Calmodulin-Cork Model of Gap Junction Channel Gating—One Molecule, Two Mechanisms
Abstract
1. Introduction
2. Why Calmodulin?
2.1. Gating is Activated by Nanomolar Calcium Concentrations [Ca2+]i
2.2. CaM Inhibitors Prevent Chemical Gating
2.3. Inhibition of CaM Expression Prevents Chemical Gating
2.4. A CaM Mutant with Higher Ca2+-Sensitivity Greatly Enhances Gating Sensitivity
- With 15 min CO2 application, in Cx43-TR257 channels, the Gj time-course is biphasic both in the presence and absence of CaMCC, as it rises before dropping (Figure 2B).
2.5. Colocalization of CaM and Connexins
2.6. Connexins Have High-Affinity CaM Binding Sites
2.7. CaM Is Linked to Connexins at Resting [Ca2+]i
2.8. Potential Role of CaM-Activated Enzymes in Chemical Gating?
3. Why Cork?
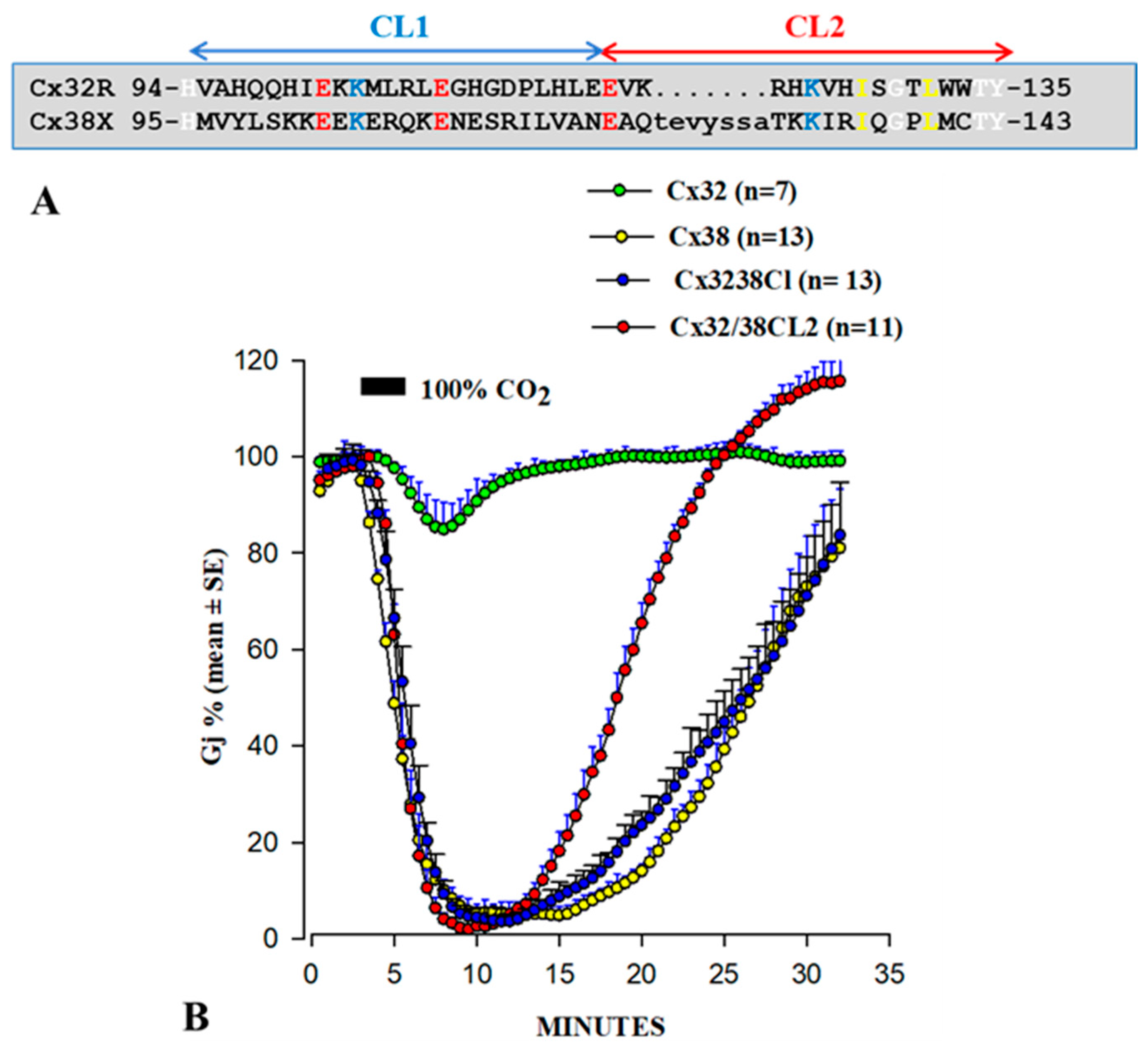
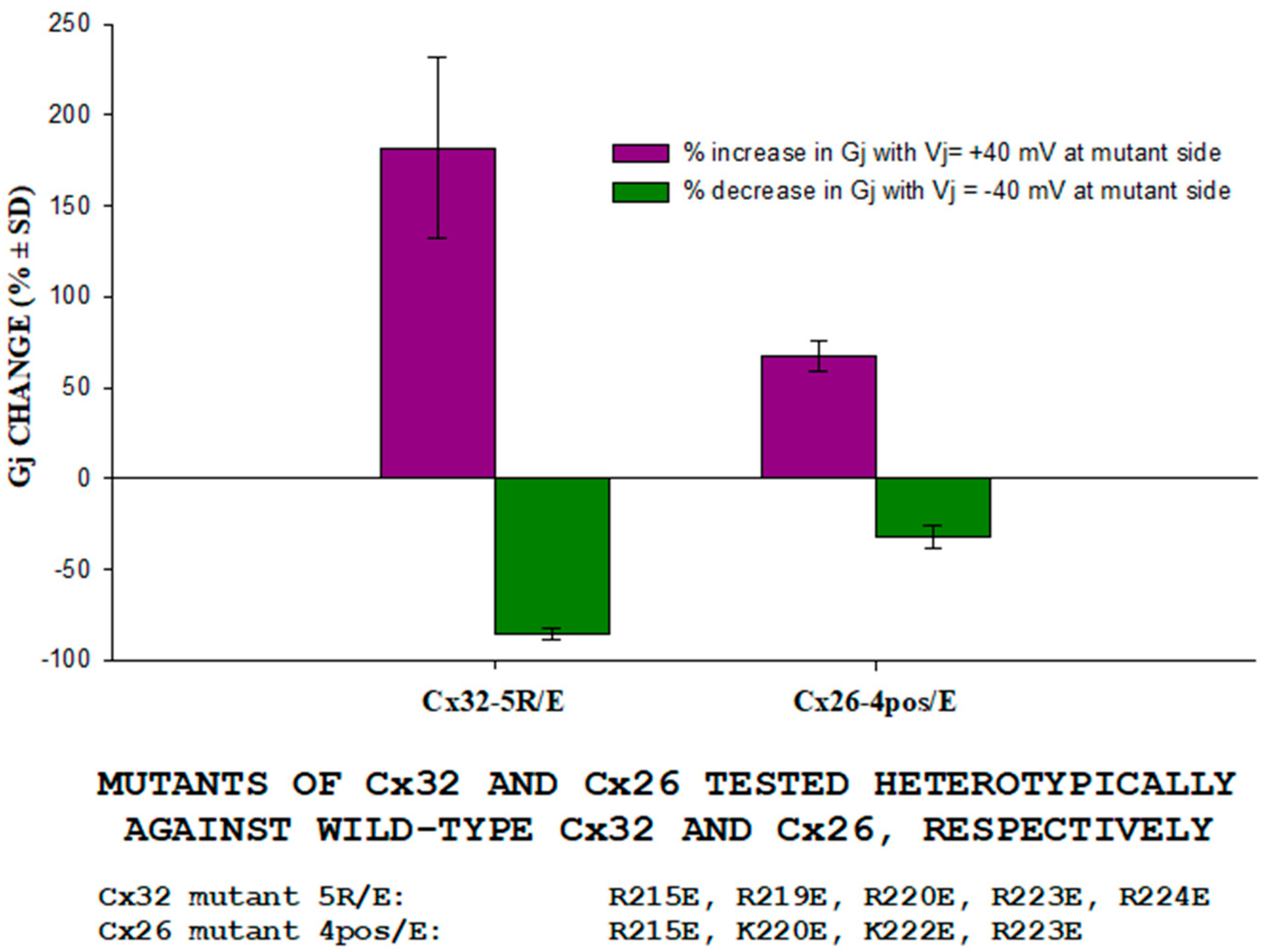
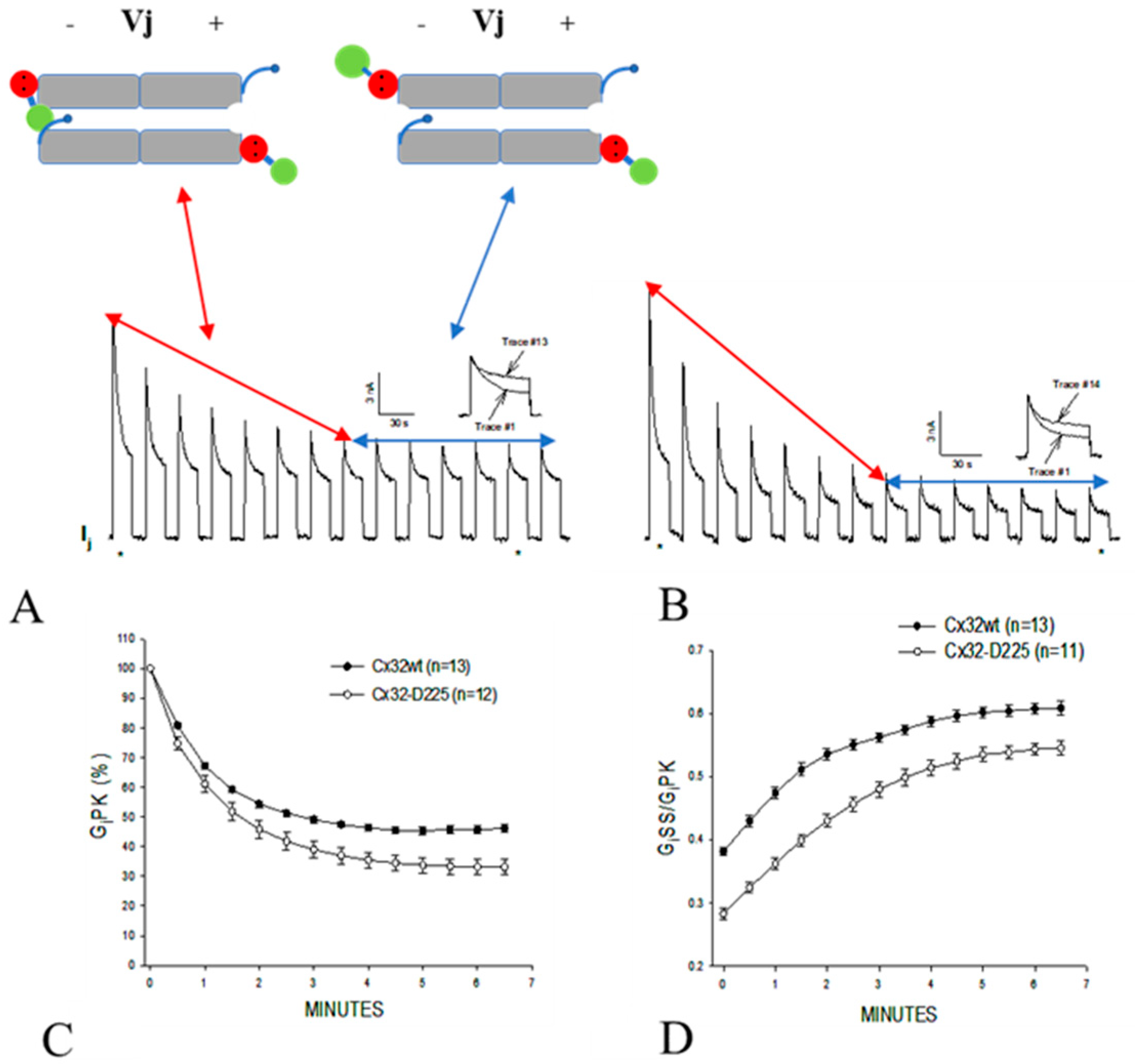
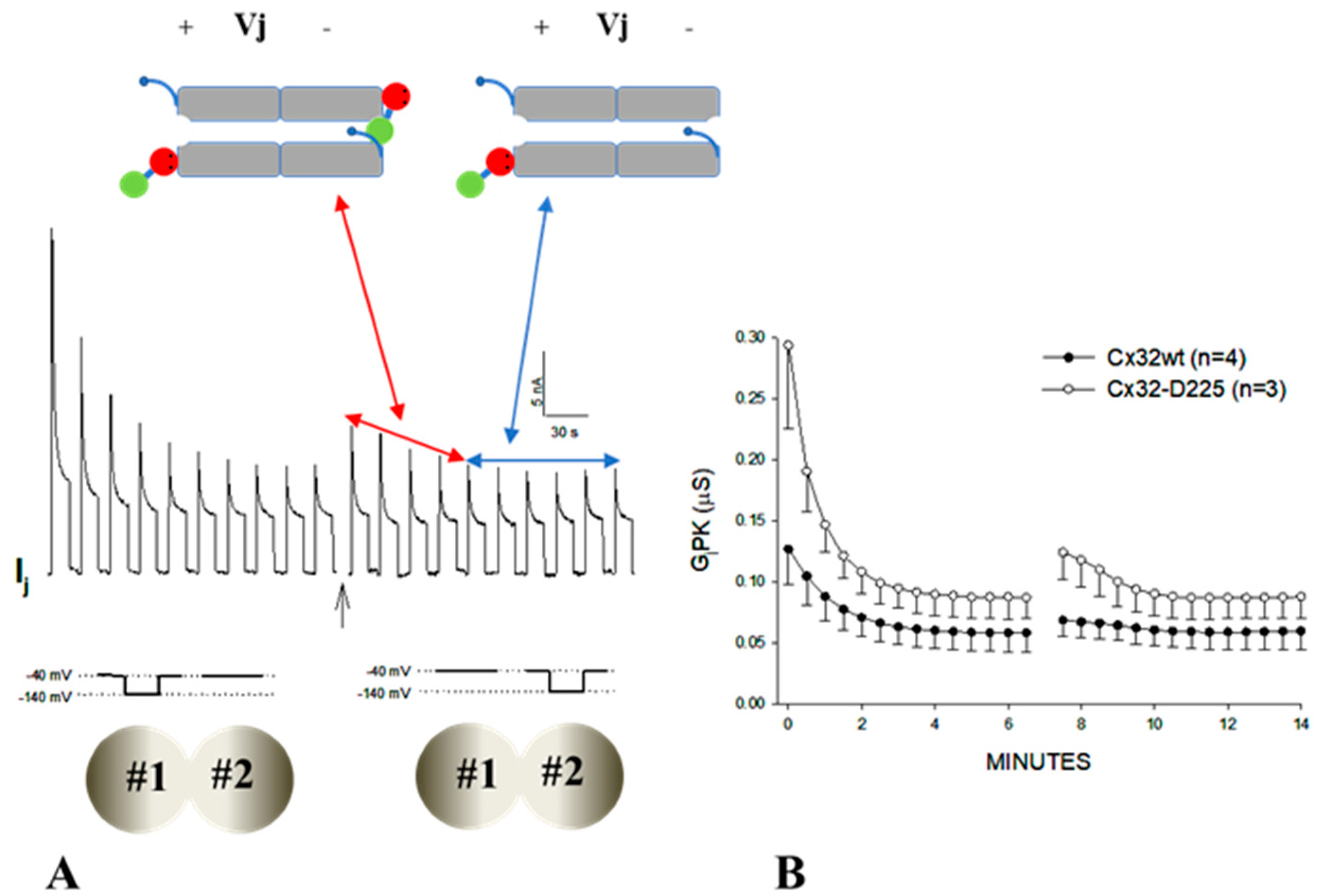
3.1. Behavior of Heterotypic Mutant-Wildtype Channels
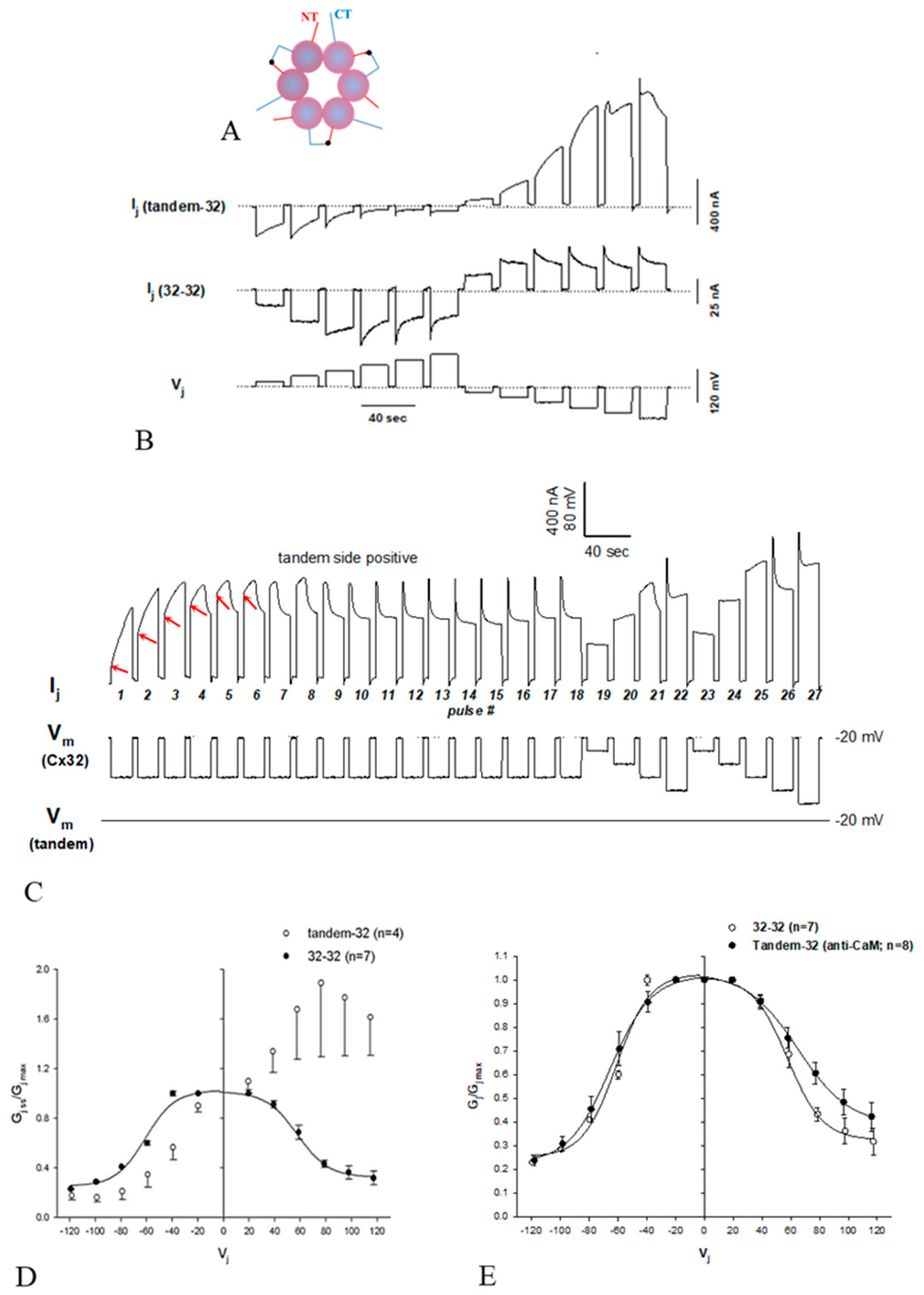
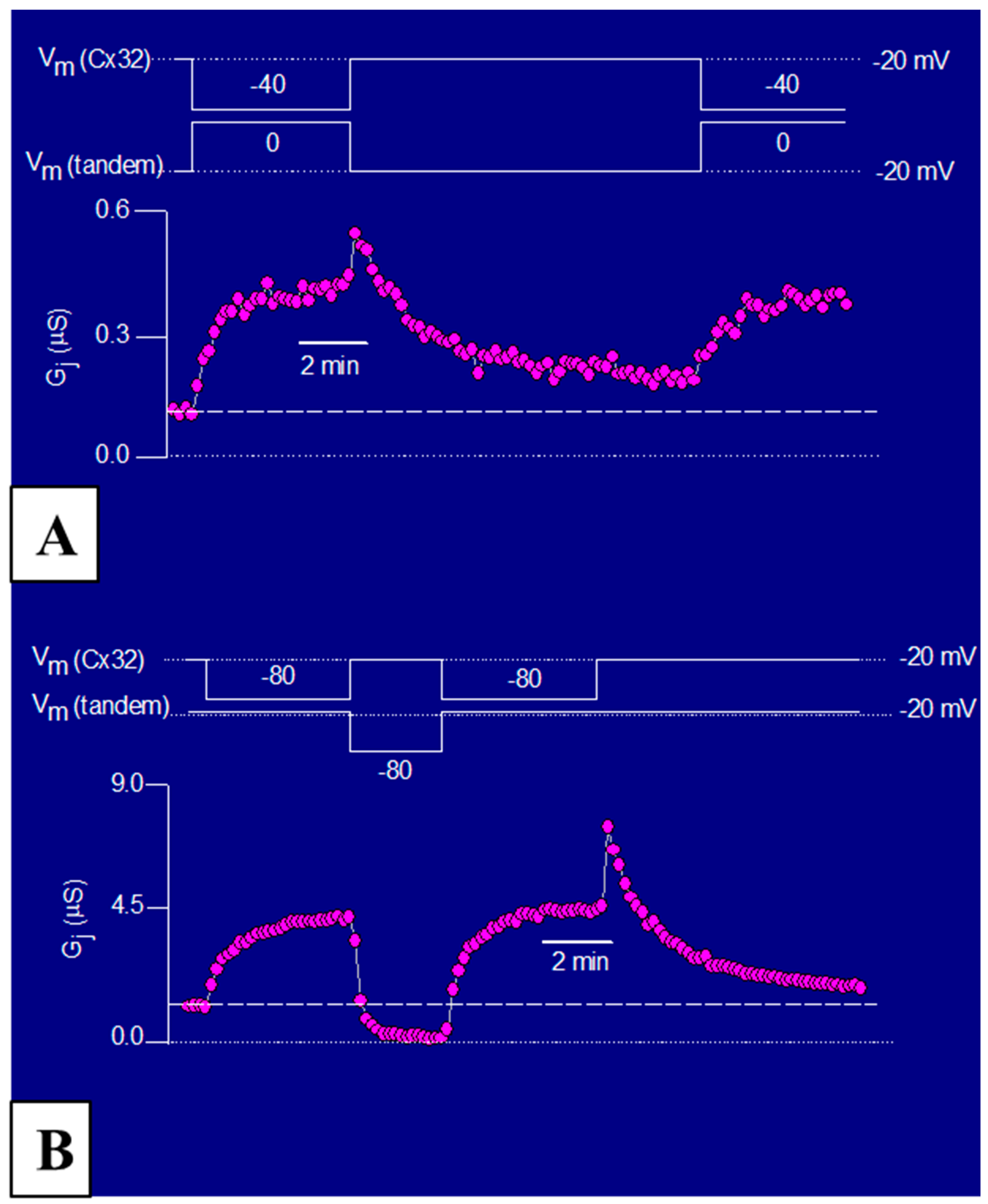
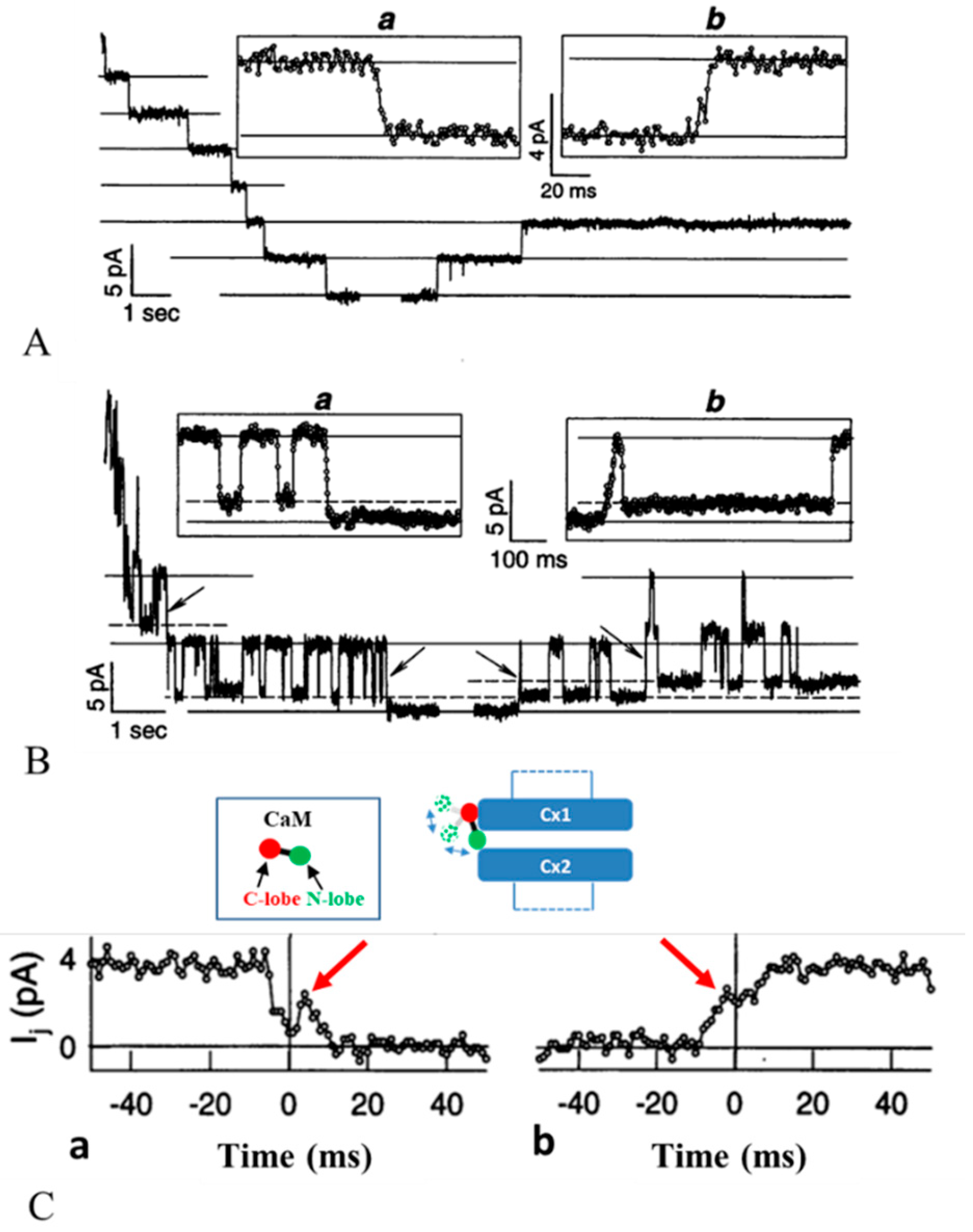
3.2. Chemical/Slow Gating Behavior at the Single-Channel Level
3.3. The Chemical/Slow Gate of Cx32 Channels Closes with Vj Gradients
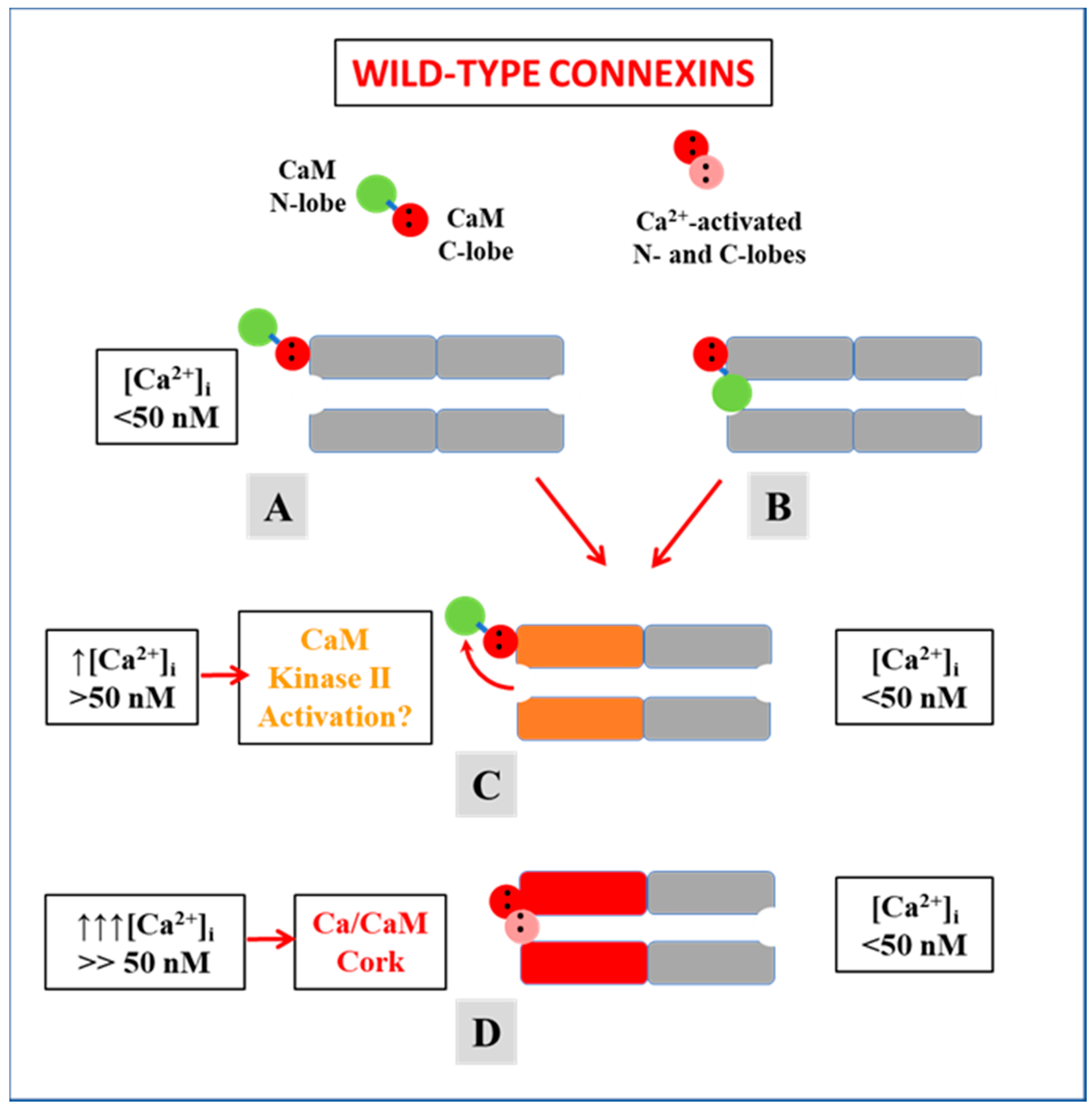
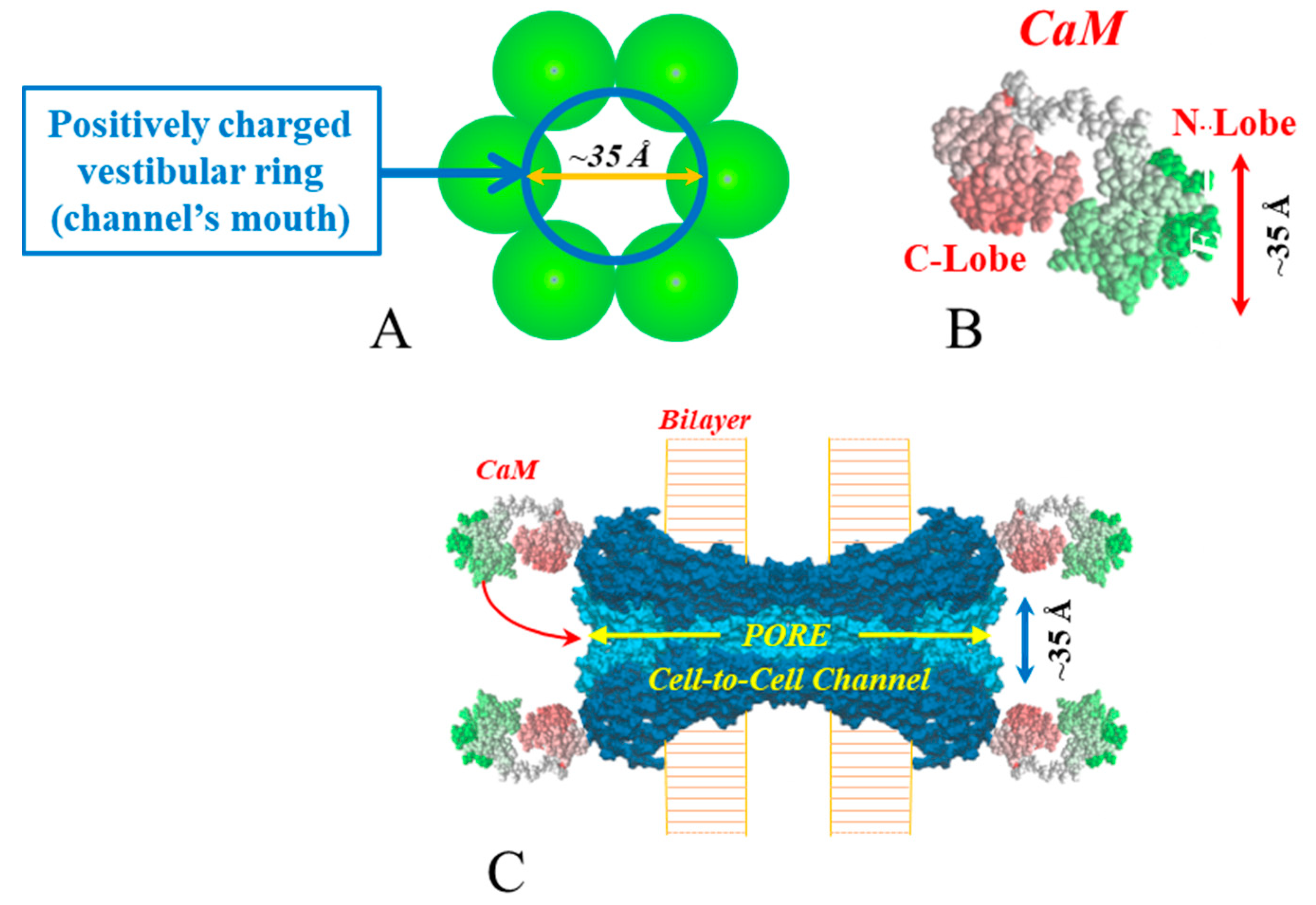
4. Calmodulin Cork Model—One Molecule, Two Mechanisms
4.1. Ca-CaM-Cork Gating
4.2. CaM-Cork Gating
5. Conclusion
- At resting [Ca2+]i, (<50nM) some channels are spontaneously closed by the CaM-Cork gating mechanism.
- With moderate [Ca2+]i rise (50–100 nM, the CaMKII cascade may be activated causing channels closed by the CaM-Cork mechanism to open.
- With greater [Ca2+]i rise (>100 nM), the channels start closing by the Ca-CaM-Cork mechanism. CaM lobe channel mouth plugging is likely to include connexin conformational changes.
- CaM-Cork gated channels could be reopened by Vj positive at gated side, but since they would close at the negative side no Gj change would occur. This is not the case with heterotypic channels between wild-type connexins paired with more gating-sensitive mutants.
- Most Ca-CaM-Cork gated channels reopen with a drop in [Ca2+]i to resting values (<50 nM). However, with prolonged exposure to high [Ca2+]i, channel gating may not be reversible.
Funding
Conflicts of Interest
References
- Peracchia, C. Gap Junction Stucture and Chemical Regulation: Direct Calmodulin Role in Cell-to-Cell Channel Gating; Elsevier: London, UK, 2019. [Google Scholar]
- Harris, A.L.; Locke, D. Connexins—A Guide; Humana Press/Springer: New York, NY, USA, 2009. [Google Scholar]
- Evans, W.H. Cell communication across gap junctions: A historical perspective and current developments. Biochem. Soc. Trans. 2015, 43, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.C.; Peracchia, L.L. Behavior of Chemical and Slow Voltage-Gates of Connexin Channels. The cork Gating Hypothesis. In Gap Junctions—Molecular Basis of Cell Comunication in Health and Disease; Peracchia, C., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 271–295. [Google Scholar]
- Noma, A.; Tsuboi, N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J. Physiol. 1987, 382, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Noma, A.; Tsuboi, N. Direct measurement of the gap junctional conductance under the influence of Ca2+ in dissociated paired myocytes of guinea-pig. Jpn. Heart J. 1986, 27, 161–166. [Google Scholar] [PubMed]
- Dekker, L.R.; Fiolet, J.W.; VanBavel, E.; Coronel, R.; Opthof, T.; Spaan, J.A.; Janse, M.J. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ. Res. 1996, 79, 237–246. [Google Scholar]
- Peracchia, C. Effects of caffeine and ryanodine on low pHi-induced changes in gap junction conductance and calcium concentration in crayfish septate axons. J. Membr. Biol. 1990, 117, 79–89. [Google Scholar] [CrossRef]
- Peracchia, C. Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J. Membr. Biol. 1990, 113, 75–92. [Google Scholar] [CrossRef]
- Peracchia, C.; Wang, X.; Li, L.; Peracchia, L.L. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. Pflüg. Arch. 1996, 431, 379–387. [Google Scholar] [CrossRef]
- Neyton, J.; Trautmann, A. Single-channel currents of an intercellular junction. Nature 1985, 317, 331–335. [Google Scholar] [CrossRef]
- Lazrak, A.; Peracchia, C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys. J. 1993, 65, 2002–2012. [Google Scholar] [CrossRef]
- Lazrak, A.; Peres, A.; Giovannardi, S.; Peracchia, C. Ca-mediated and independent effects of arachidonic acid on gap junctions and Ca-independent effects of oleic acid and halothane. Biophys. J. 1994, 67, 1052–1059. [Google Scholar] [CrossRef]
- Enkvist, M.O.; McCarthy, K.D. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J. Neurochem. 1994, 62, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Venance, L. Characterization and regulation of gap junction channels in cultured astrocytes. In Gap Junctions in the Nervous System; Spray, D.C., Dermietzel, R., Eds.; R.G Landes Medical Pub.: Austin, TX, USA, 1996; pp. 135–157. [Google Scholar]
- Cotrina, M.L.; Kang, J.; Lin, J.H.; Bueno, E.; Hansen, T.W.; He, L.; Liu, Y.; Nedergaard, M. Astrocytic gap junctions remain open during ischemic conditions. J. Neurosci. 1998, 18, 2520–2537. [Google Scholar] [CrossRef]
- Crow, J.M.; Atkinson, M.M.; Johnson, R.G. Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3332–3341. [Google Scholar]
- Dakin, K.; Zhao, Y.; Li, W.H. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat. Methods 2005, 2, 55–62. [Google Scholar] [CrossRef]
- Xu, Q.; Kopp, R.F.; Chen, Y.; Yang, J.J.; Roe, M.W.; Veenstra, R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012, 302, C1548–C1556. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.K.; Petersen, O.H. Pancreatic acinar cells: Ionic dependence of the membrane potential and acetycholine-induced depolarization. J. Physiol. 1973, 231, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Scheele, G.A.; Palade, G.E. Studies on the guinea pig pancreas. Parallel discharge of exocrine enzyme activities. J. Biol. Chem. 1975, 250, 2660–2670. [Google Scholar] [PubMed]
- Iwatsuki, N.; Petersen, O.H. Membrane potential, resistance, and intercellular communication in the lacrimal gland: Effects of acetylcholine and adrenaline. J. Physiol. 1978, 275, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, N.; Petersen, O.H. Pancreatic acinar cells: Acetylcholine-evoked electrical uncoupling and its ionic dependency. J. Physiol. 1978, 274, 81–106. [Google Scholar] [CrossRef]
- Iwatsuki, N.; Petersen, O.H. Electrical coupling and uncoupling of exocrine acinar cells. J. Cell Biol. 1978, 79, 533–545. [Google Scholar] [CrossRef]
- Mears, D.; Sheppard, N.F., Jr.; Atwater, I.; Rojas, E. Magnitude and modulation of pancreatic beta-cell gap junction electrical conductance in situ. J. Membr. Biol. 1995, 146, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Spray, D.C.; Harris, A.L.; Bennett, M.V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 1981, 211, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Possible involvement of caffeine- and rianodine-sensitive calcium stores in low pH-induced regulation of gap junction channels. In Biophysics of Gap Junction Channels; Peracchia, C., Ed.; CRS Press: Boca Raton, FL, USA, 1990; pp. 13–28. [Google Scholar]
- Peracchia, C. Calmodulin-mediated regulation of gap junction channels. Int. J. Mol. Sci. 2020, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Communicating junctions and calmodulin: Inhibition of electrical uncoupling in Xenopus embryo by calmidazolium. J. Membr. Biol 1984, 81, 49–58. [Google Scholar] [CrossRef]
- Peracchia, C.; Bernardini, G.; Peracchia, L.L. Is calmodulin involved in the regulation of gap junction permeability? Pflüg. Arch. 1983, 399, 152–154. [Google Scholar] [CrossRef]
- Peracchia, C.; Bernardini, G.; Peracchia, L.L. A calmodulin inhibitor prevents gap junction crystallization and electrical uncoupling. J. Cell Biol. 1981, 91, 124a. [Google Scholar]
- Lurtz, M.M.; Louis, C.F. Calmodulin and protein kinase C regulate gap junctional coupling in lens epithelial cells. Am. J. Physiol. Cell Physiol. 2003, 285, C1475–C1482. [Google Scholar] [CrossRef][Green Version]
- Peracchia, C. Calmodulin-like proteins and communicating junctions. Electrical uncoupling of crayfish septate axons is inhibited by the calmodulin inhibitor W7 and is not affected by cyclic nucleotides. Pflüg. Arch. 1987, 408, 379–385. [Google Scholar] [CrossRef]
- Wojtczak, J.A. Electrical uncoupling induced by general anesthetics: A calcium-independent process? In Gap Junctions; Bennett, M.V.L., Spray, D.C., Eds.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1985; pp. 167–175. [Google Scholar]
- Tuganowski, W.; Korczynska, I.; Wasik, K.; Piatek, G. Effects of calmidazolium and dibutyryl cyclic AMP on the longitudinal internal resistance in sinus node strips. Pflüg. Arch. 1989, 414, 351–353. [Google Scholar] [CrossRef]
- Gandolfi, S.A.; Duncan, G.; Tomlinson, J.; Maraini, G. Mammalian lens inter-fiber resistance is modulated by calcium and calmodulin. Curr. Eye Res. 1990, 9, 533–541. [Google Scholar] [CrossRef]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Slow gating of gap junction channels and calmodulin. J. Membr. Biol. 2000, 178, 55–70. [Google Scholar] [CrossRef]
- Peracchia, C.; Young, K.C.; Wang, X.G.; Peracchia, L.L. Is the voltage gate of connexins CO2-sensitive? Cx45 channels and inhibition of calmodulin expression. J. Membr. Biol. 2003, 195, 53–62. [Google Scholar] [PubMed]
- Peracchia, C.; Sotkis, A.; Wang, X.G.; Peracchia, L.L.; Persechini, A. Calmodulin directly gates gap junction channels. J. Biol. Chem. 2000, 275, 26220–26224. [Google Scholar] [CrossRef] [PubMed]
- Sotkis, A.; Wang, X.G.; Yasumura, T.; Peracchia, L.L.; Persechini, A.; Rash, J.E.; Peracchia, C. Calmodulin colocalizes with connexins and plays a direct role in gap junction channel gating. Cell Commun. Adhes. 2001, 8, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Persechini, A.; Gansz, K.J.; Paresi, R.J. Activation of myosin light chain kinase and nitric oxide synthase activities by engineered calmodulins with duplicated or exchanged EF hand pairs. Biochemistry 1996, 35, 224–228. [Google Scholar] [CrossRef]
- Morley, G.E.; Taffet, S.M.; Delmar, M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 1996, 70, 1294–1302. [Google Scholar] [CrossRef]
- Delmar, M.; Stergiopoulos, K.; Homma, N.; Ek-Vitorin, J.F.; Taffet, S.M. A ball-and-chain model for chemical regulation of connexin 43. In Gap Junctions; Werner, R., Ed.; IOS Press: Amsterdam, The Netherlands, 1998; pp. 8–12. [Google Scholar]
- Delmar, M.; Stergiopoulos, K.; Homma, N.; Calero, G.; Morley, G.; Ek-Vitorin, J.F.; Taffet, S.M. A molecular model for the chemical regulation of the connexin43 channels: The “ball-and-chain” hypothesis. In Gap Junctions. Molecular Basis of Cell Communication in Health and Disease; Peracchia, C., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 223–248. [Google Scholar]
- Wei, S.; Cassara, C.; Lin, X.; Veenstra, R.D. Calcium-calmodulin gating of a pH-insensitive isoform of connexin43 gap junctions. Biochem. J. 2019, 476, 1137–1148. [Google Scholar] [CrossRef]
- Pereda, A.E.; Bell, T.D.; Chang, B.H.; Czernik, A.J.; Nairn, A.C.; Soderling, T.R.; Faber, D.S. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc. Natl. Acad. Sci. USA 1998, 95, 13272–13277. [Google Scholar] [CrossRef]
- De Pina-Benabou, M.H.; Srinivas, M.; Spray, D.C.; Scemes, E. Calmodulin kinase pathway mediates the K+-induced increase in Gap junctional communication between mouse spinal cord astrocytes. J. Neurosci. 2001, 21, 6635–6643. [Google Scholar] [CrossRef]
- De Vuyst, E.; Wang, N.; Decrock, E.; De, B.M.; Vinken, M.; Van, M.M.; Lai, C.; Culot, M.; Rogiers, V.; Cecchelli, R.; et al. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 2009, 46, 176–187. [Google Scholar] [CrossRef]
- Huang, R.Y.; Laing, J.G.; Kanter, E.M.; Berthoud, V.M.; Bao, M.; Rohrs, H.W.; Townsend, R.R.; Yamada, K.A. Identification of CaMKII phosphorylation sites in Connexin43 by high-resolution mass spectrometry. J. Proteome Res. 2011, 10, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, Y. Role of intramolecular interaction in connexin50: Mediating the Ca2+-dependent binding of calmodulin to gap junction. Arch. Biochem. Biophys. 2005, 440, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Liu, Y.; Qi, Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol. Chem. 2006, 387, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.C.; Smirnova, E.; Brown, C.A.; Zoidl, C.; Spray, D.C.; Donaldson, L.W.; Zoidl, G. Structural and Functional Consequences of Connexin 36 (Cx36) Interaction with Calmodulin. Front. Mol. Neurosci. 2016, 9, 120. [Google Scholar] [CrossRef]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef]
- Zou, J.; Salarian, M.; Chen, Y.; Zhuo, Y.; Brown, N.E.; Hepler, J.R.; Yang, J. Direct Visualization of Interaction between Calmodulin and Connexin45. Biochem. J. 2017, 474, 4035–4051. [Google Scholar] [CrossRef]
- Kerruth, S.; Coates, C.; Rezavi, S.A.; Peracchia, C.; Török, K. Calmodulin interaction with gap junction intracellular loop peptides. Biophys. J 2018, 114, 468a. [Google Scholar] [CrossRef]
- Kerruth, S.; Coates, C.; Rezavi, S.A.; Peracchia, C.; Török, K. Ca2+-dependent and -independent interaction of calmodulin with gap junction cytoplasmic loop peptides. Biochem. J. 2020, submitted. [Google Scholar]
- Peracchia, C. The calmodulin hypoyhesis for gap junction regulation six years later. In Gap Junctions; Hertzberg, E., Johnson, R.G., Alan, R., Eds.; Liss: New York, NY, USA, 1988; pp. 267–282. [Google Scholar]
- Török, K.; Stauffer, K.; Evans, W.H. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem. J. 1997, 326, 479–483. [Google Scholar] [CrossRef]
- Dodd, R.; Peracchia, C.; Stolady, D.; Török, K. Calmodulin association with connexin32-derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J. Biol. Chem. 2008, 283, 26911–26920. [Google Scholar] [CrossRef] [PubMed]
- Stauch, K.; Kieken, F.; Sorgen, P. Characterization of the structure and intermolecular interactions between the connexin 32 carboxyl-terminal domain and the protein partners synapse-associated protein 97 and calmodulin. J. Biol. Chem. 2012, 287, 27771–27788. [Google Scholar] [CrossRef]
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein-protein interactions with connexin 43: Regulation and function. Int. J. Mol. Sci. 2018, 19, 1428. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.S.; Mitchell, C.K.; Keflemariam, Y.J.; Heidelberger, R.; O’Brien, J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem. Biophys. Res. Commun. 2005, 335, 1191–1198. [Google Scholar] [CrossRef][Green Version]
- Werner, R.; Levine, E.; Rabadan-Diehl, C.; Dahl, G. Gating properties of connexin32 cell-cell channels and their mutants expressed in Xenopus oocytes. Proc. Biol. Sci. 1991, 243, 5–11. [Google Scholar] [PubMed]
- Wang, X.G.; Peracchia, C. Positive charges of the initial C-terminus domain of Cx32 inhibit gap junction gating sensitivity to CO2. Biophys. J 1997, 73, 798–806. [Google Scholar] [CrossRef]
- Wang, X.G.; Peracchia, C. Connexin 32/38 chimeras suggest a role for the second half of inner loop in gap junction gating by low pH. Am. J. Physiol. 1996, 271, C1743–C1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Peracchia, L.L.; Peracchia, C. Chimeric evidence for a role of the connexin cytoplasmic loop in gap junction channel gating. Pflüg. Arch. 1996, 431, 844–852. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Ye, Y.; Huang, Y.; Lee, H.W.; Chen, Y.; Louis, C.F.; Yang, J.J. Identification of the calmodulin binding domain of connexin 43. J. Biol. Chem. 2007, 282, 35005–35017. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Lin, X.; Wong, H.C.; Xu, Q.; Jiang, J.; Wang, S.; Lurtz, M.M.; Louis, C.F.; Veenstra, R.D.; et al. Molecular interaction and functional regulation of connexin50 gap junctions by calmodulin. Biochem. J. 2011, 435, 711–722. [Google Scholar] [CrossRef]
- Zou, J.; Salarian, M.; Chen, Y.; Veenstra, R.; Louis, C.F.; Yang, J.J. Gap junction regulation by calmodulin. FEBS Lett. 2014, 588, 1430–1438. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Chen, Y.; Jiang, J.; Huang, Y.; Louis, C.F.; Yang, J.J. Calmodulin mediates the Ca2+-dependent regulation of Cx44 gap junctions. Biophys. J. 2009, 96, 2832–2848. [Google Scholar] [CrossRef] [PubMed]
- Myllykoski, M.; Kuczera, K.; Kursula, P. Complex formation between calmodulin and a peptide from the intracellular loop of the gap junction protein connexin43: Molecular conformation and energetics of binding. Biophys. Chem. 2009, 144, 130–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peracchia, C.; Chen, J.T.; Peracchia, L.L. CO2 sensitivity of voltage gating and gating polarity of gapjunction channels--connexin40 and its COOH-terminus-truncated mutant. J. Membr. Biol. 2004, 200, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Kim, J.; Truong, K.; Sherman, M.; Yuan, T.; Ikura, M. Calmodulin target database. J. Struct. Funct. Genom. 2000, 1, 8–14. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Angele, A.B.; Verselis, V.K.; Bennett, M.V. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: Properties of fast and slow gating mechanisms. Proc. Natl. Acad. Sci. USA 2002, 99, 7113–7118. [Google Scholar] [CrossRef]
- Török, K.; Trentham, D.R. Mechanism of 2-chloro-(epsilon-amino-Lys75)-[6-[4-(N,N-diethylamino)phenyl]-1,3,5-triazin-4-yl]calmodulin interactions with smooth muscle myosin light chain kinase and derived peptides. Biochemistry 1994, 33, 12807–12820. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Puranam, K.L.; Revel, J.P. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem. J. 1991, 273, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Is the chemical gate of connexins voltage sensitive? Behavior of Cx32 wild-type and mutant channels. Am. J. Physiol 1999, 276, C1361–C1373. [Google Scholar] [PubMed]
- Verselis, V.K.; Ginter, C.S.; Bargiello, T.A. Opposite voltage gating polarities of two closely related connexins. Nature 1994, 368, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.F.; Peracchia, C. Two distinct gating mechanisms in gap junction channels: CO2-sensitive and voltage-sensitive. Biophys. J. 1997, 72, 2137–2142. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Weingart, R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys. J. 1994, 67, 613–625. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Elfgang, C.; Willecke, K.; Weingart, R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys. J. 1995, 68, 2289–2298. [Google Scholar] [CrossRef][Green Version]
- Peracchia, C.; Salim, M.; Peracchia, L.L. Unusual slow gating of gap junction channels in oocytes expressing connexin32 or its COOH-terminus truncated mutant. J. Membr. Biol. 2007, 215, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Ri, Y.; Bennett, M.V.; Trexler, E.B.; Verselis, V.K.; Bargiello, T.A. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron 1997, 19, 927–938. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Jordan, K.; Bukauskiene, A.; Bennett, M.V.; Lampe, P.D.; Laird, D.W.; Verselis, V.K. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. USA 2000, 97, 2556–2561. [Google Scholar] [CrossRef]
- Astegno, A.; La Verde, V.; Marino, V.; Dell’Orco, D.; Dominici, P. Biochemical and biophysical characterization of a plant calmodulin: Role of the N- and C-lobes in calcium binding, conformational change, and target interaction. Biochim. Biophys. Acta 2016, 1864, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Peracchia, C. Molecular dissection of a basic COOH-terminal domain of Cx32 that inhibits gap junction gating sensitivity. Am. J. Physiol. 1998, 275, C1384–C1390. [Google Scholar] [CrossRef] [PubMed]







| Connexins | kD (With Ca2+) | kD (Without Ca2+) |
|---|---|---|
| Cx32 | 40 ± 4 nM | 280 ± 10 nM |
| Cx35 | 31 ± 2 nM | 2,67 ± 0.09 µM |
| Cx45 | 75 ± 4 nM | 78 ± 1 nM |
| Cx57 | 60 ± 6 nM | 32 ± 14 nM |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracchia, C. Calmodulin-Cork Model of Gap Junction Channel Gating—One Molecule, Two Mechanisms. Int. J. Mol. Sci. 2020, 21, 4938. https://doi.org/10.3390/ijms21144938
Peracchia C. Calmodulin-Cork Model of Gap Junction Channel Gating—One Molecule, Two Mechanisms. International Journal of Molecular Sciences. 2020; 21(14):4938. https://doi.org/10.3390/ijms21144938
Chicago/Turabian StylePeracchia, Camillo. 2020. "Calmodulin-Cork Model of Gap Junction Channel Gating—One Molecule, Two Mechanisms" International Journal of Molecular Sciences 21, no. 14: 4938. https://doi.org/10.3390/ijms21144938
APA StylePeracchia, C. (2020). Calmodulin-Cork Model of Gap Junction Channel Gating—One Molecule, Two Mechanisms. International Journal of Molecular Sciences, 21(14), 4938. https://doi.org/10.3390/ijms21144938





