Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K
Abstract
1. Introduction
2. Results
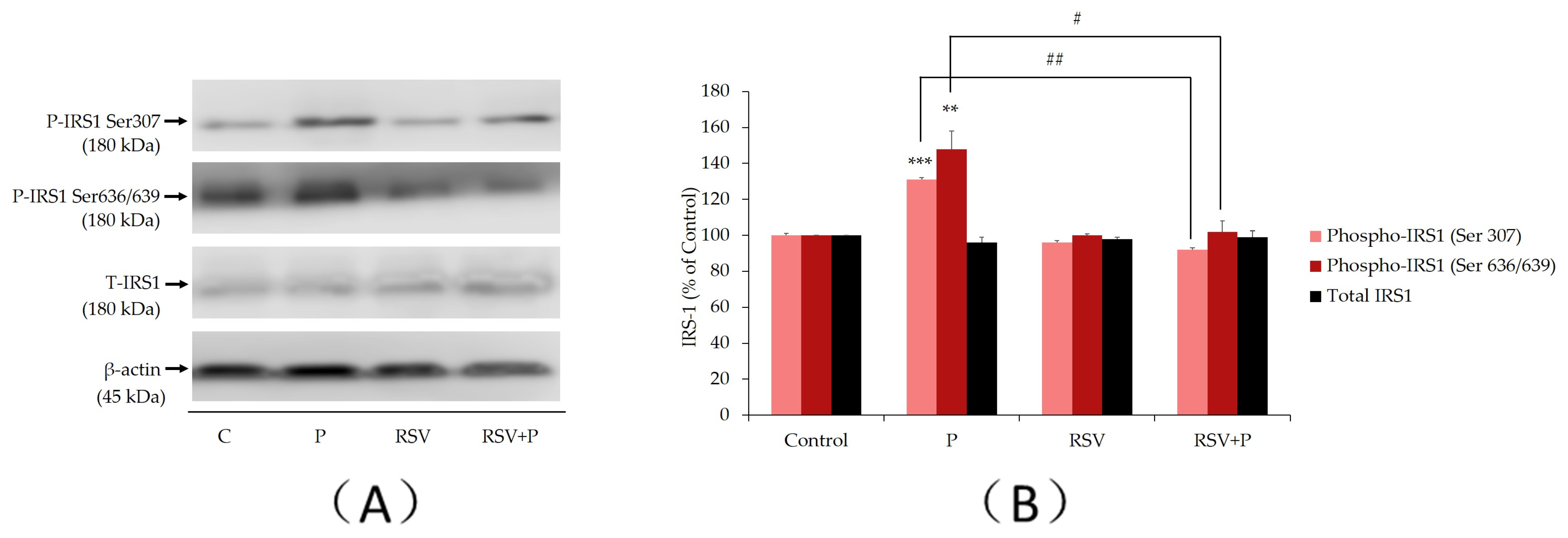
2.1. Resveratrol Abolishes the Palmitate-Induced Ser307 and Ser636/639 Phosphorylation of IRS-1
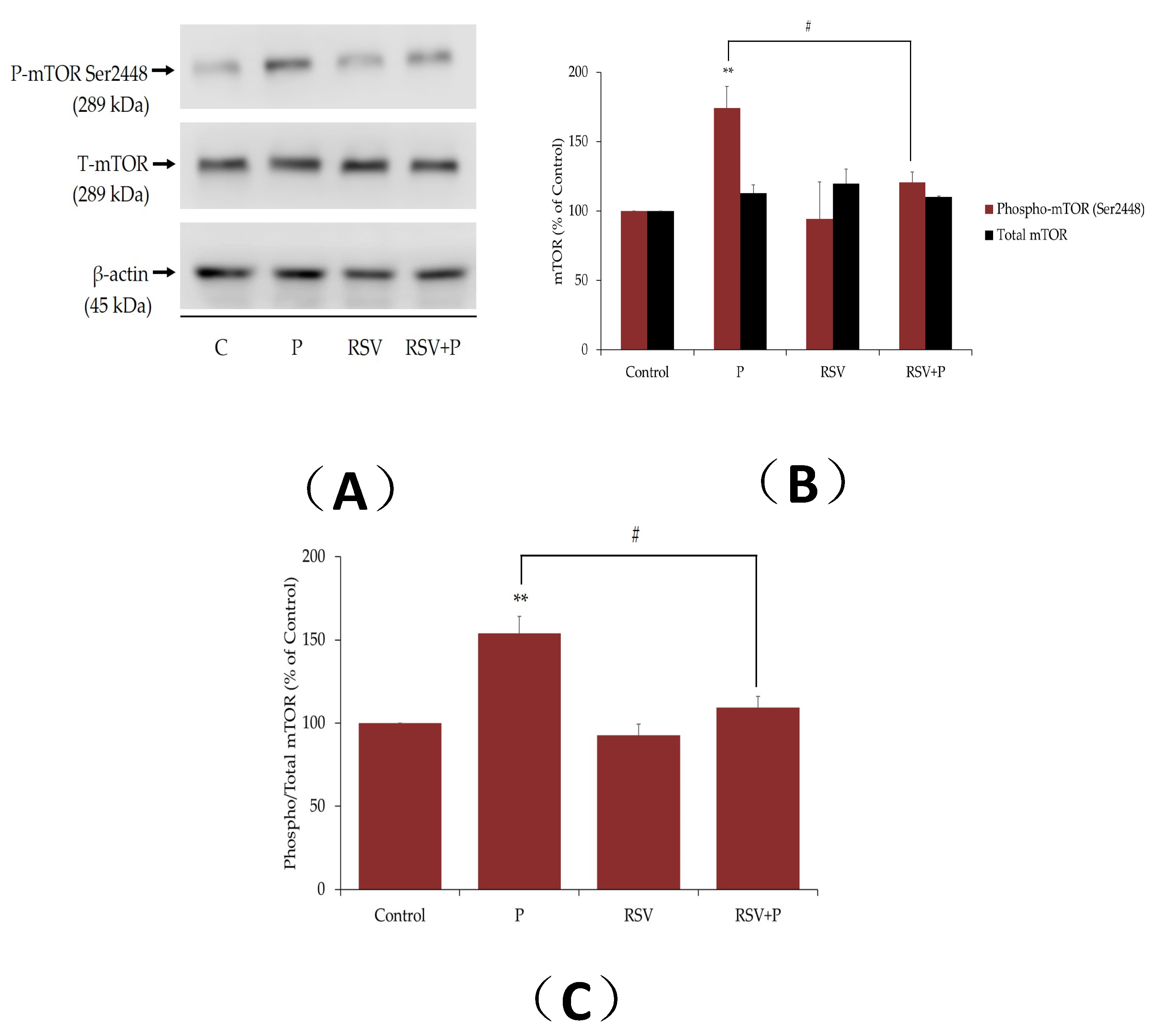
2.2. Resveratrol Attenuates the Palmitate-Induced Phosphorylation/Activation of mTOR and p70 S6K
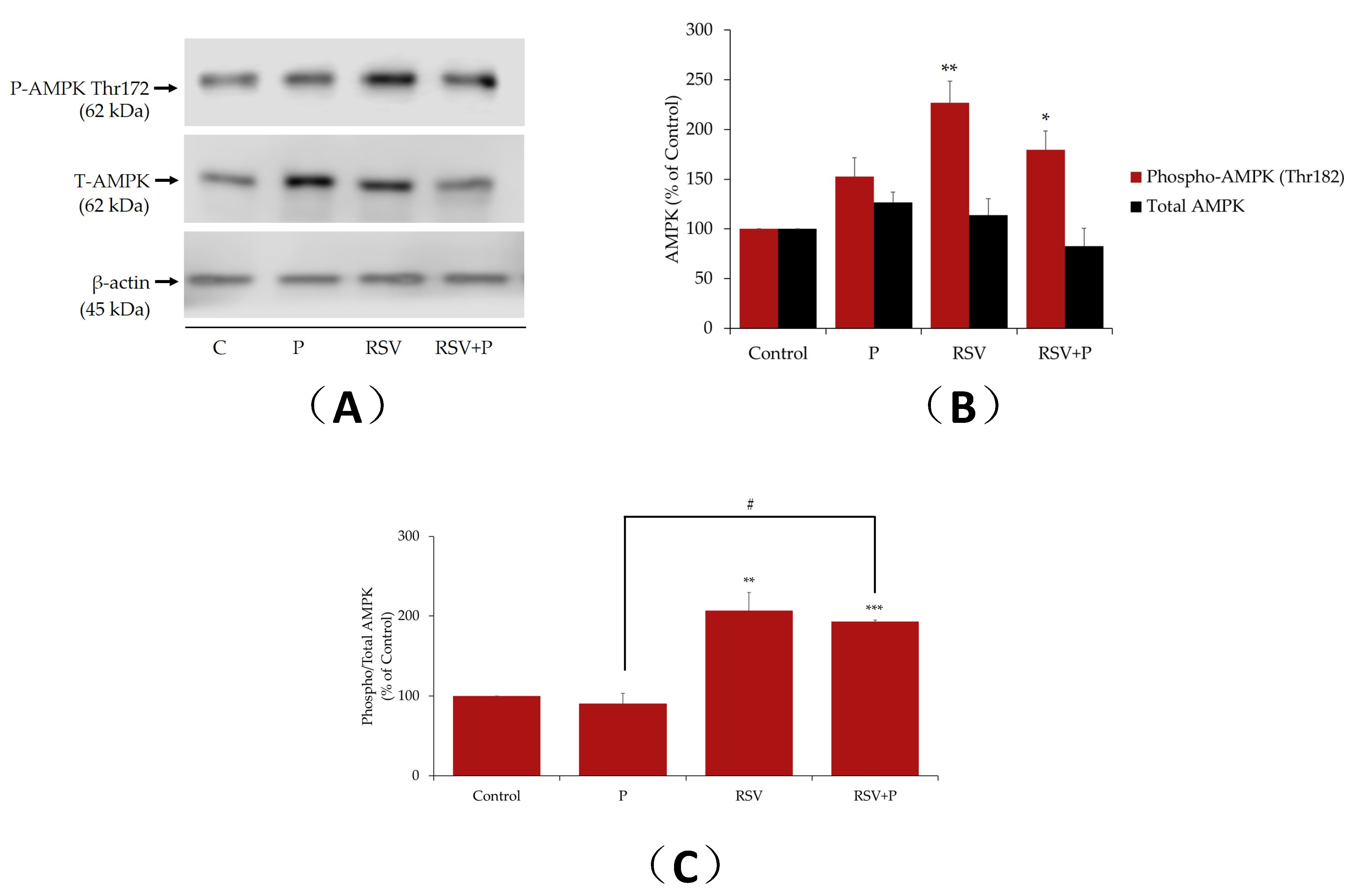
2.3. Resveratrol Increases AMPK Phosphorylation/Activation in the Presence of Palmitate
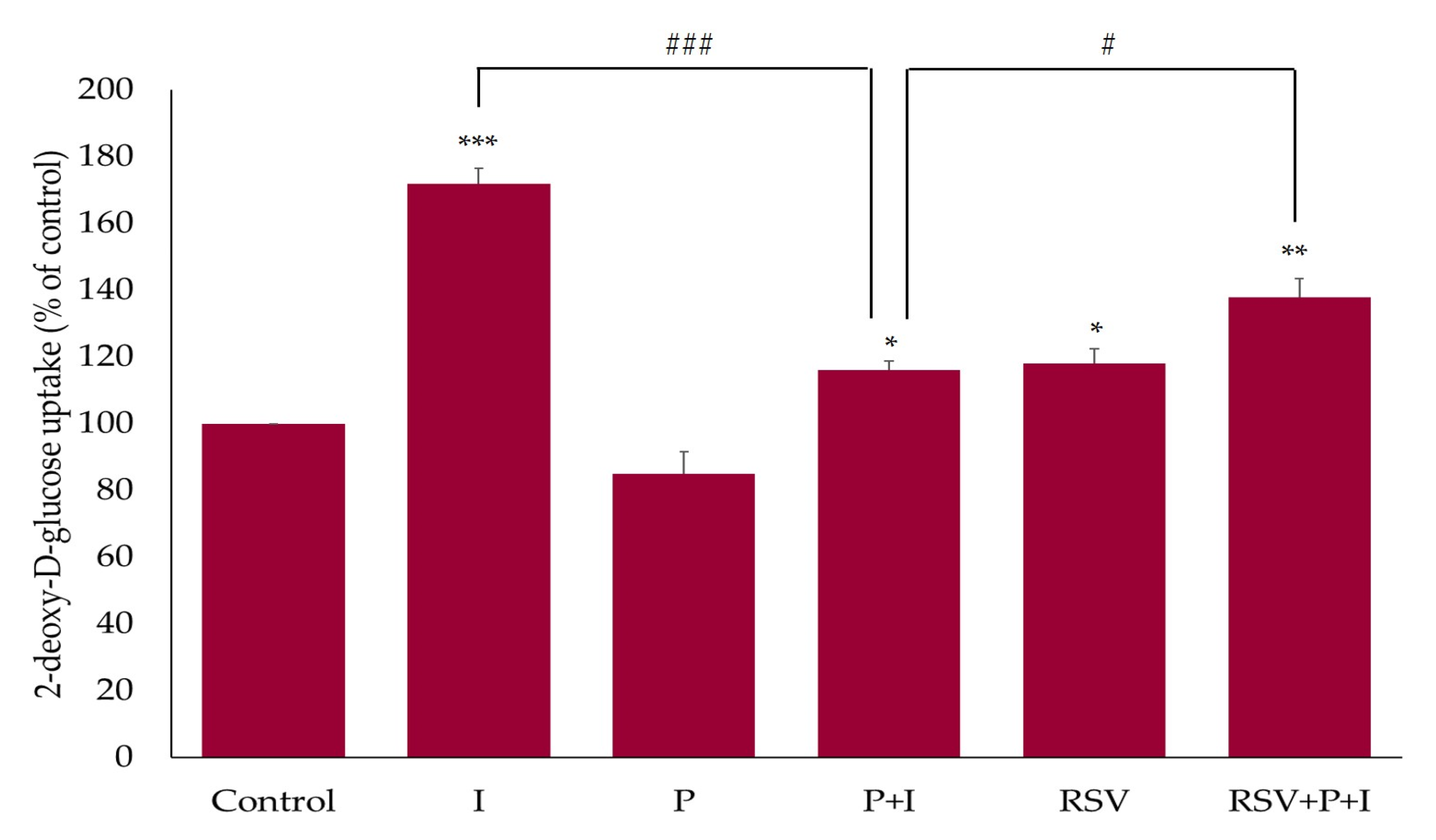
2.4. Resveratrol Restores the Insulin-Stimulated Glucose Uptake in Palmitate-Treated Muscle Cells
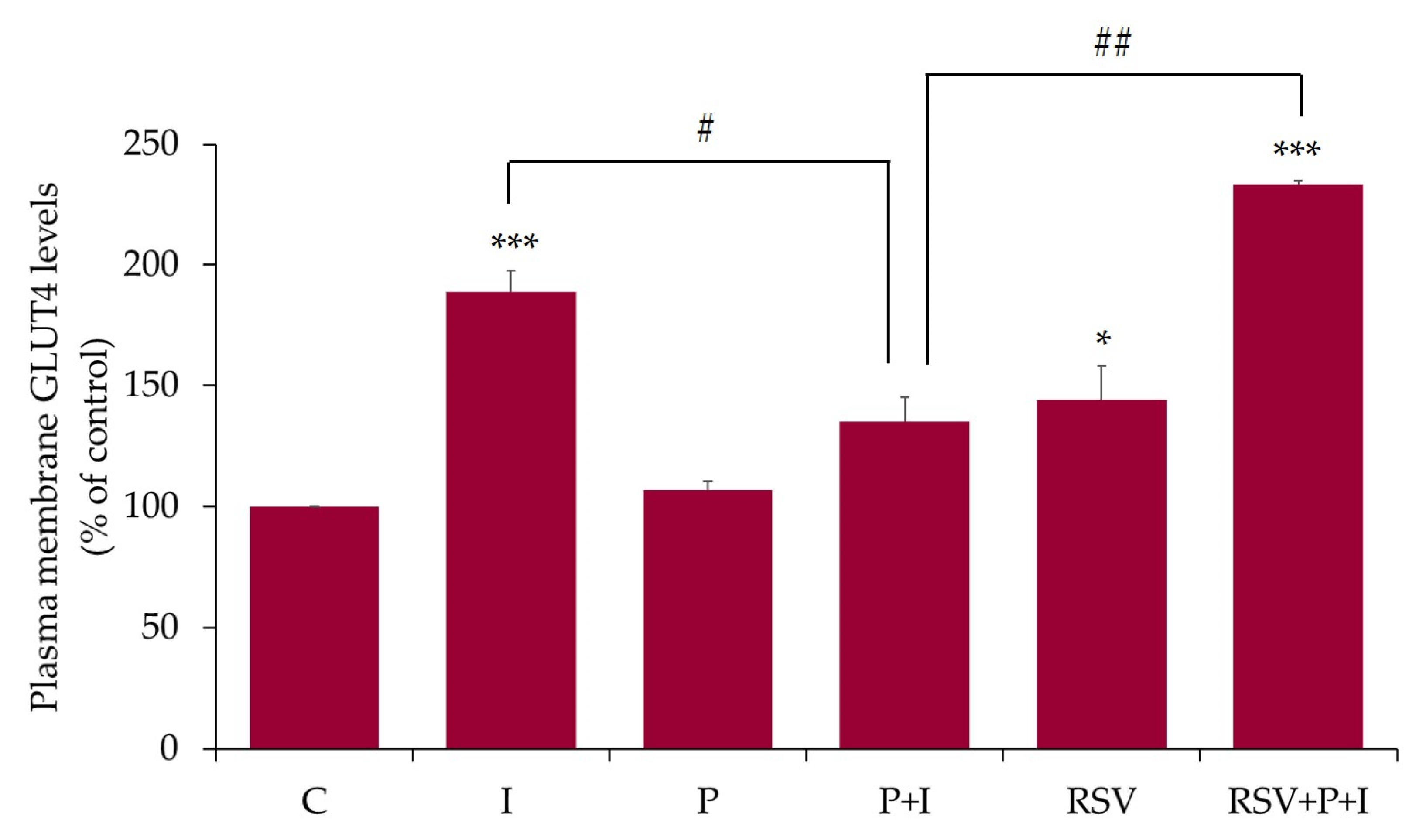
2.5. Resveratrol Restores the Insulin-Stimulated GLUT4 Translocation in Palmitate-Treated Muscle Cells
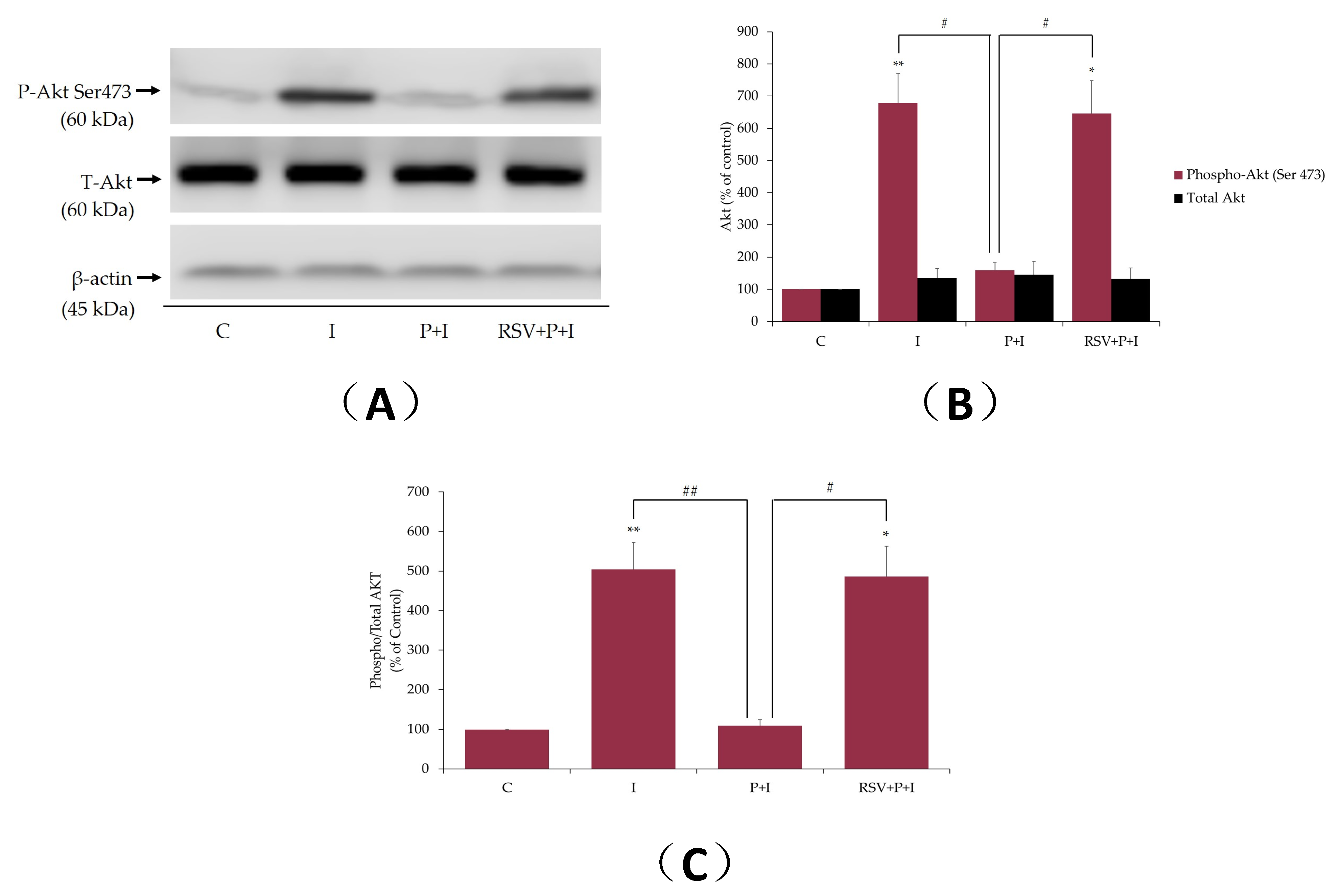
2.6. Resveratrol Restores the Insulin-Stimulated Akt Phosphorylation in Palmitate-Treated Muscle Cells
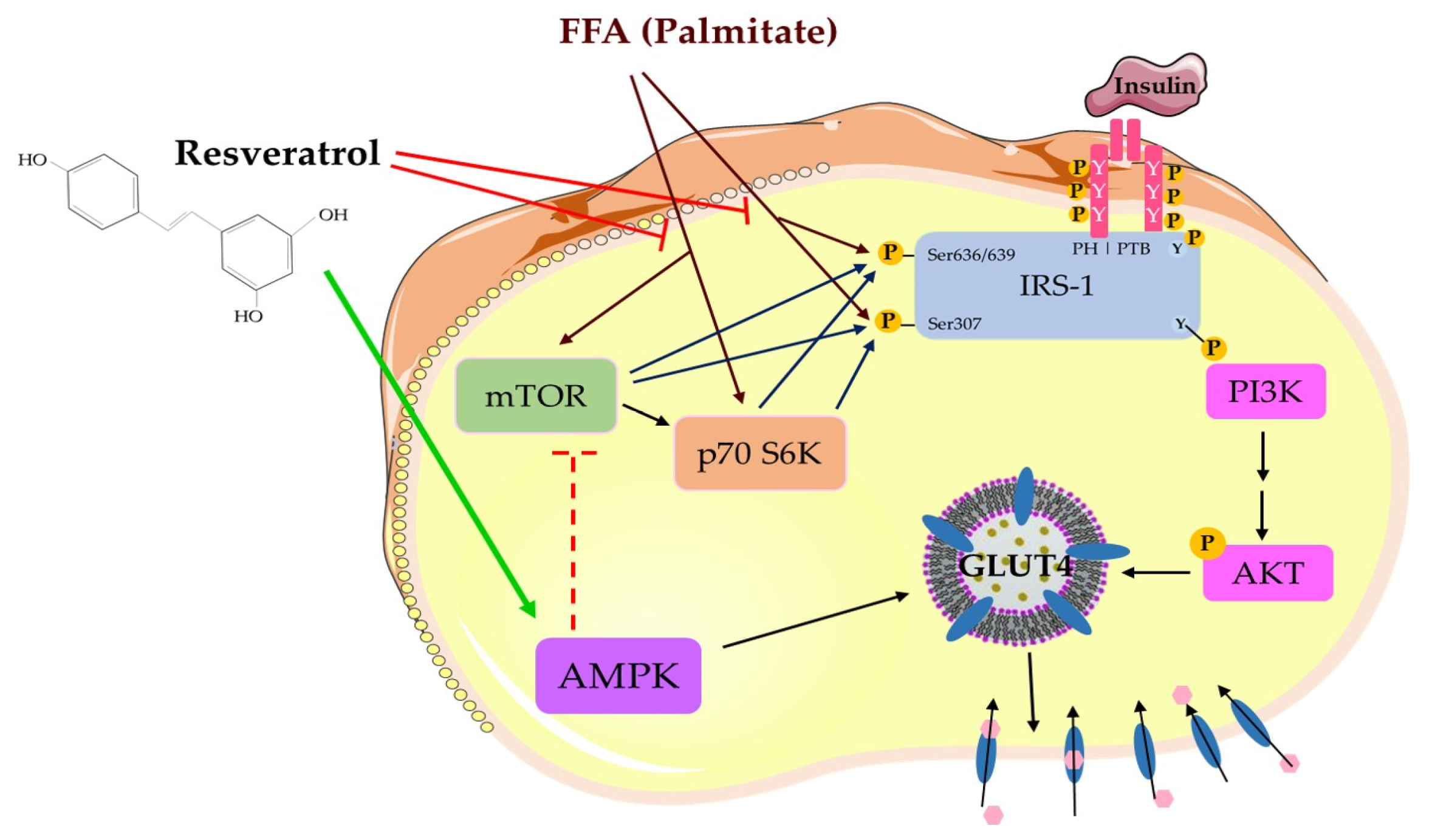
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Palmitate Solution Preparation
4.3. Cell Culture and Glucose Uptake Assay
4.4. GLUT4myc Translocation Assay
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Molecular basis of insulin resistance: The role of IRS and Foxo1 in the control of diabetes mellitus and its complications. Drug Discov. Today: Dis. Mech. 2013, 10, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Chavez, A.O. Defects in Insulin Secretion and Action in the Pathogenesis of Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Hulver, M.W.; Dohm, G.L. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc. Nutr. Soc. 2004, 63, 375–380. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Sinha, S.; Perdomo, G.; Brown, N.F.; O’Doherty, R.M. Fatty Acid-induced Insulin Resistance in L6 Myotubes Is Prevented by Inhibition of Activation and Nuclear Localization of Nuclear Factor B. J. Boil. Chem. 2004, 279, 41294–41301. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Tsiani, E. Attenuation of Free Fatty Acid-Induced Muscle Insulin Resistance by Rosemary Extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; López-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Pederson, T.M.; Kramer, D.L.; Rondinone, C.M. Serine/threonine phosphorylation of IRS-1 triggers its degradation: Possible regulation by tyrosine phosphorylation. Diabetes 2001, 50, 24–31. [Google Scholar] [CrossRef]
- Prada, P.O.; Zecchin, H.G.; Gasparetti, A.L.; Torsoni, M.A.; Ueno, M.; Hirata, A.E.; Amaral, M.E.C.D.; Höer, N.F.; Boschero, A.C.; Saad, M.J.A. Western Diet Modulates Insulin Signaling, c-Jun N-Terminal Kinase Activity, and Insulin Receptor Substrate-1 ser307 Phosphorylation in a Tissue-Specific Fashion. Endocrinology 2005, 146, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; White, M.F.; Rondinone, C.M. Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation. Biochem. Biophys. Res. Commun. 2004, 316, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, Y.; Inamitsu, T.; Chida, K.; Iemura, S.-I.; Natsume, T.; Maeda, T.; Hakuno, F.; Takahashi, S.-I. Serine Phosphorylation by mTORC1 Promotes IRS-1 Degradation through SCFβ-TRCP E3 Ubiquitin Ligase. iScience 2018, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Soos, T.J.; Wu, J.; DeGennaro, M.; Sun, X.; Littman, D.R.; Birnbaum, M.; Polakiewicz, R.D. Protein Kinase C Inhibits Insulin Signaling by Phosphorylating IRS1 at Ser1101. J. Boil. Chem. 2004, 279, 45304–45307. [Google Scholar] [CrossRef]
- Tremblay, F.; Brûlé, S.; Um, S.H.; Li, Y.; Masuda, K.; Roden, M.; Sun, X.J.; Krebs, M.; Polakiewicz, R.D.; Thomas, G.; et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc. Natl. Acad. Sci. 2007, 104, 14056–14061. [Google Scholar] [CrossRef]
- Nikoulina, S.E.; Ciaraldi, T.P.; Mudaliar, S.; Carter, L.; Johnson, K.; Henry, R.R. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes 2002, 51, 2190–2198. [Google Scholar] [CrossRef]
- Gao, Z.; Hwang, D.; Bataille, F.; Lefevre, M.; York, D.; Quon, M.; Ye, J. Serine Phosphorylation of Insulin Receptor Substrate 1 by Inhibitor κB Kinase Complex. J. Boil. Chem. 2002, 277, 48115–48121. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Boil. 2017, 19, 121–135. [Google Scholar] [CrossRef]
- Lin, S.-C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Patel, N.; Khayat, Z.A.; Ruderman, N.B.; Klip, A. Dissociation of 5′ AMP-Activated Protein Kinase Activation and Glucose Uptake Stimulation by Mitochondrial Uncoupling and Hyperosmolar Stress: Differential Sensitivities to Intracellular Ca2+ and Protein Kinase C Inhibition. Biochem. Biophys. Res. Commun. 2001, 285, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.D.; Parbu-Patel, A.; Carling, D. The Anti-diabetic Drugs Rosiglitazone and Metformin Stimulate AMP-activated Protein Kinase through Distinct Signaling Pathways. J. Boil. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Dandapani, M.; Hardie, D.G. AMPK: Mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol. Metab. 2013, 24, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A Target for Drugs and Natural Products With Effects on Both Diabetes and Cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase With Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols Stimulate AMP-Activated Protein Kinase, Lower Lipids, and Inhibit Accelerated Atherosclerosis in Diabetic LDL Receptor-Deficient Mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef]
- Breen, D.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Hartogh, D.J.D.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Gasparrini, M.; Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Bullón, P.; Battino, M. AMPK as a New Attractive Therapeutic Target for Disease Prevention: The Role of Dietary Compounds AMPK and Disease Prevention. Curr. Drug Targets 2016, 17, 865–889. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Kim, M.-J.; Lee, J.H.; Min, B.-I.; Bae, H.; Choe, W.; Kim, S.-S.; Ha, J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007, 39, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-Activated Protein Kinase–Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes 2009, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Zhao, H.; Dong, L.; Zhen, Y.-F.; Xing, H.-Y.; Ma, H.-J.; Song, G.-Y. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via ATM-AMPK axis in skeletal muscle. Eur. Rev. Med. Pharmacol. Sci 2019, 23, 9117–9125. [Google Scholar]
- IDF Diabetes Atlas, 9th edition 2019. Available online: https://www.diabetesatlas.org/en/ (accessed on 29 May 2020).
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Le Marchand-Brustel, Y.; Gual, P.; Gremeaux, T.; Gonzalez, T.; Barrès, R.; Tanti, J.-F. Fatty acid-induced insulin resistance: Role of insulin receptor substrate 1 serine phosphorylation in the retroregulation of insulin signalling. Biochem. Soc. Trans. 2003, 31, 1152–1156. [Google Scholar] [CrossRef]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.-F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef]
- Khamzina, L.; Veilleux, A.; Bergeron, S.; Marette, A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: Possible involvement in obesity-linked insulin resistance. Endocrinology 2005, 146, 1473–1481. [Google Scholar] [CrossRef]
- Tzatsos, A.; Kandror, K.V. Nutrients Suppress Phosphatidylinositol 3-Kinase/Akt Signaling via Raptor-Dependent mTOR-Mediated Insulin Receptor Substrate 1 Phosphorylation. Mol. Cell. Boil. 2006, 26, 63–76. [Google Scholar] [CrossRef]
- Rivas, D.A.; Yaspelkis, B.B.; Hawley, J.A.; Lessard, S.J. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside. J. Endocrinology 2009, 202, 441–451. [Google Scholar] [CrossRef]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef]
- Centeno-Baez, C.; Dallaire, P.; Marette, A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am. J. Physiol. Metab. 2011, 301, E922–E930. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Shulman, G.I. Etiology of insulin resistance. Am. J. Med. 2006, 119, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Stump, C.S.; Henriksen, E.J.; Wei, Y.; Sowers, J.R. The metabolic syndrome: Role of skeletal muscle metabolism. Ann. Med. 2006, 38, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Park, E.; Moore, J.; Faubert, B.; Breen, D.M.; Oprescu, A.I.; Nahle, A.; Kwan, D.; Giacca, A.; Tsiani, E. Resveratrol prevents insulin resistance caused by short-term elevation of free fatty acids in vivo. Appl. Physiol. Nutr. Metab. 2015, 40, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Thirone, A.C.P.; Huang, X.; Klip, A. Differential Contribution of Insulin Receptor Substrates 1Versus2 to Insulin Signaling and Glucose Uptake in L6 Myotubes. J. Boil. Chem. 2005, 280, 19426–19435. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef]
- Draznin, B. Molecular Mechanisms of Insulin Resistance: Serine Phosphorylation of Insulin Receptor Substrate-1 and Increased Expression of p85: The Two Sides of a Coin. Diabetes 2006, 55, 2392–2397. [Google Scholar] [CrossRef]
- Wenjun, W.; Sunyinyan, T.; Junfeng, S.; Wenwen, Y.; Shu, C.; Ruifang, B.; Zhu, D.; Bi, Y. Metformin attenuates palmitic acid-induced insulin resistance in L6 cells through the AMP-activated protein kinase/sterol regulatory element-binding protein-1c pathway. Int. J. Mol. Med. 2015, 35, 1734–1740. [Google Scholar] [CrossRef]
- Wang, X.; Yu, W.; Nawaz, A.; Guan, F.; Sun, S.; Wang, C. Palmitate Induced Insulin Resistance by PKCtheta-Dependent Activation of mTOR/S6K Pathway in C2C12 Myotubes. Exp. Clin. Endocrinol. Diabetes 2010, 118, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Shin, K.O.; Lee, Y.H.; Jang, K.S.; Bae, J.Y.; Roh, H.T. Effects of treadmill exercise on skeletal muscle mTOR signaling pathway in high-fat diet-induced obese mice. J. Phys. Ther. Sci. 2016, 28, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, X.-F.; Ke, Z.-Q.; Yao, Q.; Guo, S.; Liu, C. Resveratrol Modulates Apoptosis and Autophagy Induced by High Glucose and Palmitate in Cardiac Cells. Cell. Physiol. Biochem. 2018, 46, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Querfurth, H.W. Palmitate activates mTOR/p70S6K through AMPK inhibition and hypophosphorylation of raptor in skeletal muscle cells: Reversal by oleate is similar to metformin. Biochimie 2015, 118, 141–150. [Google Scholar] [CrossRef]
- Le Bacquer, O.; Petroulakis, E.; Paglialunga, S.; Poulin, F.; Richard, D.; Cianflone, K.; Sonenberg, N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J. Clin. Investig. 2007, 117, 387–396. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, S.S.S.; Golestani, A.; Meshkani, R. Resveratrol Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Attenuating Oxidative Stress and JNK/NF-κB Pathway in a SIRT1-Independent Mechanism. J. Cell. Biochem. 2017, 118, 2654–2663. [Google Scholar] [CrossRef]
- Szkudelska, K.; Okulicz, M.; Hertig, I.; Szkudelski, T. Resveratrol ameliorates inflammatory and oxidative stress in type 2 diabetic Goto-Kakizaki rats. Biomed. Pharmacother. 2020, 125, 110026. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Fediuc, S.; Gaidhu, M.P.; Ceddia, R.B. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J. Lipid Res. 2005, 47, 412–420. [Google Scholar] [CrossRef]
- Watt, M.J.; Steinberg, G.R.; Chen, Z.-P.; Kemp, B.E.; Febbraio, M.A. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J. Physiol. 2006, 574, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Skrobuk, P.; Von Kraemer, S.; Semenova, M.M.; Zitting, A.; Koistinen, H.A. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologial 2012, 55, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, C.; Zhang, J.; Liu, B.; Du, Q. Resveratrol inhibits inflammation and ameliorates insulin resistant endothelial dysfunction via regulation of AMP-activated protein kinase and sirtuin 1 activities. J. Diabetes 2015, 8, 324–335. [Google Scholar] [CrossRef]
- Thomas, I.; Gregg, B. Metformin; a review of its history and future: From lilac to longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Cheng, S.W.Y.; Fryer, L.G.D.; Carling, D.; Shepherd, P.R. Thr2446Is a Novel Mammalian Target of Rapamycin (mTOR) Phosphorylation Site Regulated by Nutrient Status. J. Boil. Chem. 2004, 279, 15719–15722. [Google Scholar] [CrossRef]
- Stierwalt, H.D.; Ehrlicher, S.E.; Bergman, B.C.; Robinson, M.M.; Newsom, S. Insulin-stimulated Rac1-GTP binding is not impaired by palmitate treatment in L6 myotubes. Physiol. Rep. 2018, 6, e13956. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Hatakeyama, H.; Emoto, N.; Wagatsuma, F.; Matsushita, S.; Kanzaki, M. Palmitate-induced Down-regulation of Sortilin and Impaired GLUT4 Trafficking in C2C12 Myotubes*. J. Boil. Chem. 2010, 285, 34371–34381. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2019, 242, 117212. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnar, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot Study of Resveratrol in Older Adults With Impaired Glucose Tolerance. Journals Gerontol. Ser. A: Boil. Sci. Med Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; De Ligt, M.; Phielix, E.; Van De Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-Dose Resveratrol Supplementation in Obese Men: An Investigator-Initiated, Randomized, Placebo-Controlled Clinical Trial of Substrate Metabolism, Insulin Sensitivity, and Body Composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; Da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2018, 4, 122–135. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; Tsiani, E. Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K. Int. J. Mol. Sci. 2020, 21, 4900. https://doi.org/10.3390/ijms21144900
Den Hartogh DJ, Vlavcheski F, Giacca A, Tsiani E. Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K. International Journal of Molecular Sciences. 2020; 21(14):4900. https://doi.org/10.3390/ijms21144900
Chicago/Turabian StyleDen Hartogh, Danja J., Filip Vlavcheski, Adria Giacca, and Evangelia Tsiani. 2020. "Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K" International Journal of Molecular Sciences 21, no. 14: 4900. https://doi.org/10.3390/ijms21144900
APA StyleDen Hartogh, D. J., Vlavcheski, F., Giacca, A., & Tsiani, E. (2020). Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K. International Journal of Molecular Sciences, 21(14), 4900. https://doi.org/10.3390/ijms21144900






