A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters
Abstract
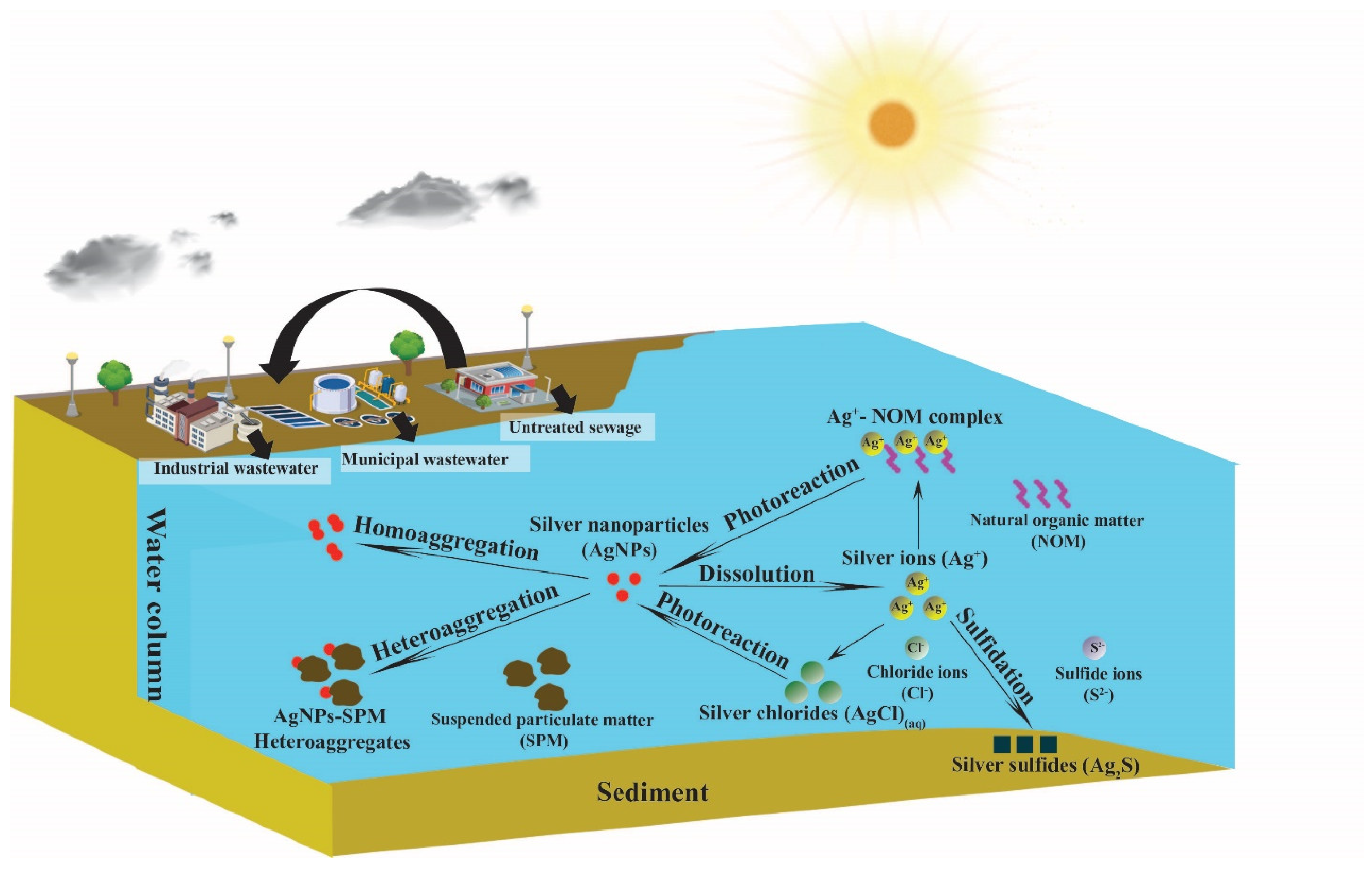
1. Introduction
2. Exposure Models for the Prediction of Engineered Nanomaterials’ (ENMs’) Concentrations in Surface Waters
2.1. Material Flow Analysis Models (MFAMs)
| Model Classification | Model Name | Model Features | Compartments Considered | Fate Processes | References |
|---|---|---|---|---|---|
| Material flow analysis models | MFAMs | Steady state, less information required, simplified structure | Air, water, soil | - | Mueller and Nowack (2008) [47] |
| P-MFAMs | Accounting for the uncertainty of model input parameters using probabilistic distribution | Air, water, soil, sediment | - | Gotschalk et al. (2009) [61], Gotschalk et al. (2010) [48], Gotschalk et al. (2011) [62], Sun et al. (2015) [49], Liu et al. (2015) [56] | |
| DP-MFAMs | Accounting for time-dependent changes in the system behavior | Air, water, soil, sediment | - | Bornhöft et al. (2016) [50], Sun et al. (2016) [63], Wang and Nowack (2018) [51] | |
| Multimedia compartmental models | MendNano | Intermedia transport processes included partitioning ratios | Air, water, soil, sediment, biota | Homoaggregation, heteroaggregation, dissolution | Liu and Cohen (2014) [54] |
| SimpleBox4 Nano (SB4N) | Steady state environmental ENM fate processes are modeled mechanistically using first-order rate constants | Air, water, soil, sediment | Heteroaggregation, dissolution | Meesters et al. (2014) [36] | |
| RedNano | A model system which combines a P-MFAMs based release model (LearNano) and a multimedia fate model (MendNano) | Air, water, soil, sediment, biota | Homoaggregation, heteroaggregation, dissolution | Liu et al. (2015) [56] | |
| SimpleBox4 Nano (SB4N) | Steady state environmental ENMs’ fate, probabilistic distribution | Air, water, soil, sediment | Heteroaggregation, dissolution | Meesters et al. (2016) [55] | |
| nanoFate | Dynamic environmental ENMs’ fate | Air, water, soil, sediment | Heteroaggregation, dissolution | Garner et al. (2017) [57] | |
| Spatial river/watershed models | Rhine river box model | Steady state box model | Water, sediment | Heteroaggregation | Praetorius et al. (2012) [35] |
| Rhone river box model | Cluster analysis, steady state box model | Water, sediment | Heteroaggregation | Sani-Kast et al. (2015) [64] | |
| Diagenesis model | 1-D sediment diagenesis model | Freshwater sediment | Dissolution, sulfidation | Dale et al. (2013) [65] | |
| GWAVA | Gridded probability distribution | Water | Dissolution | Dumont et al. (2015) [66] | |
| Nano DUFLOW | 1-D unsteady flow in open-channel systems | Water, sediment | Homoaggregation, heteroaggregation, dissolution | Quik et al. (2015) [58], Klein et al. (2016) [67] | |
| SOBEK river-DELWAQ | A model system integrates open channel hydraulics and water quality models | Water | Homoaggregation, heteroaggregation, dissolution | Markus et al. (2016) [68] | |
| WASP7–HSPF | Dynamic, mass-balance, spatially resolved differential fate and transport modeling framework | Water, sediment | Dissolution, sulfidation | Dale et al. (2015) [59] | |
| WASP8 | A detailed surface water quality model with ENM fate and transport processes | Water, sediment | Dissolution, sulfidation, heteroaggregation, photoreaction | Bouchard et al. (2017) [41], Han et al. (2019) [42] | |
| SWMM-EFDC | Suitable for urban stormwater and sewage systems; coupling both surface hydrology and hydrodynamic models | Water, sediment | Heteroaggregation, dissolution | Saharia et al. (2019) [69] |
2.2. Environmental Fate Models (EFMs)
2.2.1. Multimedia Compartmental Models (MCMs)
2.2.2. Spatial River/Watershed Models (SRWMs)
3. Engineered Nanomaterial (ENM) Fate Processes in Surface Waters
3.1. Aggregation
3.2. Dissolution
3.3. Sulfidation
3.4. Photoreaction
4. Path Forward
5. Summary
Funding
Conflicts of Interest
References
- European Commission. RECOMMENDATIONS: COMMISSION RECOMMENDATION of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU). Off. J. Eur. Union 2011, 38–40. [Google Scholar]
- Dolez, P.I. Nanomaterials Definitions, Classifications, and Applications. Nanoengineering 2015, 3–40. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- AZoNano.com. Nanotechnology and Consumer Products—Opportunities for Nanotechnology in Consumer Products. Available online: https://www.azonano.com/article.aspx?ArticleID=2364 (accessed on 13 June 2020).
- Izzyfortiz. Nano-textiles: The Fabric of the Future. Sustainable Nano. Available online: http://sustainable-nano.com/2018/11/28/nano-textiles/ (accessed on 13 June 2020).
- Says, S.W.; AZoNano.com. Sport and Nanotechnology: Are the Big Sports Looking to Go Small? Available online: https://www.azonano.com/article.aspx?ArticleID=4859 (accessed on 13 June 2020).
- Alvarez, P.J.J.; Chan, C.K.; Elimelech, M.; Halas, N.J.; Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 2018, 13, 634–641. [Google Scholar] [CrossRef]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Mauter, M.S.; Zucker, I.; Perreault, F.; Werber, J.R.; Kim, J.-H.; Elimelech, M. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 2018, 1, 166–175. [Google Scholar] [CrossRef]
- Luo, C.; Ren, X.; Dai, Z.; Zhang, Y.; Qi, X.; Pan, C. Present Perspectives of Advanced Characterization Techniques in TiO2-Based Photocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 23265–23286. [Google Scholar] [CrossRef]
- Azzouz, I.; Habba, Y.G.; Capochichi-Gnambodoe, M.; Marty, F.; Vial, J.; Leprince-Wang, Y.; Bourouina, T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoeng. 2018, 4, 17093. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, R.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and Enhanced Photocatalytic Applications of Sodium Niobate Nanoparticles Developed by Citrate Precursor Route. Sci. Rep. 2019, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, H.; Chen, Y. Emerging Nano Drug Delivery Systems Targeting Cancer-Associated Fibroblasts for Improved Antitumor Effect and Tumor Drug Penetration. Mol. Pharm. 2020, 17, 1028–1048. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G. Antimicrobial silver nanoparticles—Regulatory situation in the European Union. Mater. Today Proc. 2017, 4, S200–S207. [Google Scholar] [CrossRef]
- Potter, P.M.; Navratilova, J.; Rogers, K.R.; Al-Abed, S.R. Transformation of silver nanoparticle consumer products during simulated usage and disposal. Environ. Sci. Nano 2019, 6, 592–598. [Google Scholar] [CrossRef]
- AZoNano.com. Metal Oxide Nanoparticles—Are they Safe? Available online: https://www.azonano.com/article.aspx?ArticleID=5444 (accessed on 13 June 2020).
- Ahsan, M.d.A.; Jabbari, V.; Imam, M.A.; Castro, E.; Kim, H.; Curry, M.L.; Valles-Rosales, D.J.; Noveron, J.C. Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci.Total Environ. 2020, 698, 134214. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Ultrafast catalytic reduction of environmental pollutants in water via MOF-derived magnetic Ni and Cu nanoparticles encapsulated in porous carbon. Appl. Surf. Sci. 2019, 497, 143608. [Google Scholar] [CrossRef]
- Camboni, M.; Hanlon, J.; Pérez García, R.; Floyd, P.; European Chemicals Agency. A State of Play Study of the Market for so Called “Next Generation” Nanomaterials. 2019. Available online: https://op.europa.eu/publication/manifestation_identifier/PUB_ED0219746ENN (accessed on 11 March 2020).
- Calipinar, H.; Ulas, D. Development of Nanotechnology in the World and Nanotechnology Standards in Turkey. Procedia Comput. Sci. 2019, 158, 1011–1018. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef]
- Romeo, D.; Salieri, B.; Hischier, R.; Nowack, B.; Wick, P. An integrated pathway based on in vitro data for the human hazard assessment of nanomaterials. Environ. Int. 2020, 137, 105505. [Google Scholar] [CrossRef] [PubMed]
- Sayre, P.G.; Steinhäuser, K.G.; van Teunenbroek, T. Methods and data for regulatory risk assessment of nanomaterials: Questions for an expert consultation. NanoImpact 2017, 8, 20–27. [Google Scholar] [CrossRef]
- Nowack, B. Evaluation of environmental exposure models for engineered nanomaterials in a regulatory context. NanoImpact 2017, 8, 38–47. [Google Scholar] [CrossRef]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO Nanoparticle Dissolution and Toxicity to Wheat (Triticum aestivum) in Rhizosphere Soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Westerhoff, P.; Posner, J.D. Biological accumulation of engineered nanomaterials: A review of current knowledge. Environ. Sci. Process. Impacts. 2013, 15, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review: Nanomaterials in the environment. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling Nanomaterial Environmental Fate in Aquatic Systems. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef]
- Williams, R.J.; Harrison, S.; Keller, V.; Kuenen, J.; Lofts, S.; Praetorius, A.; Svendsen, C.; Vermeulen, L.C.; van Wijnen, J. Models for assessing engineered nanomaterial fate and behaviour in the aquatic environment. Curr. Opin. Environ. Sustain. 2019, 36, 105–115. [Google Scholar] [CrossRef]
- Praetorius, A.; Scheringer, M.; Hungerbühler, K. Development of Environmental Fate Models for Engineered Nanoparticles—A Case Study of TiO2 Nanoparticles in the Rhine River. Environ. Sci. Technol. 2012, 46, 6705–6713. [Google Scholar] [CrossRef]
- Meesters, J.A.J.; Koelmans, A.A.; Quik, J.T.K.; Hendriks, A.J.; van de Meent, D. Multimedia Modeling of Engineered Nanoparticles with SimpleBox4nano: Model Definition and Evaluation. Environ. Sci. Technol. 2014, 48, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, A.; Tufenkji, N.; Goss, K.-U.; Scheringer, M.; von der Kammer, F.; Elimelech, M. The road to nowhere: Equilibrium partition coefficients for nanoparticles. Environ. Sci. Nano 2014, 1, 317–323. [Google Scholar] [CrossRef]
- Cornelis, G. Fate descriptors for engineered nanoparticles: The good, the bad, and the ugly. Environ. Sci. Nano 2015, 2, 19–26. [Google Scholar] [CrossRef]
- Baalousha, M.; Cornelis, G.; Kuhlbusch, T.A.J.; Lynch, I.; Nickel, C.; Peijnenburg, W.; van den Brink, N.W. Modeling nanomaterial fate and uptake in the environment: Current knowledge and future trends. Environ. Sci. Nano 2016, 3, 323–345. [Google Scholar] [CrossRef]
- Bouchard, D.; Knightes, C.; Chang, X.; Avant, B. Simulating Multiwalled Carbon Nanotube Transport in Surface Water Systems Using the Water Quality Analysis Simulation Program (WASP). Environ. Sci. Technol. 2017, 51, 11174–11184. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Knightes, C.D.; Bouchard, D.; Zepp, R.; Avant, B.; Hsieh, H.-S.; Chang, X.; Acrey, B.; Henderson, W.M.; Spear, J. Simulating graphene oxide nanomaterial phototransformation and transport in surface water. Environ. Sci. Nano 2019, 6, 180–194. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Krol, M.; Khan, U.T.; Asad, M.A. Factors affecting nano scale zero valent iron (nZVI) travel distance in heterogeneous groundwater aquifers: A statistical modeling approach. In Proceedings of the AGU Fall Meeting, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Asad, M.A.; Krol, M.; Briggs, S. Nano zero valent iron (nZVI) remediation: A COMSOL modelling approach. In Proceedings of the Canadian Geotechnical Society (CGS) Conference (GeoEdmonton 2018), Edmonton, AB, Canada, 23–26 September 2018. [Google Scholar]
- Yu, Z.; Hu, L.; Lo, I.M.C. Transport of the arsenic (As)-loaded nano zero-valent iron in groundwater-saturated sand columns: Roles of surface modification and As loading. Chemosphere 2019, 216, 428–436. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Sun, T.Y.; Conroy, G.; Donner, E.; Hungerbühler, K.; Lombi, E.; Nowack, B. Probabilistic modelling of engineered nanomaterial emissions to the environment: A spatio-temporal approach. Environ. Sci. Nano 2015, 2, 340–351. [Google Scholar] [CrossRef]
- Bornhöft, N.A.; Sun, T.Y.; Hilty, L.M.; Nowack, B. A dynamic probabilistic material flow modeling method. Environ. Model. Softw. 2016, 76, 69–80. [Google Scholar] [CrossRef]
- Wang, Y.; Nowack, B. Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions. Environ. Pollut. 2018, 235, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Scheringer, M.; Praetorius, A.; Goldberg, E.S. Environmental Fate and Exposure Modeling of Nanomaterials. Front. Nanosci. 2014, 7, 89–125. [Google Scholar] [CrossRef]
- Di Guardo, A.; Gouin, T.; MacLeod, M.; Scheringer, M. Environmental fate and exposure models: Advances and challenges in 21st century chemical risk assessment. Environ. Sci. Process. Impacts 2018, 20, 58–71. [Google Scholar] [CrossRef]
- Liu, H.H.; Cohen, Y. Multimedia Environmental Distribution of Engineered Nanomaterials. Environ. Sci. Technol. 2014, 48, 3281–3292. [Google Scholar] [CrossRef]
- Meesters, J.A.J.; Quik, J.T.K.; Koelmans, A.A.; Hendriks, A.J.; van de Meent, D. Multimedia environmental fate and speciation of engineered nanoparticles: A probabilistic modeling approach. Environ. Sci. Nano 2016, 3, 715–727. [Google Scholar] [CrossRef]
- Liu, H.H.; Bilal, M.; Lazareva, A.; Keller, A.; Cohen, Y. Simulation tool for assessing the release and environmental distribution of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 938–951. [Google Scholar] [CrossRef]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the nanoFate Model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef]
- Quik, J.T.K.; de Klein, J.J.M.; Koelmans, A.A. Spatially explicit fate modelling of nanomaterials in natural waters. Water Res. 2015, 80, 200–208. [Google Scholar] [CrossRef]
- Dale, A.L.; Lowry, G.V.; Casman, E.A. Stream Dynamics and Chemical Transformations Control the Environmental Fate of Silver and Zinc Oxide Nanoparticles in a Watershed-Scale Model. Environ. Sci. Technol. 2015, 49, 7285–7293. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.16: Environmental Exposure Assessment, Version 3.0—February 2016; ISBN 978-92-9247-775-2. European Chemicals Agency (ECHA): Helsinki, Finland, 2016. [Google Scholar]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Ort, C.; Scholz, R.W.; Nowack, B. Engineered nanomaterials in rivers—Exposure scenarios for Switzerland at high spatial and temporal resolution. Environ. Pollut. 2011, 159, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef]
- Sani-Kast, N.; Scheringer, M.; Slomberg, D.; Labille, J.; Praetorius, A.; Ollivier, P.; Hungerbühler, K. Addressing the complexity of water chemistry in environmental fate modeling for engineered nanoparticles. Sci. Total. Environ. 2015, 535, 150–159. [Google Scholar] [CrossRef]
- Dale, A.L.; Lowry, G.V.; Casman, E.A. Modeling Nanosilver Transformations in Freshwater Sediments. Environ. Sci. Technol. 2013, 47, 12920–12928. [Google Scholar] [CrossRef]
- Dumont, E.; Johnson, A.C.; Keller, V.D.J.; Williams, R.J. Nano silver and nano zinc-oxide in surface waters —Exposure estimation for Europe at high spatial and temporal resolution. Environ. Pollut. 2015, 196, 341–349. [Google Scholar] [CrossRef]
- Klein, J.J.M.; de Quik, J.T.K.; Bäuerlein, P.S.; Koelmans, A.A. Towards validation of the NanoDUFLOW nanoparticle fate model for the river Dommel, The Netherlands. Environ. Sci. Nano 2016, 3, 434–441. [Google Scholar] [CrossRef]
- Markus, A.A.; Parsons, J.R.; Roex, E.W.M.; de Voogt, P.; Laane, R.W.P.M. Modelling the transport of engineered metallic nanoparticles in the river Rhine. Water Res. 2016, 91, 214–224. [Google Scholar] [CrossRef]
- Saharia, A.M.; Zhu, Z.; Aich, N.; Baalousha, M.; Atkinson, J.F. Modeling the transport of titanium dioxide nanomaterials from combined sewer overflows in an urban river. Sci. Total Environ. 2019, 696, 133904. [Google Scholar] [CrossRef]
- Shoemaker, L.; Dai, T.; Koenig, J. TMDL Model Evaluation and Research Needs; EPA/600/R-05/149; National Risk Management Research Laboratory, Office Of Research And Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2005.
- Di Toro, D.M.; Mahony, J.D.; Hansen, D.J.; Berry, W.J. A model of the oxidation of iron and cadmium sulfide in sediments. Environ. Toxicol. Chem. 1996, 15, 2168–2186. [Google Scholar] [CrossRef]
- Hou, W.-C.; Stuart, B.; Howes, R.; Zepp, R.G. Sunlight-Driven Reduction of Silver Ions by Natural Organic Matter: Formation and Transformation of Silver Nanoparticles. Environ. Sci. Technol. 2013, 47, 7713–7721. [Google Scholar] [CrossRef]
- Singh, A.; Hou, W.-C.; Lin, T.-F.; Zepp, R.G. Roles of Silver–Chloride Complexations in Sunlight-Driven Formation of Silver Nanoparticles. Environ. Sci. Technol. 2019, 53, 11162–11169. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Gregory, J.; Jia, X.; Williams, R.A. Particle Deposition and Aggregation: Measurement, Modelling and Simulation; Butterworth-Heinemann: Oxford, UK, 1998. [Google Scholar]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef]
- Quik, J.T.K.; Velzeboer, I.; Wouterse, M.; Koelmans, A.A.; van de Meent, D. Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res. 2014, 48, 269–279. [Google Scholar] [CrossRef]
- Velzeboer, I.; Quik, J.T.K.; van de Meent, D.; Koelmans, A.A. Rapid settling of nanoparticles due to heteroaggregation with suspended sediment: Nanoparticle settling due to heteroaggregation with sediment. Environ. Toxicol. Chem. 2014, 33, 1766–1773. [Google Scholar] [CrossRef]
- Bouchard, D.; Chang, X.; Chowdhury, I. Heteroaggregation of multiwalled carbon nanotubes with sediments. Environ. Nanotechnol. Monit. Manag. 2015, 4, 42–50. [Google Scholar] [CrossRef][Green Version]
- Clavier, A.; Praetorius, A.; Stoll, S. Determination of nanoparticle heteroaggregation attachment efficiencies and rates in presence of natural organic matter monomers. Monte Carlo modelling. Sci. Total Environ. 2019, 650, 530–540. [Google Scholar] [CrossRef]
- Praetorius, A.; Badetti, E.; Brunelli, A.; Clavier, A.; Gallego-Urrea, J.A.; Gondikas, A.; Hassellöv, M.; Hofmann, T.; Mackevica, A.; Marcomini, A.; et al. Strategies for determining heteroaggregation attachment efficiencies of engineered nanoparticles in aquatic environments. Environ. Sci. Nano 2020, 7, 351–367. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, Y.; Wang, Z.; Ren, W.; Wei, Y.; Cao, X.; Xing, B. Toxicity of GO to Freshwater Algae in the Presence of Al2 O3 Particles with Different Morphologies: Importance of Heteroaggregation. Environ. Sci. Technol. 2018, 52, 13448–13456. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dang, F.; Liu, C.; Wang, D.-J.; Cui, P.-X.; Yan, H.-J.; Zhou, D.-M. Heteroaggregation and dissolution of silver nanoparticles by iron oxide colloids under environmentally relevant conditions. Environ. Sci. Nano 2019, 6, 195–206. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Peretyazhko, T.S.; Zhang, Q.; Colvin, V.L. Size-Controlled Dissolution of Silver Nanoparticles at Neutral and Acidic pH Conditions: Kinetics and Size Changes. Environ. Sci. Technol. 2014, 48, 11954–11961. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Blomberg, E.; Odnevall Wallinder, I. In the Search for Nanospecific Effects of Dissolution of Metallic Nanoparticles at Freshwater-Like Conditions: A Critical Review. Environ. Sci. Technol. 2019, 53, 4030–4044. [Google Scholar] [CrossRef]
- Petersen, E.J.; Huang, Q.; Weber, W.J. Relevance of octanol-water distribution measurements to the potential ecological uptake of multi-walled carbon nanotubes. Environ. Toxicol. Chem. 2010, 29, 1106–1112. [Google Scholar] [CrossRef]
- Jafvert, C.T.; Kulkarni, P.P. Buckminsterfullerene’s (C60) Octanol−Water Partition Coefficient (Kow) and Aqueous Solubility. Environ. Sci. Technol. 2008, 42, 5945–5950. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of Metallic Silver Nanoparticles in a Pilot Wastewater Treatment Plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Ort, C.; Sinnet, B.; Thalmann, B.; Krismer, J.; Hagendorfer, H.; Elumelu, M.; Mueller, E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013, 47, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Judy, J.D.; Unrine, J.M.; Durenkamp, M.; Martin, B.; Jefferson, B.; Lowry, G.V. Fate of Zinc Oxide and Silver Nanoparticles in a Pilot Wastewater Treatment Plant and in Processed Biosolids. Environ. Sci. Technol. 2014, 48, 104–112. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Allen, A.J.; Johnston-Peck, A.C.; Pettibone, J.M. Comparing sulfidation kinetics of silver nanoparticles in simulated media using direct and indirect measurement methods. Nanoscale 2018, 10, 22270–22279. [Google Scholar] [CrossRef]
- Liu, J.; Pennell, K.G.; Hurt, R.H. Kinetics and Mechanisms of Nanosilver Oxysulfidation. Environ. Sci. Technol. 2011, 45, 7345–7353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, W.; Li, Q.; Wang, Z.; Liu, S. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ. Sci. Nano 2018, 5, 2482–2499. [Google Scholar] [CrossRef]
- He, D.; Garg, S.; Wang, Z.; Li, L.; Rong, H.; Ma, X.; Li, G.; An, T.; Waite, T.D. Silver sulfide nanoparticles in aqueous environments: Formation, transformation and toxicity. Environ. Sci. Nano 2019, 6, 1674–1687. [Google Scholar] [CrossRef]
- Hou, W.-C.; Jafvert, C.T. Photochemical Transformation of Aqueous C60 Clusters in Sunlight. Environ. Sci. Technol. 2009, 43, 362–367. [Google Scholar] [CrossRef]
- Kong, L.; Tedrow, O.; Chan, Y.F.; Zepp, R.G. Light-Initiated Transformations of Fullerenol in Aqueous Media. Environ. Sci. Technol. 2009, 43, 9155–9160. [Google Scholar] [CrossRef]
- Isaacson, C.W.; Bouchard, D. Asymmetric flow field flow fractionation of aqueous C60 nanoparticles with size determination by dynamic light scattering and quantification by liquid chromatography atmospheric pressure photo-ionization mass spectrometry. J. Chromatogr. A 2010, 1217, 1506–1512. [Google Scholar] [CrossRef]
- Hou, W.-C.; Huang, S.-H. Photochemical reactivity of aqueous fullerene clusters: C60 versus C70. J. Hazard. Mater. 2017, 322, 310–317. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Photochemical Transformation of Carboxylated Multiwalled Carbon Nanotubes: Role of Reactive Oxygen Species. Environ. Sci. Technol. 2013, 47, 14080–14088. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; BeigzadehMilani, S.; Jafvert, C.T.; Zepp, R.G. Photoreactivity of Unfunctionalized Single-Wall Carbon Nanotubes Involving Hydroxyl Radical: Chiral Dependency and Surface Coating Effect. Environ. Sci. Technol. 2014, 48, 3875–3882. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zepp, R.G. Probing Photosensitization by Functionalized Carbon Nanotubes. Environ. Sci. Technol. 2015, 49, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Chowdhury, I.; Goodwin, D.G.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical Transformation of Graphene Oxide in Sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef]
- Hou, W.-C.; Henderson, W.M.; Chowdhury, I.; Goodwin, D.G., Jr.; Chang, X.; Martin, S.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. The contribution of indirect photolysis to the degradation of graphene oxide in sunlight. Carbon 2016, 110, 426–437. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, J.; Jiang, G. Sunlight-Induced Reduction of Ionic Ag and Au to Metallic Nanoparticles by Dissolved Organic Matter. ACS Nano 2012, 6, 7910–7919. [Google Scholar] [CrossRef]
- Lee, T.-W.; Chen, C.-C.; Chen, C. Chemical Stability and Transformation of Molybdenum Disulfide Nanosheets in Environmental Media. Environ. Sci. Technol. 2019, 53, 6282–6291. [Google Scholar] [CrossRef]
- Hou, W.-C.; Kong, L.; Wepasnick, K.A.; Zepp, R.G.; Fairbrother, D.H.; Jafvert, C.T. Photochemistry of Aqueous C60 Clusters: Wavelength Dependency and Product Characterization. Environ. Sci. Technol. 2010, 44, 8121–8127. [Google Scholar] [CrossRef]
- Hou, W.-C.; Lee, P.-L.; Chou, Y.-C.; Wang, Y.-S. Antibacterial property of graphene oxide: The role of phototransformation. Environ. Sci. Nano 2017, 4, 647–657. [Google Scholar] [CrossRef]
- Chowdhury, I.; Hou, W.-C.; Goodwin, D.; Henderson, M.; Zepp, R.G.; Bouchard, D. Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment. Water Res. 2015, 78, 37–46. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water 2020, 3, 1. [Google Scholar] [CrossRef]
- Meesters, J.A.J.; Peijnenburg, W.J.G.M.; Hendriks, A.J.; Van de Meent, D.; Quik, J.T.K. A model sensitivity analysis to determine the most important physicochemical properties driving environmental fate and exposure of engineered nanoparticles. Environ. Sci. Nano 2019, 6, 2049–2060. [Google Scholar] [CrossRef]
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Zepp, R.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef]
- Harper, S.; Wohlleben, W.; Doa, M.; Nowack, B.; Clancy, S.; Canady, R.; Maynard, A. Measuring Nanomaterial Release from Carbon Nanotube Composites: Review of the State of the Science. J. Phys. Conf. Ser. 2015, 617, 012026. [Google Scholar] [CrossRef]
- Wohlleben, W.; Neubauer, N. Quantitative rates of release from weathered nanocomposites are determined across 5 orders of magnitude by the matrix, modulated by the embedded nanomaterial. NanoImpact 2016, 1, 39–45. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kingston, C.; Carter, J.; Sahle-Demessie, E.; Vázquez-Campose, S.; Acrey, B.; Chen, C.-Y.; Walton, E.; Egenolf, H.; Müller, P.; et al. NanoRelease: Pilot interlaboratory comparison of a weathering protocol applied to resilient and labile polymers with and without embedded carbon nanotubes. Carbon 2017, 113, 346–360. [Google Scholar] [CrossRef]
- Praetorius, A.; Arvidsson, R.; Molander, S.; Scheringer, M. Facing complexity through informed simplifications: A research agenda for aquatic exposure assessment of nanoparticles. Env. Sci. Process. Impacts 2013, 15, 161–168. [Google Scholar] [CrossRef]
- Geitner, N.K.; Bossa, N.; Wiesner, M.R. Formulation and Validation of a Functional Assay-Driven Model of Nanoparticle Aquatic Transport. Environ. Sci. Technol. 2019, 53, 3104–3109. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Avellan, A.; Lowry, G.V. Effect of Initial Speciation of Copper- and Silver-Based Nanoparticles on Their Long-Term Fate and Phytoavailability in Freshwater Wetland Mesocosms. Environ. Sci. Technol. 2017, 51, 12114–12122. [Google Scholar] [CrossRef]
- Ambrose, B.; Avant, B.; Han, Y.; Knightes, C.; Wool, T. Water Quality Assessment Simulation Program (WASP8): Upgrades to the Advanced Toxicant Module for Simulating Dissolved Chemicals, Nanomaterials, and Solids; EPA/600/R-17/326; Office of Research and Development, National Exposure Research Laboratory, U.S. Environmental Protection Agency: Washington, DC, USA, 2017.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhendra, E.; Chang, C.-H.; Hou, W.-C.; Hsieh, Y.-C. A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. Int. J. Mol. Sci. 2020, 21, 4554. https://doi.org/10.3390/ijms21124554
Suhendra E, Chang C-H, Hou W-C, Hsieh Y-C. A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. International Journal of Molecular Sciences. 2020; 21(12):4554. https://doi.org/10.3390/ijms21124554
Chicago/Turabian StyleSuhendra, Edward, Chih-Hua Chang, Wen-Che Hou, and Yi-Chin Hsieh. 2020. "A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters" International Journal of Molecular Sciences 21, no. 12: 4554. https://doi.org/10.3390/ijms21124554
APA StyleSuhendra, E., Chang, C.-H., Hou, W.-C., & Hsieh, Y.-C. (2020). A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. International Journal of Molecular Sciences, 21(12), 4554. https://doi.org/10.3390/ijms21124554






