Cancer Associated Endogenous Retroviruses: Ideal Immune Targets for Adenovirus-Based Immunotherapy
Abstract
1. Introduction
2. Endogenous Retrovirus in Cancer
2.1. Lessons from Rodents
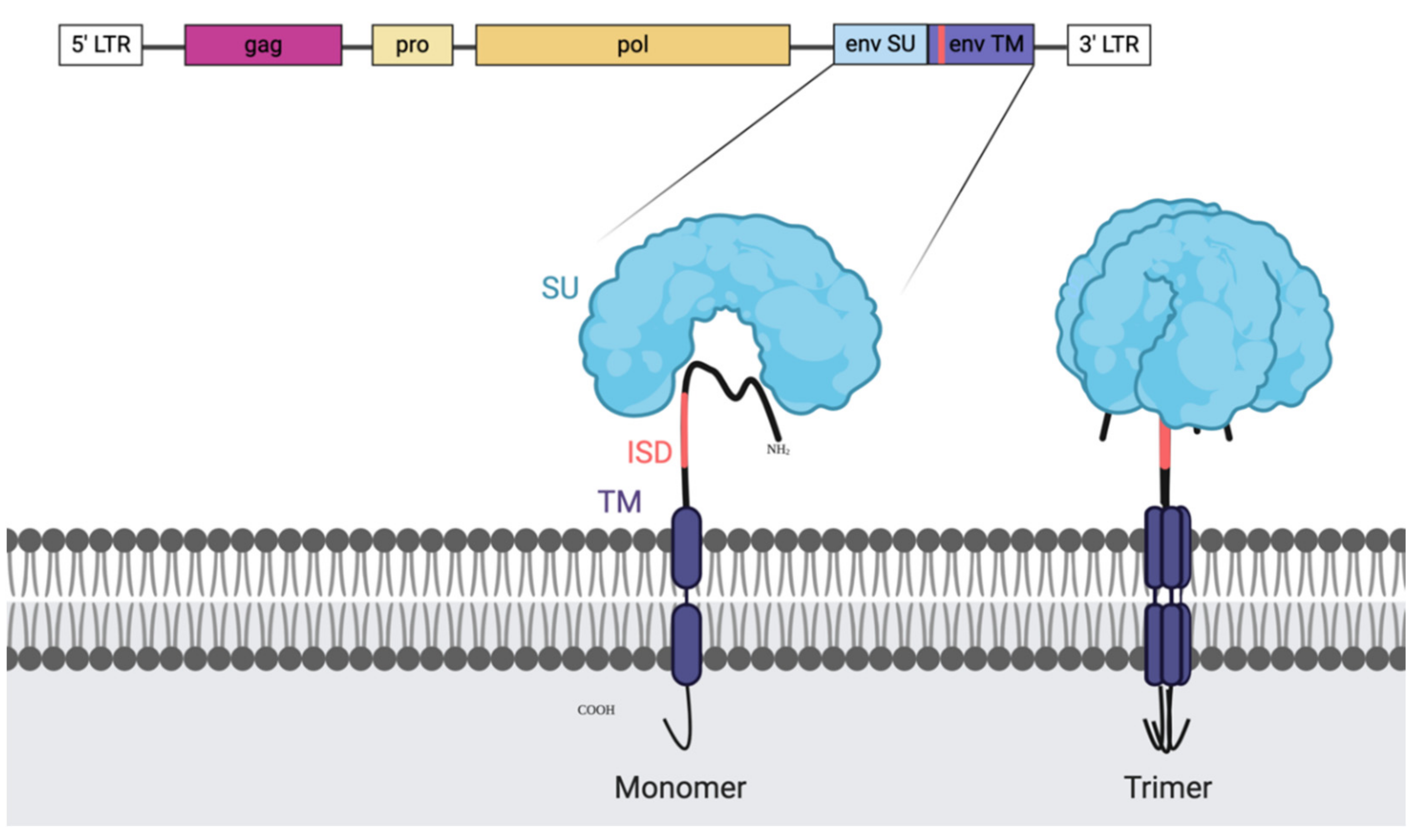
2.2. Human Endogenous Retrovirus
2.3. HERV-K
2.4. HERV-H and HERV-W
3. Endogenous Retrovirus and Immune Evasion
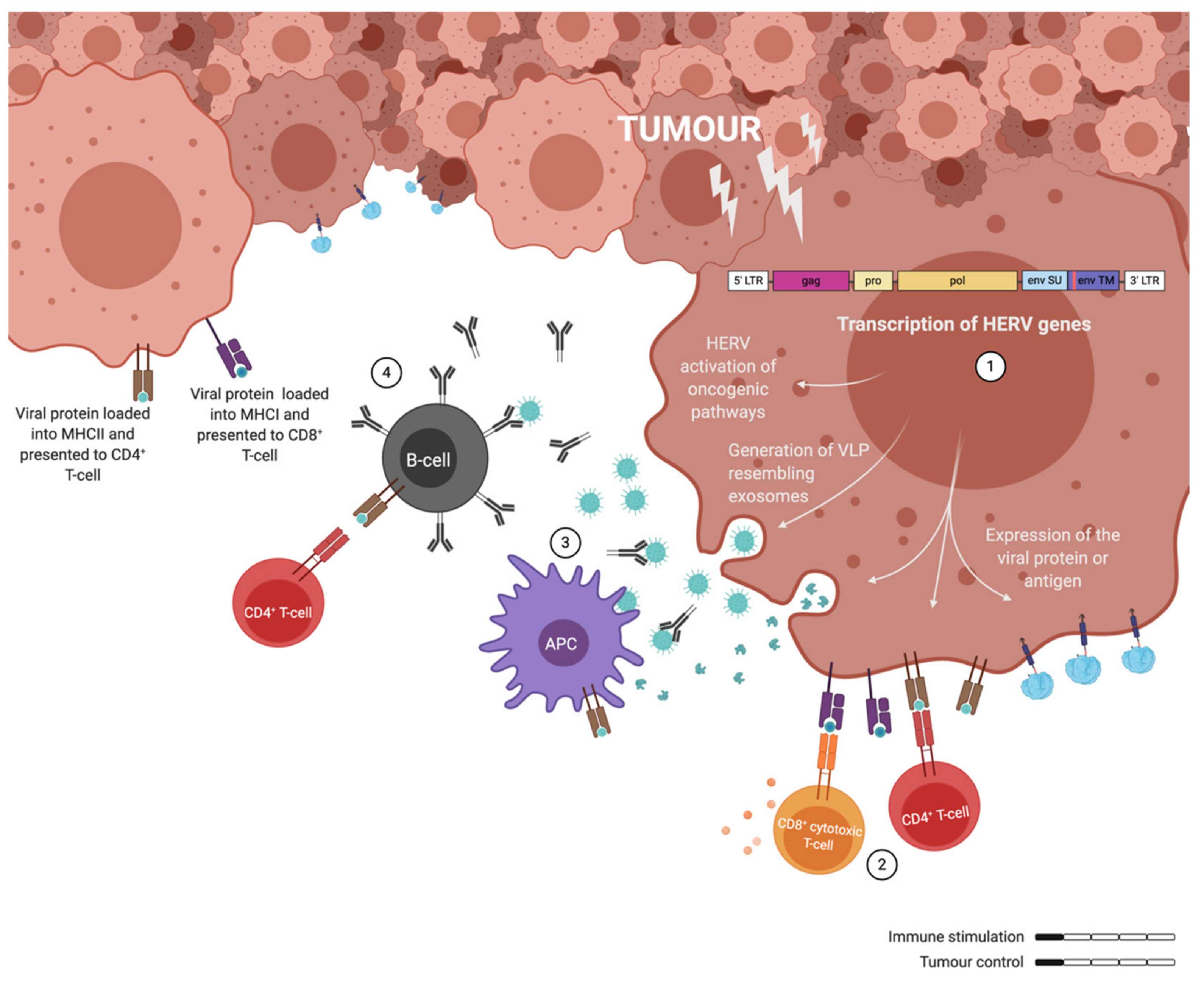
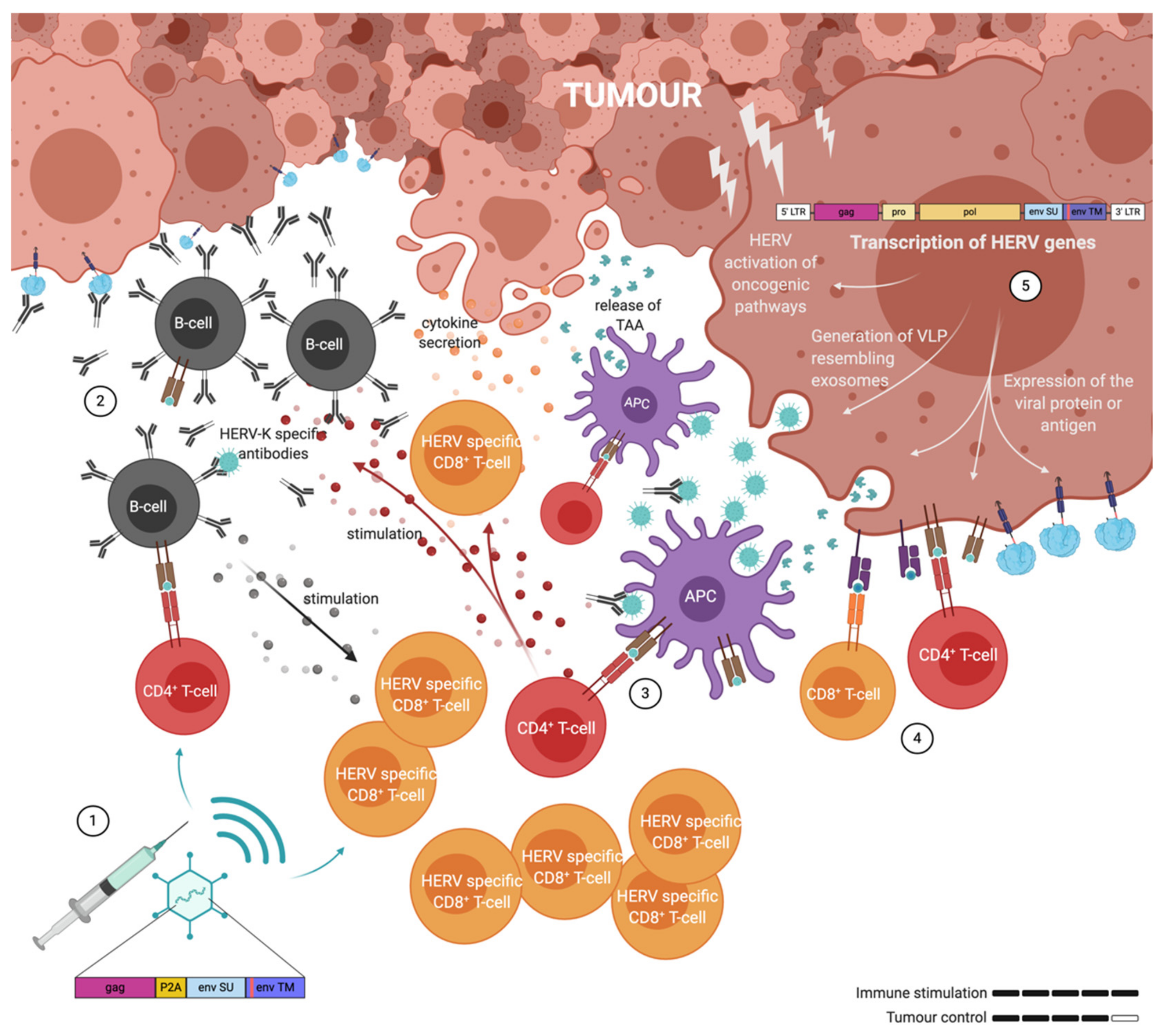
4. Targeting of Endogenous Retrovirus in Cancer
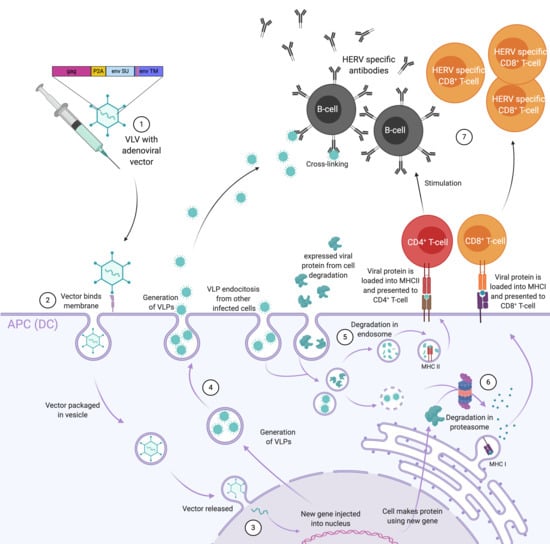
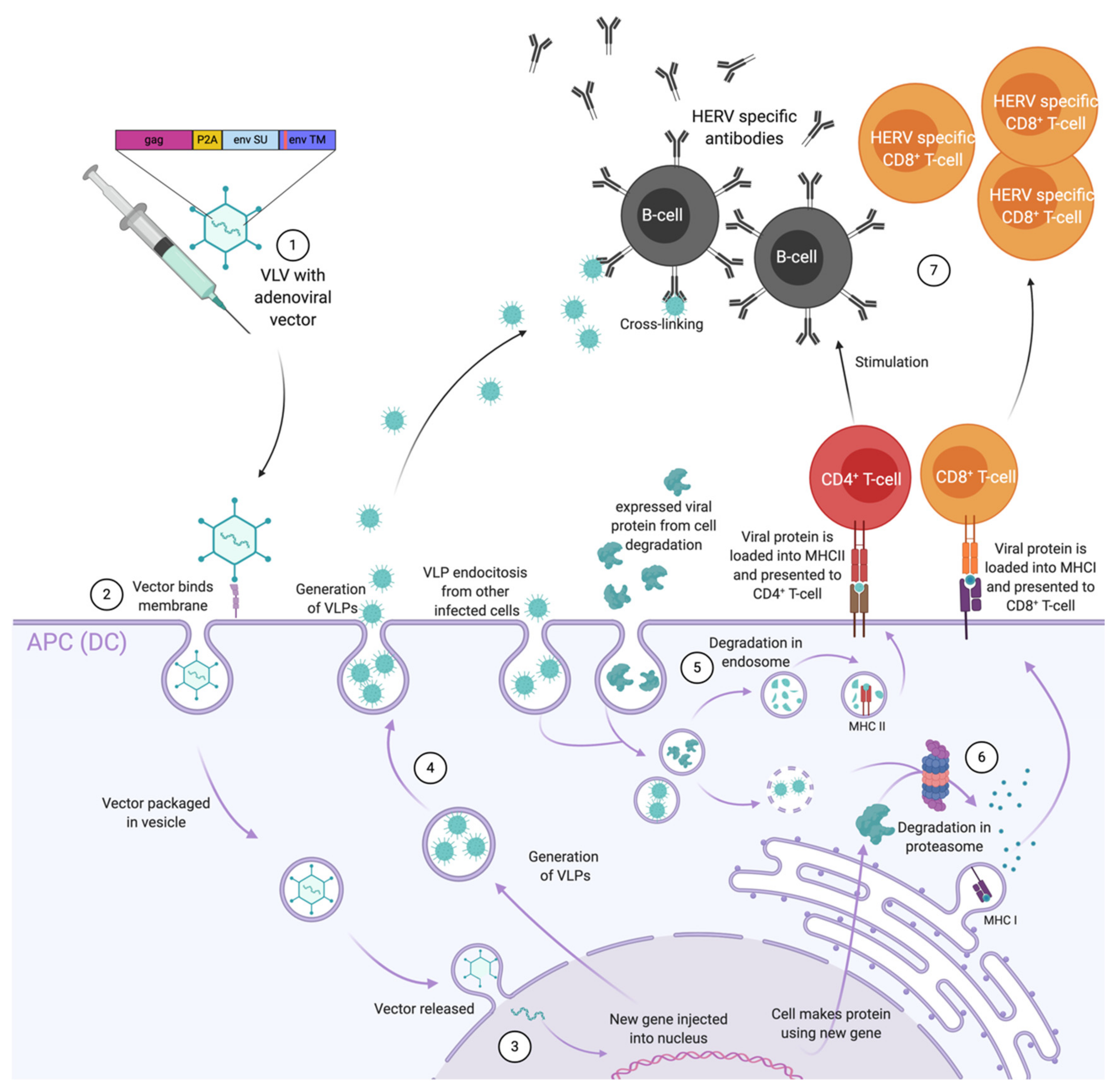
5. Adenoviral Vectors Used as Anti-Retroviral Vaccines
6. Adenovirus as Therapeutic Cancer Vaccines with ERVs as Targets
7. Discussion
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| WHO | World Health Organization |
| ERV | Endogenous retrovirus |
| HERV | Human endogenous retrovirus |
| VLV | Virus-like-vaccine |
| VLP LTR | Virus-like-particle Long terminal repeat |
| gag | Group-specific antigen |
| env pro pol | Envelope Protease Polymerase |
| HIV | Human immunodeficiency virus |
| WT | Wild type |
| HPV | Human papilloma virus |
| DC | Dendritic cell |
| SU | Surface unit |
| TM | Transmembrane |
| Treg | T regulatory |
| EMT | Epithelial to mesenchymal transition |
| HNSCC | Head and neck squamous cell carcinoma |
| TCC | Transitional cell carcinoma |
| UCC | Urothelial cell carcinoma |
| ISD | Immunosuppressive domain |
| MVA | Modified vaccinia Ankara |
| CAR | Chimeric antigen receptor |
| PASP | Pathogen associated structural pattern |
| TCR | T-cell receptor |
| MHC | Major histocompatibility complex |
| SIV | Simian immunodeficiency virus |
| NHP | Non-muman primates |
| TAA | Tumour-associated antigen |
| Ii | Invariant chain |
| LCMV | Lymphocytic choriomeningitis virus |
| Ad-MVA | Prime-boost regimen |
| Ad | Adenovirus |
References
- Milligan, I.D.; Gibani, M.M.; Sewell, R.; Clutterbuck, E.A.; Campbell, D.; Plested, E.; Nuthall, E.; Voysey, M.; Silva-Reyes, L.; McElrath, M.J.; et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines. JAMA 2016, 315, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.-M.M.C.; Schwerdtfeger, M.; Holst, P.J. Virus-Like-Vaccines against HIV. Vaccines 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 June 2020).
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; Fitzhugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Huebner, R.J.; Todaro, G.J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc. Natl. Acad. Sci. USA 1969, 64, 1087–1094. [Google Scholar] [CrossRef]
- Whitmire, C.E.; Huebner, R.J. Inhibition of Chemical Carcinogenesis by Viral Vaccines. Science 1972, 177, 60–61. [Google Scholar] [CrossRef]
- Fish, D.C.; Demarais, J.T.; Djurickovic, D.B.; Huebner, R.J. Prevention of 3-methylcholanthrene-induced fibrosarcomas in rats pre-inoculated with endogenous rat retrovirus. Proc. Natl. Acad. Sci. USA 1981, 78, 2526–2527. [Google Scholar] [CrossRef]
- Mangeney, M.; Heidmann, T. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 14920–14925. [Google Scholar] [CrossRef]
- Mangeney, M.; Pothlichet, J.; Renard, M.; Ducos, B.; Heidmann, T. Endogenous Retrovirus Expression Is Required for Murine Melanoma Tumor Growth In vivo. Cancer Res. 2005, 65, 2588–2591. [Google Scholar] [CrossRef]
- Scrimieri, F.; Askew, D.; Corn, D.J.; Eid, S.; Bobanga, I.D.; Bjelac, J.A.; Tsao, M.L.; Allen, F.; Othman, Y.S.; Wang, S.-C.G.; et al. Murine leukemia virus envelope gp70 is a shared biomarker for the high-sensitivity quantification of murine tumor burden. Oncoimmunology 2013, 2, e26889. [Google Scholar] [CrossRef]
- Callahan, R.; Chiu, I.; Wong, J.; Tronick, S.R.; Roe, B.; Aaronson, S.; Schlom, J. A new class of endogenous human retroviral genomes. Science 1985, 228, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.P.; Wildschutte, J.H.; Russo, C.; Coffin, J.M. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology 2011, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, M.; Wei, Y.; Lin, K.; Lu, Y.; Shen, J.; Johanning, G.L.; Wang-Johanning, F. Activation of HERV-K Env protein is essential for tumorigenesis and metastasis of breast cancer cells. Oncotarget 2016, 7, 84093–84117. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Zheng, X.; Weinstein, J.N.; Malouf, G.G.; Johanning, G.L.; Su, X.; Esteva, F.J. Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.; Rycaj, K.; Geng, S.; Li, M.; Plummer, J.B.; Yin, B.; Liu, H.; Xu, X.; Zhang, Y.; Yan, Y.; et al. Expression of Human Endogenous Retrovirus Type K Envelope Protein is a Novel Candidate Prognostic Marker for Human Breast Cancer. Genes Cancer 2011, 2, 914–922. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Epp, L.; Lu, D.W.; Johanning, G.L. Quantitation of HERV-K env gene expression and splicing in human breast cancer. Oncogene 2003, 22, 1528–1535. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Hao, Y.; Tan, Z. Identification of low-abundance alternatively spliced mRNA variants by exon exclusive reverse transcriptase polymerase chain reaction. Anal. Biochem. 2008, 383, 307–310. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Duan, X.; Sun, Y.; Wang, X.; Li, Z. Highly sensitive and multiplexed quantification of mRNA splice variants by the direct ligation of DNA probes at the exon junction and universal PCR amplification. Chem. Sci. 2017, 8, 3635–3640. [Google Scholar] [CrossRef]
- Lemaître, C.; Tsang, J.; Bireau, C.; Heidmann, T.; Dewannieux, M. A human endogenous retrovirus-derived gene that can contribute to oncogenesis by activating the ERK pathway and inducing migration and invasion. PLoS Pathog. 2017, 13, e1006451. [Google Scholar] [CrossRef]
- Li, M.; Radvanyi, L.; Yin, B.; Rycaj, K.; Li, J.; Chivukula, R.; Lin, K.; Lu, Y.; Shen, J.; Chang, D.Z.; et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin. Cancer Res. 2017, 23, 5892–5911. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Plummer, J.B.; Yin, B.; Li, M.; Garza, J.; Radvanyi, L.; Ramondetta, L.M.; Lin, K.; Johanning, G.L.; Tang, D.G.; et al. Cytotoxicity of human endogenous retrovirus K-specific T cells toward autologous ovarian cancer cells. Clin. Cancer Res. 2015, 21, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Liu, J.; Rycaj, K.; Huang, M.; Tsai, K.; Rosen, D.G.; Chen, D.T.; Lu, D.W.; Barnhart, K.F.; Johanning, G.L. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer 2006, 120, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Triratanachat, S.; Rattanatunyong, P.; Mutirangura, A.; Khemapech, N.; Iramaneerat, K. HERV-K Hypomethylation in Ovarian Clear Cell Carcinoma Is Associated with a Poor Prognosis and Platinum Resistance. Int. J. Gynecol. Cancer 2010, 21, 51–57. [Google Scholar] [CrossRef]
- Heidmann, O.; Berthier, R.; Paternina, J.; Heidmann, T.; Bawa, O.; Béguin, A.; Deloger, M. HEMO, an ancestral endogenous retroviral envelope protein shed in the blood of pregnant women and expressed in pluripotent stem cells and tumors. Proc. Natl. Acad. Sci. USA 2017, 114, E6642–E6651. [Google Scholar] [CrossRef] [PubMed]
- Agoni, L.; Guha, C.; Lenz, J. Detection of Human Endogenous Retrovirus K (HERV-K) Transcripts in Human Prostate Cancer Cell Lines. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef]
- Ishida, T.; Obata, Y.; Ohara, N.; Matsushita, H.; Sato, S.; Uenaka, A.; Saika, T.; Miyamura, T.; Chayama, K.; Nakamura, Y.; et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008, 8, 15. [Google Scholar]
- Wallace, T.A.; Downey, R.F.; Seufert, C.J.; Schetter, A.; Dorsey, T.H.; Johnson, C.A.; Goldman, R.; Loffredo, C.A.; Yan, P.; Sullivan, F.J.; et al. Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis 2014, 35, 2074–2083. [Google Scholar] [CrossRef]
- Goering, W.; Schmitt, K.; Dostert, M.; Schaal, H.; Deenen, R.; Mayer, J.; Schulz, W.A. Human endogenous retrovirus HERV-K(HML-2) activity in prostate cancer is dominated by a few loci. Prostate 2015, 75, 1958–1971. [Google Scholar] [CrossRef]
- Reis, B.S.; Jungbluth, A.A.; Frosina, D.; Holz, M.; Ritter, E.; Nakayama, E.; Ishida, T.; Obata, Y.; Carver, B.; Scher, H.; et al. Prostate cancer progression correlates with increased humoral immune response to a human endogenous retrovirus GAG protein. Clin. Cancer Res. 2013, 19, 6112–6125. [Google Scholar] [CrossRef]
- Florl, A.R.; Löwer, R.; Schmitz-Dräger, B.J.; Schulz, W.A. DNA methylation and expression of LINE-1 and HERV-K provirus sequences in urothelial and renal cell carcinomas. Br. J. Cancer 1999, 80, 1312–1321. [Google Scholar] [CrossRef]
- Siebenthall, K.T.; Miller, C.P.; Vierstra, J.D.; Mathieu, J.; Tretiakova, M.; Reynolds, A.; Sandstrom, R.; Rynes, E.; Haugen, E.; Johnson, A.; et al. Integrated epigenomic profiling reveals endogenous retrovirus reactivation in renal cell carcinoma. EBioMedicine 2019, 41, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; Kaplan, M.H.; Leissner, P.; Verjat, T.; Ferlenghi, I.; Bagnoli, F.; Giusti, F.; Dosik, M.H.; Hayes, D.F.; Gitlin, S.D.; et al. Human Endogenous Retrovirus K (HML-2) Elements in the Plasma of People with Lymphoma and Breast Cancer. J. Virol. 2008, 82, 9329–9336. [Google Scholar] [CrossRef]
- Chen, T.; Meng, Z.; Gan, Y.; Wang, X.; Xu, F.; Gu, Y.; Xu, X.; Tang, J.; Zhou, H.; Zhang, X.; et al. The viral oncogene Np9 acts as a critical molecular switch for co-activating β-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia 2013, 27, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Büscher, K.; Trefzer, U.; Hofmann, M.; Sterry, W.; Kurth, R.; Denner, J. Expression of human endogenous retrovirus K in melanomas and melanoma cell lines. Cancer Res. 2005, 65, 4172–4180. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Reichrath, J.; Roesch, A.; Meese, E.; Mayer, J. Transcriptional profiling of human endogenous retrovirus group HERV-K(HML-2) loci in melanoma. Genome Biol. Evol. 2013, 5, 307–328. [Google Scholar] [CrossRef]
- Giebler, M.; Staege, M.S.; Blauschmidt, S.; Ohm, L.I.; Kraus, M.; Würl, P.; Taubert, H.; Greither, T. Elevated HERV-K Expression in Soft Tissue Sarcoma Is Associated with Worsened Relapse-Free Survival. Front. Microbiol. 2018, 9, 211. [Google Scholar] [CrossRef]
- Strissel, P.L.; Ruebner, M.; Thiel, F.; Wachter, D.; Ekici, A.B.; Wolf, F.; Thieme, F.; Ruprecht, K.; Beckmann, M.W.; Strick, R. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: Emergence of new molecular targets. Oncotarget 2012, 3, 1204–1219. [Google Scholar] [CrossRef]
- Zare, M.; Mostafaei, S.; Ahmadi, A.; Azimzadeh Jamalkandi, S.; Abedini, A.; Esfahani-Monfared, Z.; Dorostkar, R.; Saadati, M. Human endogenous retrovirus env genes: Potential blood biomarkers in lung cancer. Microb. Pathog. 2018, 115, 189–193. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, Z.; Xu, R.; Wu, L.; Zheng, S. Expression Patterns of Non-Coding Spliced Transcripts from Human Endogenous Retrovirus HERV-H Elements in Colon Cancer. PLoS ONE 2012, 7, e29950. [Google Scholar] [CrossRef]
- Ferrari, L.; Cafora, M.; Rota, F.; Hoxha, M.; Iodice, S.; Tarantini, L.; Dolci, M.; Delbue, S.; Pistocchi, A.; Bollati, V. Extracellular vesicles released by colorectal cancer cell lines modulate innate immune response in zebrafish model: The possible role of human endogenous retroviruses. Int. J. Mol. Sci. 2019, 20, 3669. [Google Scholar] [CrossRef]
- Brennan, K.; Koenig, J.L.; Gentles, A.J.; Sunwoo, J.B.; Gevaert, O. Identification of an atypical etiological head and neck squamous carcinoma subtype featuring the CpG island methylator phenotype. EBioMedicine 2017, 17, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Strati, K.; Pitot, H.C.; Lambert, P.F. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 14152–14157. [Google Scholar] [CrossRef] [PubMed]
- Michna, A.; Schötz, U.; Selmansberger, M.; Zitzelsberger, H.; Lauber, K.; Unger, K.; Hess, J. Transcriptomic analyses of the radiation response in head and neck squamous cell carcinoma subclones with different radiation sensitivity: Time-course gene expression profiles and gene association networks. Radiat. Oncol. 2016, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, T.; Zhao, Z.; Chen, Y.; Zeng, J.; Liu, S.; Zhu, F. Mutations in 3’-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene 2014, 33, 3947–3958. [Google Scholar] [CrossRef] [PubMed]
- Gosenca, D.; Gabriel, U.; Steidler, A.; Mayer, J.; Diem, O.; Erben, P.; Fabarius, A.; Leib-Mösch, C.; Hofmann, W.K.; Seifarth, W. HERV-E-Mediated Modulation of PLA2G4A Transcription in Urothelial Carcinoma. PLoS ONE 2012, 7, e49341. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ye, H.; Wang, J.; Chen, S.; Chen, X.; Zhang, C. Immune Checkpoint Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 is Upregulated and Independently Predicts Unfavorable Prognosis in Bladder Urothelial Carcinoma. Nephron 2019, 141, 256–264. [Google Scholar] [CrossRef]
- Panda, A.; de Cubas, A.A.; Stein, M.; Riedlinger, G.; Kra, J.; Mayer, T.; Smith, C.C.; Vincent, B.G.; Serody, J.S.; Beckermann, K.E.; et al. Endogenous retrovirus expression is associated with response to immune checkpoint pathway in clear cell renal cell carcinoma. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Bergallo, M.; Mareschi, K.; Montanari, P.; Calvi, C.; Berger, M.; Bini, I.; Daprà, V.; Galliano, I.; Fagioli, F. Human endogenous retrovirus, HERV-P and HERV-R in pediatric leukemia patients. J. Hematop. 2019, 12, 51–56. [Google Scholar] [CrossRef]
- Maliniemi, P.; Vincendeau, M.; Mayer, J.; Frank, O.; Hahtola, S.; Karenko, L.; Carlsson, E.; Mallet, F.; Seifarth, W.; Leib-Mösch, C.; et al. Expression of Human Endogenous Retrovirus-W Including Syncytin-1 in Cutaneous T-Cell Lymphoma. PLoS ONE 2013, 8, e76281. [Google Scholar] [CrossRef]
- Fava, P.; Bergallo, M.; Astrua, C.; Brizio, M.; Galliano, I.; Montanari, P.; Tovo, P.A.; Novelli, M.; Savoia, P.; Quaglino, P.; et al. Human Endogenous Retrovirus Expression in Primary Cutaneous T-Cell Lymphomas. Dermatology 2016, 232, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Trejbalová, K.; Kovářová, D.; Vernerová, Z.; Hron, T.; Kučerová, D.; Hejnar, J. DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas. Retrovirology 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Okahara, G.; Matsubara, S.; Oda, T.; Sugimoto, J.; Jinno, Y.; Kanaya, F. Expression analyses of human endogenous retroviruses (HERVs): Tissue-specific and developmental stage-dependent expression of HERVs. Genomics 2004, 84, 982–990. [Google Scholar] [CrossRef]
- Shiroma, T.; Sugimoto, J.; Oda, T.; Jinno, Y.; Kanaya, F. Search for active endogenous retroviruses: Identification and characterization of a HERV-E gene that is expressed in the pancreas and thyroid. J. Hum. Genet. 2001, 46, 619–625. [Google Scholar] [CrossRef][Green Version]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. Evaluating the expression level of HERV-K env, np9, rec and gag in breast tissue. Infect. Agent. Cancer 2019, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.-C.; Venables, P.J.W.; Tönjes, R.R.; Scherer, J.; Eriksson, L.; Larsson, E. Developmental Expression of HERV-R (ERV3) and HERV-K in Human Tissue. Virology 2002, 297, 220–225. [Google Scholar] [CrossRef]
- Blond, J.-L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.-L. An Envelope Glycoprotein of the Human Endogenous Retrovirus HERV-W Is Expressed in the Human Placenta and Fuses Cells Expressing the Type D Mammalian Retrovirus Receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.-Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elisseeff, X.; Vargas, A.; Rassart, É.; Lafond, J.; Leduc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells. Biol. Reprod. 2019. [Google Scholar] [CrossRef]
- Morozov, V.A.; Morozov, A.V.; Semaan, M.; Denner, J. Single mutations in the transmembrane envelope protein abrogate the immunosuppressive property of HIV-1. Retrovirology 2012, 9, 67. [Google Scholar] [CrossRef]
- White, H.D.; Robbins, M.D.; Green, W.R. Mechanism of escape of endogenous murine leukemia virus emv-14 from recognition by anti-AKR/Gross virus cytolytic T lymphocytes. J. Virol. 1990, 64, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Scheeren, R.A.; Oostendorp, R.A.J.; Van der baan, S.; Keehnen, R.M.J.; Scheper, R.J.; Meijer, C.J.L.M. Distribution of retroviral p15E-related proteins in neoplastic and non-neoplastic human tissues, and their role in the regulation of the immune response. Clin. Exp. Immunol. 1992, 89, 94–99. [Google Scholar] [CrossRef]
- Morozov, V.A.; Dao Thi, V.L.; Denner, J. The Transmembrane Protein of the Human Endogenous Retrovirus-K (HERV-K) Modulates Cytokine Release and Gene Expression. PLoS ONE 2013, 8, e70399. [Google Scholar] [CrossRef]
- Kudo-Saito, C.; Yura, M.; Yamamoto, R.; Kawakami, Y. Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Res. 2014, 74, 1361–1370. [Google Scholar] [CrossRef]
- Tas, M.P.R.; Laarman, D.; Haan-Meulman, M.; Balm, A.J.M.; Snow, G.B.; Drexhage, H.A. Retroviral p15E-related serum factors and recurrence of head and neck cancer. Clin. Otolaryngol. 1993, 18, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.B.; Balm, A.J.M.; Snow, G.B.; Drexhage, H.A. Immunosuppressive retroviral-related factors in sera of patients with head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 1990, 247, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.B.; Drexhage, H.A.; Mullink, R.; Hensen-Logmans, S.; De Haan-Meulman, M.; Snow, G.B.; Balm, A.J.M. Immunohistochemical Detection of Retroviral—P15E—Related Material in Carcinomas of the Head and Neck. Otolaryngol. Neck Surg. 1987, 96, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.S.; Oostendorp, R.A.J.; Simons, P.J.; Boersma, W.; Knegt, P.; van Ewijk, W. New monoclonal antibodies against the putative immunosuppressive site of retroviral p15E. Cancer Res. 1994, 54, 1831–1836. [Google Scholar] [PubMed]
- Hummel, J.; Kämmerer, U.; Müller, N.; Avota, E.; Schneider-Schaulies, S. Human endogenous retrovirus envelope proteins target dendritic cells to suppress T-cell activation. Eur. J. Immunol. 2015, 45, 1748–1759. [Google Scholar] [CrossRef]
- Thiel, H.J.; Schwarz, H.; Fischinger, P.; Bolognesi, D.; Schafer, W. Role of antibodies to murine leukemia virus p15E transmembrane protein in immunotherapy against AKR leukemia: A model for studies in human acquired immunodeficiency syndrome. Proc. Natl. Acad. Sci. USA 1987, 84, 5893–5897. [Google Scholar] [CrossRef]
- Bronte, V.; Cingarlini, S.; Apolloni, E.; Serafini, P.; Marigo, I.; De Santo, C.; Macino, B.; Marin, O.; Zanovello, P. Effective Genetic Vaccination with a Widely Shared Endogenous Retroviral Tumor Antigen Requires CD40 Stimulation during Tumor Rejection Phase. J. Immunol. 2003, 171, 6396–6405. [Google Scholar] [CrossRef] [PubMed]
- Takeda, J.; Sato, Y.; Kiyosawa, H.; Mori, T.; Yokoya, S.; Irisawa, A.; Miyata, M.; Obara, K.; Fujita, T.; Suzuki, T.; et al. Anti-tumor immunity against CT26 colon tumor in mice immunized with plasmid DNA encoding β-galactosidase fused to an envelope protein of endogenous retrovirus. Cell. Immunol. 2000, 204, 11–18. [Google Scholar] [CrossRef]
- Rice, J.; Buchan, S.; Stevenson, F.K. Critical Components of a DNA Fusion Vaccine Able to Induce Protective Cytotoxic T Cells Against a Single Epitope of a Tumor Antigen. J. Immunol. 2002, 169, 3908–3913. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Rycaj, K.; Plummer, J.B.; Li, M.; Yin, B.; Frerich, K.; Garza, J.G.; Shen, J.; Lin, K.; Yan, P.; et al. Immunotherapeutic potential of anti-human endogenous retrovirus-k envelope protein antibodies in targeting breast tumors. J. Natl. Cancer Inst. 2012, 104, 189–210. [Google Scholar] [CrossRef]
- Kraus, B.; Fischer, K.; Büchner, S.M.; Wels, W.S.; Löwer, R.; Sliva, K.; Schnierle, B.S. Vaccination Directed against the Human Endogenous Retrovirus-K Envelope Protein Inhibits Tumor Growth in a Murine Model System. PLoS ONE 2013, 8, e72756. [Google Scholar] [CrossRef] [PubMed]
- Kraus, B.; Fischer, K.; Sliva, K.; Schnierle, B.S. Vaccination directed against the human endogenous retrovirus-K (HERV-K) gag protein slows HERV-K gag expressing cell growth in a murine model system. Virol. J. 2014, 11, 58. [Google Scholar] [CrossRef]
- Zhou, F.; Krishnamurthy, J.; Wei, Y.; Li, M.; Hunt, K.; Johanning, G.L.; Cooper, L.J.N.; Wang-Johanning, F. Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of Ras. Oncoimmunology 2015, 4, e1047582. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Rabinovich, B.A.; Mi, T.; Switzer, K.C.; Olivares, S.; Maiti, S.N.; Plummer, J.B.; Singh, H.; Kumaresan, P.R.; Huls, H.M.; et al. Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin. Cancer Res. 2015, 21, 3241–3251. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. Innate Immune Response to Adenoviral Vectors Is Mediated by both Toll-Like Receptor-Dependent and -Independent Pathways. J. Virol. 2007, 81, 3170–3180. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Lundholm-Beauchamp, U.; Horswood, R.L.; Pernis, B.; Wold, W.S.M.; Chanock, R.M.; Prince, G.A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc. Natl. Acad. Sci. USA 1989, 86, 3823–3827. [Google Scholar] [CrossRef]
- Tatsis, N.; Ertl, H.C.J. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Soloff, A.C.; Lu, X.; Montecalvo, A.; Nguyen, D.C.; Matsuoka, Y.; Robbins, P.D.; Swayne, D.E.; Donis, R.O.; Katz, J.M.; et al. Protection of Mice and Poultry from Lethal H5N1 Avian Influenza Virus through Adenovirus-Based Immunization. J. Virol. 2006, 80, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, A.T.; Koup, R.A.; Roederer, M.; Bailer, R.T.; Enama, M.E.; Moodie, Z.; Gu, L.; Martin, J.E.; Novik, L.; Chakrabarti, B.K.; et al. Phase 1 Safety and Immunogenicity Evaluation of a Multiclade HIV-1 Candidate Vaccine Delivered by a Replication-Defective Recombinant Adenovirus Vector. J. Infect. Dis. 2006, 194, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Baybutt, T.R.; Berman-Booty, L.; Magee, M.S.; Waldman, S.A.; Alexeev, V.Y.; Snook, A.E. Prime-Boost Immunization Eliminates Metastatic Colorectal Cancer by Producing High-Avidity Effector CD8+ T Cells. J. Immunol. 2017, 198, 3507–3514. [Google Scholar] [CrossRef]
- Sullivan, N.J.; Sanchez, A.; Rollin, P.E.; Yang, Z.; Nabel, G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature 2000, 408, 605–609. [Google Scholar] [CrossRef]
- Yin, X.; Langer, S.; Zhang, Z.; Herbert, K.M.; Yoh, S.; König, R.; Chanda, S.K. Sensor Sensibility—HIV-1 and the Innate Immune Response. Cells 2020, 9, 254. [Google Scholar] [CrossRef]
- Qian, C.; Liu, X.; Xu, Q.; Wang, Z.; Chen, J.; Li, T.; Zheng, Q.; Yu, H.; Gu, Y.; Li, S.; et al. Recent progress on the versatility of virus-like particles. Vaccines 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Li, M.; Bharadwaj, U.; Zhang, R.; Zhang, S.; Mu, H.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol. Cancer Ther. 2008, 7, 286–296. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Chimeric Trop2 Virus-like Particles. J. Immunother. 2011, 34, 251–263. [Google Scholar] [CrossRef]
- Barth, H. Uptake and presentation of hepatitis C virus-like particles by human dendritic cells. Blood 2005, 105, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.-Y. Therapeutic Cancer Vaccines. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peers-Adams, A.; Win, S.J.; Scullion, S.; Wilson, M.; Young, V.L.; Jennings, P.; Ward, V.K.; Baird, M.A.; Young, S.L. Antigen Incorporated In Virus-like Particles Is Delivered to Specific Dendritic Cell Subsets That Induce An Effective Antitumor Immune Response In Vivo. J. Immunother. 2013, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bayer, W.; Tenbusch, M.; Lietz, R.; Johrden, L.; Schimmer, S.; Uberla, K.; Dittmer, U.; Wildner, O. Vaccination with an Adenoviral Vector That Encodes and Displays a Retroviral Antigen Induces Improved Neutralizing Antibody and CD4+ T-Cell Responses and Confers Enhanced Protection. J. Virol. 2010, 84, 1967–1976. [Google Scholar] [CrossRef]
- De Souza, M.S.; Ratto-Kim, S.; Chuenarom, W.; Schuetz, A.; Chantakulkij, S.; Nuntapinit, B.; Valencia-Micolta, A.; Thelian, D.; Nitayaphan, S.; Pitisuttithum, P.; et al. The Thai Phase III Trial (RV144) Vaccine Regimen Induces T Cell Responses That Preferentially Target Epitopes within the V2 Region of HIV-1 Envelope. J. Immunol. 2012, 188, 5166–5176. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.J.; Dubey, S.A.; Mehrotra, D.V.; Guan, L.; Long, R.; Anderson, K.; Collins, K.; Gaunt, C.; Fernandez, R.; Cole, S.; et al. Comparison of T cell immune responses induced by vectored HIV vaccines in non-human primates and humans. Vaccine 2010, 28, 7881–7889. [Google Scholar] [CrossRef]
- Neukirch, L.; Fougeroux, C.; Andersson, A.-M.C.; Holst, P.J. The potential of adenoviral vaccine vectors with altered antigen presentation capabilities. Expert Rev. Vaccines 2020, 19, 25–41. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Holst, P. The rationale of vectored gene-fusion vaccines against cancer: Evolving strategies and latest evidence. Ther. Adv. Vaccines 2013, 1, 33–47. [Google Scholar] [CrossRef]
- Andersson, A.-M.C.M.C.; Holst, P.J. Increased t cell breadth and antibody response elicited in prime-boost regimen by viral vector encoded homologous siv gag/env in outbred cd1 mice. J. Transl. Med. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Schwerdtfeger, M.; Andersson, A.-M.C.; Neukirch, L.; Holst, P.J. Virus-like vaccines against HIV/SIV synergize with a subdominant antigen T cell vaccine. J. Transl. Med. 2019, 17, 175. [Google Scholar] [CrossRef]
- Thompson, P.J.; Macfarlan, T.S.; Lorincz, M.C. Long Terminal Repeats: From Parasitic Elements to Building Blocks of the Transcriptional Regulatory Repertoire. Mol. Cell 2016, 62, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Yang, P.; Füchtbauer, A.C.; Füchtbauer, E.-M.; Silva, A.M.; Park, C.; Wu, W.; Nielsen, A.L.; Pedersen, F.S.; Macfarlan, T.S. The KRAB zinc finger protein ZFP809 is required to initiate epigenetic silencing of endogenous retroviruses. Genes Dev. 2015, 29, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; Kaplan, M.H.; Dube, D.; Gonzalez-Hernandez, M.J.; Chan, S.; Meng, F.; Dai, M.; Omenn, G.S.; Gitlin, S.D.; Markovitz, D.M. Human Endogenous Retrovirus Type K (HERV-K) Particles Package and Transmit HERV-K—Related Sequences. J. Virol. 2015, 89, 7187–7201. [Google Scholar] [CrossRef]
- Boller, K.; Schönfeld, K.; Lischer, S.; Fischer, N.; Hoffmann, A.; Kurth, R.; Tönjes, R.R. Human endogenous retrovirus HERV-K113 is capable of producing intact viral particles. J. Gen. Virol. 2008, 89, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kassiotis, G.; Stoye, J.P. Immune responses to endogenous retroelements: Taking the bad with the good. Nat. Rev. Immunol. 2016, 16, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Tumour virus vaccines: Hepatitis B virus and human papillomavirus. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; Lambe, T.; Rollier, C.S.; Spencer, A.J.; Hill, A.V.S.; Dorrell, L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr. Opin. Immunol. 2016, 41, 47–54. [Google Scholar] [CrossRef]
- Holst, P.J.; Sorensen, M.R.; Mandrup Jensen, C.M.; Orskov, C.; Thomsen, A.R.; Christensen, J.P. MHC Class II-Associated Invariant Chain Linkage of Antigen Dramatically Improves Cell-Mediated Immunity Induced by Adenovirus Vaccines. J. Immunol. 2008, 180, 3339–3346. [Google Scholar] [CrossRef]
- Sorensen, M.R.; Holst, P.J.; Pircher, H.; Christensen, J.P.; Thomsen, A.R. Vaccination with an adenoviral vector encoding the tumor antigen directly linked to invariant chain induces potent CD4+ T-cell-independent CD8+ T-cell-mediated tumor control. Eur. J. Immunol. 2009, 39, 2725–2736. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Andersson, A.-M.C.; Mariya, S.; Pedersen, A.G.; Burk, R.D.; Folgori, A.; Colloca, S.; Cortese, R.; Nicosia, A.; Pamungkas, J.; et al. Therapeutic Vaccine Against Primate Papillomavirus Infections of the Cervix. J. Immunother. 2017, 40, 51–61. [Google Scholar] [CrossRef]
- Esposito, I.; Cicconi, P.; Capone, S.; Brown, A.; Esposito, M.L.; Mori, F.; Vassilev, V.; Siani, L.; Ghaffari, E.; Gardiner, C.; et al. GS-05-MHC-II invariant chain adjuvanted chimpanzee adenoviral and MVA hepatitis C vaccines elicit unprecedented levels of anti-viral T-cell immune responses in humans. J. Hepatol. 2019, 70, e3–e4. [Google Scholar] [CrossRef]
- Halbroth, B.R.; Sebastian, S.; Poyntz, H.C.; Bregu, M.; Cottingham, M.G.; Hill, A.V.S.; Spencer, A.J. Development of a Molecular Adjuvant to Enhance Antigen-Specific CD8+ T Cell Responses. Sci. Rep. 2018, 8, 15020. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.A.H.; Steffensen, M.A.; Nielsen, K.N.; Christensen, J.P.; Thomsen, A.R.; Holst, P.J. Co-Expression of Tumor Antigen and Interleukin-2 From an Adenoviral Vector Augments the Efficiency of Therapeutic Tumor Vaccination. Mol. Ther. 2014, 22, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Andersson, A.-M.C.; Pedersen, A.E.; Laursen, H.; Holst, P.J. An adenoviral cancer vaccine co-encoding a tumor associated antigen together with secreted 4-1BBL leads to delayed tumor progression. Vaccine 2016, 34, 2147–2156. [Google Scholar] [CrossRef]
- Neukirch, L.; Nielsen, T.K.; Laursen, H.; Daradoumis, J.; Thirion, C.; Holst, P.J. Adenovirus based virus-like-vaccines targeting endogenous retroviruses can eliminate growing colorectal cancers in mice. Oncotarget 2019, 10, 1458–1472. [Google Scholar] [CrossRef]
- D’Alise, A.M.; Leoni, G.; Cotugno, G.; Troise, F.; Langone, F.; Fichera, I.; De Lucia, M.; Avalle, L.; Vitale, R.; Leuzzi, A.; et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Kron, M.W.; Engler, T.; Schmidt, E.; Schirmbeck, R.; Kochanek, S.; Kreppel, F. High-capacity adenoviral vectors circumvent the limitations of ΔE1 and ΔE1/ΔE3 adenovirus vectors to induce multispecific transgene product-directed CD8 T-cell responses. J. Gene Med. 2011, 13, 648–657. [Google Scholar] [CrossRef]
| HERV-K | HERV-E | HERV-W | HERV-H | HEMO | HERV-FRD | HERV-R | HERV-P | |
|---|---|---|---|---|---|---|---|---|
| Breast | X | X | X | X | X | X | ||
| Lymphoma | X | X | X | |||||
| Leukaemia | X | X | ||||||
| Endometrial | X | X | X | X | X | |||
| Prostate | X | |||||||
| Seminoma | X | X | ||||||
| TCC | X | |||||||
| Ovarian | X | X | X | X | ||||
| Melanoma | X | |||||||
| Lung | X | X | X | X | X | |||
| Colon | X | X | X | X | ||||
| Pancreas | X | |||||||
| Sarcoma | X | |||||||
| Urothelial/Renal | X | X | X | X | X | X | ||
| HNSCC | X | X |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara Bermejo, A.; Ragonnaud, E.; Daradoumis, J.; Holst, P. Cancer Associated Endogenous Retroviruses: Ideal Immune Targets for Adenovirus-Based Immunotherapy. Int. J. Mol. Sci. 2020, 21, 4843. https://doi.org/10.3390/ijms21144843
Vergara Bermejo A, Ragonnaud E, Daradoumis J, Holst P. Cancer Associated Endogenous Retroviruses: Ideal Immune Targets for Adenovirus-Based Immunotherapy. International Journal of Molecular Sciences. 2020; 21(14):4843. https://doi.org/10.3390/ijms21144843
Chicago/Turabian StyleVergara Bermejo, Amaia, Emeline Ragonnaud, Joana Daradoumis, and Peter Holst. 2020. "Cancer Associated Endogenous Retroviruses: Ideal Immune Targets for Adenovirus-Based Immunotherapy" International Journal of Molecular Sciences 21, no. 14: 4843. https://doi.org/10.3390/ijms21144843
APA StyleVergara Bermejo, A., Ragonnaud, E., Daradoumis, J., & Holst, P. (2020). Cancer Associated Endogenous Retroviruses: Ideal Immune Targets for Adenovirus-Based Immunotherapy. International Journal of Molecular Sciences, 21(14), 4843. https://doi.org/10.3390/ijms21144843