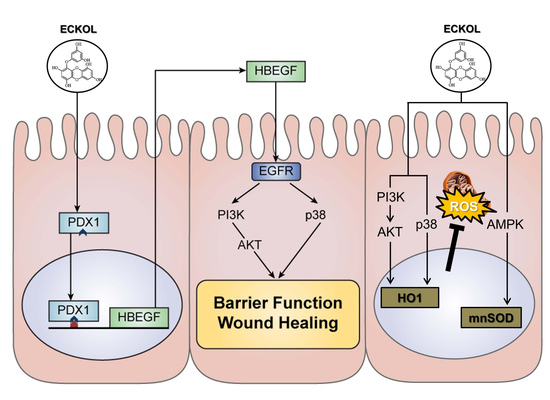
Eckol Alleviates Intestinal Dysfunction during Suckling-to-Weaning Transition via Modulation of PDX1 and HBEGF
Abstract
1. Introduction
2. Results
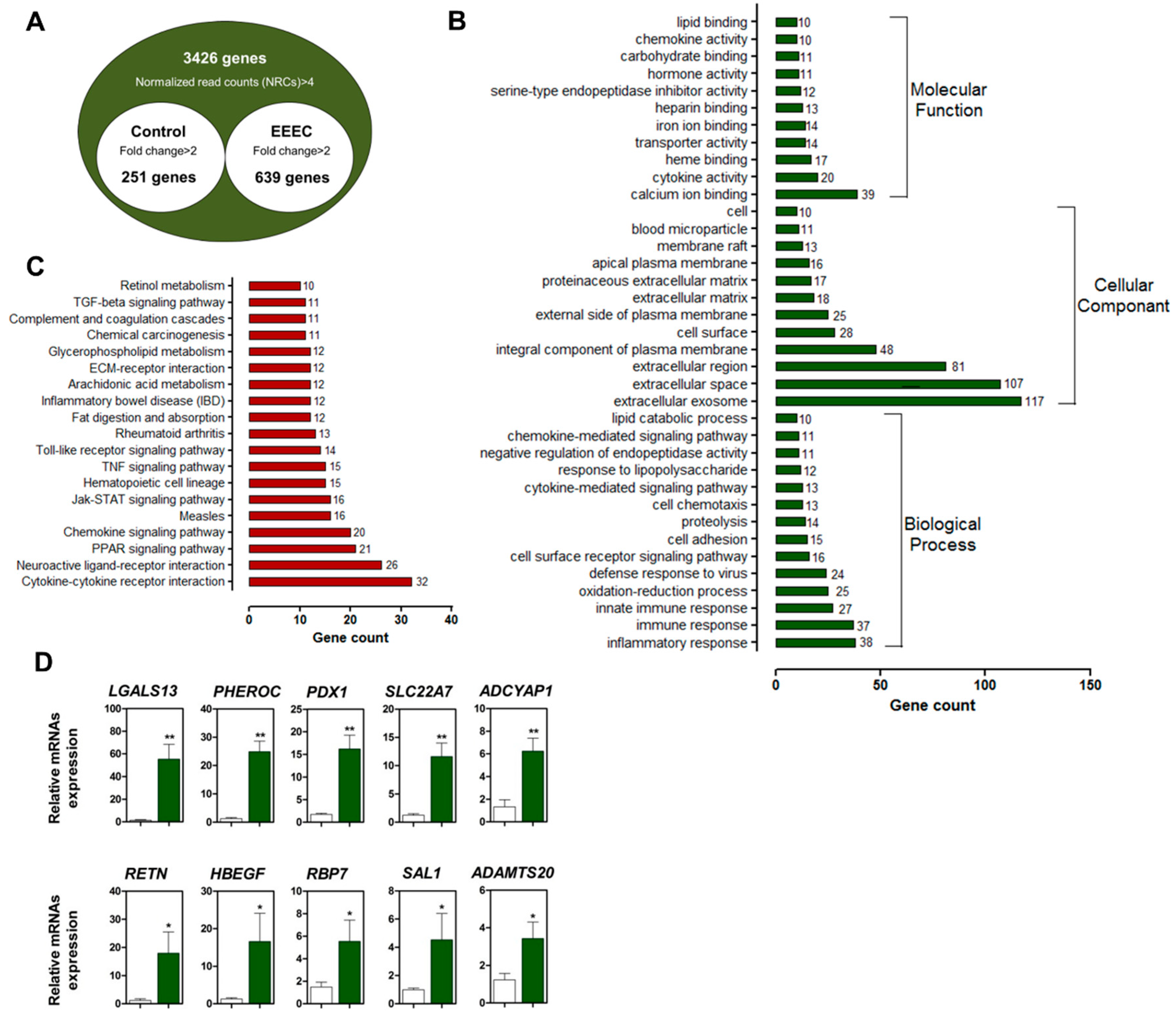
2.1. Identification and Validation of DEGs
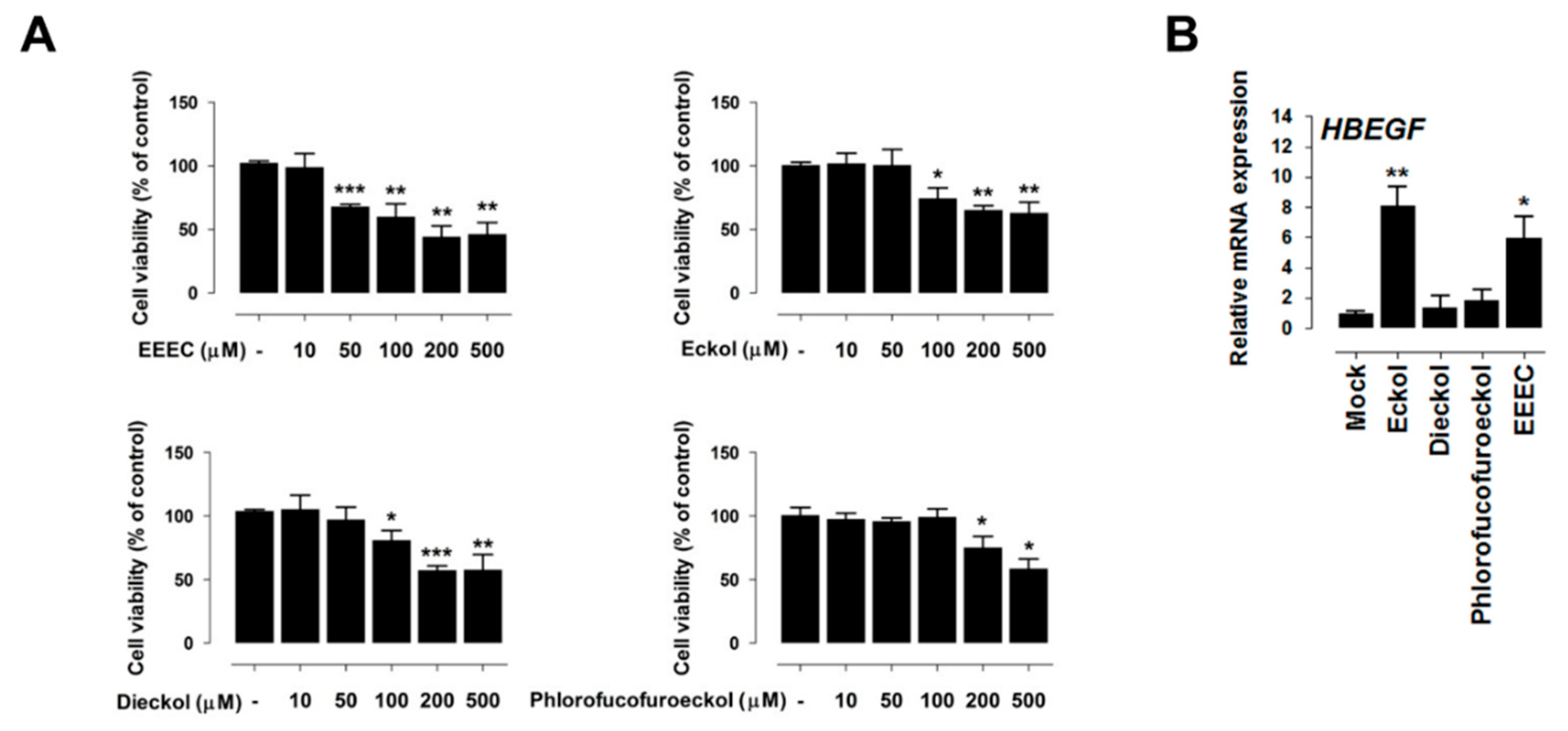
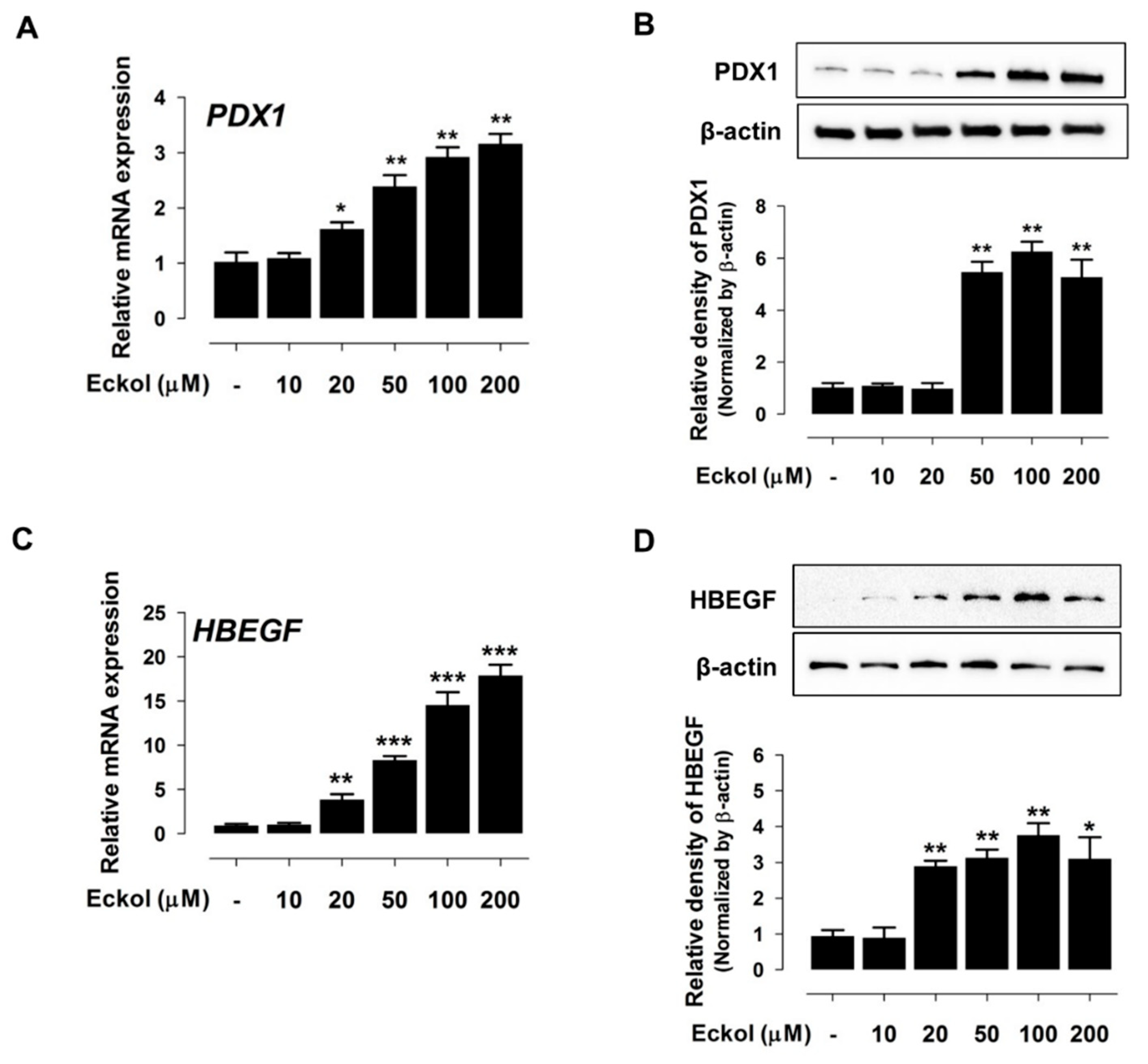
2.2. Eckol Induces PDX1 and HBEGF Expression
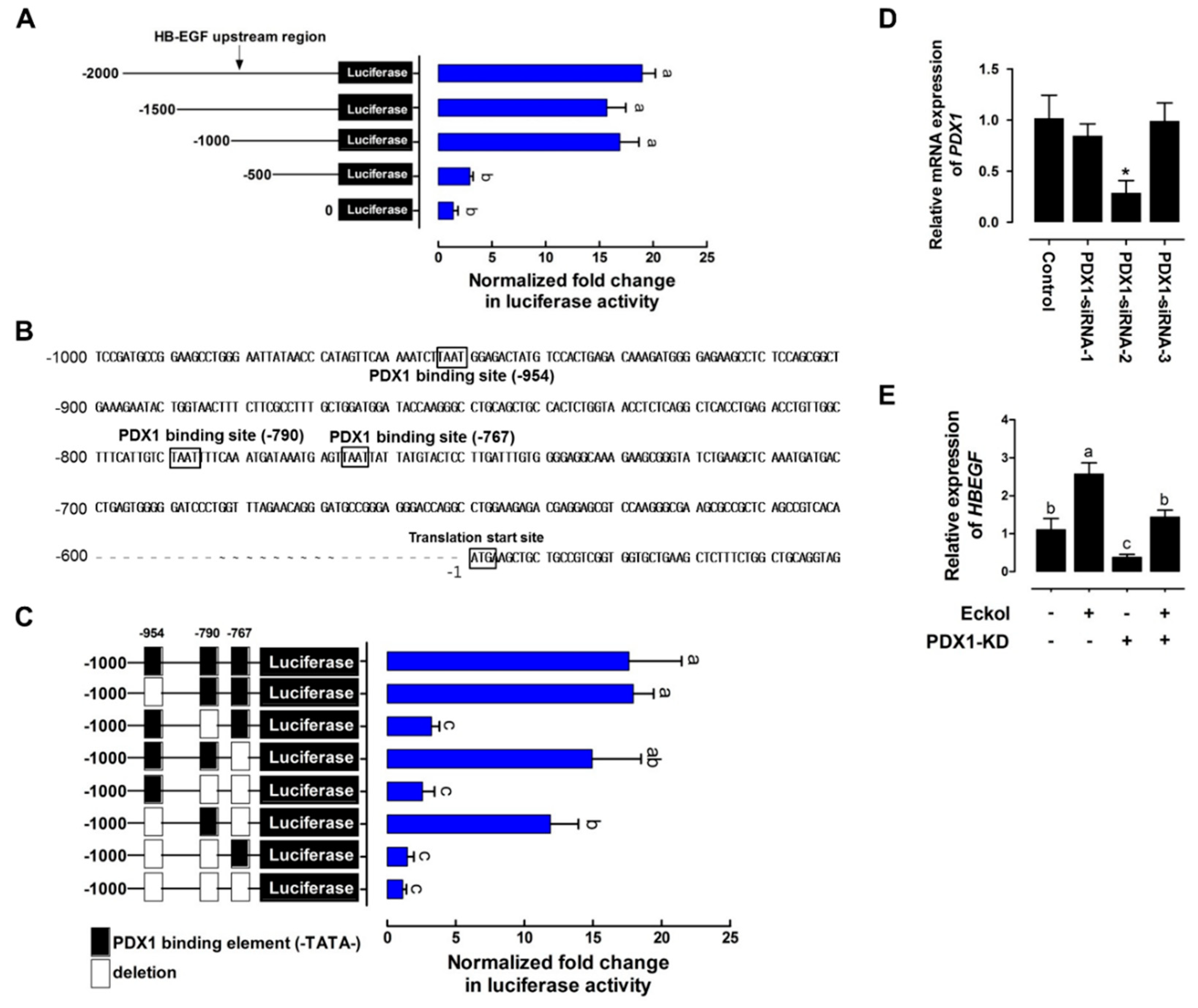
2.3. Eckol-Mediated Induction of PDX1 Regulates HBEGF Expression
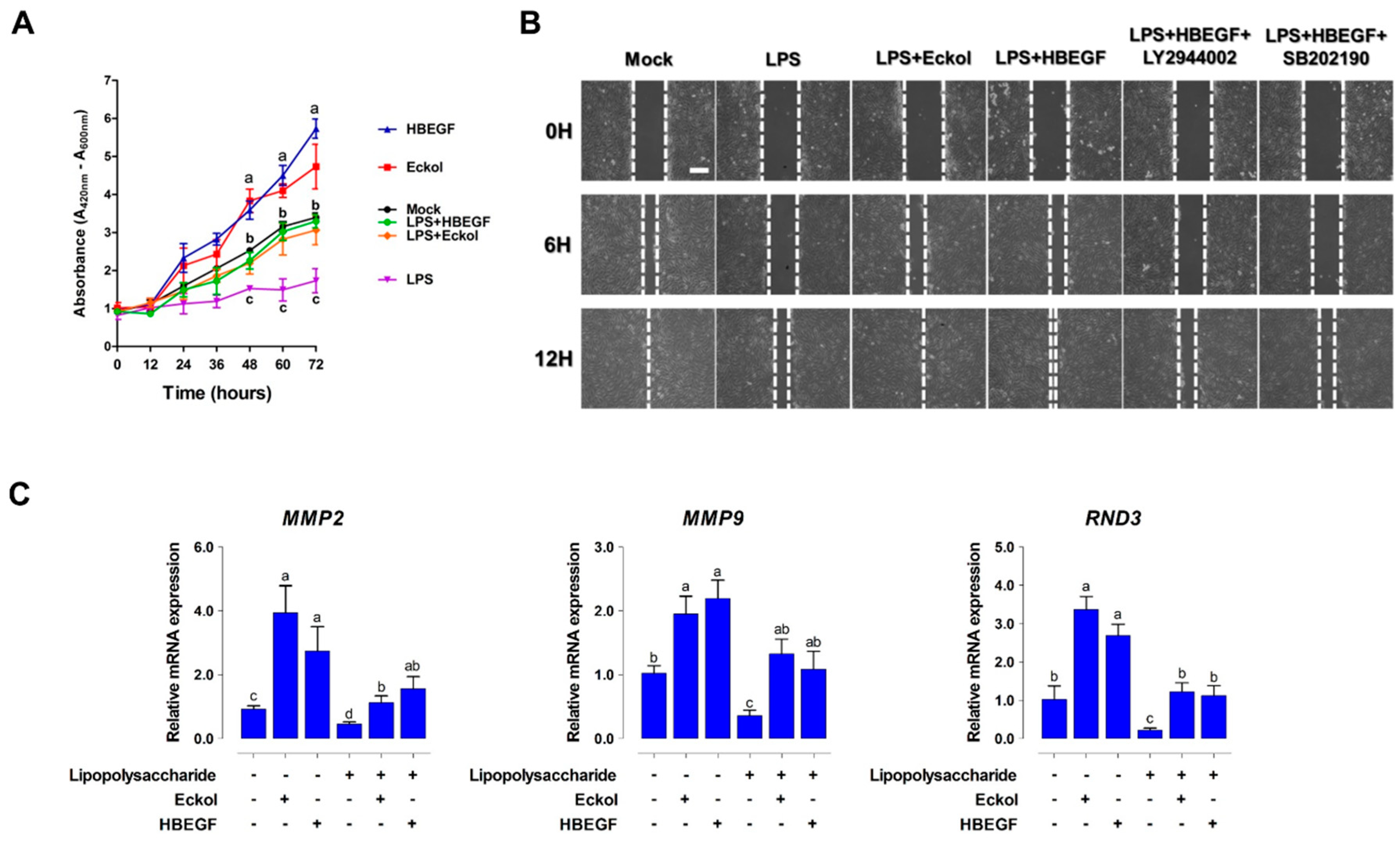
2.4. Eckol Enhances Intestinal Barrier Function and Promotes Wound Healing
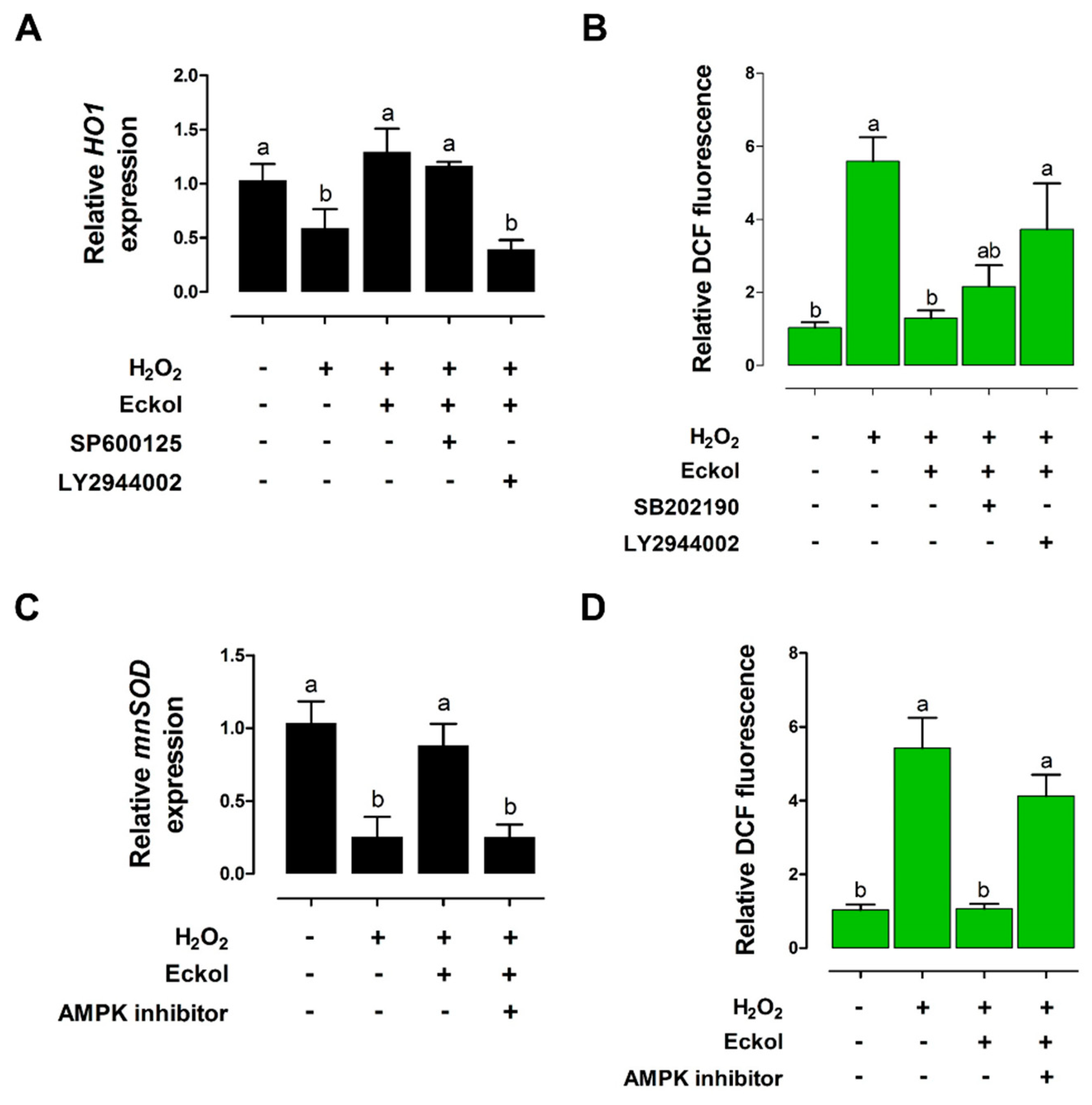
2.5. Eckol Protects against Oxidative Stress in the Intestine
2.6. EEEC Supplementation Improves Intestinal Function in Pigs during Weaning
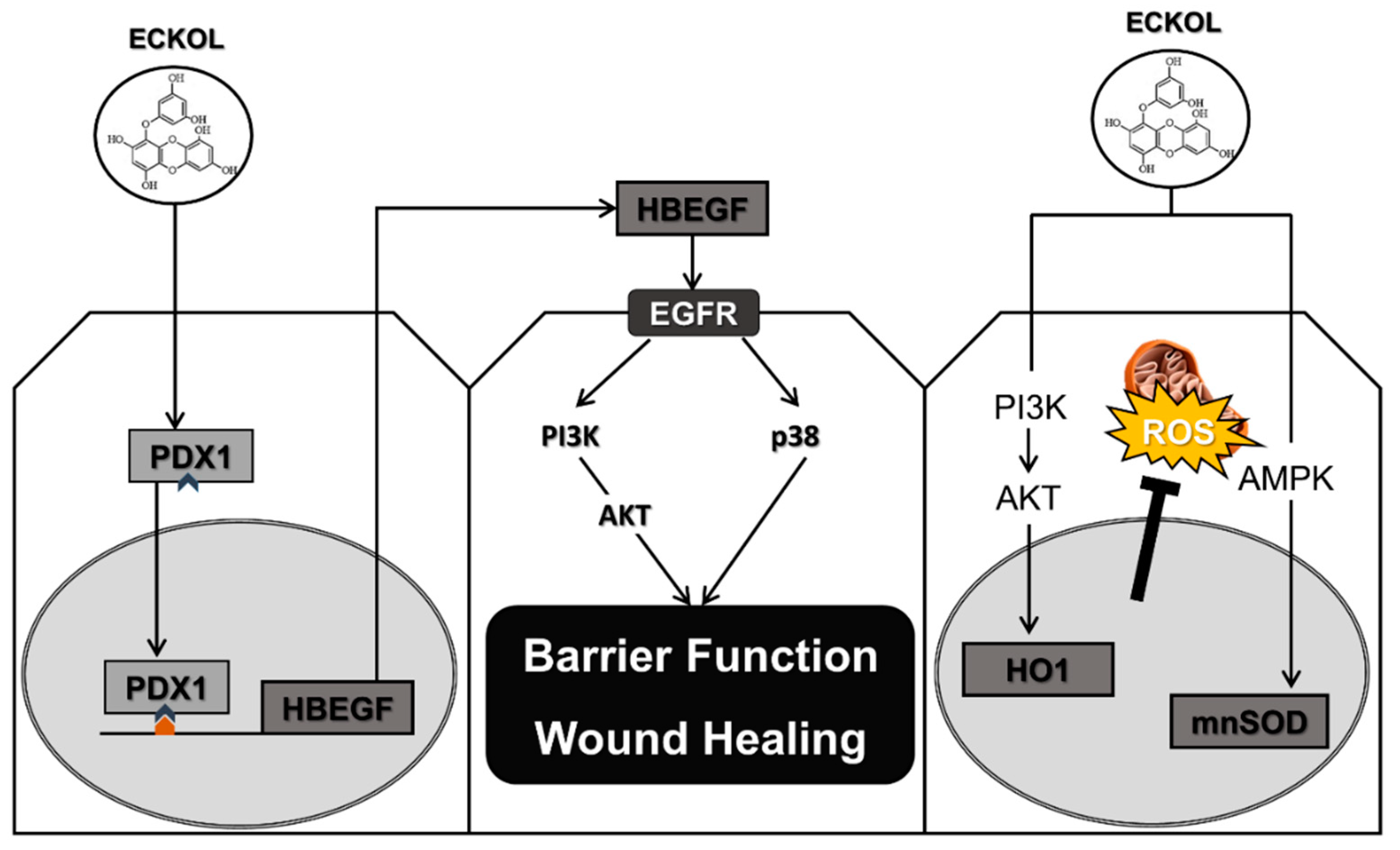
3. Discussion
4. Materials and Methods
4.1. Animals and Feeding Trial
4.2. Gene Expression Profiling
4.3. Quantitative Real-Time (qRT)-PCR and Western Blotting
4.4. Cell Culture and Treatments
4.5. Immunofluorescence
4.6. Cell Proliferation and Migration Assays
4.7. Transepithelial Electrical Resistance (TEER) and Intestinal Permeability
4.8. Cellular ROS Detection
4.9. Vector construction, Gene Silencing, and Luciferase assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADG | average daily gain |
| AMPK | amp-activated protein kinase |
| DEG | differentially expressed genes |
| EEEC | ethanol extract of dried E. cava |
| HBEGF | heparin-binding epidermal growth factor-like growth factor |
| HO-1 | heme oxygenase-1 |
| IBD | inflammatory bowel disease |
| PDX-1 | pancreatic and duodenal homeobox 1 |
| ROS | reactive oxygen species |
| JAK-STAT | Janus kinase-signal transducer and activator of transcription |
| TEER | transepithelial/transendothelial electrical resistance |
| ZO1 | zona occludens-1 |
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Hampson, D.J. Alterations in piglet small intestinal structure at weaning. Res. Veter. Sci. 1986, 40, 32–40. [Google Scholar] [CrossRef]
- Boudry, G.; Peron, V.; Le Huerou-Luron, I.; Lalles, J.P.; Seve, B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004, 134, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Boudry, G.; Favier, C.; Le Huerou-Luron, I.; Lalles, J.P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef]
- Pacha, J. Development of intestinal transport function in mammals. Physiol. Rev. 2000, 80, 1633–1667. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Henderson, P.; van Limbergen, J.E.; Schwarze, J.; Wilson, D.C. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 382–395. [Google Scholar] [CrossRef]
- Schneeman, B.O. Gastrointestinal physiology and functions. Br. J. Nutr. 2002, 88, S159–S163. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Veter. Sci. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Adams, S.; Zhao, B.; Qin, G.; Jiang, H. Effects of dietary L-arginine supplementation from conception to post-weaning in piglets. Curr. Protein Pept. Sci. 2019, 20, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kim, I.H. Nucleotide-mediated SPDEF modulates TFF3-mediated wound healing and intestinal barrier function during the weaning process. Sci. Rep. 2018, 8, 4827. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Zhao, P.Y.; Kim, I.H. Probiotics as a dietary additive for pigs: A review. J. Anim. Veter. Adv. 2011, 10, 2127–2134. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, K.Y.; Kim, I.H. Protected organic Acid blends as an alternative to antibiotics in finishing pigs. Asian-Australas. J. Anim. Sci. 2014, 27, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017, 25, 93–99. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, J.H.; Han, J.S. A phlorotannin constituent of Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Pharm. Biol. 2017, 55, 1149–1154. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kang, K.H.; Park, J.W.; Park, S.J.; Kim, S.K. Anti-HIV-1 activity of phlorotannin derivative 8,4’’’-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef]
- Kim, R.K.; Uddin, N.; Hyun, J.W.; Kim, C.; Suh, Y.; Lee, S.J. Novel anticancer activity of phloroglucinol against breast cancer stem-like cells. Toxicol. Appl. Pharmacol. 2015, 286, 143–150. [Google Scholar] [CrossRef]
- Kwon, H.J.; Ryu, Y.B.; Kim, Y.M.; Song, N.; Kim, C.Y.; Rho, M.C.; Jeong, J.H.; Cho, K.O.; Lee, W.S.; Park, S.J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorganic Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Nam, T.J. Enzyme-treated Ecklonia cava extract inhibits adipogenesis through the downregulation of C/EBPalpha in 3T3-L1 adipocytes. Int. J. Mol. Med. 2017, 39, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hosseindoust, A.; Goel, A.; Lee, S.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Effects of Ecklonia cava as fucoidan-rich algae on growth performance, nutrient digestibility, intestinal morphology and caecal microflora in weanling pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 64–70. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.D.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Hyun, C.L.; Kang, H.K.; Lee, N.H.; et al. Cytoprotective effect of eckol against oxidative stress-induced mitochondrial dysfunction: Involvement of the FoxO3a/AMPK pathway. J. Cell. Biochem. 2014, 115, 1403–1411. [Google Scholar] [CrossRef]
- Moon, C.; Kim, S.H.; Kim, J.C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lai, C.Q.; Nie, L.; Ordovas, J.; Band, M.; Moser, L.; Meydani, M. The modulation of endothelial cell gene expression by green tea polyphenol-EGCG. Mol. Nutr.Food Res. 2008, 52, 1182–1192. [Google Scholar] [CrossRef]
- Vittal, R.; Selvanayagam, Z.E.; Sun, Y.; Hong, J.; Liu, F.; Chin, K.V.; Yang, C.S. Gene expression changes induced by green tea polyphenol (-)-epigallocatechin-3-gallate in human bronchial epithelial 21BES cells analyzed by DNA microarray. Mol. Cancer Ther. 2004, 3, 1091–1099. [Google Scholar]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kamisako, T.; Kagawa, T.; Haraguchi, S.; Yagoto, M.; Takahashi, R.; Kawai, K.; Suemizu, H. Expression of pancreatic and duodenal homeobox1 (PDX1) protein in the interior and exterior regions of the intestine, revealed by development and analysis of Pdx1 knockout mice. Lab. Anim. Res. 2015, 31, 93–98. [Google Scholar] [CrossRef]
- Holland, A.M.; Garcia, S.; Naselli, G.; Macdonald, R.J.; Harrison, L.C. The Parahox gene Pdx1 is required to maintain positional identity in the adult foregut. Int. J. Dev. Biol. 2013, 57, 391–398. [Google Scholar] [CrossRef]
- Chen, C.; Sibley, E. Expression profiling identifies novel gene targets and functions for Pdx1 in the duodenum of mature mice. Am. J. Physiol. Liver Physiol. 2012, 302, G407–G419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Leavitt, T.; Sibley, E. Intestinal Pdx1 mediates nutrient metabolism gene networks and maternal expression is essential for perinatal growth in mice. Biochem. Biophys. Res. Commun. 2012, 424, 549–553. [Google Scholar] [CrossRef][Green Version]
- Kim, J.M.; Bak, E.J.; Chang, J.Y.; Kim, S.T.; Park, W.S.; Yoo, Y.J.; Cha, J.H. Effects of HB-EGF and epiregulin on wound healing of gingival cells in vitro. Oral Dis. 2011, 17, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, R.; Mekada, E. ErbB and HB-EGF signaling in heart development and function. Cell Struct. Funct. 2006, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Su, Y.; Zhou, Y.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) therapy for intestinal injury: Application and future prospects. Pathophysiology 2014, 21, 95–104. [Google Scholar] [CrossRef]
- Kuhn, M.A.; Xia, G.; Mehta, V.B.; Glenn, S.; Michalsky, M.P.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid. Redox Signal. 2002, 4, 639–646. [Google Scholar] [CrossRef]
- Michalsky, M.P.; Kuhn, A.; Mehta, V.; Besner, G.E. Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. J. Pediatr. Surg. 2001, 36, 1130–1135. [Google Scholar] [CrossRef]
- Feng, J.; El-Assal, O.N.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 167–174. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Z.; Yang, H.; Zhang, F.; Reinach, P.S. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 485–493. [Google Scholar] [CrossRef]
- Kaneto, H.; Miyagawa, J.; Kajimoto, Y.; Yamamoto, K.; Watada, H.; Umayahara, Y.; Hanafusa, T.; Matsuzawa, Y.; Yamasaki, Y.; Higashiyama, S.; et al. Expression of heparin-binding epidermal growth factor-like growth factor during pancreas development. A potential role of PDX-1 in transcriptional activation. J. Biol. Chem. 1997, 272, 29137–29143. [Google Scholar] [CrossRef]
- Miyata, K.; Yotsumoto, F.; Nam, S.O.; Kuroki, M.; Miyamoto, S. Regulatory mechanisms of the HB-EGF autocrine loop in inflammation, homeostasis, development and cancer. Anticancer Res. 2012, 32, 2347–2352. [Google Scholar] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann. N. Y. Acad. Sci. 2000, 915, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Radulescu, A.; Chen, C.L.; Zhang, H.Y.; James, I.O.; Besner, G.E. Heparin-binding epidermal growth factor-like growth factor improves intestinal barrier function and reduces mortality in a murine model of peritonitis. Surgery 2013, 153, 52–62. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Radulescu, A.; Besner, G.E. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery 2009, 146, 334–339. [Google Scholar] [CrossRef]
- Ran, X.; Li, Y.; Chen, G.; Fu, S.; He, D.; Huang, B.; Wei, L.; Lin, Y.; Guo, Y.; Hu, G. Farrerol ameliorates TNBS-induced colonic inflammation by inhibiting ERK1/2, JNK1/2, and NF-kappaB signaling pathway. Int. J. Mol. Sci. 2018, 19, 2037. [Google Scholar] [CrossRef]
- El-Assal, O.N.; Besner, G.E. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 2005, 129, 609–625. [Google Scholar] [CrossRef]
- Lam, G.; Apostolopoulos, V.; Zulli, A.; Nurgali, K. NADPH oxidases and inflammatory bowel disease. Curr. Med. Chem. 2015, 22, 2100–2109. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; McClain, C.J.; de Villiers, W.J. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed]
- Schierack, P.; Nordhoff, M.; Pollmann, M.; Weyrauch, K.D.; Amasheh, S.; Lodemann, U.; Jores, J.; Tachu, B.; Kleta, S.; Blikslager, A.; et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell Biol. 2006, 125, 293–305. [Google Scholar] [CrossRef] [PubMed]


| Gene Symbol | Description | Accession No. | Primer Sequence (5’−>3’) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| LGALS13 | lectin, galactoside-binding, soluble, 13 | NM_001142841 | CTCTCGCCACAATCTGTGAA | ATCCCGTTTGTGAACTCAGG |
| PHEROC | pheromaxein C subunit | NM_001123161 | CCAGTGATTCCAGCGTAACC | CACTTGCATAAACACGCTGAA |
| PDX1 | pancreatic and duodenal homeobox 1 | NM_001141984 | TGAAATTGATGCTGGTGGAA | CATGGGGAGTACAGGCACTT |
| SLC22A7 | solute carrier family 22 member 7 | NM_001044617 | GGGAAGGGTTTTTCTGAAGC | TGACAGCCATACTCCATCCA |
| ADCYAP1 | adenylate cyclase activating polypeptide 1 | NM_001001544 | ACAGCAGCGTCTACTGCTCA | TCTCTTTCTTCCGCTGGGTA |
| RETN | resistin | NM_213783 | CTCAGGCTTTGCTGTCACTG | GATGCGCAGATGCAAACTTA |
| HBEGF | heparin-binding EGF like growth factor | NM_214299 | CCTACCGAATCTACGGACCA | CTTTCTTTTCCCTCGCTCCT |
| RBP7 | retinol binding protein 7 | NM_001145222 | TGCTGGCCCTAGGTATTGAC | CCAGGCCTTTGTTATCCTCA |
| SAL1 | salivary lipocalin | NM_213814 | ATGTCAATGGCGACAAAACA | AGTTGGAAGCAGCGATCAAT |
| ADAMTS20 | ADAM metallopeptidase with thrombospondin type 1 motif 20 | NM_001257275 | CATCAGCTGTGGCCTGTAGA | CGGCCATACATTCCACTCTT |
| HO1 | HMOX1, heme oxygenase 1 | NM_001004027 | AGCTGTTTCTGAGCCTCCAA | GAACGAAGAGTGGCTCCAAC |
| mnSOD | SOD2, superoxide dismutase 2 | NM_214127 | TTTGGGGCTGTTTTTGTAGG | TGATGGTTTGGGATGGTTTT |
| MMP2 | matrix metallopeptidase 2 | NM_214192 | ACTCCCACTTTGACGACGAT | CGTACTTGCCATCCTTGTCG |
| MMP9 | matrix metallopeptidase 9 | NM_001038004 | TGAAGACGCAGAAGGTGGAT | TTCAGGAGGTCGAAGGTCAC |
| CDH1 | cadherin 1 | NM_001163060 | CTGTTGCAGGTCTCATCGTG | AACATAGACCGTCCTTGGCA |
| RND3 | Rho family GTPase 3 | NM_214296 | GAGAGAAGAGCCAGCCAGAA | TGTCCCACAGGCTCAATTCT |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001206359 | ACACCGAGCATCTCCTGACT | GACGAGGCAGGTCTCCCTAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.I.; Kim, I.H. Eckol Alleviates Intestinal Dysfunction during Suckling-to-Weaning Transition via Modulation of PDX1 and HBEGF. Int. J. Mol. Sci. 2020, 21, 4755. https://doi.org/10.3390/ijms21134755
Lee SI, Kim IH. Eckol Alleviates Intestinal Dysfunction during Suckling-to-Weaning Transition via Modulation of PDX1 and HBEGF. International Journal of Molecular Sciences. 2020; 21(13):4755. https://doi.org/10.3390/ijms21134755
Chicago/Turabian StyleLee, Sang In, and In Ho Kim. 2020. "Eckol Alleviates Intestinal Dysfunction during Suckling-to-Weaning Transition via Modulation of PDX1 and HBEGF" International Journal of Molecular Sciences 21, no. 13: 4755. https://doi.org/10.3390/ijms21134755
APA StyleLee, S. I., & Kim, I. H. (2020). Eckol Alleviates Intestinal Dysfunction during Suckling-to-Weaning Transition via Modulation of PDX1 and HBEGF. International Journal of Molecular Sciences, 21(13), 4755. https://doi.org/10.3390/ijms21134755