Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes
Abstract
1. Introduction
2. Results
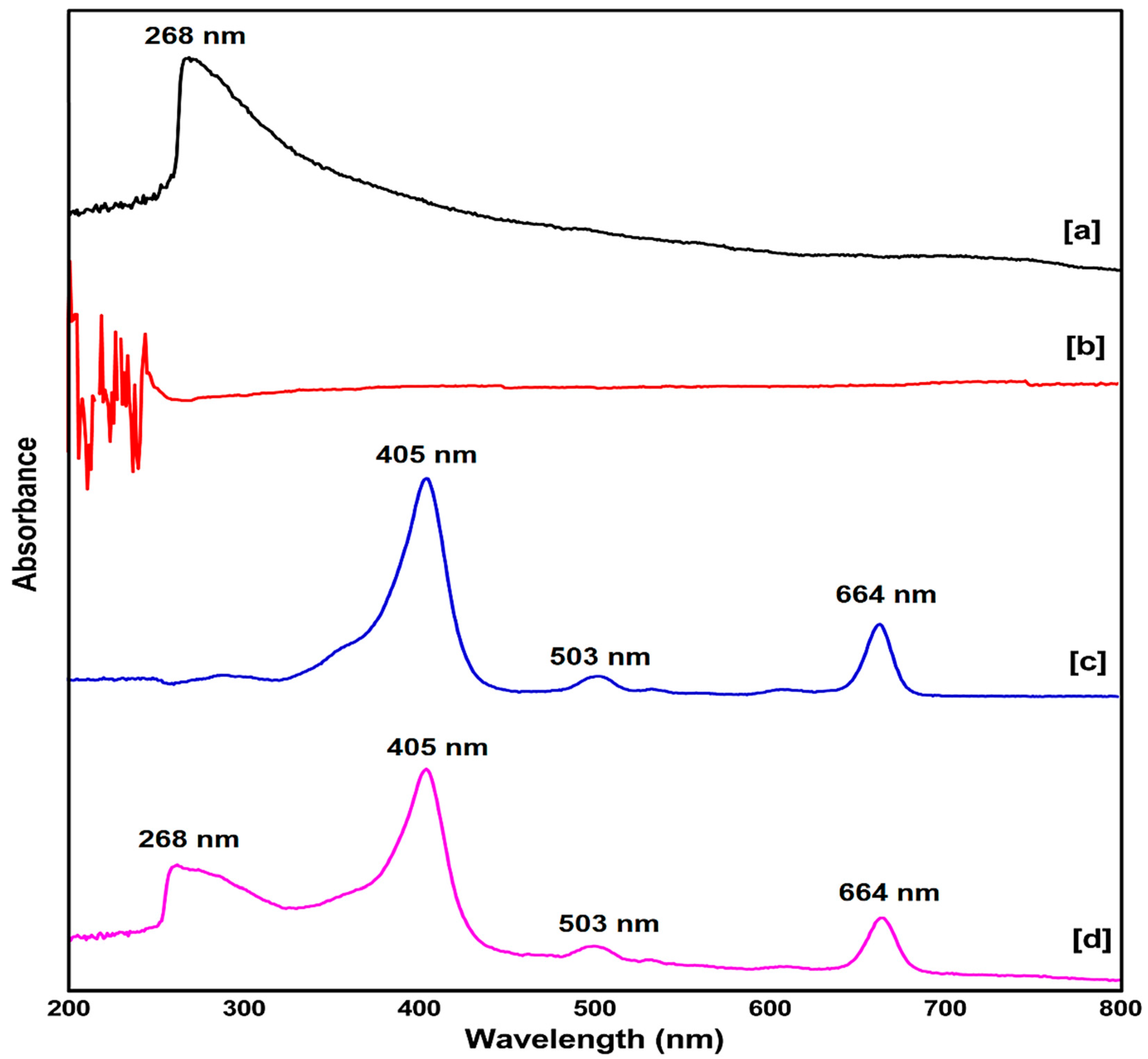
2.1. UV-Vis Spectroscopy Study
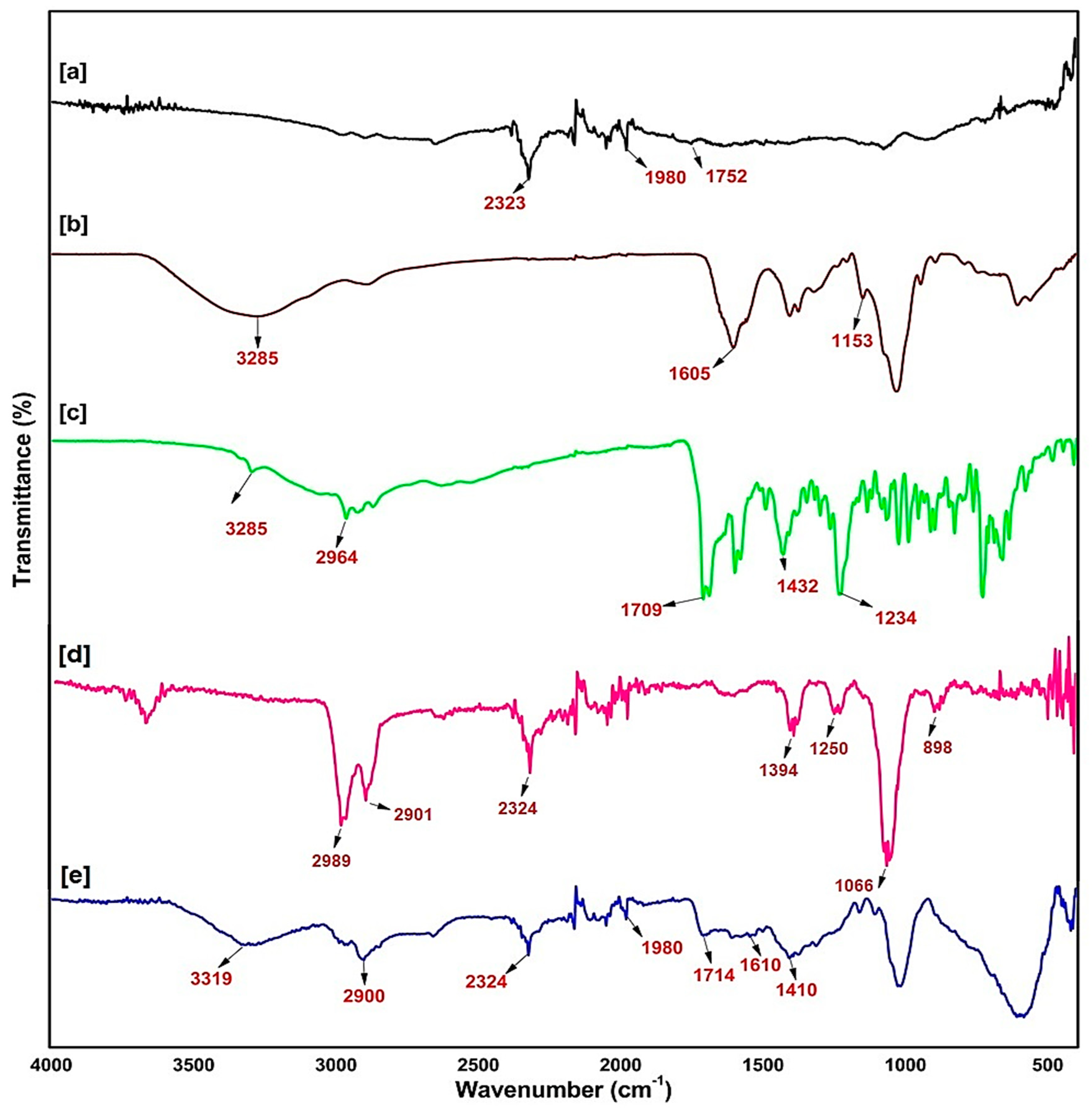
2.2. Fourier Transmittance of Infra-Red Spectroscopy of the Synthesised Nanobiocomposite
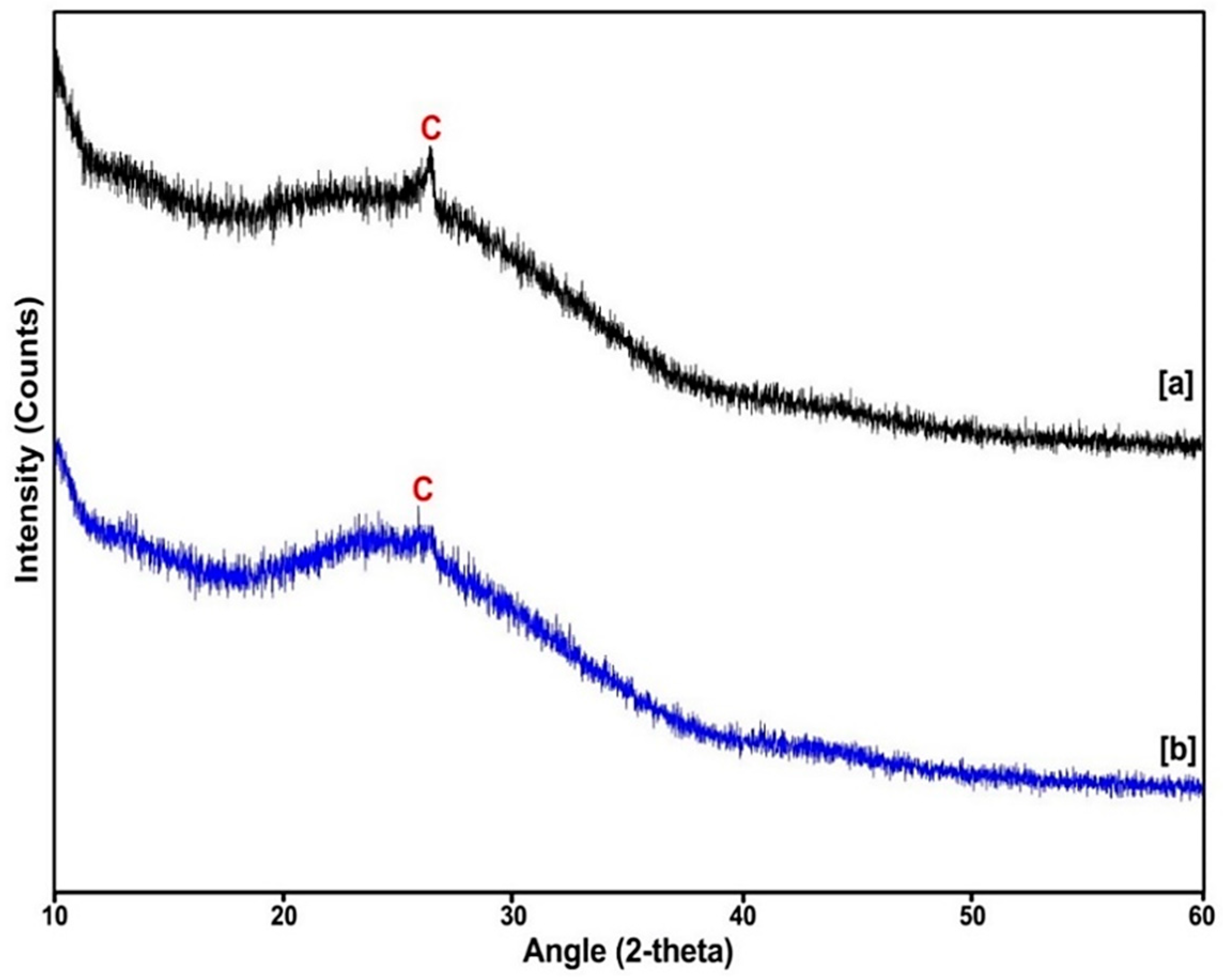
2.3. X-ray Diffraction (XRD) Analysis
2.4. Particle Size Distribution and Zeta Potential of the Synthesised Nanobiocomposite
2.5. Nanobiocomposite PDT Effects on Colon Cancer Cells
2.5.1. Morphology Studies
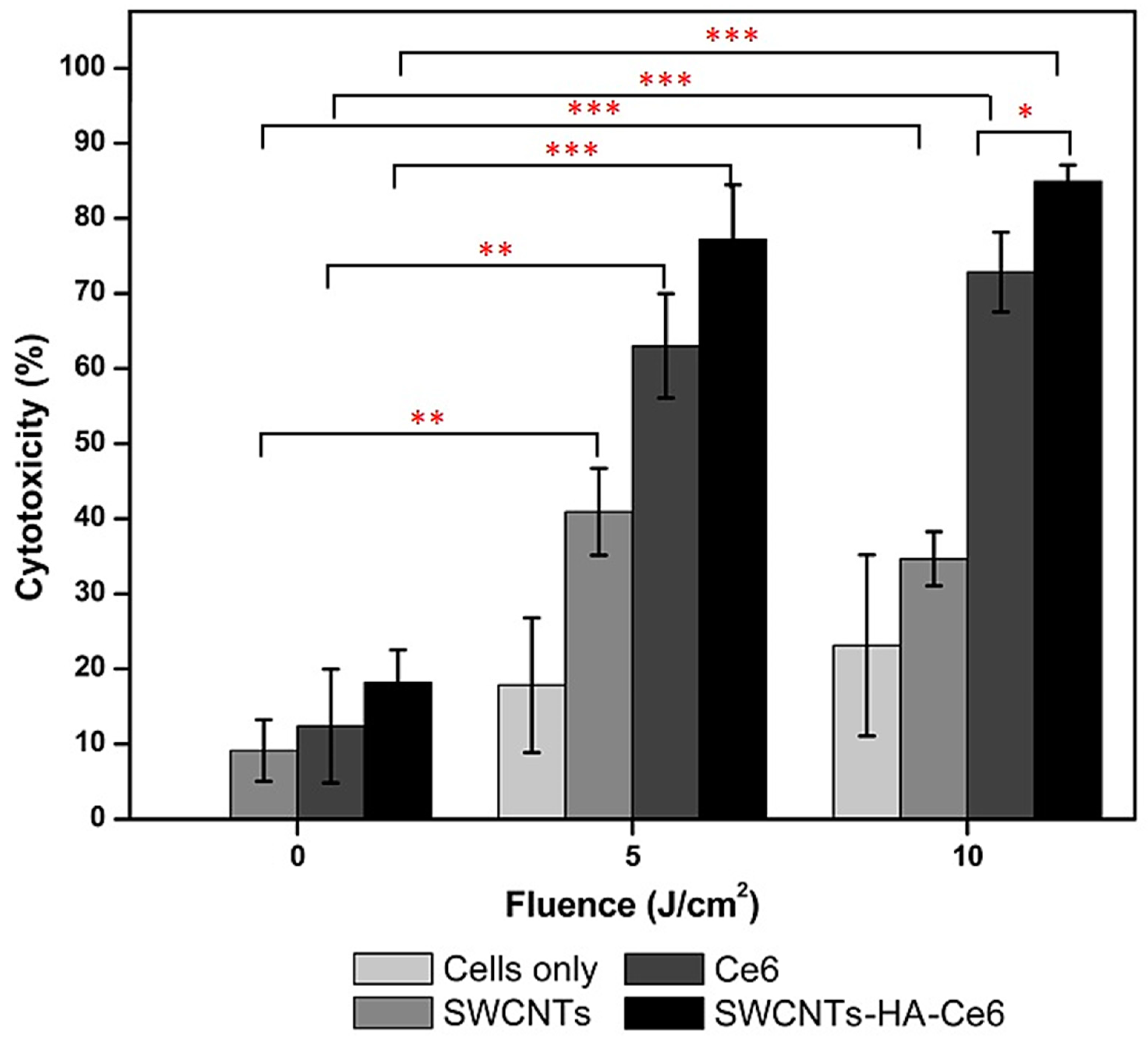
2.5.2. In Vitro Cytotoxicity Assay
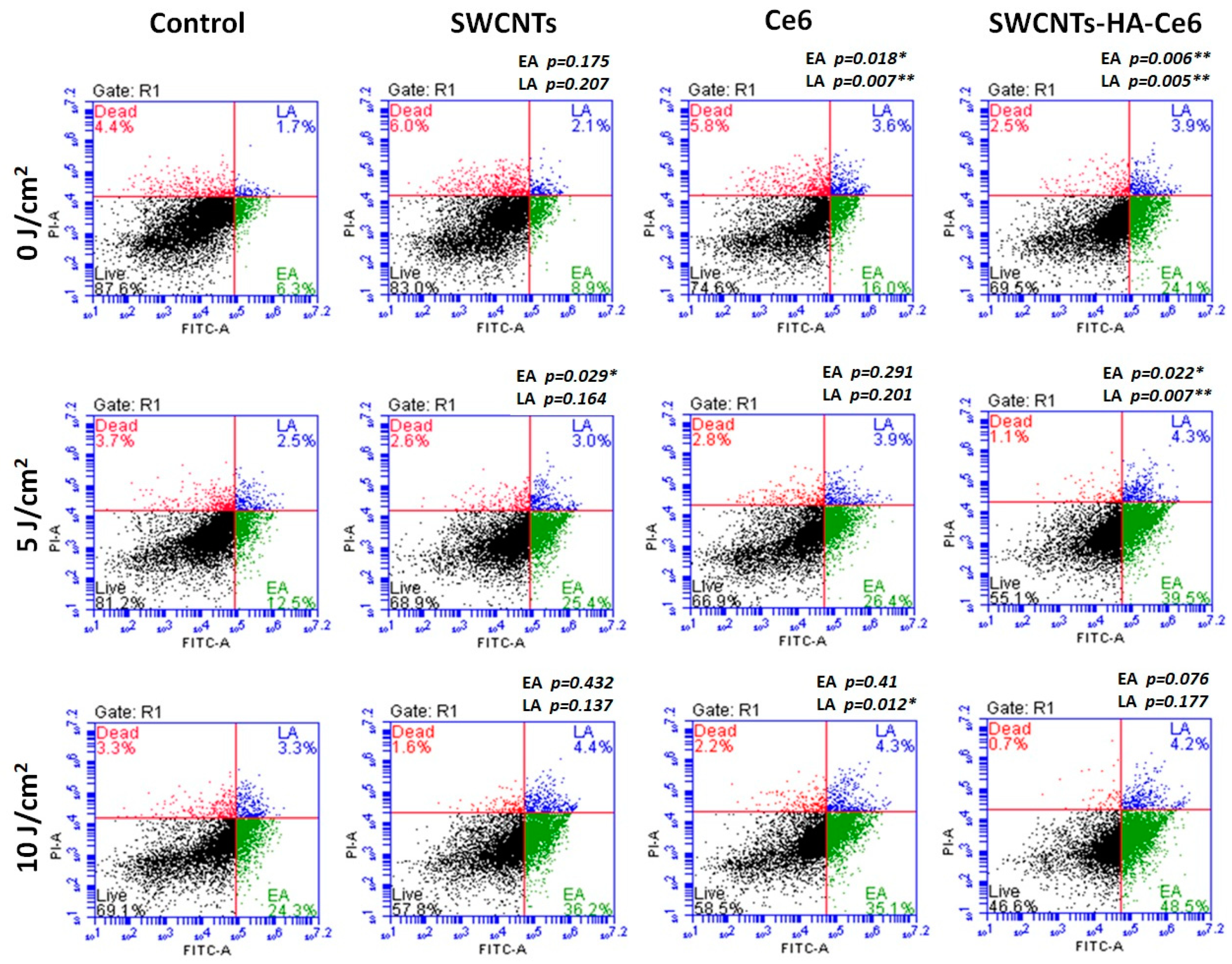
2.5.3. Cell Death Analysis
3. Discussion
4. Materials and Methods
4.1. Experiment
4.2. Synthesis of the Nanobiocomposite
4.3. Spectrum Analysis of Synthesised the Nanobiocomposite
4.4. Fourier Transform Electron Microscopy (FTIR) of the Nanobiocomposite
4.5. X-ray Diffraction (XRD) of the Nanobiocomposite
4.6. Particle Size and Zeta Potential (DLS Method) of Nanobiocomposite
4.7. Cell Culture
4.8. Photodynamic Effects (PDT)
4.8.1. Morphology
4.8.2. Cytotoxicity
4.8.3. Cell Death
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SWCNTs | Single walled carbon nanotubes |
| HA | Hyaluronic acid |
| Ce6 | Chlorin e6 |
| PDT | Photodynamic therapy |
| PS | Photosensitizers |
| DMF | Dimethylsulfoxide |
| EDC.HCl | N-ethylcarbodiimde hydrochloride |
| NHS | N-hydroxysuccinimide |
| UV | Ultraviolet |
| FTIR | Fourier transform infra-red |
| XRD | X-ray diffraction |
| DLS | Dynamic laser scattering |
| PI | Propidium idode |
| DMEM | Dulbecco’s Modified Eagle Medium |
| °C | Degree Celsius |
| μL | Microlitre |
| mL | Millilitre |
| mA | Milli ampere |
| kV | Kilo voltage |
| J | Joules |
| h | Hours |
| min | Minutes |
| s | Seconds |
| rpm | Rotation peer minute |
| nm | Nanometer |
| mV | Milli voltage |
| cm | Centimetre |
| RT | Room temperature |
References
- Lopez-Lazaro, M. What is the main cause of cancer? J. Cancer Stud. Ther. 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Rebecca, L.S.; Kimberly, D.M.; Ahmedin, J. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Badea, N.; Craciun, M.M.; Dragomir, A.S.; Balas, M.; Dinischiotu, A.; Nistor, C.; Gavan, C.; Ionita, D. Systems based on carbon nanotubes with potential in cancer therapy. Mater. Chem. Phys. 2020, 241, 1224–1235. [Google Scholar] [CrossRef]
- Moan, J.; Peng, Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar] [PubMed]
- Juzeniene, A. Chlorin e6-based photosensitizers for photodynamic therapy and photodiagnosis. Photodiagnosis Photodyn. Ther. 2009, 6, 26630–26637. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, V.F.; Barnhart, T.E.; Liu, Z.; Cai, W.B. Cerenkov radiation induced photodynamic therapy using chlorin e6-loadedhollow mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodunamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharm. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Pramuala, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiria, N. Polymer-lipid-PEG hybrid nanoparticles as photosensitizer carrier for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2017, 173, 12–22. [Google Scholar] [CrossRef]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Rompicharla, S.V.K.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Lipid and poly (ethylene glycol)-conjugated bi-functionalized chlorine e6 micelles for NIR-light induced photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101633. [Google Scholar] [CrossRef]
- Marangon, I.; Ménard-Moyon, C.; Silva, A.K.A.; Bianco, A.; Luciani, N.; Gazeau, F. Synergic mechanisms of photothermal and photodynamic therapies mediated by photosensitizer/carbon nanotube complexes. Carbon 2016, 97, 110–123. [Google Scholar] [CrossRef]
- Alea-Reyes, M.E.; Soriano, J.; Mora-Espi, I.; Rodirgues, M.; Russell, D.A.; Barrios, L.; Perez-Garcia, L. Amphiphilic gemini pyridinium-mediated incorporation of Zn(II)meso-tetrakis(4-carboxyphenyl)porphyrin into water-soluble gold nanoparticles for photodynamic therapy. Colloids Surf. B Biointerfaces 2017, 158, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Broughton, D.L.; Yan, B. Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: Developing a model for cell uptake. Nano Lett. 2009, 9, 4370–4375. [Google Scholar] [CrossRef]
- Karimi, M.; Solati, N.; Ghasemi, A.; Estiar, M.A.; Hashemkhani, M.; Kiani, P.; Mohamed, E.; Saeidi, A.; Taheri, M.; Avci, P.; et al. Carbon nanotubes part II: A remarkable carrier for drug and gene delivery. Expert Opin. Drug Deliv. 2019, 12, 1089–1105. [Google Scholar] [CrossRef]
- Tuncel, D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale 2011, 3, 3545–3554. [Google Scholar] [CrossRef]
- Tan, J.M.; Arulselvan, P.; Fakurazi, S.; Ithnin, H.; Hussein, M.Z. A Review on Characterizations and Biocompatibility of Functionalized Carbon Nanotubes in Drug Delivery Design. J. Nanomater. 2014, 917024. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Nyokong, T. The effect of ascorbic acid on the photophysical properties and photodynamic therapy activities of zinc phthalocyanine-single walled carbon nanotube conjugate on MCF-7 cancer cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 174–183. [Google Scholar] [CrossRef] [PubMed]
- De Adhikari, A.; Tiwari, S.K.; Ha, S.K.; Nayak, G.C. Boosted electrochemical performance of TiO2 decorated RGO/CNT hybrid nanocomposite by UV irradiation. Vacuum 2009, 160, 421–428. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Wang, Y.; Wang, L.; Yang, X.; Zhang, Z. Hyaluronic acid-functionalized single-walled carbon nanotubes as tumor-targeting MRI contrast agent. Int. J. Nanomed. 2015, 10, 4507–4520. [Google Scholar] [CrossRef]
- Xiao, H.; Zhu, B.; Wang, D.; Pang, Y.; He, L.; Ma, X.; Wang, R.; Jin, C.; Chen, Y.; Zhu, X. Photodynamic effects of chlorin e6 attached to single wall carbon nanotubes through noncovalent interactions. Carbon 2012, 50, 1681–1689. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Hajian, A.; Shahriyari, A. Protein capperd Cu nanoclusters-SWCNT nanocomposite as a novel candidate of high performance platform for organophosphates enzymeless biosensor. Biosens. Bioelectron. 2017, 89, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, O.L.; Parkhats, M.V.; Gorbachova, A.N.; Terekhow, S.N. FTIR spectra and normal-mode analysis of chlorin e6 and its degradation-induced impurities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Antoniraj, M.G.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex ffor the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74. [Google Scholar] [CrossRef]
- Kumar, T.H.V.; Sundramoorthy, A.K. Non-enzymatic electrochemical detection of urea on silver nanoparticles anchored nitrogen-doped single-walled carbon nanotube modified electrode. J. Electrochem. Soc. 2018, 165, B3006–B3016. [Google Scholar] [CrossRef]
- Pham, T.; Bui, L.; Kim, G.; Hoang, D.; Tran, T.; Hoang, M. Cancers in Vietnam Burden and Control Efforts: A Narrative Scoping Review. Cancer Control 2019, 26, 1–14. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment. Technol. Cancer Res. Treat. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, L.; Li, W.; Xie, J. Novel biomaterials and biotechnology for nanomedicine. Eur. J. Med. Res. 2015, 1, 1–2. [Google Scholar] [CrossRef]
- Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent progress in metal-based nanoparticles mediated photodynamic therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef]
- Abrahamse, H.; Tynga, I. Nano-mediated photodynamic therapy for cancer: Enhancement of cancer specificity and therapeutic effects. Nanomaterials 2018, 923, 1–14. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. Biomed Res. Int. 2013, 578290, 1–12. [Google Scholar] [CrossRef]
- Mishra, V.; Kesharwani, P.; Jain, N.K. Biomedical applications and toxicological aspects of functionalized carbon nanotubes. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 293–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, J.; Ji, J.; Shen, J. A novel biomimetic polymer as amphiphilic surfactant for soluble and biocompatible carbon nanotubes (CNTs). Colloids Surf. B Biointerfaces 2008, 67, 67–72. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Functionalization of carbon nanomaterials for biomedical applications. J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef]
- Yu, C.; Cheng, K.; Ding, J.; Deng, C.; Xue, Z.; Wu, X.; Zhu, H. Synthesis and characterisation of Al4O4C nanorod/CNT composites. Ceram. Int. 2017, 43, 11415–11420. [Google Scholar] [CrossRef]
- Nowis, D.; Makowski, M.; Stoklosa, T.; Legat, M.; Issat, T.; Golab, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef]
- Xie, L.; Wang, G.; Zhou, H.; Zhang, F.; Guo, Z.; Liu, C.; Zhang, X.; Zhu, L. Functional long circulating single walled carbon nanotubes for fluorescent/photoacoustic imaging-guided enhanced phototherapy. Biomaterias 2016, 103, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Choi, K.Y.; Mertz, E.L.; Huang, X.; Zhang, F.; Zhu, L.; Yoon, H.Y.; Park, J.H.; Bhirde, A.; Lee, S.; et al. A Facile, One-Step Nanocarbon Functionalization for Biomedical Applications. Nano Lett. 2012, 12, 3613–3620. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Park, S.-J.; Ling, D.; Park, W.; Han, J.Y.; Na, K.; Char, K. Hyaluronic acid-conjugated graphene oxide/photosensitizer nanohybrids for cancer targeted photodynamic therapy. J. Mater. Chem. B 2013, 1, 1678–1686. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef]


| PARAMETERS | |
|---|---|
| Laser type semiconductor | Diode |
| Wavelength (nm) | 660 |
| Wave emission | Continuous |
| Fluences (J/cm2) | 5 and 10 |
| Irradiation time | 7 min 16s 14 min 32 s |
| Photosensitizer | Chlorin e6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. https://doi.org/10.3390/ijms21134745
Sundaram P, Abrahamse H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. International Journal of Molecular Sciences. 2020; 21(13):4745. https://doi.org/10.3390/ijms21134745
Chicago/Turabian StyleSundaram, Prabhavathi, and Heidi Abrahamse. 2020. "Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes" International Journal of Molecular Sciences 21, no. 13: 4745. https://doi.org/10.3390/ijms21134745
APA StyleSundaram, P., & Abrahamse, H. (2020). Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. International Journal of Molecular Sciences, 21(13), 4745. https://doi.org/10.3390/ijms21134745






