Post-Treatment M2BPGi Level and the Rate of Autotaxin Reduction are Predictive of Hepatocellular Carcinoma Development after Antiviral Therapy in Patients with Chronic Hepatitis C
Abstract
1. Introduction
2. Results
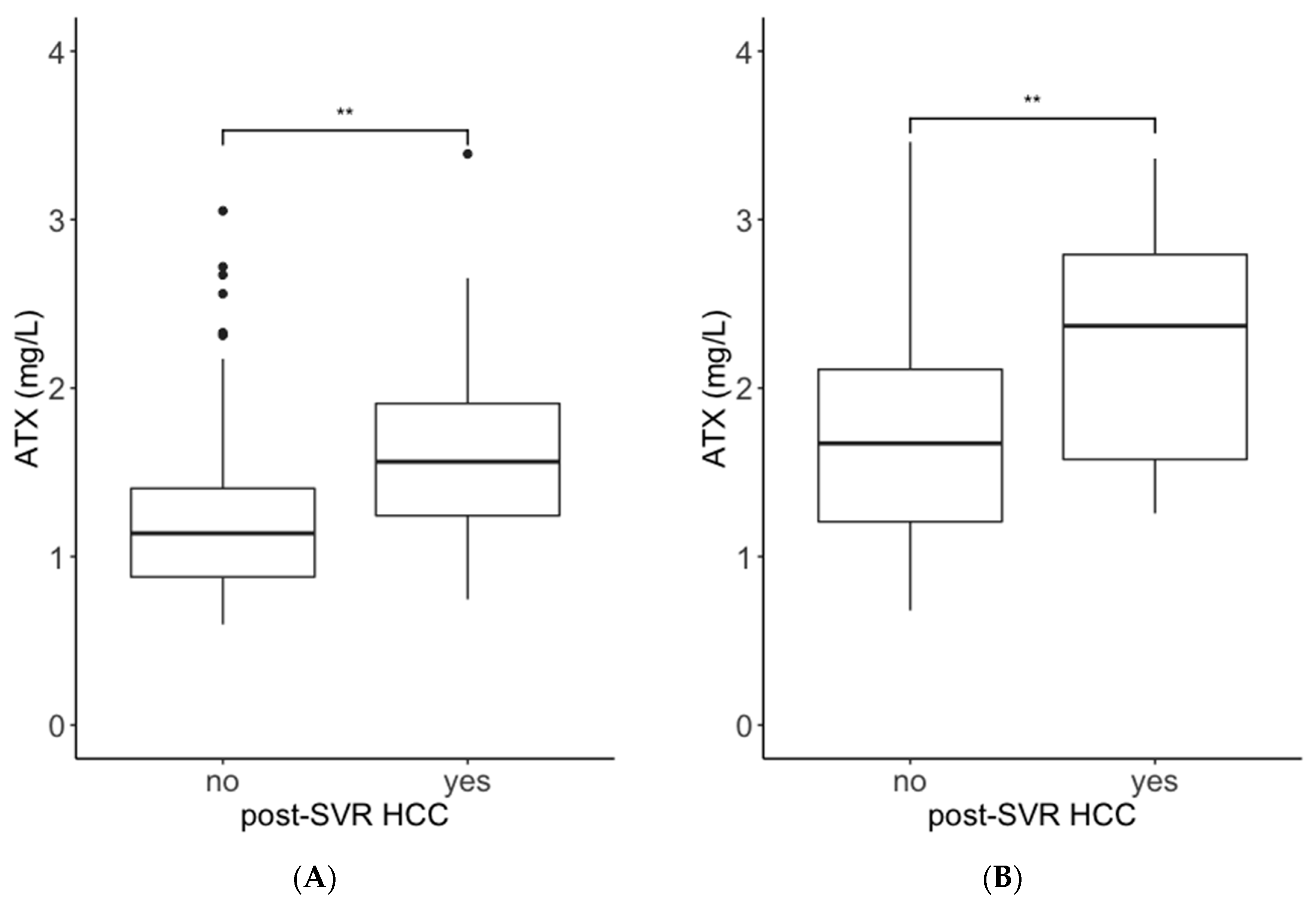
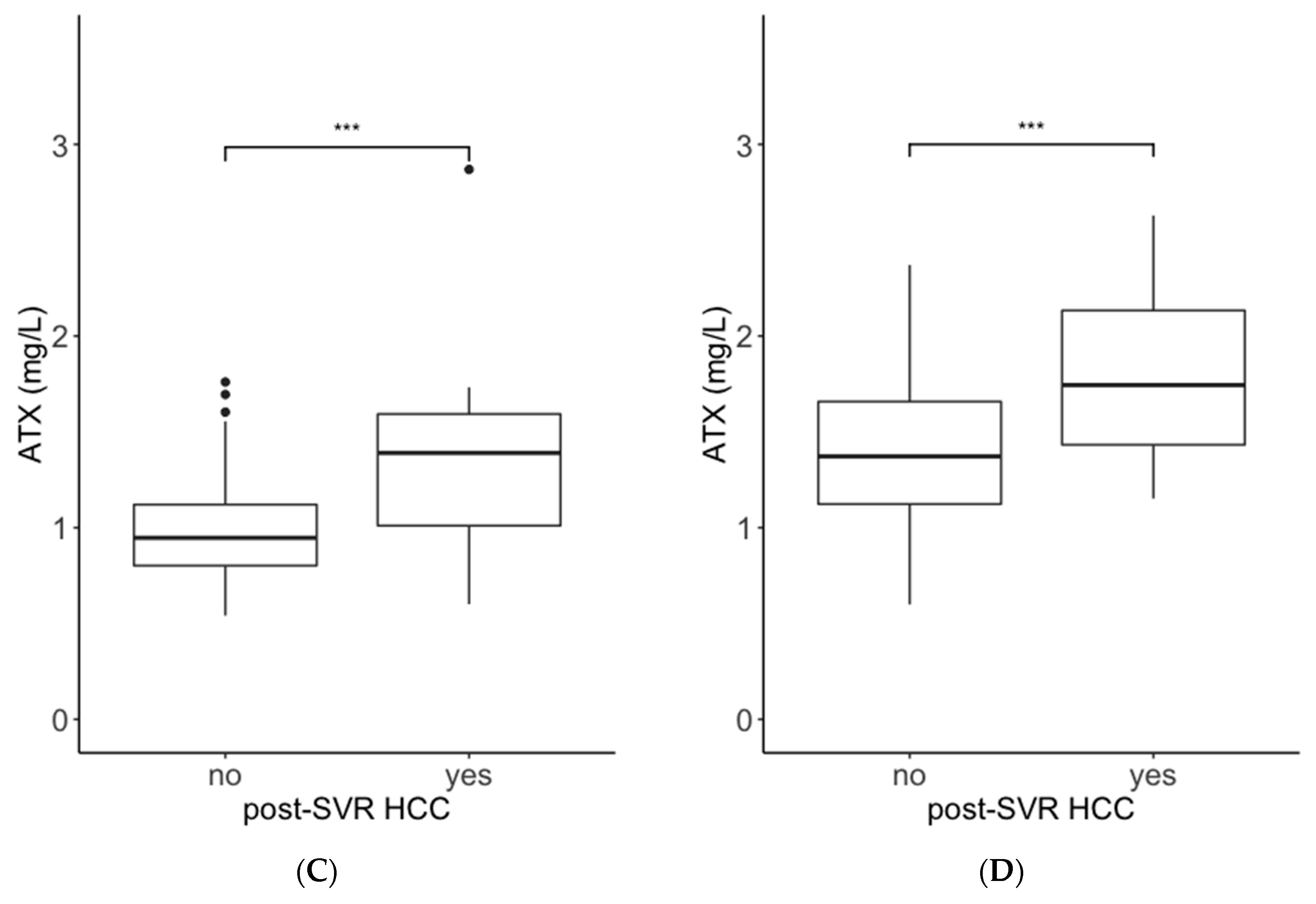
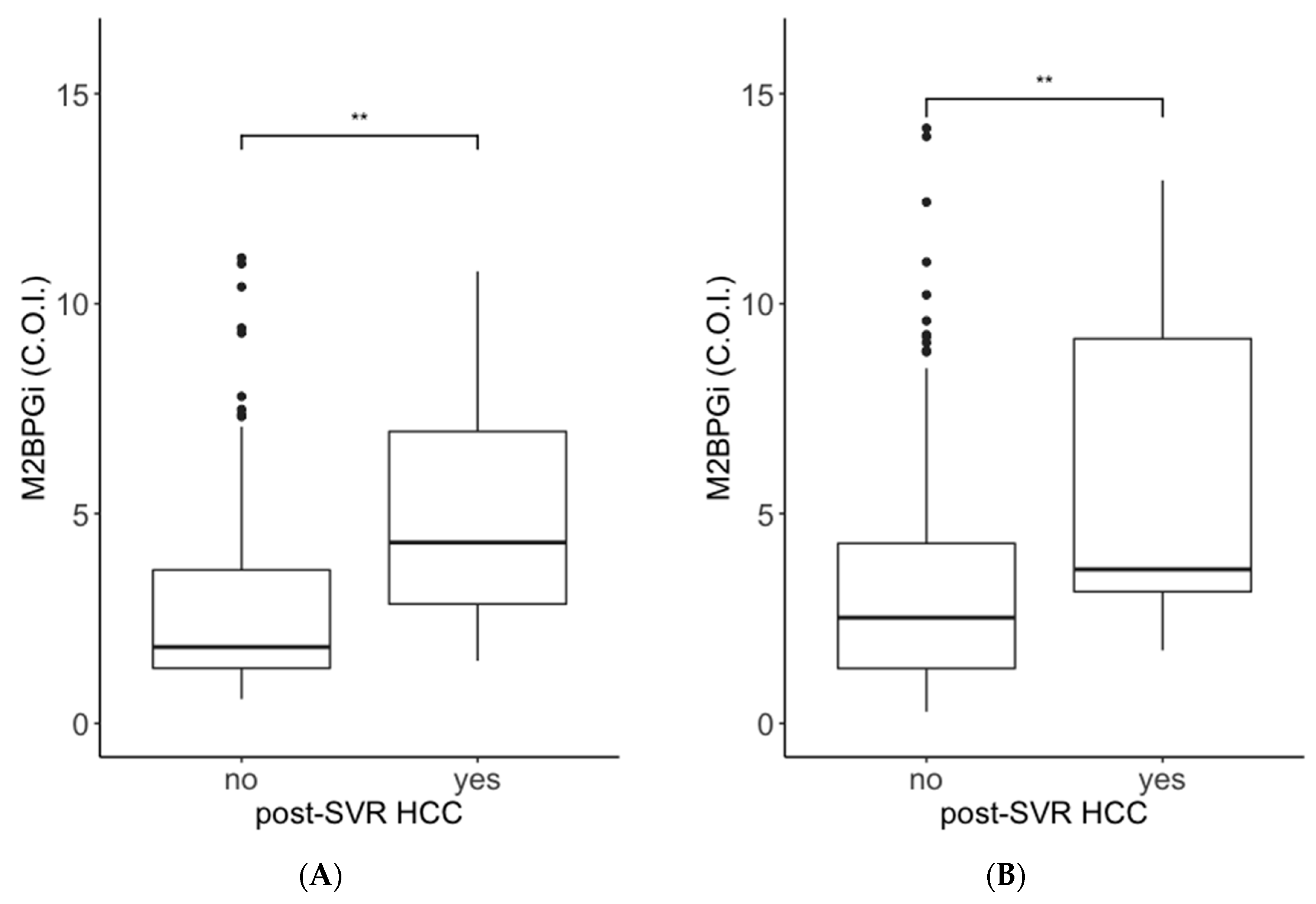
2.1. Comparisons of ATX and M2BPGi Levels by the Presence or Absence of Post-SVR HCC
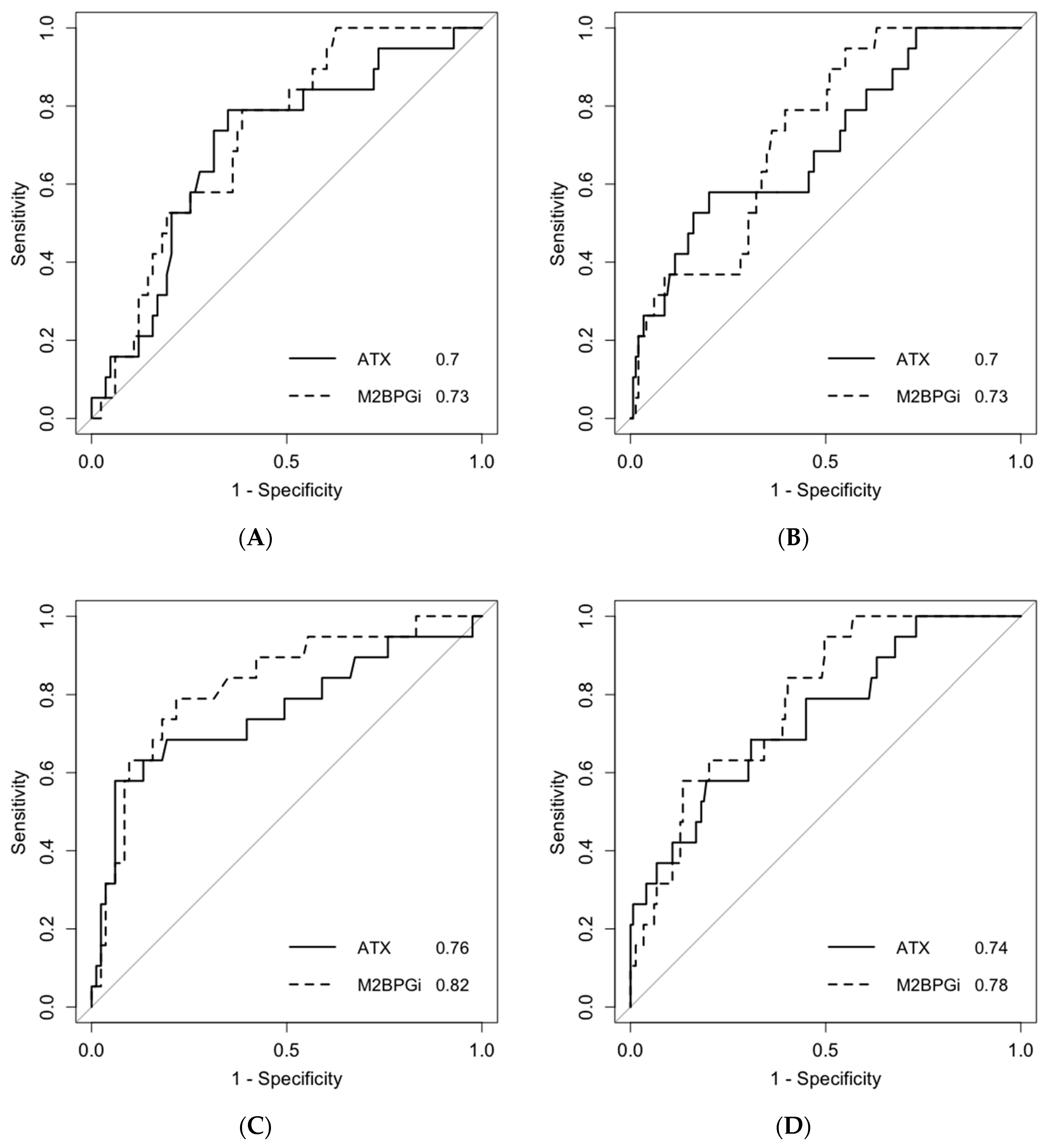
2.2. Predictive Ability for Post-SVR HCC Development within 3 Years after Antiviral Treatment
2.3. Multivariate Analysis of Post-SVR HCC Development
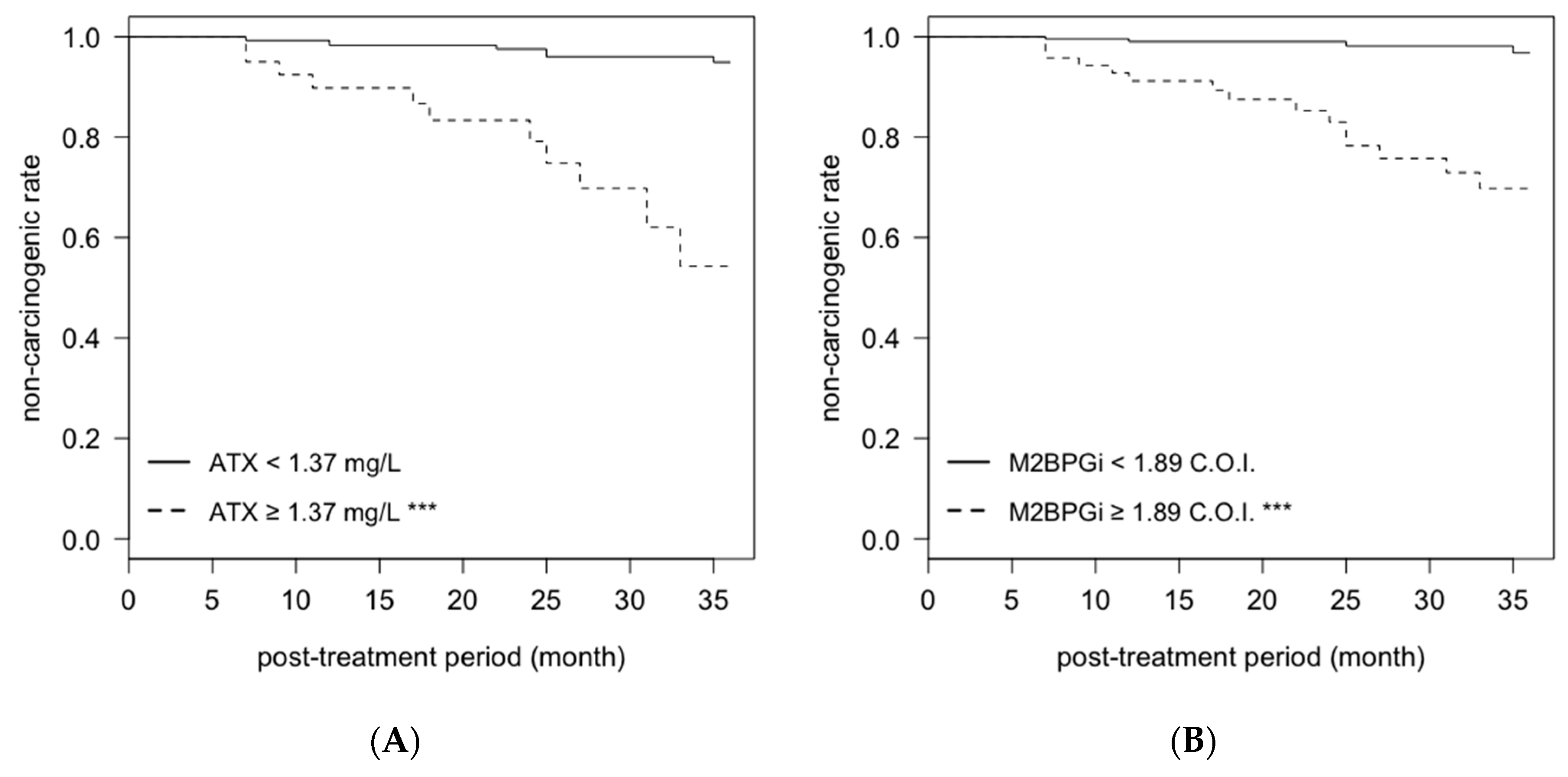
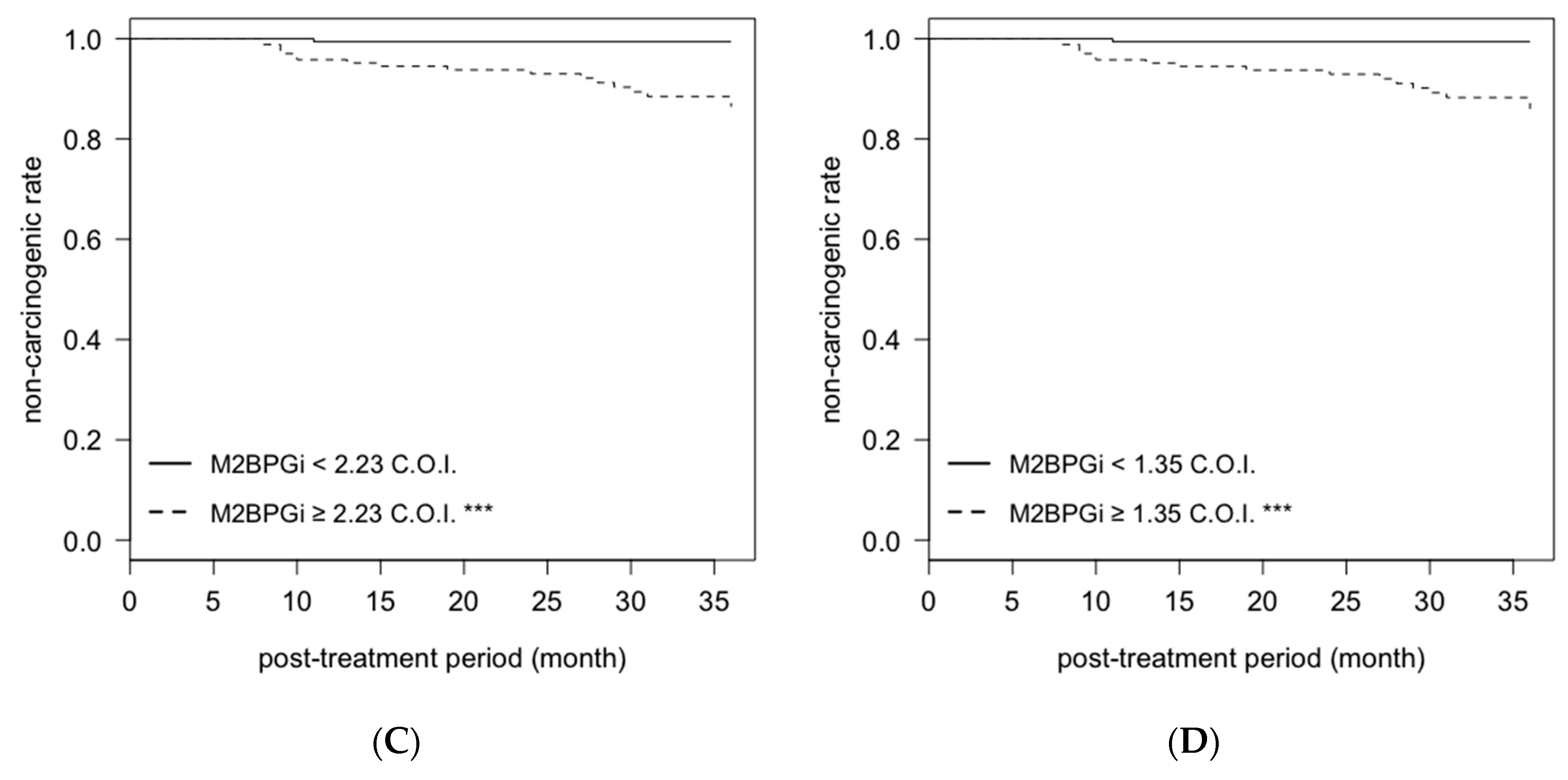
2.4. Cumulative Non-Carcinogenic Rate after Antiviral Treatment
2.5. Association between the Rate of ATX Change and Post-SVR HCC Development
3. Discussion
4. Materials and Methods
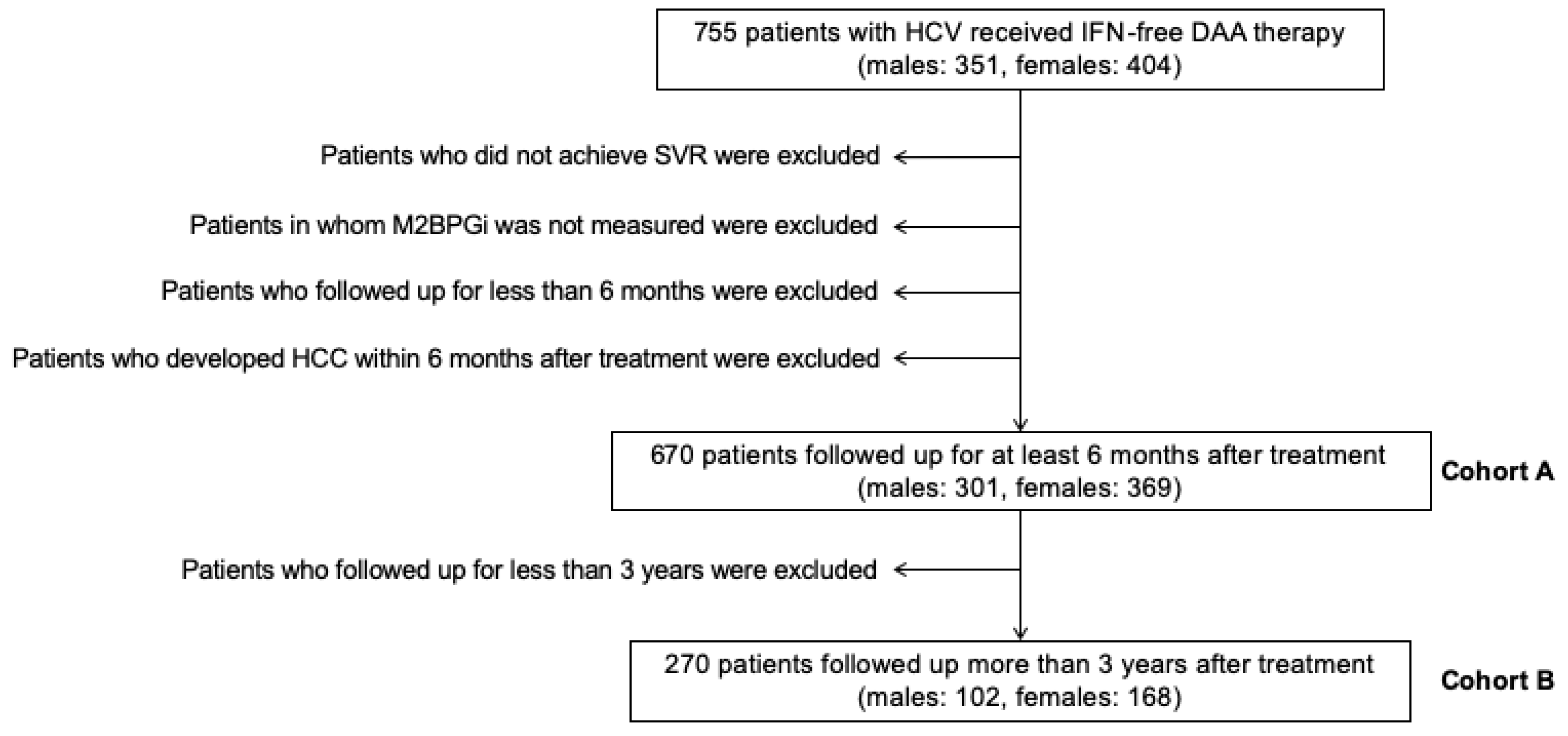
4.1. Subjects
4.2. Measurement of Serum ATX Levels
4.3. Laboratory Data
4.4. Surveillance after Antiviral Treatment and Diagnosis of HCC
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCV | Hepatitis C virus |
| HCC | Hepatocellular carcinoma |
| DAA | Direct-acting antiviral |
| SVR | Sustained viral response |
| US | Ultrasonography |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| AST | Aspartate aminotransferase |
| M2BPGi | Wisteria floribunda agglutinin positive Mac-2 binding protein |
| ATX | Autotaxin |
| LPA | Lysophosphatidic acid |
| HBV | Hepatitis B virus |
| NAFLD | Non-alcoholic fatty liver disease |
| IFN | Interferon |
| C.O.I. | Cutoff index |
| AUROC | Area under the receiver operating characteristic |
| FIB-4 | Fibrosis-4 |
| Gd-EOB-DTPA-MRI | Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced dynamic magnetic resonance imaging |
References
- Spearman, C.W.; Dusheiko, G.M.; Hellard, M.; Sonderup, M. Hepatitis C. Lancet 2019, 394, 1451–1466. [Google Scholar] [CrossRef]
- Waziry, R.; Hajarizadeh, B.; Grebely, J.; Amin, J.; Law, M.; Danta, M.; George, J.; Dore, G.J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017, 67, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Ren, Y.; Fierer, D.S.; Rutledge, S.; Shaikh, O.S.; Lo Re, V., III; Simon, T.; Abou-Samra, A.-B.; Chung, R.T.; Butt, A.A. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 2018, 67, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 1477–1492. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H.; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Ikeda, M.; Fujiyama, S.; Tanaka, M.; Sata, M.; Ide, T.; Yatsuhashi, H.; Watanabe, H. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J. Gastroenterol. 2005, 40, 148–156. [Google Scholar] [CrossRef]
- Arase, Y.; Kobayashi, M.; Suzuki, F.; Suzuki, Y.; Kawamura, Y.; Akuta, N.; Kobayashi, M.; Sezaki, H.; Saito, S.; Hosaka, T.; et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology 2013, 57, 964–973. [Google Scholar] [CrossRef]
- Yamashita, N.; Ohho, A.; Yamasaki, A.; Kurokawa, M.; Kotoh, K.; Kajiwara, E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: Significance of lifelong periodic cancer screening for improving outcomes. J. Gastroenterol. 2013, 49, 1504–1513. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F.; Richardson, P.; Kramer, J. Risk of Hepatocellular Carcinoma After Sustained Virological Response in Veterans with Hepatitis C Virus Infection. Hepatology 2016, 64, 130–137. [Google Scholar] [CrossRef]
- Nagata, H.; Nakagawa, M.; Nishimura-Sakurai, Y.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Goto, F.; Otani, S.; Kawai-Kitahata, F.; et al. Serial measurement of Wisteria floribunda agglutinin positive Mac-2-binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol. Int. 2016, 10, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels of Wisteria floribundaagglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014, 60, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Nakagawa, M.; Asahina, Y.; Sato, A.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Otani, S.; Kawai-Kitahata, F.; et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017, 67, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Stracke, M.L.; Krutzsch, H.C.; Unsworth, E.J.; Arestad, A.; Cioce, V.; Schiffmann, E.; Liotta, L.A. Identification, Purification, and Partial Sequence Analysis of Autotaxin, a Novel Motility-stimulating Protein*. J. Biol. Chem. 1992, 267, 2524–2529. [Google Scholar] [PubMed]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, W.H. Lysophospholipids in the limelight: Autotaxin takes center stage. J. Cell Biol. 2002, 158, 197–199. [Google Scholar] [CrossRef]
- Aikawa, S.; Hashimoto, T.; Kano, K.; Aoki, J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem. 2015, 157, 81–89. [Google Scholar] [CrossRef]
- Watanabe, N.; Ikeda, H.; Nakamura, K.; Ohkawa, R.; Kume, Y.; Aoki, J.; Hama, K.; Okudaira, S.; Tanaka, M.; Tomiya, T.; et al. Both Plasma Lysophosphatidic Acid and Serum Autotaxin Levels are Increased in Chronic Hepatitis C. J. Clin. Gastroenterol. 2007, 41, 616–623. [Google Scholar] [CrossRef]
- Yamazaki, T.; Joshita, S.; Umemura, T.; Usami, Y.; Sugiura, A.; Fujimori, N.; Shibata, S.; Ichikawa, Y.; Komatsu, M.; Matsumoto, A.; et al. Association of Serum Autotaxin Levels with Liver Fibrosis in Patients with Chronic Hepatitis C. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Joshita, S.; Ichikawa, Y.; Umemura, T.; Usami, Y.; Sugiura, A.; Shibata, S.; Yamazaki, T.; Fujimori, N.; Komatsu, M.; Matsumoto, A.; et al. Serum Autotaxin Is a Useful Liver Fibrosis Marker in Patients with Chronic Hepatitis B Virus Infection. Hepatol. Res. 2017, 1–28. [Google Scholar] [CrossRef]
- Fujimori, N.; Umemura, T.; Kimura, T.; Tanaka, N.; Sugiura, A.; Yamazaki, T.; Joshita, S.; Komatsu, M.; Usami, Y.; Sano, K.; et al. Serum autotaxin levels are correlated with hepatic fibrosis and ballooning in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Andries, M.; Vekemans, K.; Vanbilloen, H.; Verbruggen, A.; Bollen, M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009, 284, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ikeda, H.; Nakamura, K.; Ohkawa, R.; Masuzaki, R.; Tateishi, R.; Yoshida, H.; Watanabe, N.; Tejima, K.; Kume, Y.; et al. Autotaxin as a novel serum marker of liver fibrosis. Clin. Chim. Acta 2011, 412, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Kaffe, E.; Katsifa, A.; Xylourgidis, N.; Ninou, I.; Zannikou, M.; Harokopos, V.; Foka, P.; Dimitriadis, A.; Evangelou, K.; Moulas, A.N.; et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology 2017, 65, 1369–1383. [Google Scholar] [CrossRef]
- Nakagawa, S.; Wei, L.; Song, W.M.; Higashi, T.; Ghoshal, S.; Kim, R.S.; Bian, C.B.; Yamada, S.; Sun, X.; Venkatesh, A.; et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell 2016, 30, 879–890. [Google Scholar] [CrossRef]
- Erstad, D.J.; Tager, A.M.; Hoshida, Y.; Fuchs, B.C. The autotaxin-lysophosphatidic acid pathway emerges as a therapeutic target to prevent liver cancer. Mol. Cell. Oncol. 2017, 4, 1–2. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.-H.; Lee, M.-H.; Korenaga, M.; Jen, C.-L.; Batrla-Utermann, R.; Lu, S.-N.; Wang, L.-Y.; Mizokami, M.; Chen, C.-J.; et al. Serum Levels of M2BPGi as Short- Term Predictors of Hepatocellular Carcinoma in Untreated Chronic Hepatitis B Patients. Sci. Rep. 2017, 1–10. [Google Scholar] [CrossRef]
- Su, T.-H.; Peng, C.-Y.; Tseng, T.-C.; Yang, H.-C.; Liu, C.-J.; Liu, C.-H.; Chen, P.-J.; Chen, D.-S.; Kao, J.-H. Serum Mac-2-Binding Protein Glycosylation Isomer at Virological Remission Predicts Hepatocellular Carcinoma and Death in Chronic Hepatitis B-Related Cirrhosis. J. Infect. Dis. 2019, 36, 1755–1759. [Google Scholar] [CrossRef]
- Kawanaka, M.; Tomiyama, Y.; Hyogo, H.; Koda, M.; Shima, T.; Tobita, H.; Hiramatsu, A.; Nishino, K.; Okamoto, T.; Sato, S.; et al. Wisteria floribundaagglutinin-positive Mac-2 binding protein predicts the development of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2018, 48, 521–528. [Google Scholar] [CrossRef]
- Kuno, A.; Sato, T.; Shimazaki, H.; Unno, S.; Saitou, K.; Kiyohara, K.; Sogabe, M.; Tsuruno, C.; Takahama, Y.; Ikehara, Y.; et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Prot. Clin. Appl. 2013, 7, 642–647. [Google Scholar] [CrossRef]
- Yamazaki, T.; Joshita, S.; Umemura, T.; Usami, Y.; Sugiura, A.; Fujimori, N.; Kimura, T.; Matsumoto, A.; Igarashi, K.; Ota, M.; et al. Changes in serum levels of autotaxin with direct-acting antiviral therapy in patients with chronic hepatitis C. PLoS ONE 2018, 13, e0195632-20. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Fujii, T.; Iwasawa, Y.; Nakamura, K.; Ohkawa, R.; Igarashi, K.; Okudaira, S.; Ikeda, H.; Kozuma, S.; Aoki, J.; et al. Serum autotaxin measurements in pregnant women: Application for the differentiation of normal pregnancy and pregnancy-induced hypertension. Clin. Chim. Acta 2011, 412, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Nakamura, K.; Izutsu, K.; Igarashi, K.; Ohkawa, R.; Jona, M.; Higashi, K.; Yokota, H.; Okudaira, S.; Kishimoto, T.; et al. Serum autotaxin measurement in haematological malignancies: A promising marker for follicular lymphoma. Br. J. Haematol. 2008, 143, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Kobayashi, M.; Kumada, H.; Enooku, K.; Koike, K.; Kurano, M.; Sato, M.; Nojiri, T.; Kobayashi, T.; Ohkawa, R.; et al. Performance of autotaxin as a serum marker of liver fibrosis. Ann. Clin. Biochem. 2018, 55, 469–477. [Google Scholar] [CrossRef]
- Reig, M.A.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]


| Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|
| Factor | Category | Hazard Ratio | 95% CI | p-Value | ||
| male | pretreatment | ATX | ≥1.21 mg/L | 3.26 | 0.82–12.89 | 0.092 |
| M2BPGi | ≥2.28 C.O.I. | 2.38 | 0.60–9.40 | 0.217 | ||
| N = 301 | post-treatment | ATX | ≥1.37 mg/L | 3.75 | 1.32–10.70 | 0.013 |
| M2BPGi | ≥1.89 C.O.I. | 6.43 | 1.83–22.52 | 0.004 | ||
| female | pretreatment | ATX | ≥2.26 mg/L | 2.50 | 0.97–6.41 | 0.057 |
| M2BPGi | ≥2.23 C.O.I. | 11.76 | 1.47–94.07 | 0.02 | ||
| N = 369 | post-treatment | ATX | ≥1.73 mg/L | 2.34 | 0.92–5.97 | 0.08 |
| M2BPGi | ≥1.35 C.O.I. | 13.07 | 1.66–103.22 | 0.015 | ||
| No | First-Onset | No Relapse | Relapse | p-Value | ||
|---|---|---|---|---|---|---|
| male N = 102 | Group A | 28 | 6 | 7 | 7 | 0.006 |
| Group B | 45 | 0 | 3 | 6 | ||
| female N = 168 | Group A | 57 | 8 | 8 | 5 | 0.034 |
| Group B | 81 | 3 | 3 | 3 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Cohort A | Cohort B | p Value | Cohort A | Cohort B | p-Value | |
| N | 301 | 102 | − | 369 | 168 | − |
| age (years) | 67 (56–73) | 70 (62–75) | 0.01 | 70 (62–76) | 71 (62–75) | 0.95 |
| therapy régimen † (A/B/C/D/E/F) | 47/102/61/35/6/50 | 41/39/17/2/2/1 | − | 68/167/49/31/13/41 | 58/77/25/3/5/0 | − |
| ATX (mg/L) | 1.14 (0.86–1.47) | 1.17 (0.91–1.56) | 0.28 | 1.62 (1.20-2.10) | 1.71 (1.27–2.25) | 0.15 |
| M2BPGi (C.O.I.) | 1.82 (1.17–3.62) | 2.15 (1.41–4.25) | 0.04 | 2.10 (1.30–4.22) | 2.63 (1.46–4.34) | 0.10 |
| PLT (×104/μL) | 17.1 (12.9–21.8) | 14.4 (10.6–20.1) | 0.004 | 16.2 (11.5–21.4) | 14.9 (10.7–19.5) | 0.06 |
| AST (U/L) | 44 (30–68) | 47 (33–72) | 0.20 | 40 (28–61) | 41 (32–61) | 0.27 |
| ALT (U/L) | 41 (26–70) | 42 (27–70) | 0.67 | 35 (23–54) | 37 (27–54) | 0.31 |
| FIB-4 index | 2.67 (1.77–4.25) | 3.28 (2.29-5.33) | <0.001 | 3.03 (1.86–4.95) | 3.51 (2.09–5.26) | 0.10 |
| follow-up period (months) | 25 (13–39) | − | − | 34 (18–42) | − | − |
| HCC history before HCV treatment (Yes/No) | 41/260 | 23/79 | − | 30/339 | 19/149 | − |
| HCC development within 3 years after HCV treatment (Yes/No) | 19/282 | 19/83 | − | 19/350 | 19/149 | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takemura, K.; Takizawa, E.; Tamori, A.; Nakamae, M.; Kubota, H.; Uchida-Kobayashi, S.; Enomoto, M.; Kawada, N.; Hino, M. Post-Treatment M2BPGi Level and the Rate of Autotaxin Reduction are Predictive of Hepatocellular Carcinoma Development after Antiviral Therapy in Patients with Chronic Hepatitis C. Int. J. Mol. Sci. 2020, 21, 4517. https://doi.org/10.3390/ijms21124517
Takemura K, Takizawa E, Tamori A, Nakamae M, Kubota H, Uchida-Kobayashi S, Enomoto M, Kawada N, Hino M. Post-Treatment M2BPGi Level and the Rate of Autotaxin Reduction are Predictive of Hepatocellular Carcinoma Development after Antiviral Therapy in Patients with Chronic Hepatitis C. International Journal of Molecular Sciences. 2020; 21(12):4517. https://doi.org/10.3390/ijms21124517
Chicago/Turabian StyleTakemura, Kazuya, Etsuko Takizawa, Akihiro Tamori, Mika Nakamae, Hiroshi Kubota, Sawako Uchida-Kobayashi, Masaru Enomoto, Norifumi Kawada, and Masayuki Hino. 2020. "Post-Treatment M2BPGi Level and the Rate of Autotaxin Reduction are Predictive of Hepatocellular Carcinoma Development after Antiviral Therapy in Patients with Chronic Hepatitis C" International Journal of Molecular Sciences 21, no. 12: 4517. https://doi.org/10.3390/ijms21124517
APA StyleTakemura, K., Takizawa, E., Tamori, A., Nakamae, M., Kubota, H., Uchida-Kobayashi, S., Enomoto, M., Kawada, N., & Hino, M. (2020). Post-Treatment M2BPGi Level and the Rate of Autotaxin Reduction are Predictive of Hepatocellular Carcinoma Development after Antiviral Therapy in Patients with Chronic Hepatitis C. International Journal of Molecular Sciences, 21(12), 4517. https://doi.org/10.3390/ijms21124517






