The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells
Abstract
1. Introduction
2. The Reprogramming Treatments of Cancer Stem-Like Cells: The Results of the Experiments In Vitro and In Vivo
3. SCDSFs: Results from Clinical Trials on Intermediate-Advanced Hepatocellular Carcinoma (HCC) and on Colon Cancer
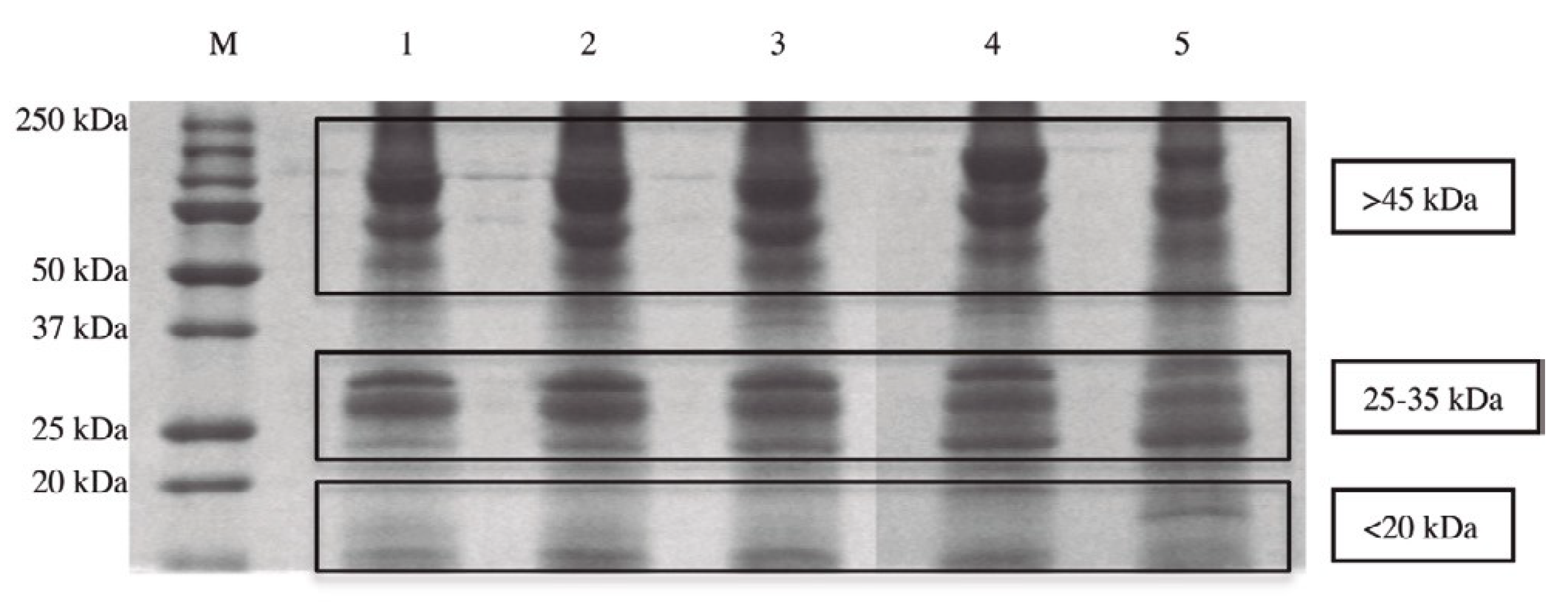
4. The Role of SCDSFs in Addressing the Fate of Human Adipose-Derived Stem Cells (hASCs)
Funding
Acknowledgments
Conflicts of Interest
References
- Einhorn, L. Are there factors preventing cancer development during embryonic life? Oncodev. Biol. Med. 1983, 4, 219–229. [Google Scholar] [PubMed]
- Lakshmi, M.S.; Sherbet, I. Embryonic and Tumor Cell Interactions; Sherbet, G.V., Ed.; Karger Basel: New York, NY, USA, 1974; pp. 380–399. [Google Scholar]
- Brent, R.L. Radiation Teratogenesis. Teratology 1980, 21, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.B. The cancer cell and its control by the embryo. Rous-Whipple Award lecture. Am. J. Pathol. 1983, 113, 117–124. [Google Scholar]
- Yu, C.L.; Tsai, M.H. Fetal fetuin selectively induces apoptosis in cancer cell lines and shows anti-cancer activity in tumor animal models. Cancer Lett. 2001, 166, 173–184. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; McBurney, M.V.; Gardner, R.L.; Evans, M.J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature 1975, 258, 70–73. [Google Scholar] [CrossRef]
- Biava, P.M.; Canaider, S.; Facchin, F.; Bianconi, E.; Ljungberg, L.; Rotilio, D.; Burigana, F.; Ventura, C. Stem Cell Differentiation Stage Factors from Zebrafish Embryo: A Novel Strategy to Modulate the Fate of Normal and Pathological Human (Stem) Cells. Curr. Pharm. Biotechnol. 2015, 16, 782–792. [Google Scholar] [CrossRef][Green Version]
- Facchin, F.; Canaider, S.; Bianconi, E.; Maioli, M.; Santoro, U.; Santaniello, S.; Basoli, V.; Biava, P.M.; Ventura, C. Zebrafish embryo extract counteracts human cell senescence. Front. Biosci. Sch. Ed. 2019, 11, 89–104. [Google Scholar]
- Facchin, F.; Bianconi, E.; Canaider, S.; Basoli, V.; Biava, P.M.; Ventura, C. Tissue Regeneration without Stem Cell Transplantation: Self-Healing Potential from Ancestral Chemistry and Physical Energies. Stem Cells Int. 2018. [Google Scholar] [CrossRef]
- Facchin, F.; Alviano, F.; Canaider, S.; Bianconi, E.; Rossi, M.; Bonsi, L.; Casadei, R.; Biava, P.M.; Ventura, C. Early Developmental Zebrafish Embryo Extract to Modulate Senescence in Multisource Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2646. [Google Scholar] [CrossRef]
- Biava, P.M.; Bonsignorio, D. Cancer and Cell Differentiation: A Model to Explain Malignancy. J. Tumor Marker Oncol. 2002, 17, 47–54. [Google Scholar]
- Biava, P.M.; Bonsignorio, D.; Hoxa, M. Cell Proliferation Curve od Different Human Tumor Lines after in Vitro Treatment with Zebrafish embryonic extracts. J. Tumor Marker Oncol. 2001, 16, 195–202. [Google Scholar]
- Biava, P.M.; Bonsignorio, D.; Hox, A.M.; Larese, F.; Negro, C. Mother-Embryo Cross-Talk: The Anticancer Substances Produced by Mother and Embryo during Cell Differentiation. A Review of Experimental Data. J. Tumor Marker Oncol. 2002, 17, 55–58. [Google Scholar]
- Biava, P.M.; Carluccio, A. Activation of anti-oncogene p53 produced by embryonic extracts in vitro tumor cells. J. Tumor Marker Oncol. 1977, 12, 9–15. [Google Scholar]
- Biava, P.M.; Bonsignorio, D.; Hoxa, M.; Impagliazzo, M.; Facco, R.; Ielapi, T.; Frati, L.; Bizzarri, M. Post translational modification of the retinoblastoma protein (pRb) induced by in vitro administration of Zebrafish embryonic extracts on human kidney adenocarcinoma cell line. J. Tumor Marker Oncol. 2002, 17, 59–64. [Google Scholar]
- Cucina, A.; Biava, P.M.; D’Anselmi, F.; Coluccia, P.; Conti, F.; di Clemente, R.; Miccheli, A.; Frati, L.; Gulino, A.; Bizzarri, M. Zebrafish Embryo Proteins Induce Apoptosis in Human Colon Cancer Cells (Caco2). Apoptosis 2006, 11, 1617–1628. [Google Scholar] [CrossRef]
- Proietti, S.; Cucina, A.; Pensotti, A.; Biava, P.M.; Minini, M.; Monti, N.; Catizone, A.; Ricci, G.; Leonetti, E.; Harrath, A.H.; et al. Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-Regulation of TCTP and Modulation of E-cadherin/Beta-catenin Pathway. Int. J. Mol. Sci. 2019, 20, 2151. [Google Scholar] [CrossRef]
- D’Anselmi, F.; Cucina, A.; Biava, P.M.; Proietti, S.; Coluccia, P.; Frati, L.; Bizzarri, M. Zebrafish stem cell differentiation stage factors suppress Bcl-X release and enhance 5-Fu mediated apoptosis in colon cancer cells. Curr. Pharm. Biotechnol. 2011, 12, 261–267. [Google Scholar]
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef]
- Gauthaman, K.; Yee, F.C.; Cheyyatraivendran, S.; Biswas, A.; Choolani, M.; Bongso, A. Umbilical cord Wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J. Cell Biochem. 2012, 113, 2027–2039. [Google Scholar] [CrossRef]
- Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; Pyle, M.M.; Maurya, D.K.; Troyer, D.; Tamura, M. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009, 280, 31–37. [Google Scholar] [CrossRef]
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.; O’Driscoll, L. miR-134 in extracellular vesicles reduces triple-negative breast cance7 aggression and increases drug sensitivity. Oncotarget 2015, 6, 32774–32789. [Google Scholar] [CrossRef] [PubMed]
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.A.; Richard, D.J.; O’Byrne, K.J. Functions and therapeutic roles of exosomes in cancer. Front. Oncol. 2014, 4, 127. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Inhibit Tumor Growth. Stem Cells Dev. 2013, 22, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.J.; Seftor, E.A.; Bonde, G.; Cornell, R.A.; Hendrix, M.J.C. The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. 2005, 233, 1560–1570. [Google Scholar] [CrossRef]
- Kulesa, P.M.; Kasemeier-Kulesa, J.C.; Teddy, J.M.; Margaryan, N.V.; Seftor, E.A.; Hendrix, M.J. Reprogramming metastatic melanoma cells to assume a neural crest-like phentype in a embryonic microenvironment. Proc. Natl. Acad. Sci. USA 2006, 103, 3752–3757. [Google Scholar] [CrossRef]
- Postovit, L.M.; Margaryan, N.V.; Seftor, E.A.; Kirschmann, D.A.; Lipavsky, A.; Wheaton, W.W.; Abbott, D.E.; Seftor, R.E.; Hendrix, M.J. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc. Natl. Acad. Sci. USA 2008, 105, 4329–4334. [Google Scholar] [CrossRef]
- Díez-Torre, A.; Andrade, R.; Eguizábal, C.; López, E.; Arluzea, J.; Silió, M.; Aréchaga, J. Reprogramming of melanoma cells by embryonic microenvironments. Int. J. Dev. Biol. 2009, 53, 1563–1568. [Google Scholar]
- Giuffrida, D.; Rogers, I.; Nagy, A.; Calogero, A.; Brown, T.; Casper, R.F. Human embryonic stem cells secrete soluble factors that inhibit cancer cell growth. Cell Prolif. 2009, 42, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Ishii, H.; Nagai, K.; Hoshino, H.; Mimori, K.; Tanaka, F.; Hiroak, I.N.; Mitongu, S.; Yuichino, D.; Musaki, M. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 40–45. [Google Scholar] [CrossRef]
- Boulanger, C.A.; Bruno, R.D.; Mack, D.L.; Gonzales, M.; Castro, N.P.; Salomon, D.S.; Smitth, G.H. Embryonic stem cells are redirected to non-tumorigenic epithelial cell fate by interaction with the mammary microenvironment. PLoS ONE 2013, 8, e62019. [Google Scholar] [CrossRef]
- No, J.G.; Choi, M.K.; Kwon, D.J.; Yoo, J.G.; Yang, B.C.; Park, J.K.; Dong, H.K. Cell-free extract from porcine induced pluripotent stem cells can affect porcine somatic cell nuclear reprogramming. J. Reprod. Dev. 2015, 61, 90–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Livraghi, T.; Meloni, F.; Frosi, A.; Lazzaroni, S.; Bizzarri, T.M.; Frati, L.; Biava, P.M. Treatment with stem cell differentiation stage factors of intermediate-advanced hepatocellular carcinoma: An open randomized clinical trial. Oncol. Res. 2005, 15, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Ceriani, R.; Palmisano, A.; Pedicini, V.; Pich, M.G.; Tommasini, M.A.; Torzilli, G. Complete response in 5 out of 38 patients with advanced hepatocellular carcinoma treated with stem cell differentiation stage factors: Case reports from a single centre. Curr. Pharm. Biotechnol. 2011, 12, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Giuliani, A.; Verna, R.; Palombi, E.; Biava, P.M.; Pensotti, A. Fish protein extract enhances clinical response to salvage chemotherapy in colon cancer patients. Org. J. Biol. Sci. 2018, 2, 81–90. [Google Scholar]
- Lugnani, F.; Simone, G.; Biava, P.M.; Ablin, R.J. The role of neuroendocrine cells in prostate cancer: A comprehensive review of current literature and subsequent rationale to broaden and integrate current treatment modalities. Curr. Med. Chem. 2014, 21, 1082–1092. [Google Scholar] [CrossRef]
- Biava, P.M.; Nicolini, A.; Ferrari, P.; Carpi, A.; Sell, S. A systemic approach to cancer treatment: Tumor cell reprogramming focused on endocrine-related cancers. Curr. Med. Chem. 2014, 21, 1072–1081. [Google Scholar] [CrossRef][Green Version]
- Sell, S.; Nicolini, A.; Ferrari, P.; Biava, P.M. Cancer: A problem of developmental biology; scientific evidence for reprogramming and differentiation therapy. Curr. Drug.Target. 2016, 17, 1103–1110. [Google Scholar] [CrossRef]
- Laszlo, E.; Biava, P.M. Chapter 2: Addendum: Declaration of a Committee of Oncologists. In Information Medicine; Healing Arts Press: Rochester, VT, USA, 2019; pp. 39–50. [Google Scholar]
- Norata, G.D.; Biava, P.M.; Di Pierro, F. The Zebrafish embryo derivative affects cell viability of epidermal cells: A possible role in the treatment of psoriasis. G. Ital. Dermatol. Venereol. 2013, 148, 479–483. [Google Scholar]
- Calzavara-Pinton, P.; Rossi, M. A topical remedy in association with phototherapy. Efficacy evaluation in patients suffering from moderate psoriasis. HiTech Dermo 2012, 1, 41–47. [Google Scholar]
- Di Pierro, F.; Negri, M.; Bollero, C. Terapia della psoriasi. Efficacia clinica di un preparato multicomponente. Cosmet. Technol. 2009, 12, 13–17. [Google Scholar]
- Harak, H.; Frosi, A.; Biava, P.M. Studio clinico sull’efficacia e tollerabilita’ di una crema per uso topico nel trattamento della psoriasi. Med. Biol. 2012, 3, 27–31. [Google Scholar]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A new non enzymatic method and device to obtain a fat tissue derivative high enriched in pericyte-like elements by mild mechanical forces from human kipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Canaider, S.; Maioli, M.; Facchin, F.; Bianconi, E.; Santaniello, S.; Pigliaru, G.; Ljungberg, L.; Burigana, F.; Bianchi, F.; Olivi, E.; et al. Human Stem Cell Exposure to Developmental Stage Zebrafish Extracts: A Novel Strategy for Tuning Stemness and Senescence Patterning. CellR4 2014, 2, e1226. [Google Scholar]
- Feng, R.; Zhou, S.; Liu, Y.; Song, D.; Luan, Z.; Dai, X.; Li, Y.; Tang, N.; Wen, J.; Li, L. Sox2 protects neural stem cells from apoptosis via up regulating survivin expression. Biochem. J. 2013, 450, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Qian, D.; Kiel, M.; Becker, M.W.; Pihalja, M.; Weissman, I.L.; Morrison, S.J.; Clarke, M.F. Bmi-1 is required for maintenance of adult self-renewing hematopioetic stem cells. Nature 2003, 423, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, F.; Bellone, C.; Viviani, B.; Marinovich, M.; Meli, E.; Pellegrini-Giampietro, D.E.; Cattabeni, F.; Di Luca, M. Lack of PSD-95 drives hippocampal neuronal cell death through activation of an alpha CaMKII transduction pathway. Eur. J. Neurosci. 2002, 16, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Biava, P.M.; Bonizzoni, E.; Zafiropoulus, S.; Laudani, A.; Burigana, F.; Burian, L.I.; Lotti, T. Stem cell growth and differentiation factors from Zebrafish embryo and their role as epigenetic regulators in hair regeneration: Results after transdermal administration using cryopass laser treatment. Aesthetic Med. 2020, 6, 11–19. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biava, P.M. The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells. Int. J. Mol. Sci. 2020, 21, 4914. https://doi.org/10.3390/ijms21144914
Biava PM. The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells. International Journal of Molecular Sciences. 2020; 21(14):4914. https://doi.org/10.3390/ijms21144914
Chicago/Turabian StyleBiava, Pier Mario. 2020. "The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells" International Journal of Molecular Sciences 21, no. 14: 4914. https://doi.org/10.3390/ijms21144914
APA StyleBiava, P. M. (2020). The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells. International Journal of Molecular Sciences, 21(14), 4914. https://doi.org/10.3390/ijms21144914



