1. Introduction
Large numbers of studies conducted over the previous decades have increased knowledge of pain mechanisms at both the cellular and molecular level. Several membrane-bound receptors and ion channels have been found to play a key role in the transmission or attenuation of painful stimuli. Among them, the µ-opioid receptor (MOR) has attracted great attention as the primary molecular target of opioid drugs, a group of most effective analgesics [
1,
2]. MOR belongs to the family of G protein-coupled receptors (GPCRs). MOR is linked to the inhibitory G proteins, whose major signaling pathway leads to inhibition of adenylyl cyclase or modulation of mitogen activated protein kinases (MAPKs). The properties of these receptors and their signaling systems have been extensively studied not only in connection with nociception but also with a potential risk of tolerance and dependence associated with long-term use or abuse of opioids [
3,
4,
5]. Interestingly, it has been observed that MOR-initiated signaling can be modulated by other GPCRs or some ion channels. In the case of antinociception, communication between the MOR and TRPV1 (transient receptor potential vanilloid 1) receptors appears particularly noteworthy [
6,
7].
TRPV1 receptors, which play a central role in thermal nociception and inflammation-induced hyperalgesia, belong to the large superfamily of TRP ion channels [
8]. These are ligand-driven, non-selective cation channels that are present at low levels in different types of cells and tissues but are primarily expressed in the posterior spinal neurons and trigeminal ganglia [
9]. TRPV1 receptors are considered to be important molecular integrators of nociceptive stimuli because they respond not only to elevated temperature but also to reduced pH, vanilloids (e.g., capsaicin), and other pain-inducing substances (e.g., arachidonic acid metabolites). Furthermore, these receptors serve as the final target structure of intracellular signaling pathways triggered by inflammatory mediators, thereby potentiating their activity and contributing to elevated hyperexcitability and nociception [
10]. Thus, the function of TRPV1 receptors can be regulated through their direct activation by “external” pain stimuli or through “intrinsic” sensitization induced by intracellular signaling cascades that are linked to GPCRs or receptor tyrosine kinases (RTKs). The sensitization of TRPV1 receptors is based on their post-translational modifications, such as protein kinase A (PKA)- and protein kinase C (PKC)-mediated phosphorylation [
11].
Previous studies have demonstrated natural co-expression of MOR and TRPV1 receptors in different regions of the central nervous system and pointed to possible functional interactions between these receptors [
7,
12]. In the recent past, Bao et al. [
13] have noticed that MOR and TRPV1 receptor agonists may have conflicting effects on antinociception, tolerance, and dependence. It has been repeatedly observed that administration of morphine can induce antinociceptive effects by modulating the activity of TRPV1 receptors. As indicated above, TRPV1 receptors can be activated in a variety of ways. However, the potential specific effects of MOR agonists on TRPV1 receptors and vice versa have not yet been fully explored.
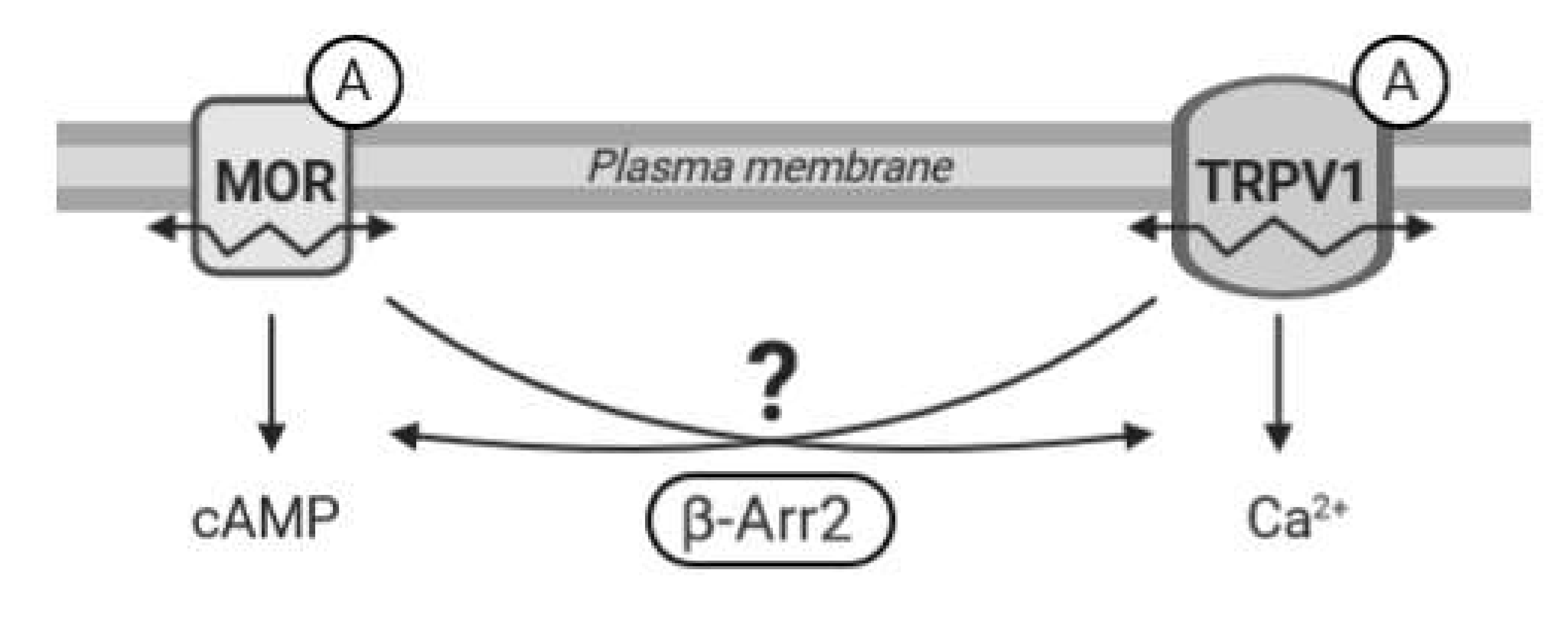
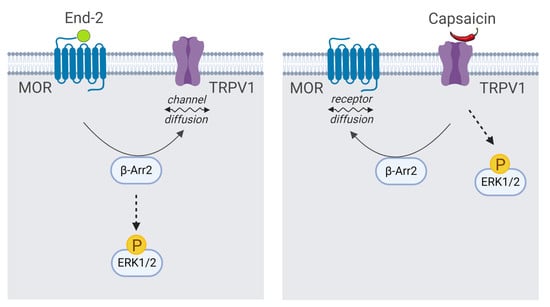
Recent evidence suggests that functional communication between MOR and TRPV1 receptors is bilateral and highly dependent on specific conditions; the mechanism and duration of activation of these receptors seem particularly important. However, many intriguing questions regarding MOR–TRPV1 interactions, crosstalk between their signaling pathways, and respective feedback loops still remain unresolved. Therefore, in the present study we set out to investigate behavior and functional properties of these receptors and their signaling pathways in defined in vitro conditions and examine the role of key components of both these important cellular signaling systems in their mutual communication (
Figure 1).
3. Discussion
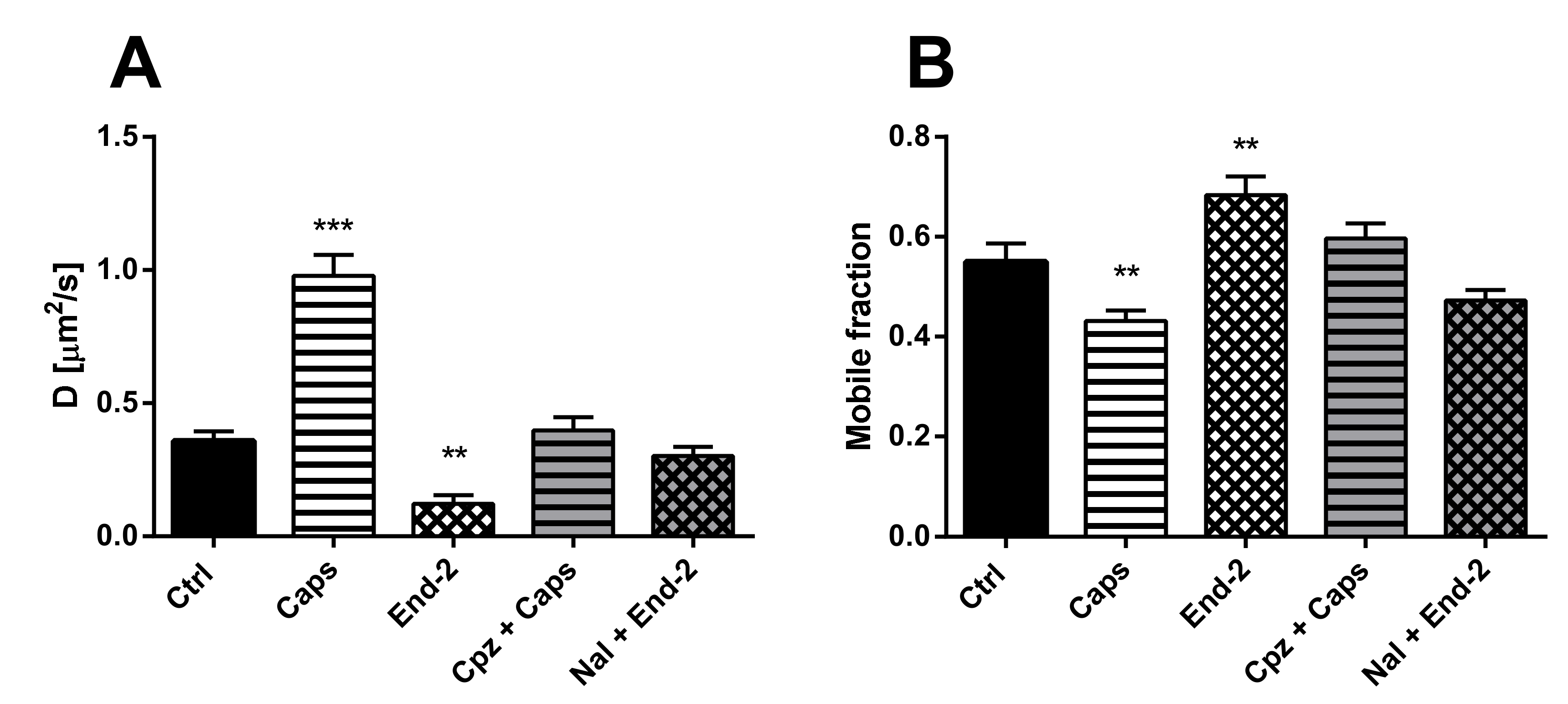
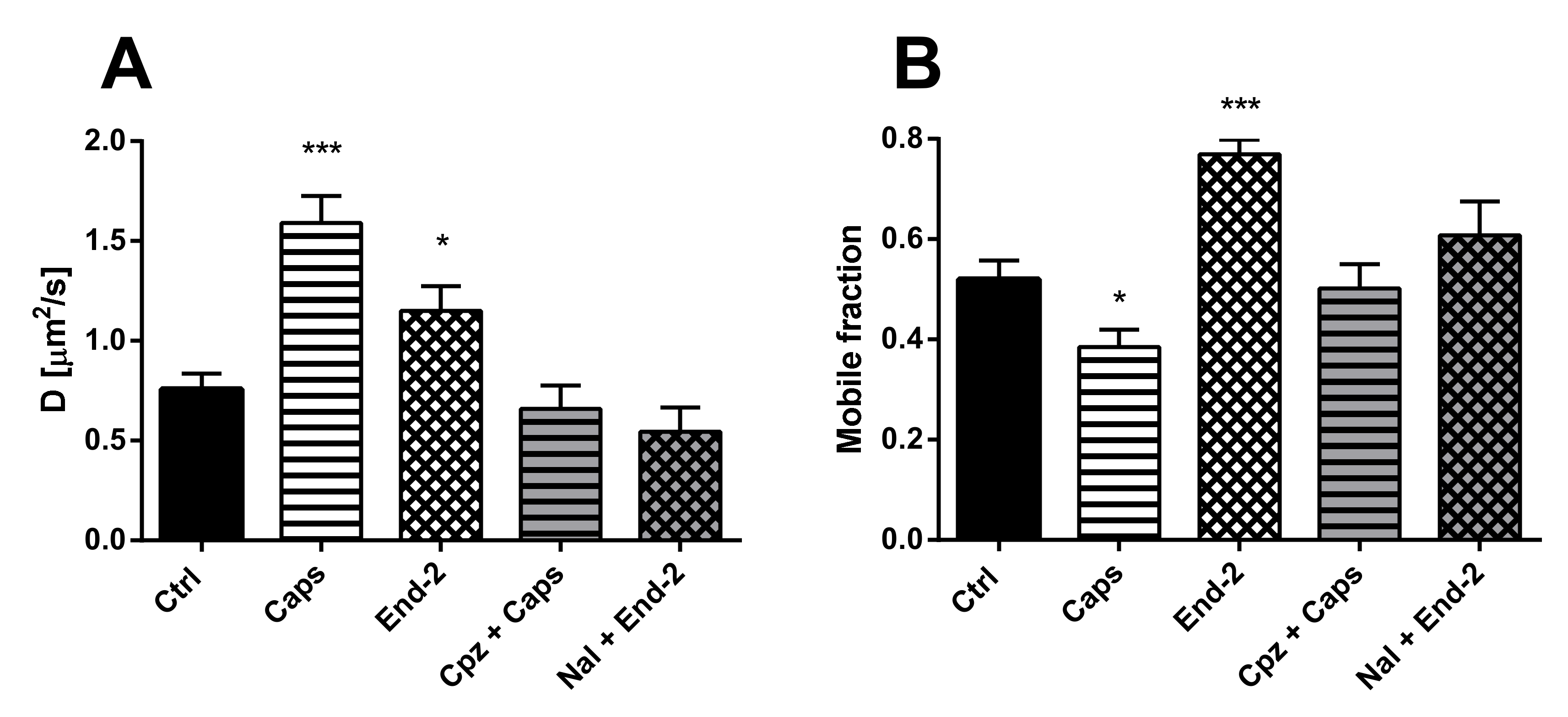
The purpose of this study was to investigate the relationship between MOR and TRPV1 and potential crosstalk between their signaling pathways. Using Fluorescent recovery after photobleaching (FRAP), we compared the mobility of both these receptors in the plasma membrane after their activation by capsaicin and endomorphin-2. Capsaicin is a naturally occurring vanilloid that exhibits TRPV1 agonistic activity [
15]. Endomorphin-2 is an endogenous MOR agonist biased towards β-arrestin 2-dependent signaling [
16,
17]. We have previously found that the lateral mobility of MOR in the plasma membrane was significantly increased by DAMGO and decreased by endomorphin-2 [
14]. Our current results indicate that the exogenous expression of TRPV1 in HMY-1 cells did not affect the mobility of MOR. However, the activation of TRPV1 with capsaicin markedly increased the mobility of MOR and decreased the number of mobile receptors in the plasma membrane. On the other hand, we have also observed that the diffusion rate of TRPV1 was changed not only after activation of TRPV1 with capsaicin but also after activation of MOR with endomorphin-2. Interestingly, there were similar changes in mobile fractions of both MOR and TRPV1 after activation with endomorphin-2 or capsaicin. The mobile fractions of both these receptors were decreased after treatment with capsaicin and increased after treatment with endomorphin-2. To date, there is not much information about TRPV1 diffusion in the plasma membrane. It has been reported that the mobility of TRPV1 decreased within seconds upon channel activation in the presence of Ca
2+ [
18]. We therefore presume that the decrease in the mobile fraction observed after activation of TRPV1 with capsaicin is due to the certain number of active channels in the plasma membrane.
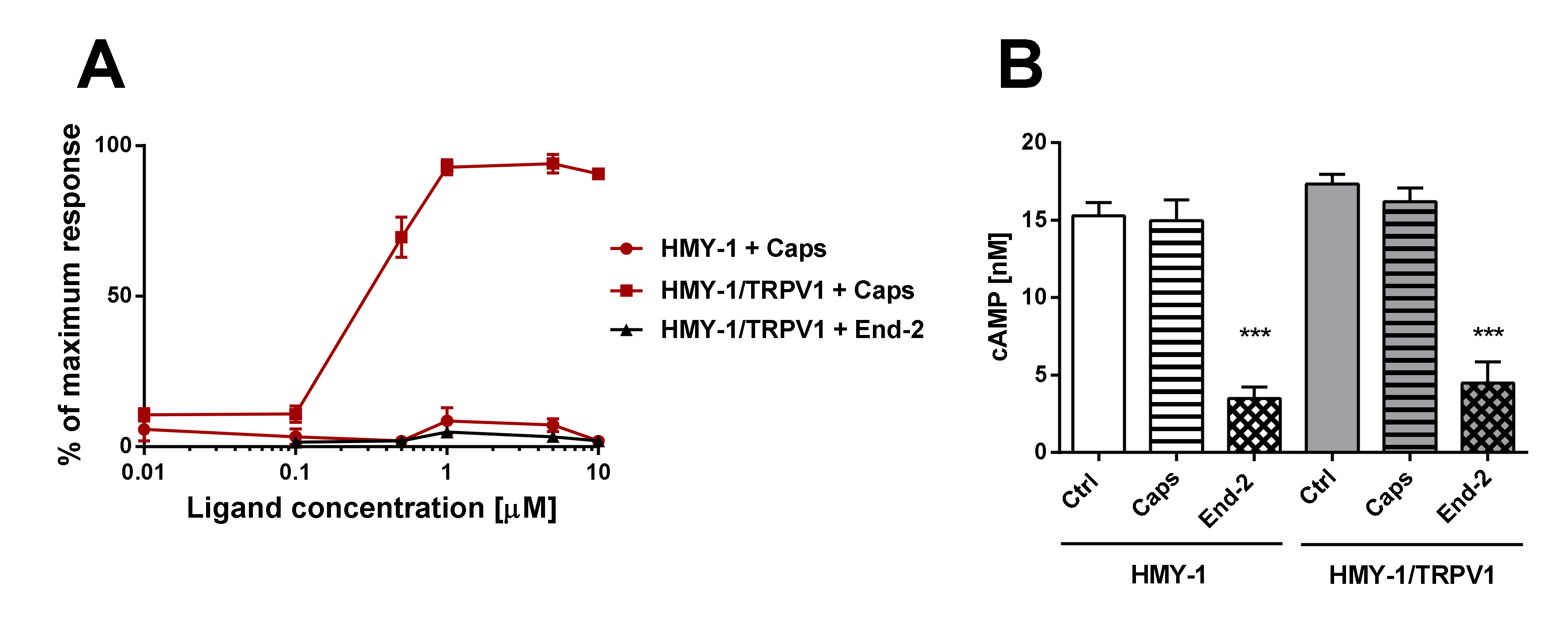
Our functional studies confirmed that the activation of TRPV1 by capsaicin leads to calcium influx and the activation of MOR by endomorphin-2 suppresses AC activity as reflected by a decrease in cAMP production. Although capsaicin did not affect AC activity and endomorphin-2 did not trigger calcium influx, both these ligands were able to change the mobility of their cognate as well as noncognate receptors in the plasma membrane. These data suggest that these receptors may communicate with each other, but the mechanism is not yet known.
β-Arrestins are important proteins that can form scaffolding complexes with a wide variety of proteins in order to regulate diverse signaling pathways [
19]. β-Arrestin 2 was first identified as a protein capable of mediating desensitization of β
2-adrenergic receptor signaling after agonist stimulation [
20]. It is nowadays obvious that β-arrestins play an important role in cell signaling including GPCR desensitization and, rather curiously, may also participate in desensitization of TRPV1 [
21]. It was recently shown that the activation of TRPV1 results in nuclear translocation of GRK5, which blocks its ability to phosphorylate MOR, and that this interaction leaves the G protein-mediated analgesic signaling of MOR intact but inhibits β-arrestin 2-mediated internalization and desensitization of MOR [
22]. These authors hypothesized that MOR and TRPV1 may compete for GRK5 and β-arrestin 2. Our current observations indicate that β-arrestin 2 is crucially implicated in mediating the relationship between MOR and TRPV1. According to the classical scenario, activation of MOR with endomorphin-2 initiates GRK-catalyzed receptor phosphorylation, which is followed by β-arrestin 2 recruitment to the plasma membrane and binding to the receptor. Consequently, the amount of β-arrestin 2 in the cytoplasm is lowered, which may rather limit its scaffolding function. This could likely explain the observed changes in the diffusion coefficient and mobile fraction of TRPV1.
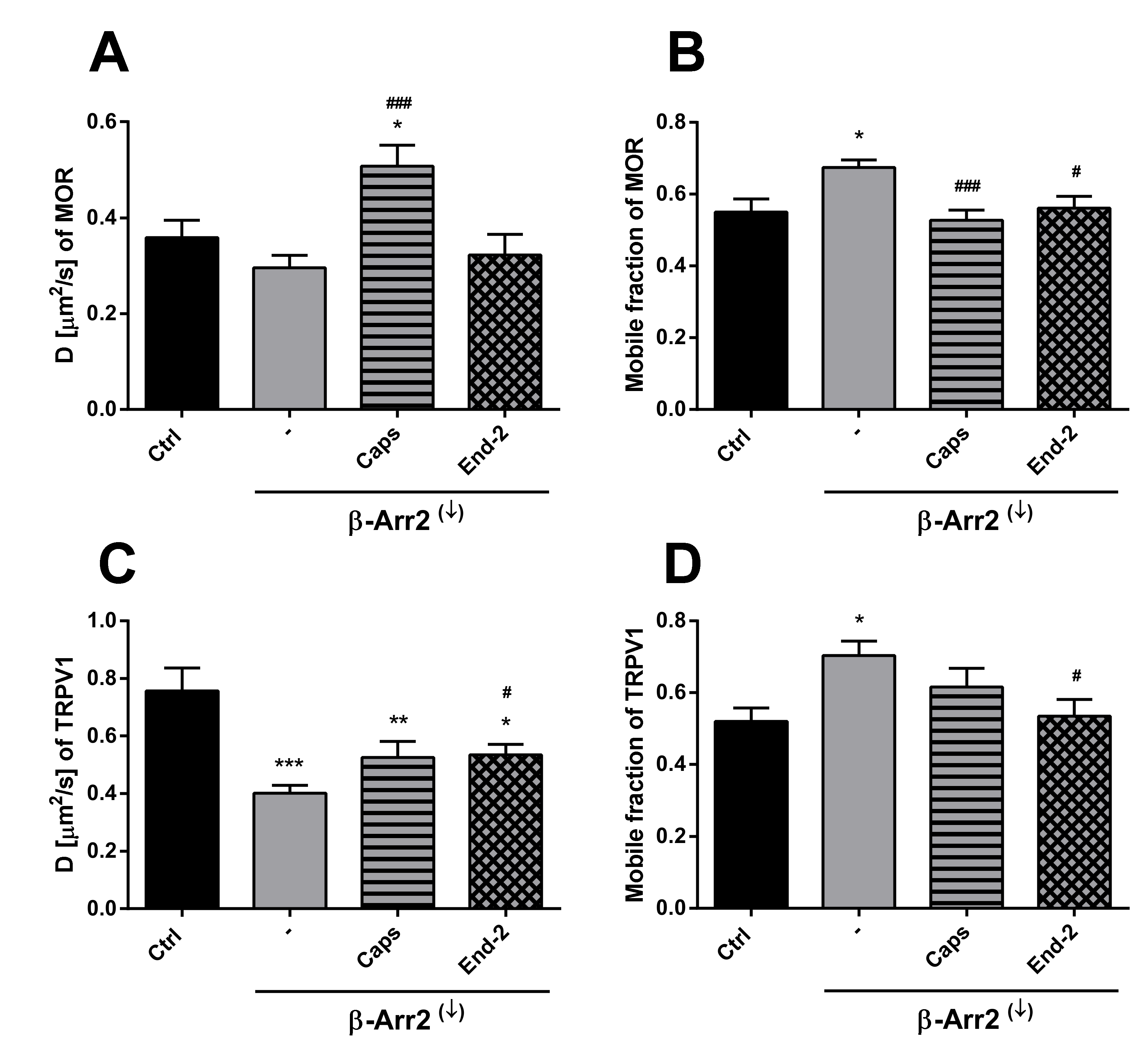
In order to assess the role of β-arrestin 2 in TRPV1 and MOR cross-communication, we investigated receptors mobility after β-arrestin 2 knockdown. As expected, this intervention increased the mobile fraction of both TRPV1 and MOR in plasma membranes of control (unstimulated) cells. In parallel, the diffusion coefficient of TRPV1 was markedly reduced and there was a downward tendency in the diffusion rate of MOR. The effects of capsaicin and endomorphin-2 on the mobility of both TRPV1 and MOR were notably diminished or altered after β-arrestin 2 knockdown. Interestingly, the study of Por and colleagues [
23] demonstrated increased association of unactivated TRPV1 and β-arrestin 2 in CHO cells under normal serum media conditions and reduced association of these two proteins in serum-free media. Here, we observed that treatment of HMY-1/TRPV1 cells with capsaicin for 5 min resulted in a significant decrease in the amount of β-arrestin 2 attached to the plasma membrane. It has been demonstrated that the activation of MOR by some ligands including endomorphin-2 leads to internalization of the MOR–β-arrestin 2 complex [
17]. Although this event is quite fast, it does not occur within 5 min after MOR activation [
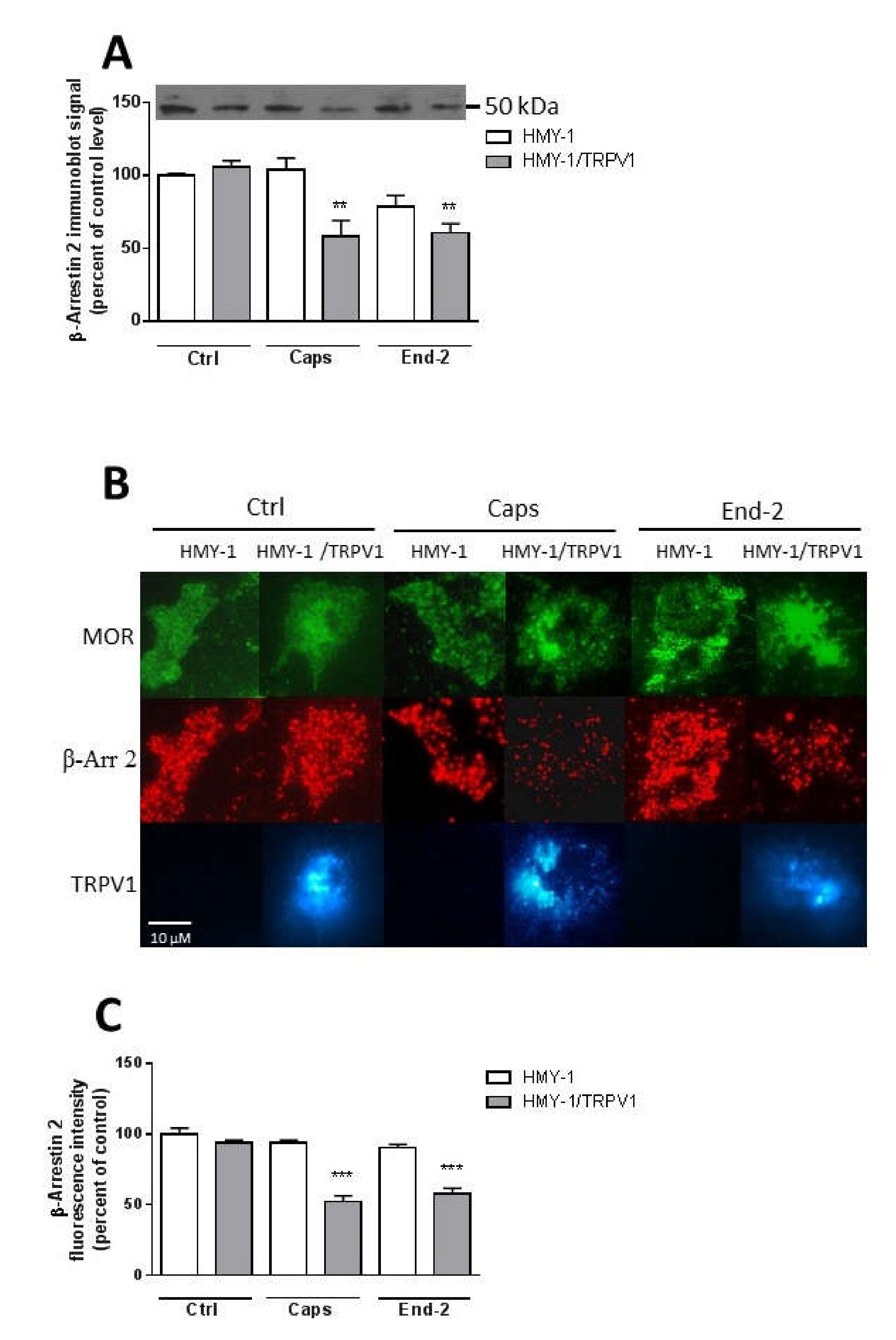
24]. It therefore seems plausible that the observed decrease in plasma membrane β-arrestin 2 level is somehow connected with TRPV1 signaling. Our data supports this view: the activation of MOR with endomorfin-2 in HMY-1 cells (not expressing TRPV1) did not reduce the amount of β-arrestin 2 in the plasma membrane. Surprisingly, stimulation of HMY-1/TRPV1 cells with endomorfin-2, similarly as with capsaicin, led to a significant decrement in β-arrestin 2 in the plasma membrane. These observations endorse our hypothesis that there is crosstalk between MOR- and TRPV1-mediated pathways which can play an important role in modulating the receptor signaling.
The MAPK family is a diverse group of proteins involved in ensuring a wide variety of cell physiological processes. They include extracellular signal-regulated kinase (ERK), p38-mitogen activated protein kinase (p38), and c-Jun N-terminal kinase (JNK) [
25]. It has been found that the activation of MAPK may participate in generating pain hypersensitivity and that MEK inhibitors known to suppress phosphorylation of ERK can effectively alleviate pain at various time points in several animal models of neuropathic pain [
26,
27]. It has been reported that stimulation of TRPV1 with capsaicin leads to a rapid phosphorylation and activation of ERK1/2 [
28]. Morphine was found to induce ERK activation in CHO cells stably transfected with MOR [
29]. It seems evident that MOR can mediate responses in an agonist dependent manner. Agonists such as morphine and methadone activate ERKs via the protein kinase C-dependent pathway but not the β-arrestin-dependent pathway. Contrarily, agonists such as etorphine and fentanyl activate ERKs in a β-arrestin-dependent manner [
30]. Previous studies have indicated that morphine and methadone are G protein biased agonists, whereas etorphine, fentanyl, and endomorphin-2 are β-arrestin biased agonists [
17]. Interestingly, β-arrestin 2 is not a regulator of phosphorylation of ERK1/2 initiated by activation of some GPCRs [
31,
32]. Here we show that the cross-communication between the TRPV1 and MOR signaling pathways after activation either by capsaicin or by endomorphin-2 proceeds to ERK1/2. This is clearly evident from our results showing the increase in ERK1/2 phosphorylation after activation of TRPV1 with capsaicin in cells expressing TRPV1. Endomorphin-2 elicited a significant increase in ERK1/2 phosphorylation in cells expressing MOR but, rather surprisingly, a more pronounced increase in ERK1/2 phosphorylation was detected in cells expressing both MOR and TRPV1. Interestingly, Popiolek-Barczyk et al. [
33] reported that inhibition of ERK1/2 phosphorylation through inhibiting MEK1/2 (kinase phosphorylating ERK1/2) significantly enhanced the analgesic effects of morphine. It means that phosphorylation of ERK1/2 attenuates analgesia induced by opioids. On the other hand, the p38 MAPK pathway was found to be responsible for more effective analgesia after morphine administration [
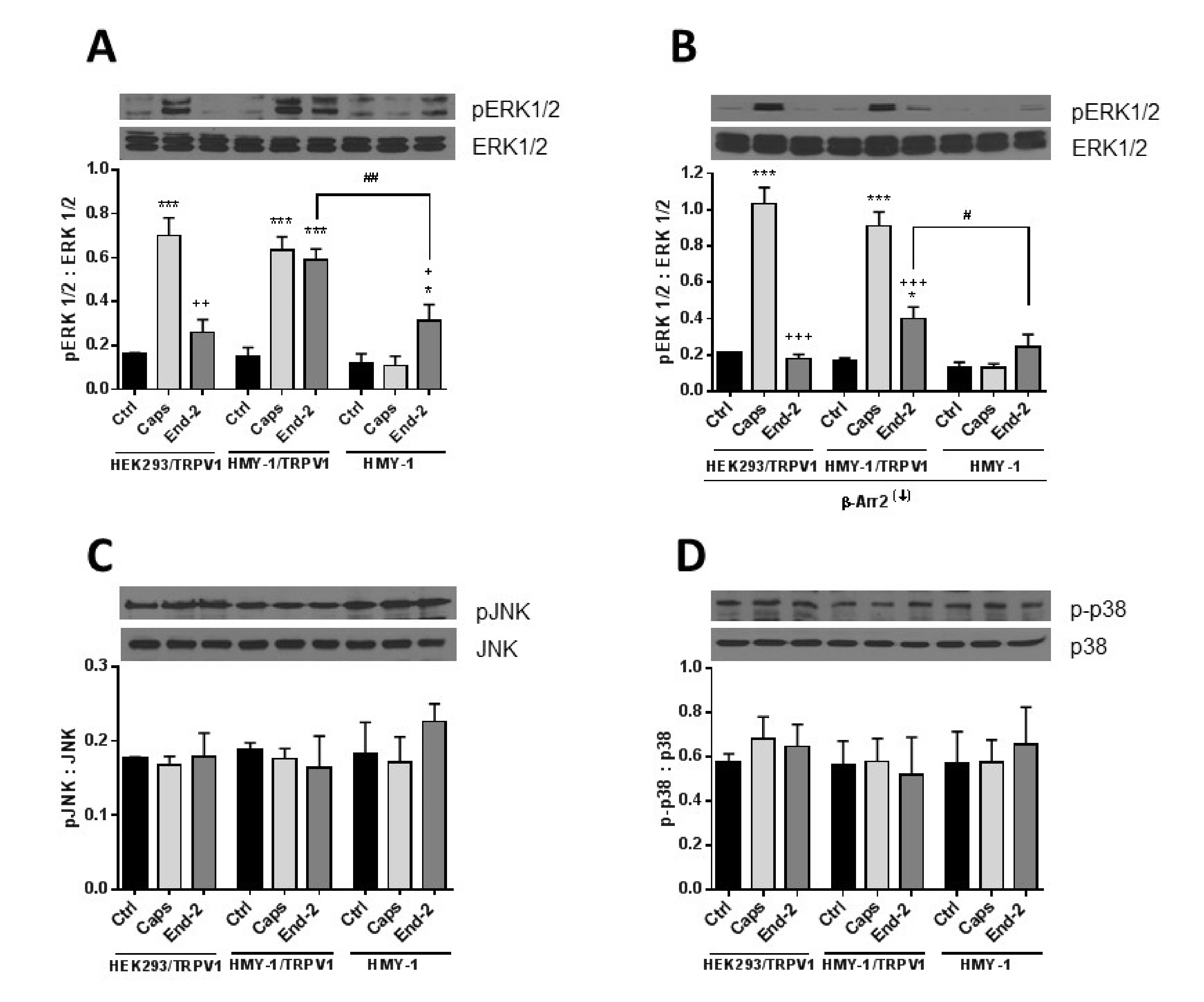
34]. Here, we observed that TRPV1, which plays a central role in nociception, is of high importance for enhanced phosphorylation of ERK1/2 but not for p38 or JNK phosphorylation in HMY-1/TRPV1 cells following activation of MOR with endomorphin-2. Interestingly, β-arrestin 2 appears to be especially important for MOR-induced ERK1/2 phosphorylation because its downregulation strongly attenuated the ability of endomorphin-2 to increase ERK1/2 phosphorylation. On the other hand, the ability of capsaicin to increase ERK1/2 phosphorylation was not affected by β-arrestin 2 knockdown. These observations suggest that the mechanism of receptor-induced ERK1/2 activation somewhat differ between TRPV1 and MOR.
4. Materials and Methods
4.1. Cell Culture, Transient Transfection, and Drug Treatment
The human embryonic kidney (HEK) 293 cell line was purchased from Sigma-Aldrich (St. Louis, MI, USA). The HMY-1 cell line (HEK293 cells stably expressing MOR) was prepared and characterized previously [
14]. Both cell lines were cultured in Dulbeco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic antimycotic solution (AAS, Sigma-Aldrich) at 37 °C in a humidified atmosphere containing 5% CO
2. For transient transfection, cells were plated in the appropriate multi-well plates and cultivated in the above-mentioned medium. After 24 h, on reaching 60–70% confluence, cells were transfected using Lipofectamine 3000 reagent (Invitrogene, Waltham, MA, USA) according the manufacturer’s instructions. Briefly, Lipofectamine 3000 diluted in Opti-MEM medium was mixed with DNA or siRNA diluted in Opti-MEM with P3000 reagent. Final mixture was incubated for 5 min at room temperature and then added directly to the cell culture. After 24 h of incubation, cells were used in further experiments. The plasmid containing TRPV1 with CFP fused to the C-terminus was a generous gift from Dr. Leon D Islas (National Autonomous University of Mexico [
35]. β-Arrestin 2 siRNA (sc-29208) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). HMY-1 cells carrying the TRPV1–CFP fusion receptor were denoted HMY-1/TRPV1.
The function of MOR and TRPV1 was modulated by the MOR agonist endomorphin-2 or antagonist naloxone and the TRPV1 agonist capsaicin or antagonist capsazepine. These ligands were dissolved in phosphate-buffered saline (PBS). Before starting the measurement, cells were incubated for 5 min either with endomorphin-2 (1 µM) or capsaicin (0.5 µM) or naloxone (10 µM) or capsazepine (5 µM). In some cases, cells were pretreated for 10 min with naloxone (10 µM) or capsazepine (5 µM) and then exposed for 5 min to endomorphin-2 (1 µM) or capsaicin (0.5 µM), respectively.
4.2. Total Internal Reflection (TIRF)
Cells were plated on a 35 mm glass-bottom dishes in phenol red-free DMEM supplemented with 10% FBS and 1% AAS. After 24 h, cells were transfected with plasmid cDNA encoding the CFP-tagged TRPV1 receptor as described above. One day after transfection, cells were visualized using Zeiss Elyra SP.1 nanoscope equipped with TIRF technology.
4.3. Fluorescent Recovery after Photobleaching
HMY-1/TRPV1 cells were seeded on glass-bottom dishes and maintained in phenol red-free DMEM supplemented with 10% FCS, 1% AAS, and 0.8 mg/mL geneticin. FRAP experiments on living cells were performed on an inverted Zeiss LSM 880 confocal laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany) equipped with 40×/1.2 WDICIII C Apochromat objective lens and back-thinned CCD camera (Zeiss Axio Cam). For excitation of fluorophores, we used the 514-nm laser for visualizing YFP and 405-nm laser for visualizing CFP. Images were acquired using ZEN Black software (Carl Zeiss AG). During all FRAP experiments, the cells were placed in a chamber providing a stable temperature 37 °C and 5% CO
2. All the diffusion data was always assessed by FRAP measurements in plasma membrane areas adjacent to the glass support. Bleaching (six iterations per bleach) was accomplished with a circular spot with 2-μm radius using the 488- and 514-nm for YFP and 458- and 488-nm for CFP laser pulse from a 40-mW Argon laser operating at 100% power. The time course of the fluorescence recovery signal after photobleaching was monitored at low laser intensity (2% of maximum power) and 512 × 512 pixels resolution with sampling rate of 2 ms. In each FRAP series, 15 prebleach images were collected and, immediately after photobleaching, 400 successive postbleach images were recorded to monitor the fluorescence redistribution. FRAP curves were normalized to the prebleach value of the respective pulse train. The data obtained from at least 50 cells (screened in three individual runs) during each FRAP experiment were analyzed using easyFRAP, a MATLAB platform-based tool [
36]. Recovery curves were calculated according to the following equation:
where I
bleach is the fluorescence intensity of the bleached spot, I
bckg is the fluorescence intensity of the background, and I
ref is the fluorescence intensity of the control regions in other cells or regions far remote from the target cell. The values of the apparent diffusion coefficients (D) for both receptors were obtained from the following equation:
where ω is the diameter of the selected bleached spot and t½ is the half-life of the fluorescence recovery [
37].
The potential effects of agonists on the lateral mobility of MOR–YFP or TRPV1–CFP were investigated using endomorphin-2 and capsaicin. HMY1/TRPV1 cells were pretreated for 5 min with individual agonists and the agonists were left in the medium during the whole experiment. Antagonists of MOR and TRPV1 receptors (naloxone and capsazepine, respectively) were applied to cells 10 min before adding agonists and they were used at final concentrations 10 times higher than those of agonists.
4.4. Time Resolved Fluorescence Assay for cAMP
The direct quantitative determination of cAMP in cells affected by receptor ligands was performed using cAMP–Gi kit (Cisbio Bioassays, Parc Marcel Boiteux, Codolet, France) based on HTRF® technology (Homogenous Time Resolved Fluorescence). Cells were seeded in a 384-well plate (8000 cells per well), transfected in 24 h, and the assay was performed after 24 h of incubating the cells with complex of Lipofectamine 3000-TRPV1 DNA plasmid. Cells were incubated for 20 min at 37 °C and 5% CO2 in the presence of ligands diluted to a final concentration 1 µM in stimulation buffer. Forskolin at a concentration 2 µM was added before incubating the cells for 45 min at 37 °C. Then cryptate-labeled cAMP and monoclonal anti-cAMP-d2 antibody diluted in lysis and detection buffer were added. The plate was incubated for 1 h at room temperature before transferring the final lysate to a white 96-well plate and reading absorbance at 620 and 665 nm in Tecan Safire 2 reader.
4.5. Endpoint Calcium Assay
Cells were seeded in a 384-well plate (8000 cells per well) and after 24 h transfected with the TRPV1–CFP plasmid. After the next 24 h, cells were used for the assay. We used Cell MeterTM No Wash and Probenecid-Free Endpoint Calcium Assay kit (AAT Bioquest). Fluo-8ETM AM dye solution was prepared according to the manufacturer’s instructions and mixed with assay buffer. Then, 25 µL per well of the mixture was added to the plate and incubated for 45 min at 37 °C. After the incubation, agonist resuspended in HHBS buffer was added and the calcium flux assay was run immediately. The fluorescence intensity at Ex/Em = 490/525 nm was monitored on a microplate reader (bottom read mode).
4.6. Isolation of a Plasma Membrane Fraction
Transfected and non-transfected (control) cells were incubated in the presence or absence of ligands at 37 °C for 5 min. The cells were immediately chilled in an ice bath and harvested in TMES buffer (20 mM Tris, 3 mM MgCl2, 1 mM EDTA, 250 mM sucrose; pH 7.4). The plasma membrane fraction was isolated using Percoll
® self-forming gradient as previously described with some modifications [
38]. Cell homogenates (3 mL) were loaded on the top of 23 mL of 18% Percoll solution in TMES buffer and centrifuged in a Beckman Ti50 rotor at 60,000× g for 15 min. The resulting upper layer enriched in plasma membranes was collected, diluted in TME buffer (20 mM Tris, 3 mM MgCl
2, 1 mM EDTA; pH 7.4), and centrifuged at 150,000×
g for 1 h. The pellet was resuspended in TME buffer, frozen in liquid nitrogen, and stored at −80 °C.
4.7. SDS-PAGE and Immunoblotting
Samples were solubilized in Laemmli buffer and loaded on standard 10% acrylamide gels for SDS-PAGE. The resolved proteins were transferred to nitrocellulose membrane, blocked with 5% non-fat dry milk in TBS buffer (10 mM Tris, 150 mM NaCl; pH 8.0) for 30 min, and then incubated in the presence of relevant primary antibodies with gentle agitation overnight at 4 °C. After three 10-min washes in TBS containing 0.3% Tween 20 (TBS-Tween), the secondary antibodies labeled with horseradish peroxidase were applied for 1 h at room temperature. After another three 10-min washes in TBS-Tween, the blots were visualized by enhanced chemiluminescence technique according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL, USA). The immunoblots were scanned and quantitatively analyzed by ImageJ software.
4.8. Immunofluorescence
Cells were plated in 35 mm glass bottom dishes and were cultured in phenol red-free DMEM supplemented with 10% FBS and 1% AAS for 24 h prior to transfection with TRPV1-CFP construct as described above. The following day, cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were washed three times in PBS and permeabilized with 0.2% Triton in PBS for 5 min. Samples were blocked with 10% donkey serum and 1% BSA in PBS for 1 h and then washed three times and incubated with primary antibody against β-arrestin 2 for 1 h. Cells were washed three times with PBS and incubated for 1 h with secondary donkey anti-rabbit antibody (Alexa Fluor 594, Life Technologies). After incubation, cells were washed three times with PBS and visualized using Zeiss Elyra SP.1 nanoscope enabling live-cell imaging and TIRF illumination.
4.9. Materials
Lipofectamine 3000 was purchased from Invitrogen (Carlsbad, CA, USA) and fetal bovine serum (FBS) was from Thermo Fisher Scientific (Waltham, MA, USA). HTRF cAMP Gi Assay kit was purchased from Cisbio Bioassays (Barford, MA, USA) and Cell MeterTM No Wash and Probenecid-Free Endpoint Calcium Assay kit was from AAT Bioquest (Sunnyvale, CA, USA). β-Arrestin 2 antibody was from ThermoFisher Scientific, ERK1/2, p-ERK1/2, JNK, and pJNK antibodies were from Cell Signaling Technology (Beverly, MA) and p38 and p-p38 antibodies were from Santa Cruz Biotechnology. All the ligands and other chemicals were purchased from Sigma-Aldrich (St. Louis, MI, USA) and they were of the highest purity available.
4.10. Statistics
Data are expressed as mean values ± standard error of the mean (S.E.M.) of at least three independent experiments. All statistical analyses were conducted using GraphPad Prism, Version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). The differences between the means of relevant groups were statistically evaluated by one-way ANOVA followed by the Bonferroni post hoc test. Significance level was set at p ≤ 0.05.