New Insight into the Composition of Wheat Seed Microbiota
Abstract
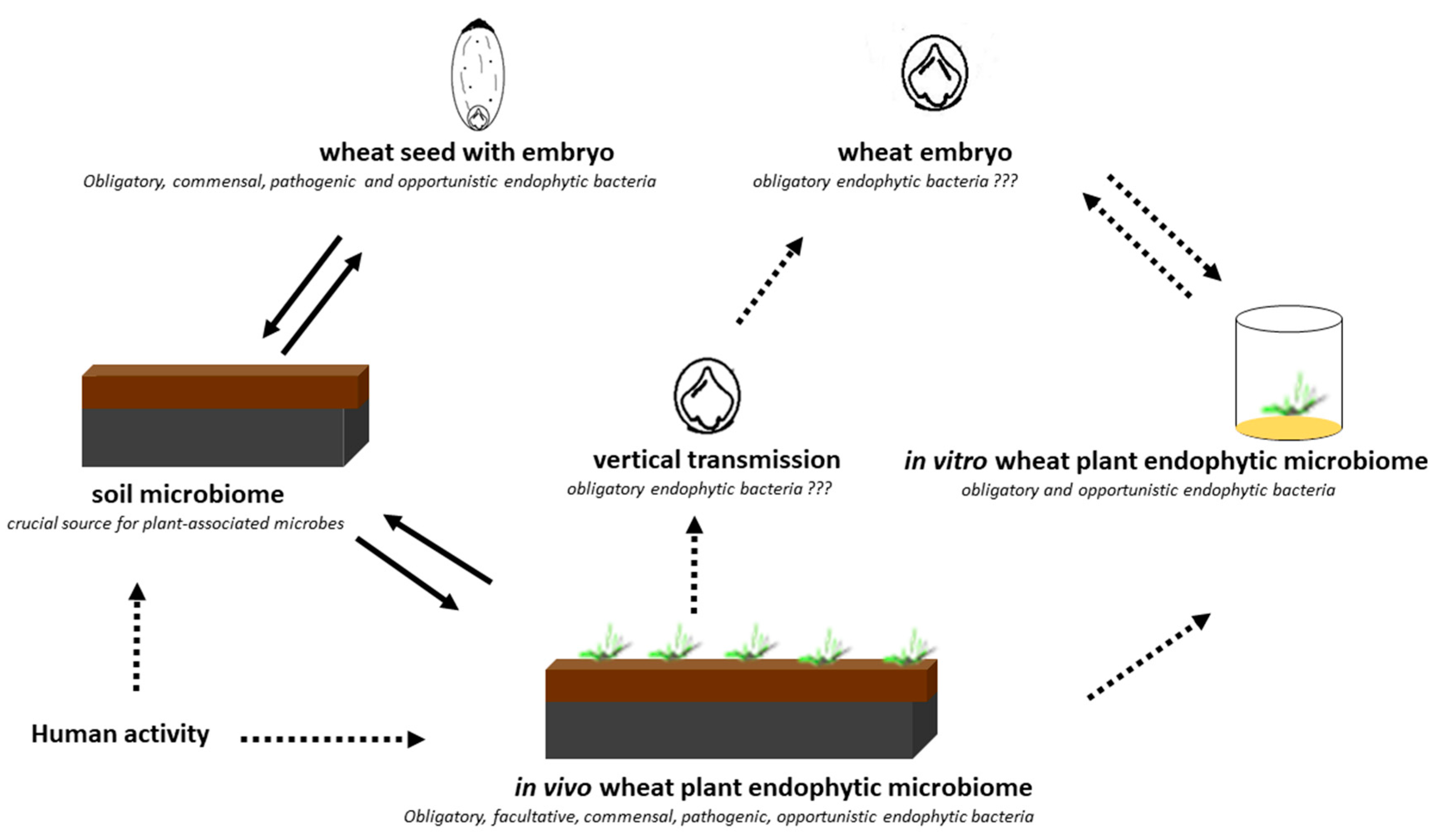
1. Introduction
2. Results
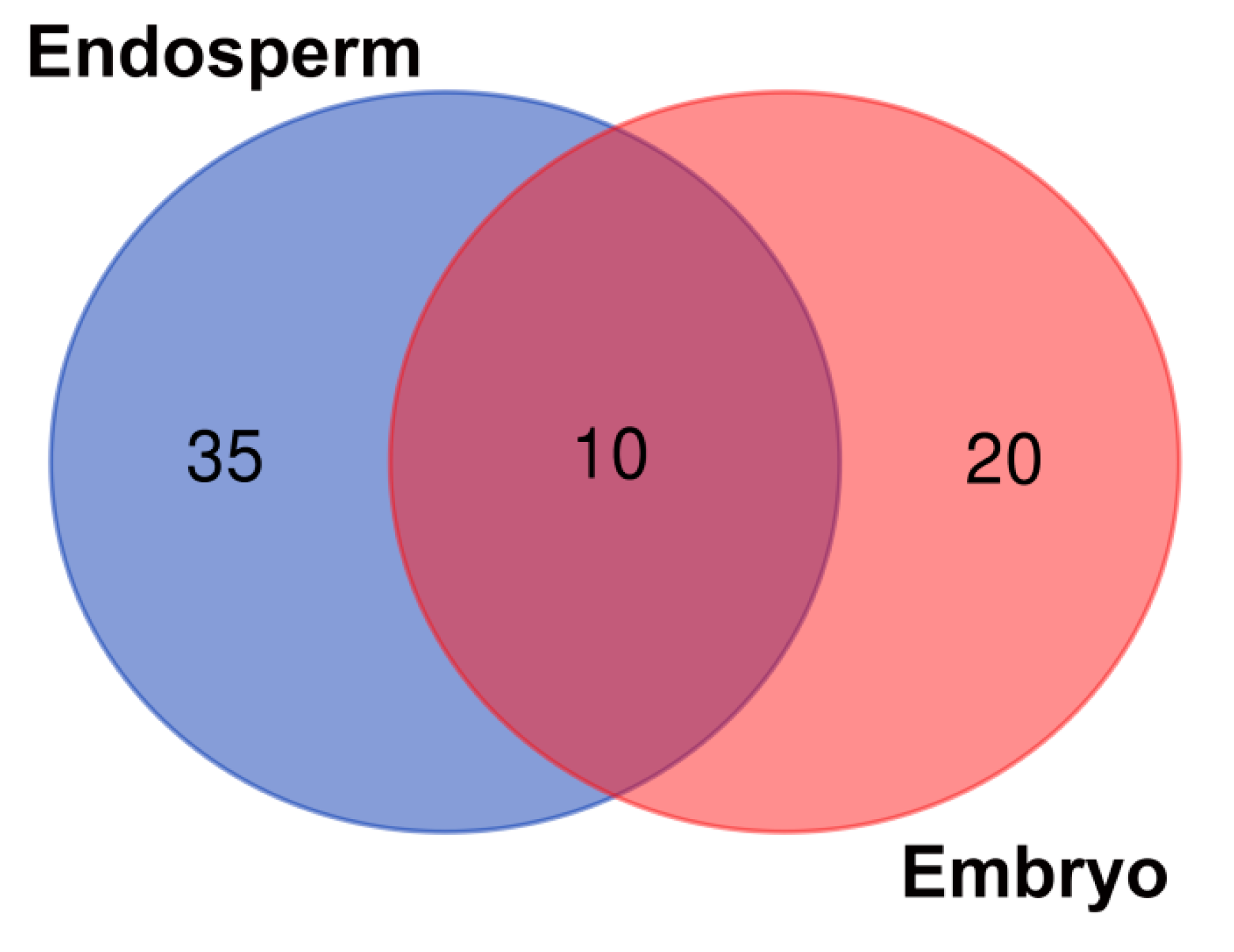
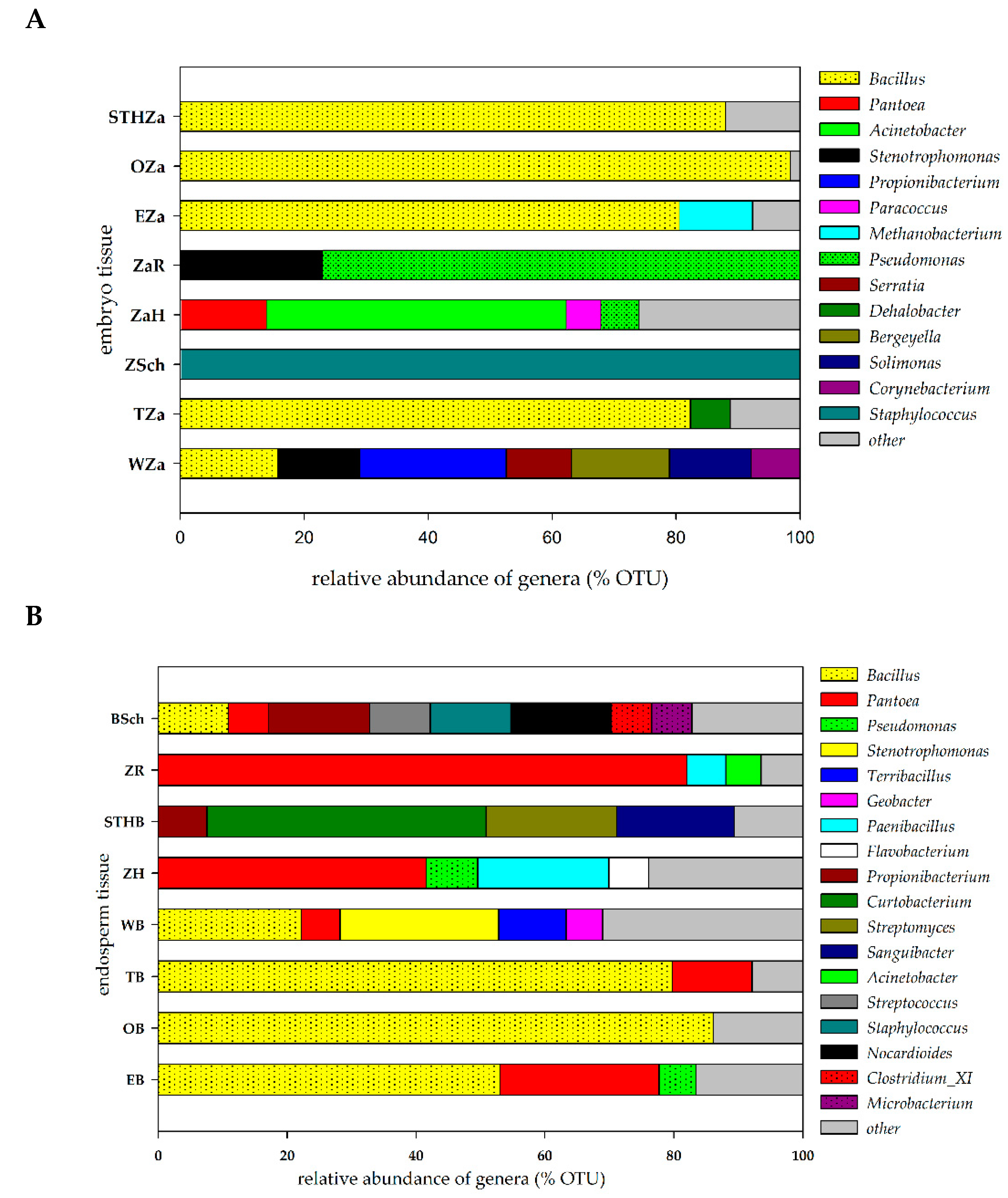
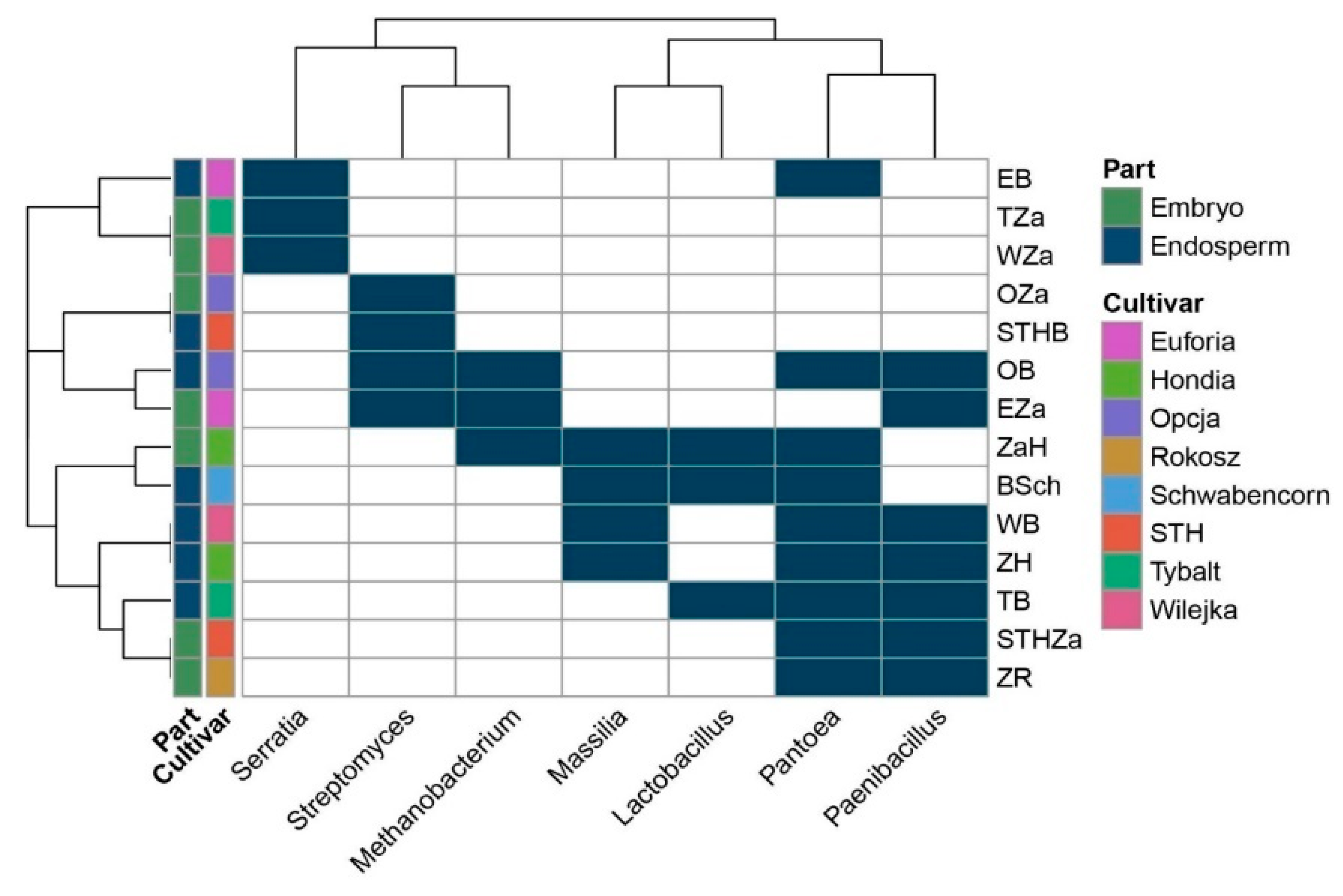
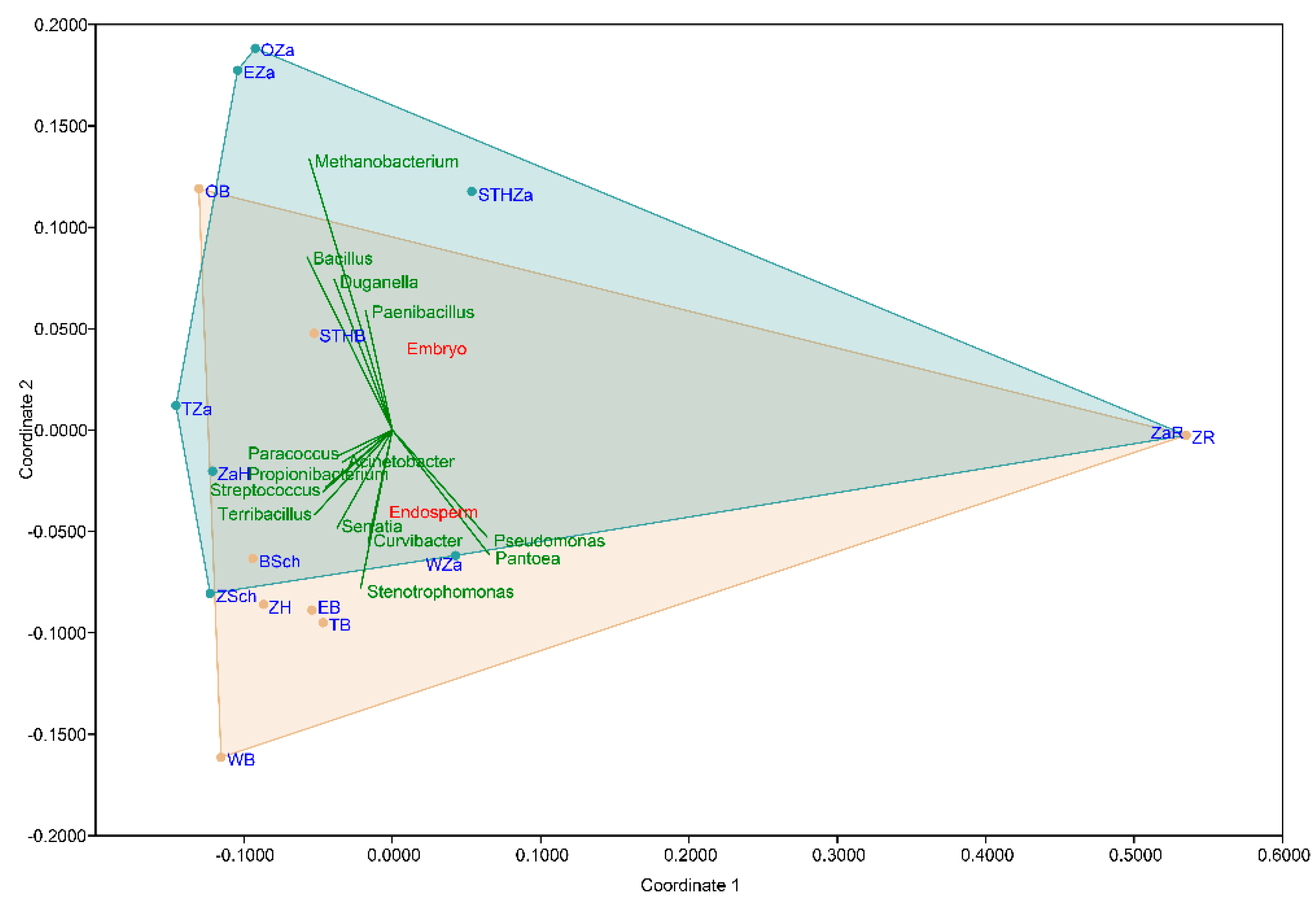
2.1. Seed-Borne Microbiome of Endosperms and Embryos of Different Wheat Cultivars
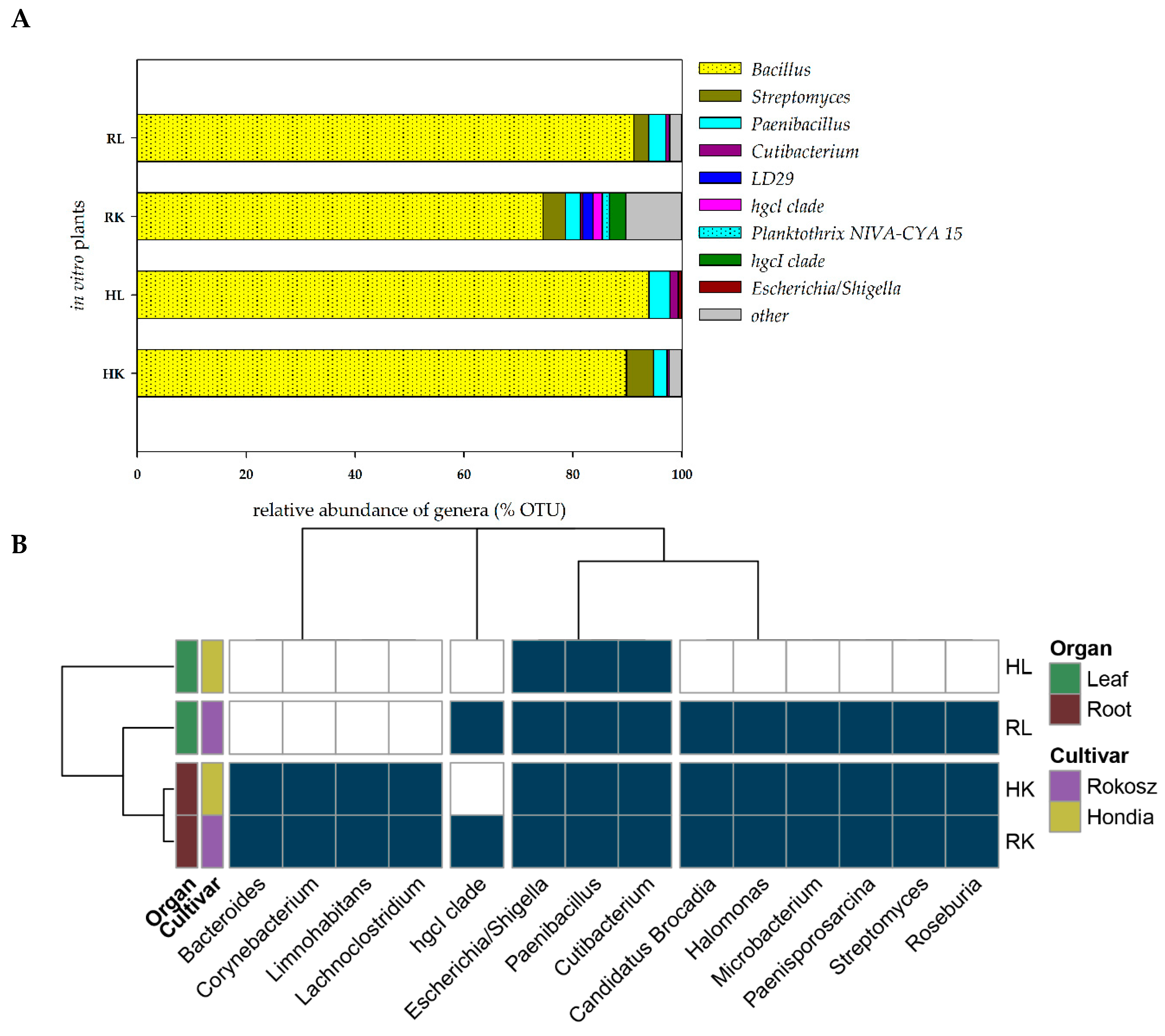
2.2. In Vitro Experiment—Endophytic Microbiome Colonizing the Roots and Leaves of Hondia and Rokosz Cultivars
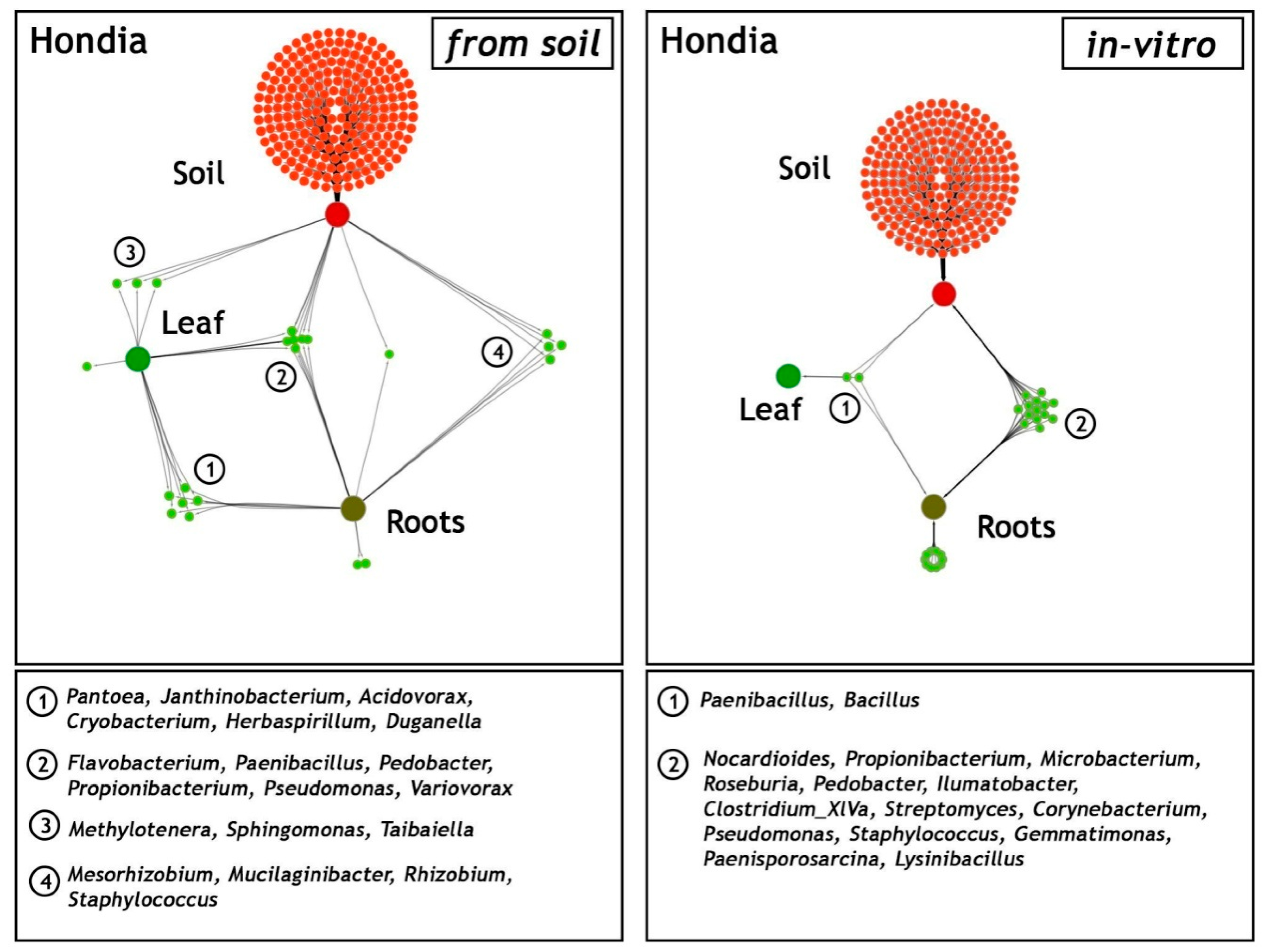
2.3. Comparison of Shared and Unique Endophytic Genera Inhabiting Three Niches: Rhizospheric Soil and the Leaves and Roots of T. aestivum L. Hondia—Field and In Vitro Experiments
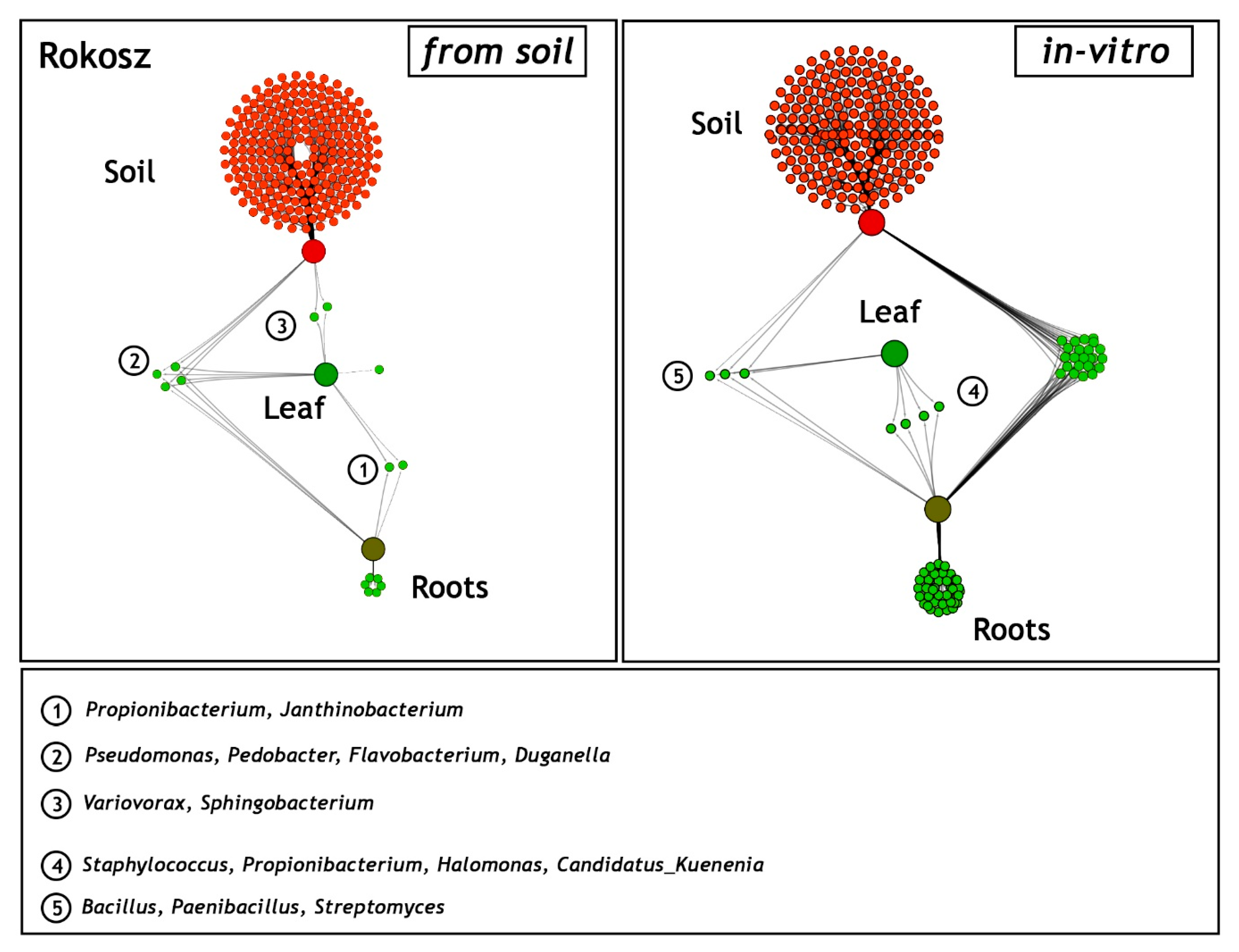
2.4. Comparison of Shared and Unique Endophytic Genera Inhabiting Three Niches: Rhizospheric Soil and the Leaves and Roots of T. spelta L. Rokosz—Field and In Vitro Experiments
3. Discussion
4. Materials and Methods
4.1. Seed Material
4.2. In Vitro Plant Experiment
4.3. In Vivo Plant Experiment
4.4. Soil Sampling
4.5. DNA Extraction and Next Generation Sequencing (NGS)
4.6. Bioinformatic Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.J.; Fraaije, B.A.; Clark, I.M.; Jackson, R.W.; Hirsch, P.R.; Mauchline, T.H. Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant Soil 2016, 45, 381–396. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.A.; Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, Y.J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Kuźniar, A.; Włodarczyk, K.; Grządziel, J.; Goraj, W.; Gałązka, A.; Wolińska, A. Culture-independent analysis of an endophytic core microbiome in two species of wheat: Triticum aestivum L. (cv. ‘Hondia’) and the first report of microbiota in Triticum spelta L. (cv. ‘Rokosz’). Syst. Appl. Microbiol. 2020, 43, 126025. [Google Scholar] [CrossRef]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Nelson, E.B.; Simoneau, P.; Barret, M.; Mitter, B.; Compant, S. Editorial special issue: The soil, the seed, the microbes and the plant. Plant Soil 2018, 422, 1–5. [Google Scholar] [CrossRef]
- Geisen, S.; Kostenko, O.; Cnossen, M.C.; ten Hooven, F.C.; Vres, B.; van der Putten, W.H. Seed and root endophytic fungi in a range expanding and a related plant species. Front. Microbiol. 2017, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Goggin, D.E.; Emery, R.J.; Kurepin, L.V.; Powles, S.B. A potential role for endogenous microflora in dormancy release, cytokinin metabolism and the response to fluridone in Loliumrigidum seeds. Ann. Bot. 2015, 115, 293–301. [Google Scholar] [CrossRef]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of grapevine flowers, berries, and seeds: Identification of cultivable bacteria, comparison with other plant parts, and visualisation of niche colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manuslis-Sasson, S.; Smart, C.D. Tomato fruit and seed colonization by Clavibactermichiganensis subsp. michiganensis through external and internal routes. Appl. Environ. Microbiol. 2013, 79, 6948–6957. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Al-Hosni, K.; Kang, S.M.; Seo, C.W.; Lee, I.-J. Indoleacetic acid production and plant growth promoting potential of bacterial endophytes isolated from rice (Oryza sativa L.) seeds. Acta Biol. Hung. 2017, 68, 175–186. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Li, N.; Wang, W.; Cheng, C. Identification of endophytic bacterial strain RSE1 from seeds of super hybrid rice Shenliangyou 5814 (Oryza sativa L.) and evaluation of its antagonistic activity. Plant Growth Reg. 2017, 82, 403–408. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.N.; White, J.F. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 2007, 53, 802–808. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Lopez-Lopez, A.; Martinez, J.; Rogel, M.A.; Toledo, I.; Martinez-Romero, E. Seed bacterial endophytes: Common genera, seed-to-seed variability and their possible role in plants. Acta Horticultural 2012, 938, 39–48. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Zou, Y.; Wang, J.; Song, W. Investigation on diversity and population succession dynamics of endophytic bacteria from seeds of maize (Zea mays L.; Nongda 108) at different growth stages. Ann. Microbiol. 2013, 63, 71–79. [Google Scholar] [CrossRef]
- Herrera, D.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef]
- Cope-Selby, N.; Cookson, A.; Squance, M.; Donnison, I.; Flavell, R.; Farrar, K. Endophytic bacteria in Miscanthus seed: Implications for germination, vertical inheritance of endophytes, plant evolution and breeding. GCB Bioenerg. 2016, 9, 57–77. [Google Scholar] [CrossRef]
- Frank, A.C.; SaldiernaGuzmán, J.P.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Soares, H.M.V.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant-growth promoting properties and microbial safety aspect. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Agaras, B.; Werra, P.; Wall, L.G.; Valverde, C. Characterization and screening of plant probiotic traits of bacteria isolated from rice seeds cultivated in Argentina. J. Microbiol. 2011, 49, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.T.; Franco, C.M. Visualization of an endophytic Streptomyces species in wheat seed. Appl. Environ. Microbiol. 2003, 69, 4260–4262. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Kloepper, J.W. Effect of host genotype on indigenous bacterial endophytes of cotton (Gossypiumhirsutum, L.). Plant Soil 2002, 240, 181–189. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Robinson, R.J.; Hayat, R.; Clarck, I.M.; Hughes, D.; Rossmann, M.; Hirsch, P.R.; Mendes, R.; Mauchline, T.H. Land management and microbial seed load effect on rhizosphere and endosphere bacterial community assembly in wheat. Front. Microbiol. 2019, 10, 2625. [Google Scholar] [CrossRef]
- Glassner, H.; Zchori-Fein, E.; Yaron, S.; Sessitsch, A.; Sauer, U.; Compant, S. Bacterial niches inside seeds of Cucumismelo L. Plant Soil 2018, 422, 101–113. [Google Scholar] [CrossRef]
- Darrase, A.; Barret, M.; Cesbron, S.; Compant, S.; Jacques, M.A. Niches and routes of transmission of Xanthomonascitripv. fuscans to bean seeds. Plant Soil 2018, 422, 115–128. [Google Scholar] [CrossRef]
- Sanchez-Lopez, A.S.; Thijs, S.; Beckers, B.; Gonzales-Chavez, M.C.; Weyens, N.; Carillo-Gonzalez, R.; Vangronsveld, J. Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 2018, 422, 51–66. [Google Scholar] [CrossRef]
- Tyc, O.; Putra, R.; Gols, R.; Harvey, J.A.; Garbeva, P. The ecological role of bacterial seed endophytes associated with wild cabbage in the United Kingdom. Microbiol. Op. 2020, 9, e954. [Google Scholar] [CrossRef]
- Sinnesael, A.; Leroux, O.; Janssens, S.B.; Smets, E.; Panis, B.; Verstraete, B. Is the bacterial leaf nodule symbiosis obligate for Psychotriaumbellata? The development of a Burkholderia-free host plant. PLoS ONE 2019, 14, e0219863. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Tanaka, F.; Watanabe, A.; Kaga, H.; Okunishi, S.; Morisaki, H. Culturable surface and endophytic bacterial flora of the maturing seeds of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ. 2006, 21, 86–100. [Google Scholar] [CrossRef]
- Žiarovská, J.; Medo, J.; Kysel, M.; Zamiešková, L.; Kačániová, M. Endophytic Bacterial Microbiome Diversity in Early Developmental Stage Plant Tissues of Wheat Varieties. Plants 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Delmont, T.O.; Post, A.F.; Belkin, S. Metagenomic signatures of bacterial adaptation to life in the phyllosphere of a salt-decreasing desert tree. Appl. Environ. Microbiol. 2016, 82, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.K.; Yuan, L.; Layghifrad, M.; Wang, P.W.; Guttman, D.S. Seasonal community succession of the phyllopshere microbiome. Mol. Plant Microb. Interac. 2015, 25, 274–285. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. New Approach to Modify Plant Microbiomes and Traits by Introducing Beneficial Bacteria at Flowering into Progeny Seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gałązka, A.; Grzęda, E.; Jończyk, K. Changes of microbial diversity in rhizosphere soils of new quality varieties of winter wheat cultivation in organic farming. Sustainability 2019, 11, 4057. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Meth. 2020, 170, 105860. [Google Scholar] [CrossRef]
- Soliman, T.; Yang, S.Y.; Yamazaki, T.; Jenke-Kodama, H. Profiling soil microbial communities with next-generation sequencing: The influence of DNA kit selection and technician technical expertise. Peer J. 2017, 5, e4178. [Google Scholar] [CrossRef]
- Song, Z.; Schlatter, D.; Kennedy, P.; Kinkel, L.L.; Kistler, H.C.; Nguyen, N.; Bates, S.T. Effort versus Reward: Preparing Samples for Fungal Community Characterization in High-Throughput Sequencing Surveys of Soils. PLoS ONE 2015, 10, e0127234. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Meth. 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna, Austria, 2018. Available online: https://www.R-project.org/ (accessed on 26 May 2020).
- Murali, A.; Bhargava, A.; Wright, E.S. IDTAXA: A novel approach for accurate taxonomic classification of microbiome sequences. Microbiome 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Mcmurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
| Endosperms | Embryos | Shared Genera |
|---|---|---|
| Acidovorax, Achromobacter, Actinoallomurus, Actinomyces, Alloprevotella, Burkholderia, Clostridium_IX, Curvibacter, Dechloromonas, Desulfitobacterium, Dialister, Duganella, Ethanoligenens, Geobacter, Herbaspirillum, Hyphomicrobium, Longilinea, Lysinibacillus, Melioribacter, Methylobacterium, Microbacterium, Nitrosomonas, Nocardioides, Prevotella, Roseburia, Sanguibacter, Smithella, Sphingobacterium, Sphingomonas, Sphingopyxis, Solibacillus, Subdoligranulum, Taibaiella, Terribacillus, Verrucosispora | Anaerococcus, Anaeromyxobacter, Bergeyella, Brevundimonas, Caulobacter, Ciceribacter, Clostridium-sensu-stricto, Comamonas, Dehalobacter, Enhydrobacter, Fusobacterium, Granulicatella, Haemophilus, Ignavibacterium, Kocuria, Melaminivora, Paracoccus, Soonwooa, Solimonas, Turicella | Chryseobacterium, Curtobacterium, Lactobacillus, Massilia, Methanobacterium, Neisseria, Paenibacillus, Pantoea, Serratia, Streptomyces |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźniar, A.; Włodarczyk, K.; Grządziel, J.; Woźniak, M.; Furtak, K.; Gałązka, A.; Dziadczyk, E.; Skórzyńska-Polit, E.; Wolińska, A. New Insight into the Composition of Wheat Seed Microbiota. Int. J. Mol. Sci. 2020, 21, 4634. https://doi.org/10.3390/ijms21134634
Kuźniar A, Włodarczyk K, Grządziel J, Woźniak M, Furtak K, Gałązka A, Dziadczyk E, Skórzyńska-Polit E, Wolińska A. New Insight into the Composition of Wheat Seed Microbiota. International Journal of Molecular Sciences. 2020; 21(13):4634. https://doi.org/10.3390/ijms21134634
Chicago/Turabian StyleKuźniar, Agnieszka, Kinga Włodarczyk, Jarosław Grządziel, Małgorzata Woźniak, Karolina Furtak, Anna Gałązka, Ewa Dziadczyk, Ewa Skórzyńska-Polit, and Agnieszka Wolińska. 2020. "New Insight into the Composition of Wheat Seed Microbiota" International Journal of Molecular Sciences 21, no. 13: 4634. https://doi.org/10.3390/ijms21134634
APA StyleKuźniar, A., Włodarczyk, K., Grządziel, J., Woźniak, M., Furtak, K., Gałązka, A., Dziadczyk, E., Skórzyńska-Polit, E., & Wolińska, A. (2020). New Insight into the Composition of Wheat Seed Microbiota. International Journal of Molecular Sciences, 21(13), 4634. https://doi.org/10.3390/ijms21134634









