Imakawa, K. Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem. Biophys. Res. Commun. 2018, 495, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Barceló, M.; Mata, A.; Bassas, L.; Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum. Reprod. 2018, 33, 1087–1098. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Carrell, D.T.; Jenkins, T.G.; Yeste, M. The role of miRNAs in male human reproduction: A systematic review. Andrology 2020, 8, 7–26. [Google Scholar] [CrossRef]
- Caballero, I.; Parrilla, I.; Almiñana, C.; del Olmo, D.; Roca, J.; Martínez, E.A.; Vázquez, J.M. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod. Domest. Anim. 2012, 47, 12–21. [Google Scholar] [CrossRef]
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; Mocé, E.; Mercado, E. Is sperm freezability related to the post-thaw lipid peroxidation and the formation of reactive oxygen species in boars? Reprod. Domest. Anim. 2013, 48, 177–182. [Google Scholar] [CrossRef]
- Okazaki, T.; Abe, S.; Yoshida, S.; Shimada, M. Seminal plasma damages sperm during cryopreservation, but its presence during thawing improves semen quality and conception rates in boars with poor post-thaw semen quality. Theriogenology 2009, 71, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.C.; Dominguez, J.C.; Pena, F.J.; Alegre, B.; Gonzalez, R.; Castro, M.J.; Habing, G.G.; Kirkwood, R.N. Thawing boar semen in the presence of seminal plasma: Effects on sperm quality and fertility. Anim. Reprod. Sci. 2010, 119, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Ravagnani, G.M.; Leal, D.F.; Martins, S.M.M.K.; Muro, B.B.D.; Meirelles, F.V.; Papa, F.O.; Dell’aqua Junior, J.A.; Alvarenga, M.A.; Moretti, A.S.; et al. Seminal plasma arising from the whole boar sperm-rich fraction increases the stability of sperm membrane after thawing. J. Anim. Sci. 2016, 94, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Chutia, T.; Biswas, R.K.; Tamuli, M.K.; Deka, B.C.; Sinha, S.; Goswami, J.; Banik, S.; Kayastha, R.B. Effect of holding of semen and washing of seminal plasma on quality and fertility of Hampshire boar semen preserved at liquid state. Anim. Reprod. Sci. 2014, 145, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.F.; Torres, M.A.; Ravagnani, G.M.; Martins, S.M.M.K.; Meirelles, F.V.; Andrade, A.F.C. Absence of seminal plasma from sperm-rich fraction decreases boar sperm quality characteristics during the course of liquid storage. Anim. Reprod. Sci. 2018, 198, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pavaneli, A.P.P.; Passarelli, M.S.; Freitas, F.V.; Ravagnani, G.M.; Torres, M.A.; Martins, S.M.M.K.; Yeste, M.; Andrade, A.F.C. Removal of seminal plasma prior to liquid storage of boar spermatozoa: A practice that can improve their fertilizing ability. Theriogenology 2019, 125, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. PNAS 2009, 106, 667–668. [Google Scholar] [CrossRef]
- Visconti, P.E.; Krapf, D.; Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef]
- Ramió-Lluch, L.; Yeste, M.; Fernández-Novell, J.M.; Estrada, E.; Rocha, L.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Concha, I.I.; Ramírez, A.; Rodríguez-Gil, J.E. Oligomycin A-induced inhibition of mitochondrial ATP-synthase activity suppresses boar sperm motility and in vitro capacitation achievement without modifying overall sperm energy levels. Reprod. Fertil. Dev. 2014, 26, 883–897. [Google Scholar] [CrossRef]
- Yeste, M.; Fernández-Novell, J.M.; Ramió-Lluch, L.; Estrada, E.; Rocha, L.G.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Concha, I.I.; Ramírez, A.; Rodríguez-Gil, J.E. Intracellular calcium movements of boar spermatozoa during ‘in vitro’ capacitation and subsequent acrosome exocytosis follow a multiple-storage place, extracellular calcium-dependent model. Andrology 2015, 3, 729–747. [Google Scholar] [CrossRef]
- Vadnais, M.L.; Roberts, K.P. Seminal plasma proteins inhibit in vitro-and cooling-induced capacitation in boar spermatozoa. Reprod. Fertil. Dev. 2010, 22, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Maree, L.; van der Horst, G. Quantification and identification of sperm subpopulations using computer-aided sperm analysis and species-specific cut-off values for swimming speed. Biotech. Histochem. 2013, 88, 181–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luna, C.; Yeste, M.; Rivera del Alamo, M.M.; Domingo, J.; Casao, A.; Rodriguez-Gil, J.E.; Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Effect of seminal plasma proteins on the motile sperm subpopulations in ram ejaculates. Reprod. Fertil. Dev. 2015, 29, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.M.; Bragado, M.J.; Gil, M.C.; Garcia-Herreros, M.; Gonzalez-Fernandez, L.; Tapia, J.A.; Garcia-Marin, L.J. Porcine sperm motility is regulated by serine phosphorylation of the glycogen synthase kinase-3α. Reproduction 2007, 134, 435–444. [Google Scholar] [CrossRef]
- Reid, A.T.; Anderson, A.L.; Roman, S.D.; McLaughlin, E.A.; McCluskey, A.; Robinson, P.J.; Aitken, R.J.; Nixon, B. Glycogen synthase kinase 3 regulates acrosomal exocytosis in mouse spermatozoa via dynamin phosphorylation. FASEB J. 2015, 29, 2872–2882. [Google Scholar] [CrossRef]
- Belenky, M.; Breitbart, H. Role and regulation of glycogen synthase kinase-3 beta in bovine spermatozoa. Mol. Reprod. Dev. 2017, 84, 8–18. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Olson, S.D.; Fauci, L.J.; Suarez, S.S. Mathematical modeling of calcium signaling during sperm hyperactivation. Mol. Hum. Reprod. 2011, 17, 500–510. [Google Scholar] [CrossRef]
- Harayama, H. Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa. J. Reprod. Dev. 2013, 59, 421–430. [Google Scholar] [CrossRef]
- Gunter, T.E.; Yule, D.I.; Gunter, K.K.; Eliseev, R.A.; Salter, J.D. Calcium and mitochondria. FEBS Lett. 2004, 567, 96–102. [Google Scholar] [CrossRef]
- Gunter, T.E.; Sheu, S.S. Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. Biochim. Biophys. Acta 2009, 1787, 1291–1308. [Google Scholar] [CrossRef] [PubMed]
- Ramió-Lluch, L.; Fernández-Novell, J.M.; Peña, A.; Colás, C.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Ramírez, A.; Concha, I.I.; Rigau, T.; Rodríguez Gil, J.E. ‘In vitro’ capacitation and acrosome reaction are concomitant with specific changes in mitochondrial activity in boar sperm: Evidence for a nucleated mitochondrial activation and for the existence of a capacitation-sensitive subpopulational structure. Reprod. Domest. Anim. 2011, 46, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, H. Intracellular calcium regulation in sperm capacitation and acrosome reaction. Mol. Cell. Endocrinol. 2002, 187, 139–144. [Google Scholar] [CrossRef]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Ou, J.X.; Chen, G.W.; Wu, J.P.; Shi, H.J.; O, W.S.; Martin-DeLeon, P.A.; Chen, H. Does prohibitin expression regulate sperm mitochondrial membrane potential, sperm motility, and male fertility? Antioxid. Redox. Signal. 2012, 17, 513–519. [Google Scholar] [CrossRef]
- Kasai, T.; Ogawa, K.; Mizuno, K.; Nagai, S.; Uchida, Y.; Ohta, S.; Fujie, M.; Suzuki, K.; Hirata, S.; Hoshi, K. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J. Androl. 2002, 4, 97–103. [Google Scholar]
- Schmidt, H.; Kamp, G. Induced hyperactivity in boar spermatozoa and its evaluation by computer-assisted sperm analysis. Reproduction 2004, 128, 171–179. [Google Scholar] [CrossRef]
- Pavaneli, A.P.P.; Torres, M.A.; Ravagnani, G.M.; Passarelli, M.S.; Martins, S.M.M.K.; Andrade, A.F.C. Caracterização do espermatozoide suíno hiperativado através do sistema computadorizado de análise de sêmen. Anais do XVIII Congresso da Abraves, Goiânia, Goiás, Brasil, 17 à 19 outubro de 2017; Embrapa Suínos e Aves: Concórdia, Santa Catarina, Brasil, 2017; pp. 235–236. [Google Scholar]
- Olson, S.D.; Suarez, S.S.; Fauci, L.J. A model of Catsper channel mediated calcium dynamics in mammalian spermatozoa. Bull. Math. Biol. 2010, 72, 1925–1946. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 2001, 65, 1606–1615. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol. Reprod. 2003, 68, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Lackey, B.R.; Gray, S.L. Identification of kinases, phosphatases, and phosphorylation sites in human and porcine spermatozoa. Syst. Biol. Reprod. Med. 2015, 61, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Woodgett, J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.; Mohan, J.; Gray, H.; Khatra, B.; Carr, D.W. A role for phosphorylation of glycogen synthase kinase-3α in bovine sperm motility regulation. Biol. Reprod. 2000, 62, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Somanath, P.R.; Jack, S.L.; Vijayaraghavan, S. Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. J. Androl. 2004, 25, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Frame, S.; Cohen, P.; Biondi, R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell. 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Stambolic, V.; Woodgett, J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 1994, 303, 701–704. [Google Scholar] [CrossRef]
- Sayas, C.L.; Avila, J.; Wandosell, F. Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J. Neurosci. 2002, 22, 6863–6875. [Google Scholar] [CrossRef]
- Pérez, M.; Rojo, A.I.; Wandosell, F.; Díaz-Nido, J.; Avila, J. Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase 3. Biochem. J. 2003, 372, 129–136. [Google Scholar] [CrossRef]
- Simón, D.; Benítez, M.J.; Giménez-Cassina, A.; Jurado, J.J.G.; Bhat, R.V.; Díaz-Nido, J.; Wandosell, F. Pharmacological inhibition of GSK-3 is not strictly correlated with a decrease in tyrosine phosphorylation of residues 216/279. J. Neurosci. Res. 2008, 86, 668–674. [Google Scholar] [CrossRef]
- Jiménez, I.; González-Márquez, H.; Ortiz, R.; Herrera, J.A.; García, A.; Betancourt, M.; Fierro, R. Changes in the distribution of lectin receptors during capacitation and acrosome reaction in boar spermatozoa. Theriogenology 2003, 59, 1171–1180. [Google Scholar] [CrossRef]
- Ramió, L.; Rivera, M.M.; Ramírez, A.; Concha, I.I.; Peña, A.; Rigau, T.; Rodríguez-Gil, J.E. Dynamics of motile-sperm subpopulation structure in boar ejaculates subjected to “in vitro” capacitation and further “in vitro” acrosome reaction. Theriogenology 2008, 69, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Briz, M.; Pinart, E.; Sancho, S.; Garcia-Gil, N.; Badia, E.; Bassols, J.; Pruneda, A.; Bussalleu, E.; Casas, I.; et al. Boar spermatozoa and prostaglandin F2α: Quality of boar sperm after the addition of prostaglandin F2α to the short-term extender over cooling time. Anim. Reprod. Sci. 2008, 108, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Estrada, E.; Casas, I.; Bonet, S.; Rodríguez-Gil, J.E. Good and bad freezability boar ejaculates differ in the integrity of nucleoprotein structure after freeze-thawing but not in ROS levels. Theriogenology 2013, 79, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Waberski, D.; Bollwein, H.; Sieme, H. Identifying non-sperm particles during flow cytometric physiological assessment: A simple approach. Theriogenology 2010, 73, 995–1000. [Google Scholar] [CrossRef]
- Rocco, M.; Betarelli, R.; Placci, A.; Fernández-Novell, J.M.; Spinaci, M.; Casao, A.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Peña, A.; Rigau, T.; et al. Melatonin affects the motility and adhesiveness of in vitro capacitated boar spermatozoa via a mechanism that does not depend on intracellular ROS levels. Andrology 2018, 6, 720–736. [Google Scholar] [CrossRef]
- Yeste, M.; Llavanera, M.; Pérez, G.; Scornik, F.; Puig-Parri, J.; Brugada, R.; Bonet, S.; Pinart, E. Elucidating the role of K+ channels during in vitro capacitation of boar spermatozoa: Do SLO1 channels play a crucial role? Int. J. Mol. Sci. 2019, 20, 6330. [Google Scholar] [CrossRef]
- Harrison, R.A.P.; Ashworth, P.J.C.; Miller, N.G.A. Bicarbonate/CO2, an effector of capacitation, induces a rapid and reversible change in the lipid architecture of boar sperm plasma membranes. Mol. Reprod. Dev. 1996, 45, 378–391. [Google Scholar] [CrossRef]
- Harrison, R.A.P.; Mairet, B.; Miller, N.G.A. Flow cytometric studies of bicarbonate-mediated Ca2+ influx in boar sperm populations. Mol. Reprod. Dev. 1993, 35, 197–208. [Google Scholar] [CrossRef]
- Kadirvel, G.; Kumar, S.; Kumaresan, A.; Kathiravan, P. Capacitation status of fresh and frozen-thawed buffalo spermatozoa in relation to cholesterol level, membrane fluidity and intracellular calcium. Anim. Reprod. Sci. 2009, 116, 244–253. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Evans, G.; Maxwell, W.M.C. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology 2005, 63, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Martínez, N.; Morató, R.; Vilagran, I.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Aquaporins in boar spermatozoa. Part II: Detection and localisation of aquaglyceroporin 3. Reprod. Fertil. Dev. 2015, 29, 703–711. [Google Scholar] [CrossRef]
- Vilagran, I.; Castillo, J.; Bonet, S.; Sancho, S.; Yeste, M.; Estanyol, J.M.; Oliva, R. Acrosin-binding protein (ACRBP) and triosephosphate isomerase (TPI) are good markers to predict boar sperm freezing capacity. Theriogenology 2013, 80, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Estrada, E.; Rivera Del Álamo, M.M.; Rodríguez-Gil, J.E.; Yeste, M. The addition of reduced glutathione to cryopreservation media induces changes in the structure of motile subpopulations of frozen-thawed boar sperm. Cryobiology 2017, 78, 56–64. [Google Scholar] [CrossRef] [PubMed]

| Principal Component | Variance | Parameter | aij2 |
|---|---|---|---|
| Component 1 | 58.04% | LIN | 0.94 |
| WOB | 0.81 | ||
| STR | 0.79 | ||
| VSL | 0.62 | ||
| VAP | 0.48 | ||
| BCF | 0.44 | ||
| MAD | 0.28 | ||
| Component 2 | 22.16% | ALH | 0.88 |
| VCL | 0.78 | ||
| DANCE | 0.93 | ||
| Total | 80.20% |
| SP1 | SP2 | SP3 | |
|---|---|---|---|
| N | 10,254 | 7458 | 3078 |
| VCL (µm/s) | 63.27 ± 0.27 | 92.87 ± 0.41 | 4.52 ± 0.19 |
| VSL (µm/s) | 49.91 ± 0.25 | 42.84 ± 0.44 | 2.86 ± 0.05 |
| VAP (µm/s) | 56.27 ± 0.27 | 61.39 ± 0.43 | 2.33 ± 0.10 |
| LIN (%) | 75.60 ± 0.15 | 41.49 ± 0.31 | 3.54 ± 0.16 |
| STR (%) | 86.47 ± 0.12 | 60.92 ± 0.33 | 6.91 ± 0.32 |
| WOB (%) | 86.94 ± 0.10 | 62.78 ± 0.25 | 8.76 ± 0.37 |
| ALH (µm) | 1.90 ± 0.01 | 3.57 ± 0.01 | 0.26 ± 0.01 |
| BCF (Hz) | 8.08 ± 0.03 | 7.79 ± 0.04 | 0.56 ± 0.03 |
| DANCE (µm2/s) | 129.28 ± 0.79 | 357.99 ± 2.72 | 7.60 ± 0.35 |
| MAD (°) | 64.78 ± 0.39 | 107.85 ± 0.50 | 90.35 ± 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavaneli, A.P.P.; Recuero, S.; Chaves, B.R.; Garcia-Bonavila, E.; Llavanera, M.; Pinart, E.; Bonet, S.; De Andrade, A.F.C.; Yeste, M. The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. Int. J. Mol. Sci. 2020, 21, 4520. https://doi.org/10.3390/ijms21124520
Pavaneli APP, Recuero S, Chaves BR, Garcia-Bonavila E, Llavanera M, Pinart E, Bonet S, De Andrade AFC, Yeste M. The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. International Journal of Molecular Sciences. 2020; 21(12):4520. https://doi.org/10.3390/ijms21124520
Chicago/Turabian StylePavaneli, Ana Paula Pinoti, Sandra Recuero, Bruna Resende Chaves, Estela Garcia-Bonavila, Marc Llavanera, Elisabeth Pinart, Sergi Bonet, André Furugen Cesar De Andrade, and Marc Yeste. 2020. "The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis" International Journal of Molecular Sciences 21, no. 12: 4520. https://doi.org/10.3390/ijms21124520
APA StylePavaneli, A. P. P., Recuero, S., Chaves, B. R., Garcia-Bonavila, E., Llavanera, M., Pinart, E., Bonet, S., De Andrade, A. F. C., & Yeste, M. (2020). The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. International Journal of Molecular Sciences, 21(12), 4520. https://doi.org/10.3390/ijms21124520









