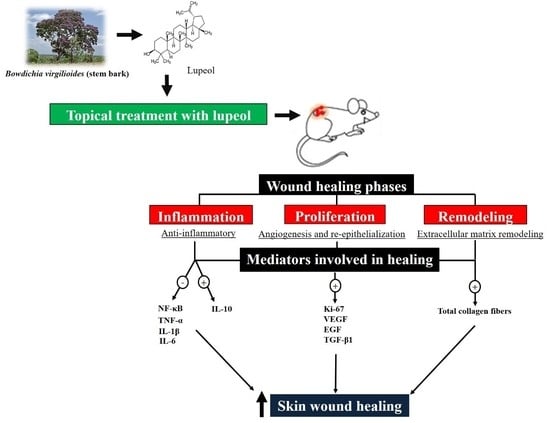
From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers
Abstract
1. Introduction
2. Results
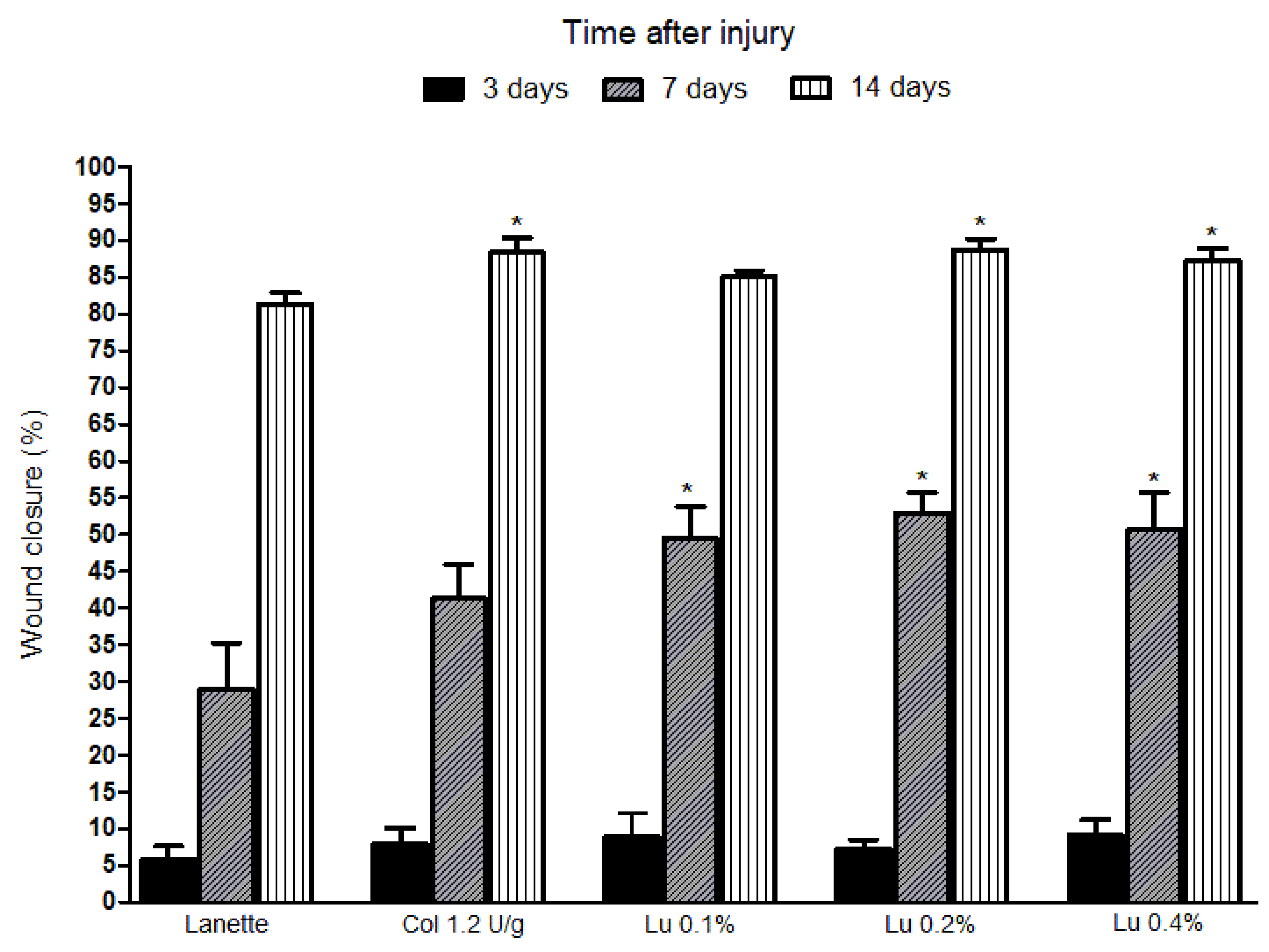
2.1. Macroscopic Analysis of Lupeol Treatment in Cutaneous Wounds
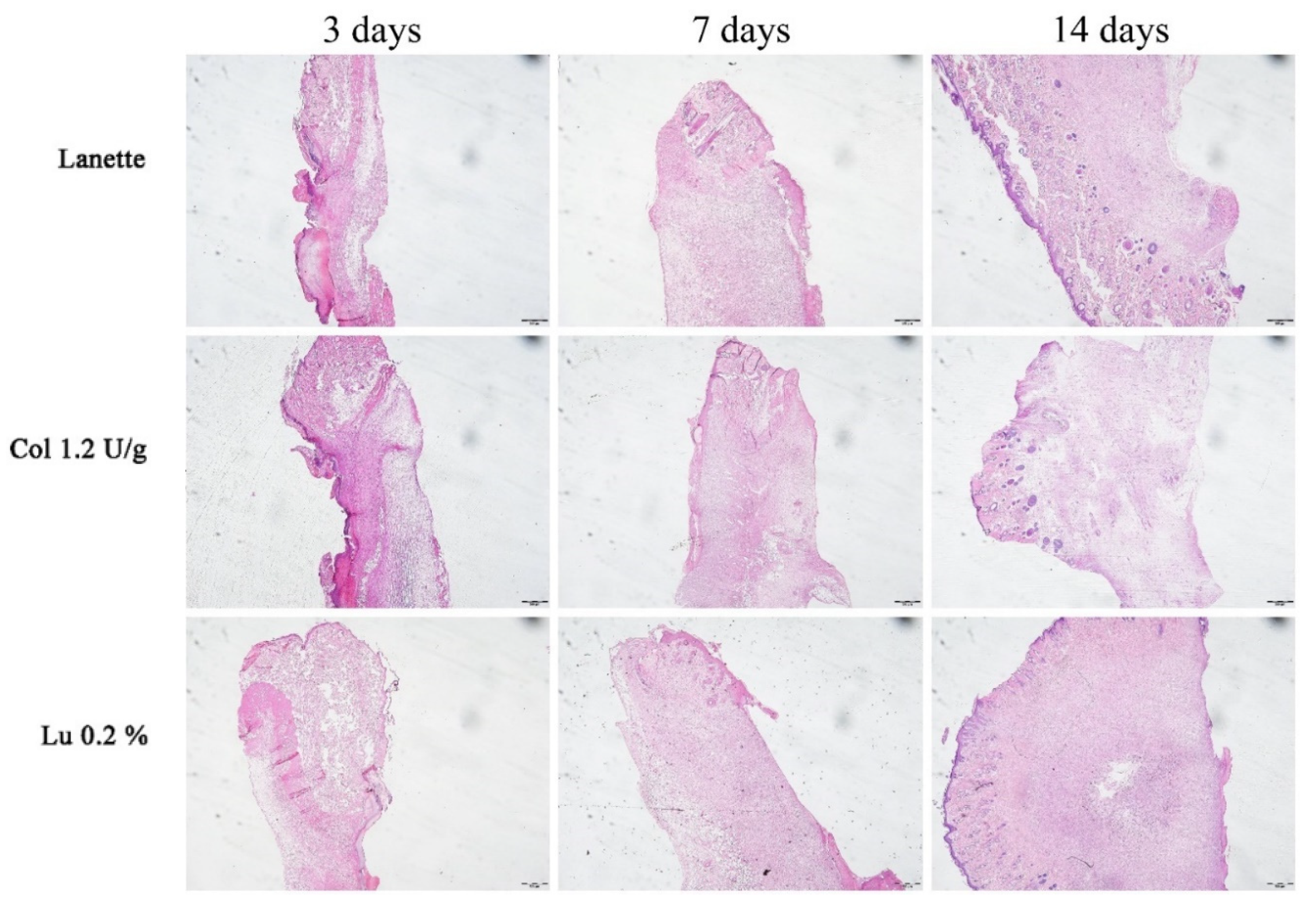
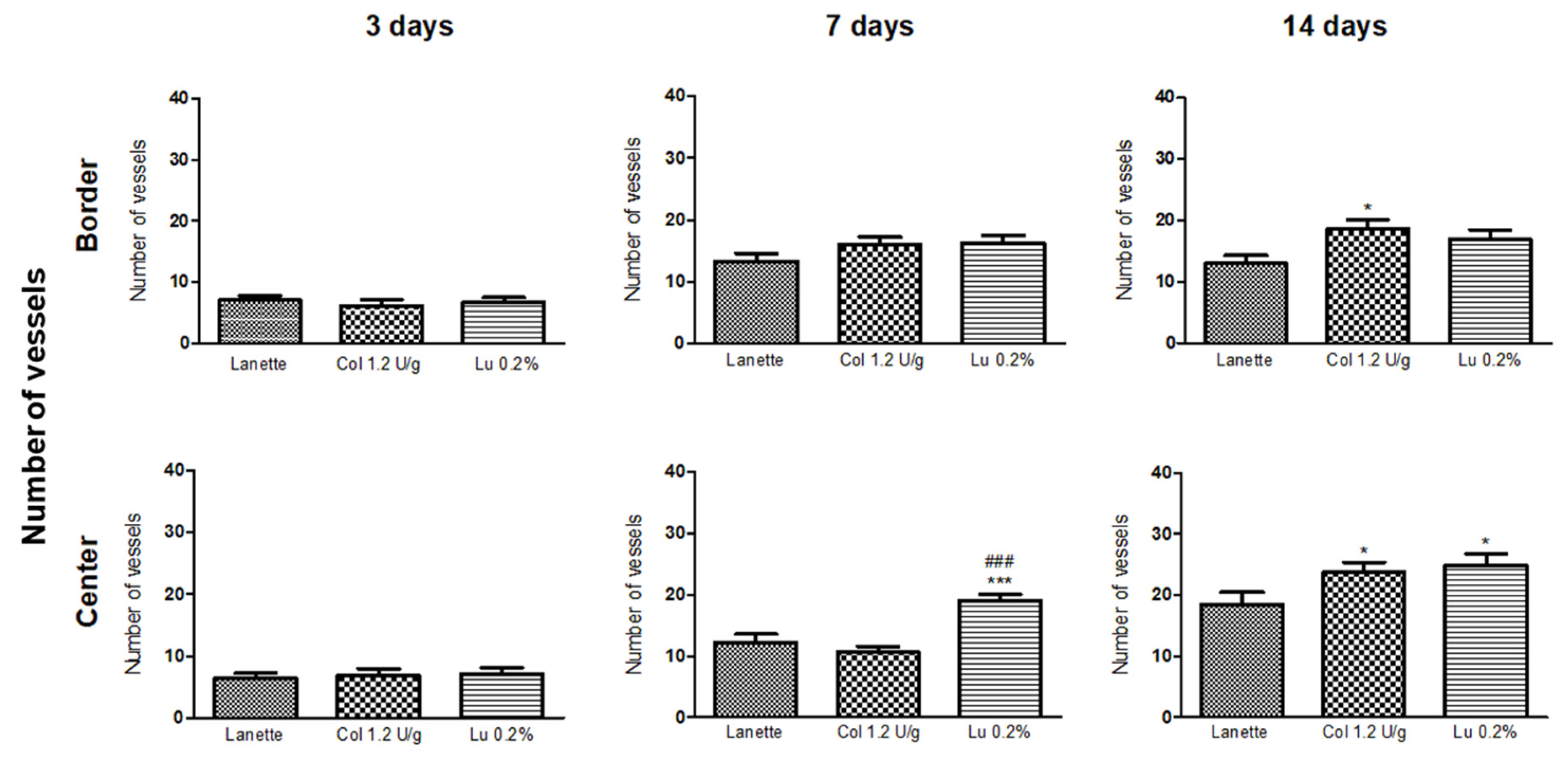
2.2. Histological Examination
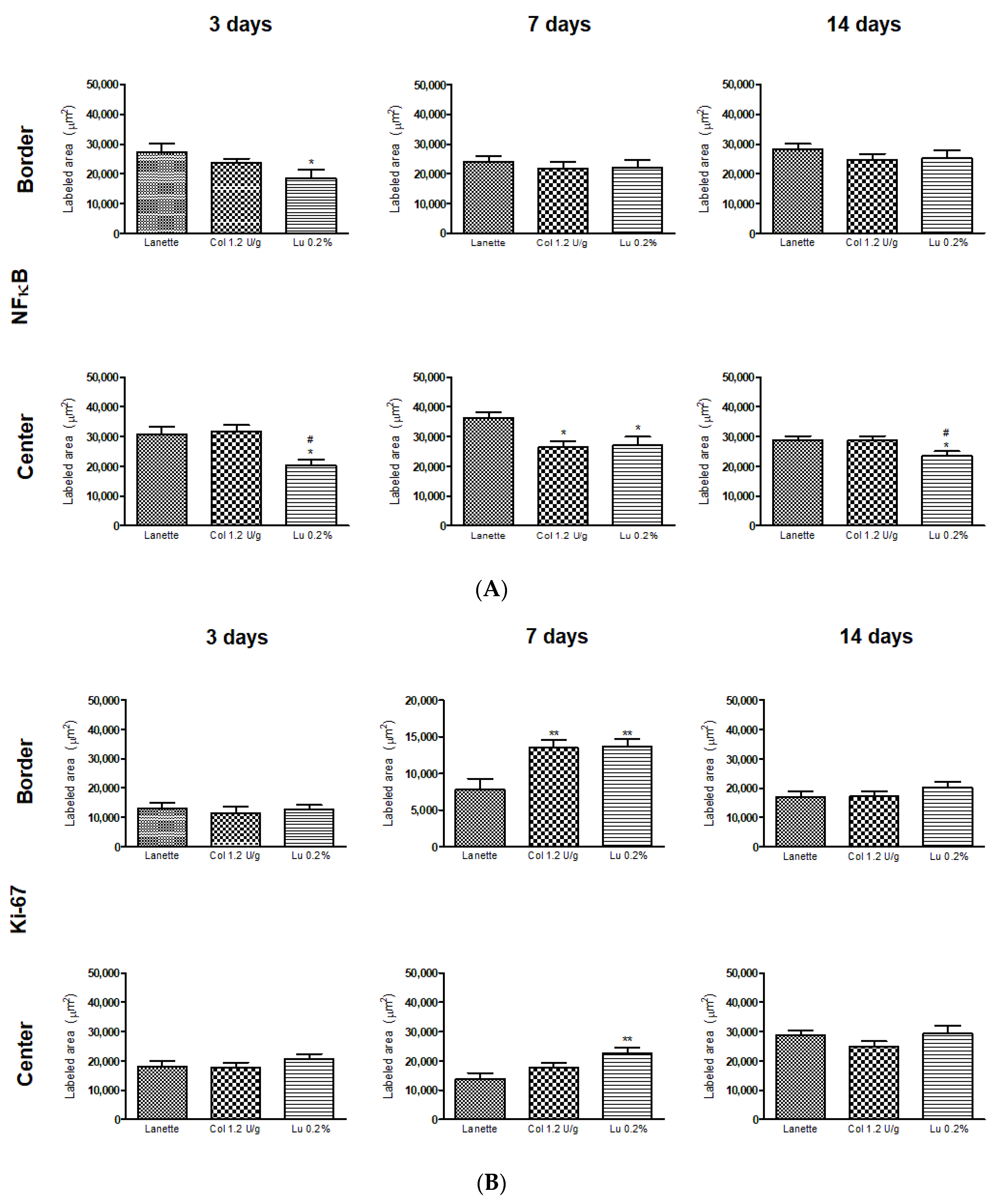
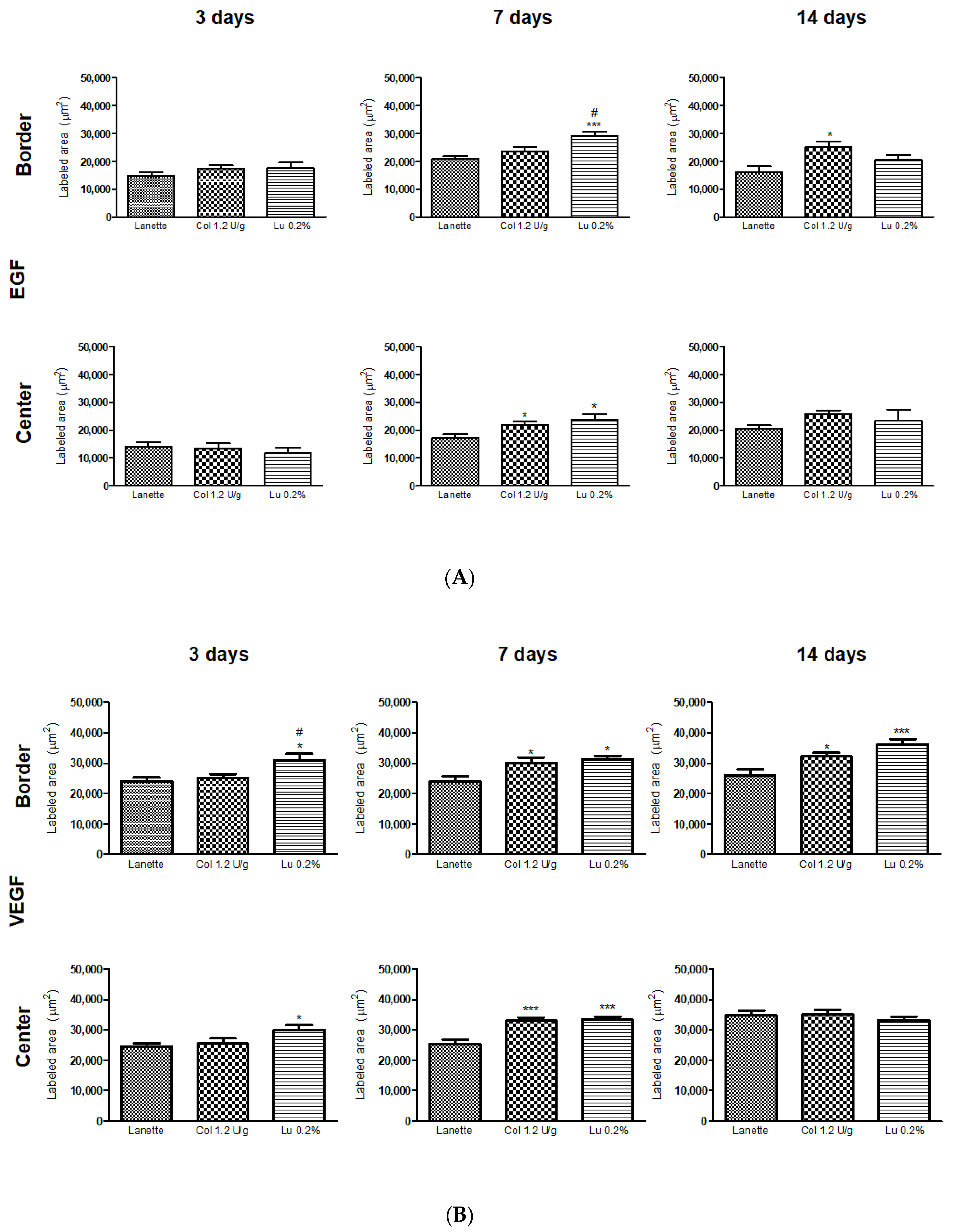
2.3. Effect of Lupeol on the Immunostaining of NF-κB, Ki-67, EGF, and VEGF
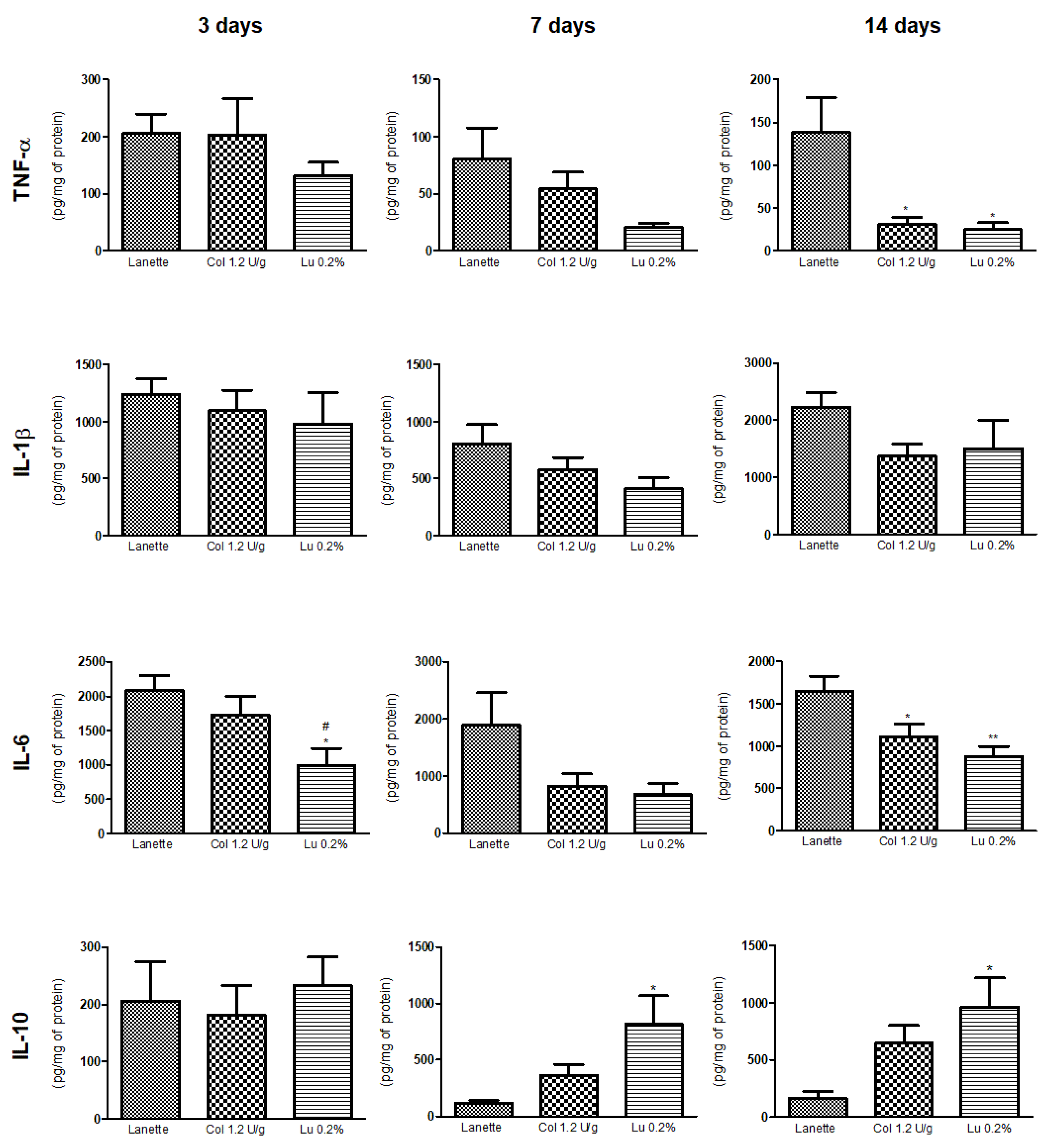
2.4. Effect of Lupeol on Pro- and Anti-Inflammatory Cytokine Levels
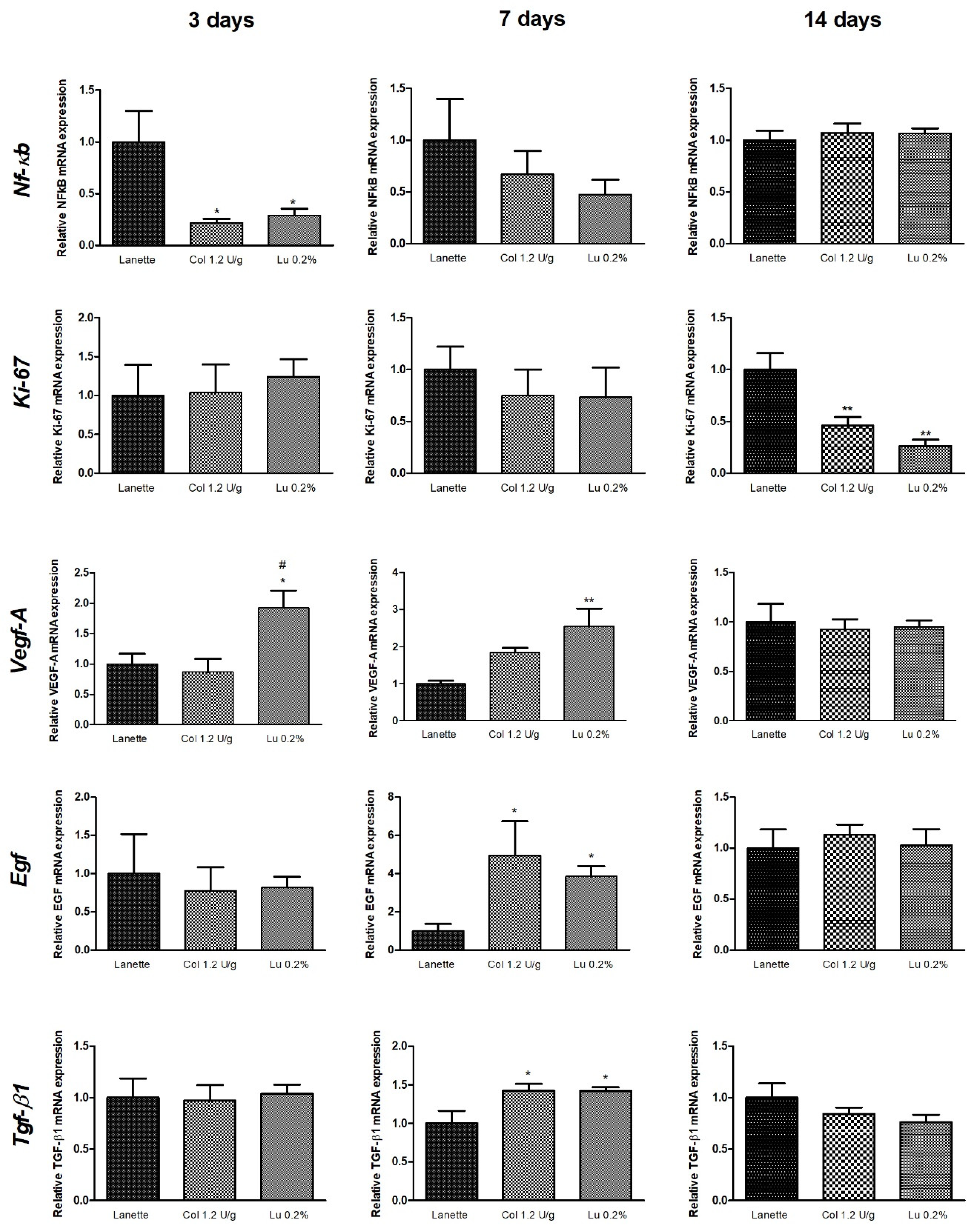
2.5. Effect of Lupeol on Nf-κb, Ki-67, Egf, Vegf-A, and Tgf-β1 mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction and Isolation of Lupeol
4.2. Animals
4.3. Excision Wound Model
4.4. Grouping and Topical Treatment
- Group I—Topically treated with Lanette cream (vehicle)
- Group II—Topically treated with collagenase 1.2 U/g (reference drug)
- Group III—Topically treated with 0.1% w/w lupeol cream (substance test)
- Group IV—Topically treated with 0.2% w/w lupeol cream (substance test)
- Group V—Topically treated with 0.4% w/w lupeol cream (substance test)
4.5. Determination of Wound Retraction Percentage
4.6. Macroscopic Examination
4.7. Histological Analysis
4.8. Immunohistochemistry Analysis
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. RNA Extraction and Reverse Transcription Quantitative PCR (RT-qPCR)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| ANOVA | One-way analysis of variance |
| cDNA | Complementary DNA |
| Cq | Quantification cycle |
| Ct | Cycle threshold |
| ddCT | Overall average of cycle threshold |
| ERK | Extracellular-signal-regulated kinase |
| EGF | Epidermal growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| EtOHE | Ethanolic extract |
| FGF-2 | Fibroblast growth factor-2 |
| FW | Forward |
| HE | Hematoxylin and eosin |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin 6 |
| Ki-67 | Antigen Ki-67 |
| MAPK | Mitogen-activated protein kinase kinase kinases |
| mRNA | Messenger RNA |
| NCBI | National Center for Biotechnology Information |
| NF-κB | Factor nuclear kappa B |
| PCR | Polymerase chain reaction |
| PDGF | Platelet-derived growth factor |
| PI3K | Phosphatidylinositol-3-kinase |
| RT-qPCR | Quantitative reverse transcription PCR |
| RV | Reverse |
| SC | Subcutaneous |
| SEM | Standard error of the mean |
| TGF-β1 | Transforming growth factor-beta 1 |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
References
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Morin, C.; Roumegous, A.; Carpentier, G.; Barbier-Chassefière, V.; Garrigue-Antar, L.; Caredda, S.; Courty, J. Modulation of inflammation by Cicaderma ointment accelerates skin wound healing. J. Pharmacol. Exp. Ther. 2012, 343, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.O.; Yesil-Celiktas, O.; Cavusoglu, T.; Demirel-Sezer, E.; Akdemir, O.; Uyanikgil, Y. Incision wound healing activity of pine bark extract containing topical formulations: A study with histopathological and biochemical analyses in albino rats. Pharmazie 2013, 68, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chattopadhyay, P.; Goyary, D.; Mazumder, P.M.; Veer, V. Eleutherine indica L. accelerates in vivo cutaneous wound healing by stimulating Smad-mediated collagen production. J. Ethnopharmacol. 2013, 146, 490–494. [Google Scholar] [CrossRef]
- Thomé, R.G.; Santos, H.B.; Santos, F.V.; Oliveira, R.J.S.O.; Camargos, L.F.; Pereira, M.N.; Longatti, T.R.; Souto, C.M.; Franco, C.S.; Schüffner, R.O.A.; et al. Evaluation of healing wound and genotoxicity potentials from extracts hydroalcoholic of Plantago major and Siparuna guianensis. Exp. Biol. Med. 2012, 237, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Alam, A.A.S.; Shan, M.S.; Ahmed, B.; Sultana, S. Lupeol, a triterpene, inhibits early responses of tumor promotion induced by benzoyl peroxide in murine skin. Pharmacol. Res. 2001, 43, 127–134. [Google Scholar] [CrossRef]
- Sudhahar, V.; Kumar, S.A.; Varalakshmi, P. Role of lupeol and lupeol linoleate on lipemic-oxidative stress in experimental hypercholesterolemia. Life Sci. 2006, 78, 1329–1335. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [CrossRef]
- Yokoe, I.; Azuma, K.; Hata, K.; Mukaiyama, T.; Goto, T.; Tsuka, T.; Imagawa, T.; Itoh, N.; Murahata, Y.; Osaki, T.; et al. Clinical systemic lupeol administration for canine oral malignant melanoma. Mol. Clin. Oncol. 2015, 3, 89–92. [Google Scholar] [CrossRef]
- Badshah, H.; Ali, T.; Rehman, S.U.; Amin, F.U.; Ullah, F.; Kim, T.H.; Kim, M.O. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol. 2016, 11, 48–60. [Google Scholar] [CrossRef]
- Beserra, F.P.; Xue, M.; Maia, G.L.A.; Rozza, A.L.; Pellizzon, C.H.; Jackson, C.J. Lupeol, a Pentacyclic Triterpene, Promotes Migration, Wound Closure, and Contractile Effect In Vitro: Possible Involvement of PI3K/Akt and p38/ERK/MAPK Pathways. Molecules 2018, 23, 23–2819. [Google Scholar] [CrossRef] [PubMed]
- Harish, B.G.; Krishna, V.; Santosh Kumar, H.S.; Khadeer, A.B.M.; Sharath, R.; Kumara, S.H.M. Wound healing activity and docking of glycogen-synthase-kinase-3-β-protein with isolated triterpenoid lupeol in rats. Phytomedicine 2008, 15, 763–767. [Google Scholar] [CrossRef]
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.S.; Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.M.; Jackson, C.J.; Maia, G.L.A.; et al. Lupeol, a Dietary Triterpene, Enhances Wound Healing in Streptozotocin-Induced Hyperglycemic Rats with Modulatory Effects on Inflammation, Oxidative Stress, and Angiogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.; Angeli-Gamba, T.; Alessandra-Perini, J.; Ferreira, L.C.; Nasciutti, L.E.; Machado, D.E. Topical application of Acheflan on rat skin injury accelerates wound healing: A histopathological, immunohistochemical and biochemical study. BMC Complement. Altern. Med. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tallis, A.; Motley, T.A.; Wunderlich, R.P.; Dickerson, J.E., Jr.; Waycaster, C.; Slade, H.B.; Collagenase Diabetic Foot Ulcer Study Group. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: Results of a randomized controlled study. Clin. Ther. 2013, 35, 1805–1820. [Google Scholar] [CrossRef] [PubMed]
- Mekkes, J.R.; Zeegelaar, J.E.; Westerhof, W. Quantitative and objective evaluation of wound debriding properties of collagenase and fibrinolysin/desoxyribonuclease in a necrotic ulcer animal model. Arch. Dermatol. Res. 1998, 290, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Durmus, A.S.; Han, M.C.; Yaman, I. Comperative evaluation of collagenase and silver sulfadiazine on burned wound healing in rats. Firat Universitesi Saglik Bilimleri Veteriner Dergisi 2009, 23, 135–139. [Google Scholar]
- Agha, R.; Ogawa, R.; Pietramaggiori, G.; Orgill, D.P. A Review of the Role of Mechanical Forces in Cutaneous Wound Healing. J. Surg. Res. 2011, 171, 700–708. [Google Scholar] [CrossRef]
- Karin, M. Inflammation and cancer: The long reach of Ras. Nat. Med. 2005, 11, 20–21. [Google Scholar] [CrossRef]
- Wagner, W.; Wehrmann, M. Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin. J. Cell. Mol. Med. 2007, 11, 1342–1351. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Sato, Y.; Ohshima, T.; Kondo, T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem. Biophys. Res. Commun. 1999, 265, 194–199. [Google Scholar] [CrossRef]
- Silvestre, J.S.; Mallat, Z.; Duriez, M.; Tamarat, R.; Bureau, M.F.; Scherman, D.; Duverger, N.; Branellec, D.; Tedgui, A.; Levy, B.I. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 2000, 87, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Nguemfo, E.L.; Dimo, T.; Dongmo, A.B.; Azebaze, A.G.; Alaoui, K.; Asongalem, A.E.; Cherrah, Y.; Kamtchouing, P. Anti-oxidative and anti-inflammatory activities of some isolated constituents from the stem bark of Allanblackia monticola Staner L.C (Guttiferae). Inflammopharmacology 2009, 17, 37–41. [Google Scholar] [CrossRef]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, S.; Lim, Y.M.; Gwon, H.J.; Park, J.S.; Nho, Y.C.; Kwon, J. Hydrogel incorporated with chestnut honey accelerates wound healing and promotes early HO-1 protein expression in diabetic (db/db) mice. J. Tissue Eng. Regen. Med. 2012, 9, 36–42. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Yoshida, S.; Matsumoto, K.; Tomioka, D.; Bessho, K.; Itami, S.; Yoshikawa, K.; Nakamura, T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 2004, 22, 111–119. [Google Scholar] [CrossRef]
- Chou, E.; Suzuma, I.; Way, K.J.; Opland, D.; Clermont, A.C.; Naruse, K.; Suzuma, K.; Bowling, N.L.; Vlahos, C.J.; Aiello, L.P.; et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states. Circulation 2002, 105, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef]
- Canesso, M.C.C.; Vieira, A.T.; Castro, T.B.R.; Sechirmer, B.A.; Cisalpino, D.; Martins, F.S.; Rachid, M.A.; Nicoli, J.R.; Teixeira, M.M.; Barcelos, L.S. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J. Immunol. 2014, 193, 5171–5180. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Asai, J.; Ii, M.; Thorne, T.; Losordo, D.W.; D’Amore, P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. [Google Scholar] [CrossRef]
- Suryati, S.; Nurdin, H.; Dachriyanus, D.; Lajis, M.N.H. Structure elucidation of antibacterial compound from Ficus deltoidea Jack leaves. Indones. J. Chem. 2011, 11, 67–70. [Google Scholar] [CrossRef]
- Beserra, F.P. Mechanisms Involved in the Cutaneous Wound Healing Effect of Lupeol Isolated from the Stem Bark of Bowdichia virgilioides Kunth. (Fabaceae) in Experimental Models in vivo and in vitro. Doctoral Thesis, São Paulo State University, Botucatu, SP, Brazil, 1 March 2019. [Google Scholar]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Karaman, E.F.; Aydoğmuş, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Hussni, C.A.; Bastos, J.K.; Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Padovani, C.R.; Lemos, M.; Polizello-Junior, M.; da Silva, J.J.M.; et al. Skin Wound Healing Potential and Mechanisms of the Hydroalcoholic Extract of Leaves and Oleoresin of Copaifera langsdorffii Desf. Kuntze in Rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 16. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Bezerra, B.M.; Leite, L.O.; Girão, V.C.; Nunes-Pinheiro, D.C. Topical continuous use of Lippia sidoides Cham. essential oil induces cutaneous inflammatory response, but does not delay wound healing process. J. Ethnopharmacol. 2014, 153, 283–289. [Google Scholar] [CrossRef]
- Krohn, R.I. The colorimetric detection and quantitation of total protein. Curr. Protoc. Cell Biol. 2002, 15, A.3H.1–A.3H.28. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de Franca, L.R.; Schulz, R.W. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar] [CrossRef] [PubMed]
- Vischer, H.F.; Teves, A.C.; Ackermans, J.C.; van Dijk, W.; Schulz, R.W.; Bogerd, J. Cloning and spatiotemporal expression of the follicle-stimulating hormone beta subunit complementary DNA in the African catfish (Clarias gariepinus). Biol. Reprod. 2003, 68, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
 ) sebaceous glands.
) sebaceous glands.
 ) sebaceous glands.
) sebaceous glands.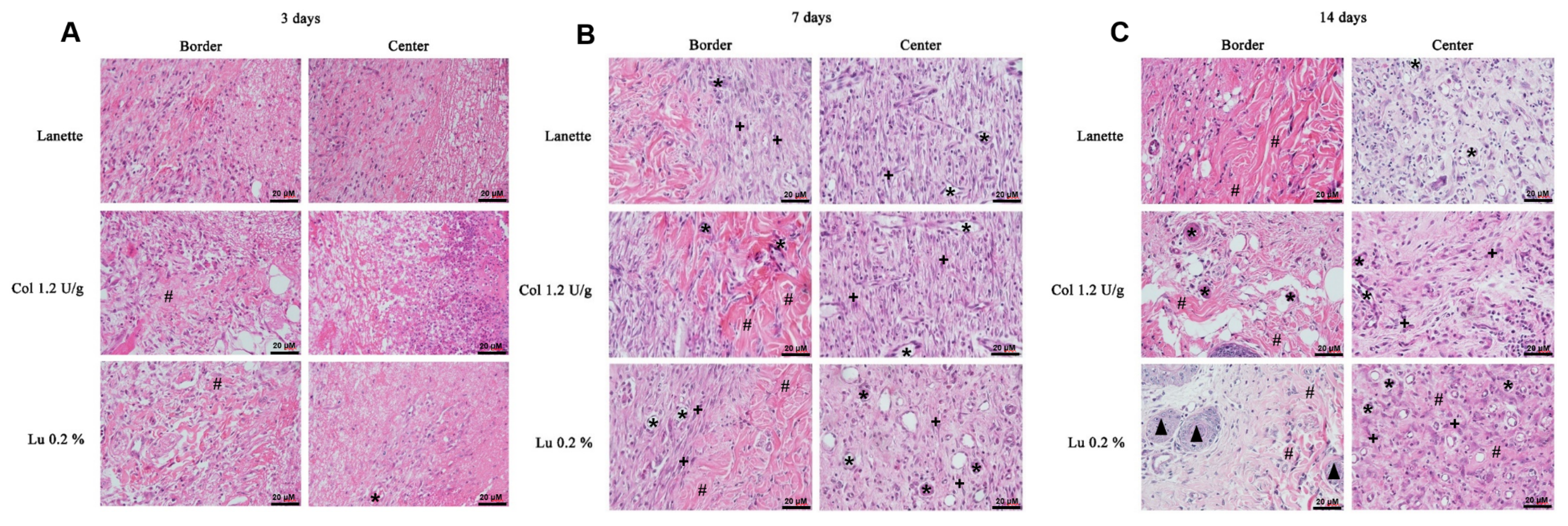
 ) indicates the presence of total collagen fibers and (
) indicates the presence of total collagen fibers and ( ) sebaceous glands.
) sebaceous glands.
 ) indicates the presence of total collagen fibers and (
) indicates the presence of total collagen fibers and ( ) sebaceous glands.
) sebaceous glands.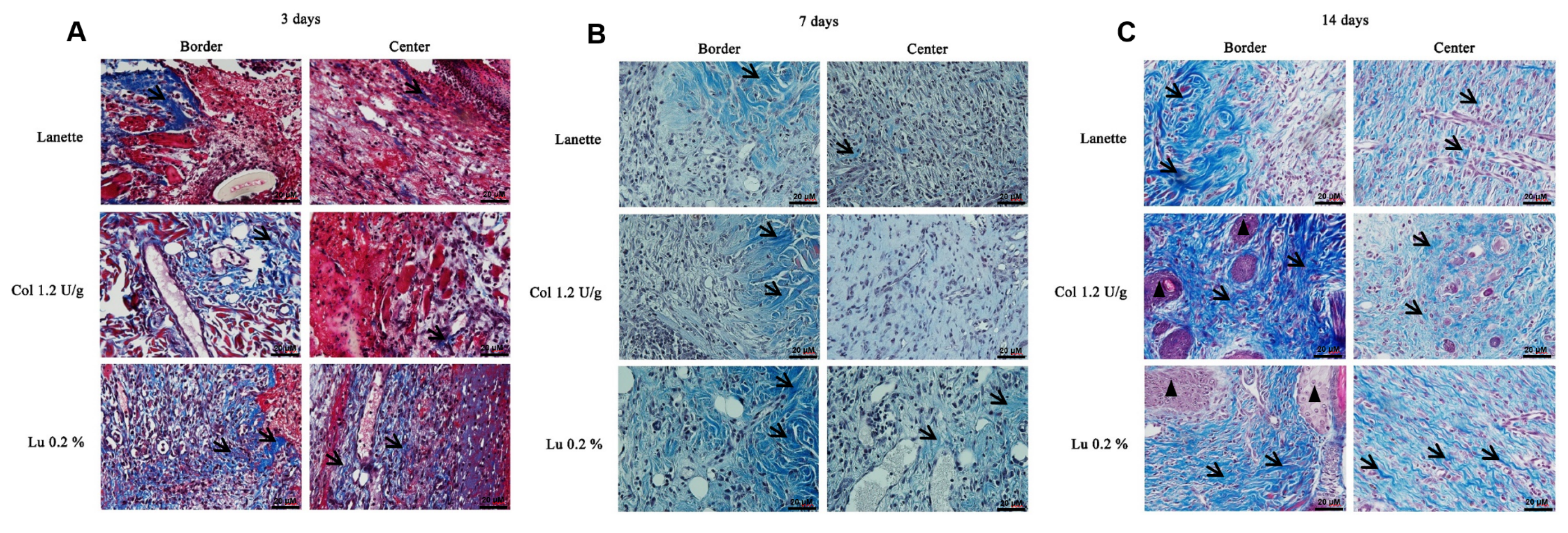



| Parameters | Groups | Days after Wound Induction | ||
|---|---|---|---|---|
| 3 Days | 7 Days | 14 Days | ||
| Exudation | Lanette | 2 (2, 3) | 1 (0, 3) | – |
| Collagenase | 1 (0, 2) * | 0 (0, 1) | – | |
| Lupeol 0.1% | 1 (1, 2) | 0 (0, 1) | – | |
| Lupeol 0.2% | 1 (0, 3) * | 0 (0, 1) | – | |
| Lupeol 0.4% | 1 (0, 3) * | 0 (0, 1) | – | |
| Edema | Lanette | 2 (1, 3) | – | – |
| Collagenase | 1 (0, 2) | – | – | |
| Lupeol 0.1% | 1 (1, 3) | – | – | |
| Lupeol 0.2% | 1 (0, 2) | – | – | |
| Lupeol 0.4% | 1 (0, 3) | – | – | |
| Hemorrhage | Lanette | 1 (0, 3) | 1 (0, 2) | – |
| Collagenase | 0.5 (0, 1) | 0 (0, 1) | – | |
| Lupeol 0.1% | 1 (0, 2) | 0.5 (0, 2) | – | |
| Lupeol 0.2% | 0 (0, 2) | 0 (0, 1) | – | |
| Lupeol 0.4% | 0 (0, 1) | 0 (0, 2) | – | |
| Presence of crust | Lanette | 0 (0, 2) | 1.5 (0, 3) | 1 (1, 3) |
| Collagenase | 1 (0, 3) | 2 (1, 3) | 1 (0, 3) | |
| Lupeol 0.1% | 0.5 (0, 2) | 2.5 (1, 3) | 0.5 (0, 2) | |
| Lupeol 0.2% | 1 (0, 3) | 3 (2, 3) | 0.5 (0, 1) * | |
| Lupeol 0.4% | 1.5 (0, 3) | 3 (2, 3) * | 0.5 (0, 1) * | |
| Granulation | Lanette | 0 (0, 1) | 1 (0, 2) | 2 (1, 3) |
| Collagenase | 1 (0, 2) | 2 (1, 3) | 1 (1, 3) | |
| Lupeol 0.1% | 1 (0, 2) | 2 (1, 3) | 1 (0, 2) | |
| Lupeol 0.2% | 1 (0, 1) | 2 (2, 3) * | 1 (0, 2) | |
| Lupeol 0.4% | 1 (0, 2) | 2.5 (1, 3) * | 1 (0, 2) | |
| Gene | Primers Sequence 5′-3′ | Product Size | Melting Temperature | Access Number * |
|---|---|---|---|---|
| β-actin | FW: CCCTGGCTCCTAGCACCAT RV: GATAGAGCCACCAATCCACACA | 80 pb | 60 °C | NM_031144.3 |
| Nf-κb | FW: CCTCATCTTTCCCTCAGAGCC RV: CGCACTTGTAACGGAAACGC | 98 pb | 60 °C | NM_199267.2 |
| Ki-67 | FW: GGGTTTCCAGACACCAGACC RV: CCAGGAAGACCAGTTAGAACC | 100 pb | 60 °C | NM_001271366.1 |
| Egf | FW: CTCAGGCCTCTGACTCCGAA RV: ATGCCGACGAGTCTGAGTTG | 93 pb | 60 °C | NM_012842.1 |
| Vegf-A | FW: TGCGGATCAAACCTCACCAA RV: GGCTCACAGTGATTTTCTGGC | 115 pb | 60 °C | NM_001110333.2 |
| Tgf-β1 | FW: GGGCTACCATGCCAACTTCTG RV: GAGGGCAAGGACCTTGCTGTA | 82 bp | 60 °C | NM_021578.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira Beserra, F.; Sérgio Gushiken, L.F.; Vieira, A.J.; Augusto Bérgamo, D.; Luísa Bérgamo, P.; Oliveira de Souza, M.; Alberto Hussni, C.; Kiomi Takahira, R.; Henrique Nóbrega, R.; Monteiro Martinez, E.R.; et al. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. Int. J. Mol. Sci. 2020, 21, 4952. https://doi.org/10.3390/ijms21144952
Pereira Beserra F, Sérgio Gushiken LF, Vieira AJ, Augusto Bérgamo D, Luísa Bérgamo P, Oliveira de Souza M, Alberto Hussni C, Kiomi Takahira R, Henrique Nóbrega R, Monteiro Martinez ER, et al. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. International Journal of Molecular Sciences. 2020; 21(14):4952. https://doi.org/10.3390/ijms21144952
Chicago/Turabian StylePereira Beserra, Fernando, Lucas Fernando Sérgio Gushiken, Ana Júlia Vieira, Danilo Augusto Bérgamo, Patrícia Luísa Bérgamo, Mariana Oliveira de Souza, Carlos Alberto Hussni, Regina Kiomi Takahira, Rafael Henrique Nóbrega, Emanuel Ricardo Monteiro Martinez, and et al. 2020. "From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers" International Journal of Molecular Sciences 21, no. 14: 4952. https://doi.org/10.3390/ijms21144952
APA StylePereira Beserra, F., Sérgio Gushiken, L. F., Vieira, A. J., Augusto Bérgamo, D., Luísa Bérgamo, P., Oliveira de Souza, M., Alberto Hussni, C., Kiomi Takahira, R., Henrique Nóbrega, R., Monteiro Martinez, E. R., John Jackson, C., Lemos de Azevedo Maia, G., Leite Rozza, A., & Helena Pellizzon, C. (2020). From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. International Journal of Molecular Sciences, 21(14), 4952. https://doi.org/10.3390/ijms21144952