Mapping of Major Fusarium Head Blight Resistance from Canadian Wheat cv. AAC Tenacious
Abstract
1. Introduction
2. Results
2.1. Phenotypic Analysis: Varietal Resistance, Days to Anthesis and Plant Height
2.2. Genetic Map
2.3. QTL Analysis
2.3.1. FHB Resistance and DON Content
2.3.2. Days to Anthesis and Plant Height
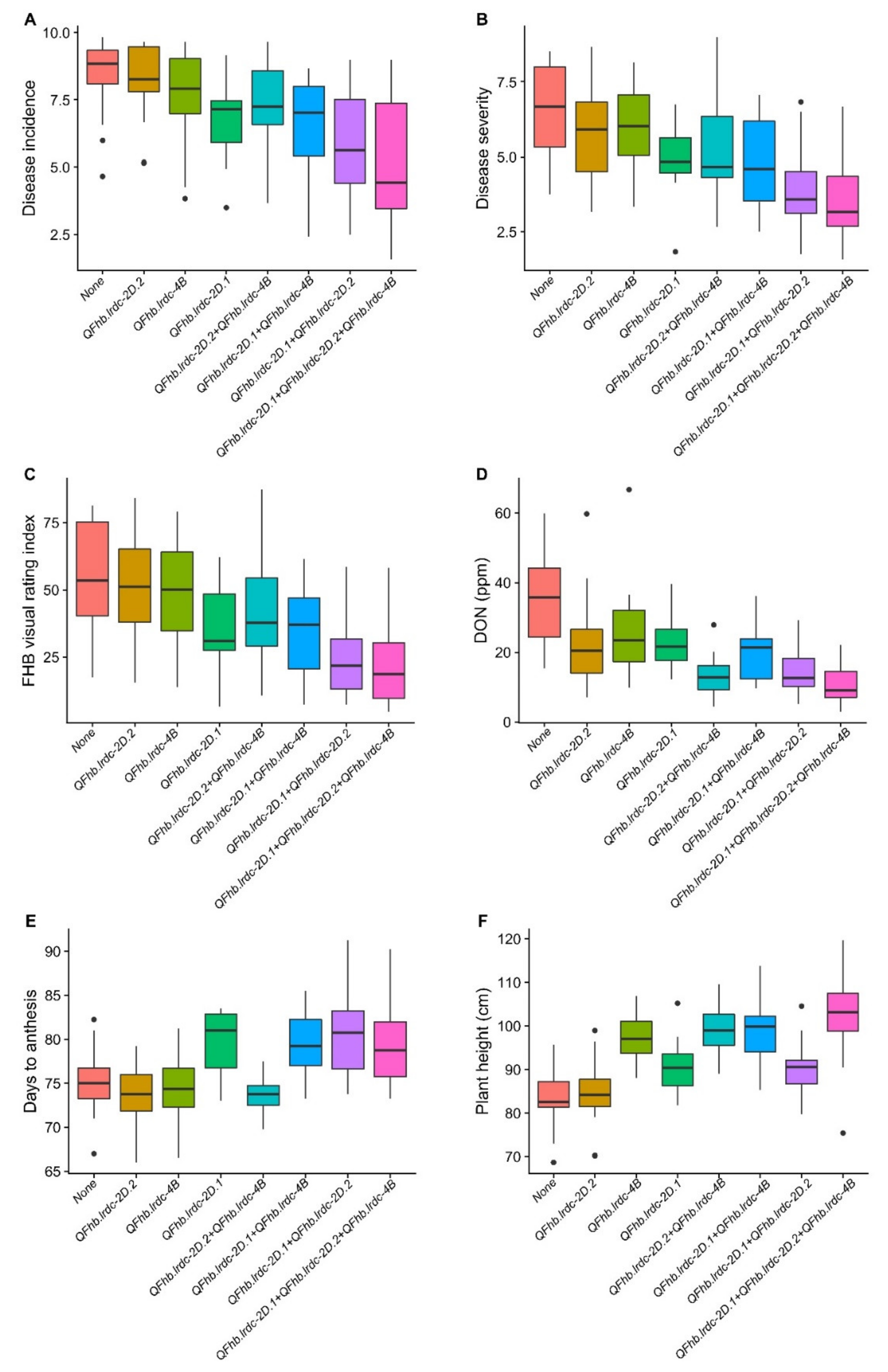
2.3.3. Pleiotropic QTL
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fusarium Head Blight (FHB) Phenotyping and Deoxynivalenol (DON) Assessment
4.3. Days to Anthesis and Plant Height Phenotyping
4.4. Phenotypic Data Evaluation
4.5. Genotyping and Linkage and Quantitative Trait Loci (QTL) Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Additive × additive (epistatic) effect |
| AE | Additive effect |
| A*E | QTL × environment interaction |
| ANOVA | Analysis of variance |
| Chr | Chromosome |
| CIM | Composite interval mapping |
| cM | centi-Morgan |
| cm | Centimetre |
| CPS | Canada Prairie Spring wheat class |
| CWSP | Canada Western Special Purpose wheat class |
| cv | Cultivar |
| CV | Coefficient of variation |
| df | Degrees of freedom |
| DH | Doubled haploid |
| DI | Disease incidence |
| DON | Deoxynivalenol |
| DS | Disease severity |
| DTA | Days to anthesis |
| E | Environment |
| FDK | Fusarium damaged kernels |
| Fg | Fusarium graminearum (Schwabe) |
| FHB | Fusarium head blight |
| G | Genotype |
| GA | Gibberellic acid |
| GDD | Growing degree days |
| His | Histidine-rich calcium-binding protein |
| I | Intermediate |
| JD | Julian calendar days |
| LG | Linkage group |
| LOD | Logarithm of the odds |
| MR | Moderately resistant |
| MS | Moderately susceptible |
| PFT | Pore-forming toxin-like protein |
| PHT | Plant height |
| QTL | Quantitative trait loci |
| R | Resistant |
| R2 | Phenotypic variation |
| Rht | Reduced height |
| S | Susceptible |
| SNP | Single nucleotide polymorphism |
| SSR | Simple sequence repeat |
| VRI | Visual rating index |
References
- Niwa, S.; Kazama, Y.; Abe, T.; Ban, T. Tracking haplotype for QTLs associated with Fusarium head blight resistance in Japanese wheat (Triticum aestivum L.) lineage. Agric. Food Secur. 2018, 7, 4. [Google Scholar] [CrossRef]
- Gilbert, J.; Tekauz, A. Review: Recent developments in research on Fusarium head blight of wheat in Canada. Can. J. Plant. Pathol. 2000, 22, 1–8. [Google Scholar] [CrossRef]
- Sieusahai, G. Alberta Fusarium Graminearum Management Plan. Available online: https://www.alberta.ca/alberta-fusarium-graminearum-management-plan.aspx (accessed on 8 November 2019).
- Heikkila, R. Economic Cost of Fusarium: Farm-level and Regional Economic Impact of Fusarium in Alberta. (External Release); Alberta Agriculture and Forestry: Edmonton, AB, Canada, 2015; p. 6. [Google Scholar]
- Komirenko, Z. Economic Cost of Fusarium: Farm-Level and Regional Economic Impact of Fusarium in Alberta. (External Release); Alberta Agriculture and Forestry: Edmonton, AB, Canada, 2018; p. 8. [Google Scholar]
- Jia, H.Y.; Zhou, J.Y.; Xue, S.L.; Li, G.Q.; Yan, H.S.; Ran, C.F.; Zhang, Y.D.; Shi, J.X.; Jia, L.; Wang, X.; et al. A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai. Crop. J. 2018, 6, 48–59. [Google Scholar] [CrossRef]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant. Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Bartok, T.; Mirocha, C.G.; Komoroczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant. Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Bai, G.H.; Shaner, G. Scab of wheat-prospects for control. Plant. Dis 1994, 78, 760–766. [Google Scholar]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant. Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Lu, Q.X.; Lillemo, M.; Skinnes, H.; He, X.Y.; Shi, J.R.; Ji, F.; Dong, Y.H.; Bjornstad, A. Anther extrusion and plant height are associated with type I resistance to Fusarium head blight in bread wheat line ‘Shanghai-3/Catbird’. Theor. Appl. Genet. 2013, 126, 317–334. [Google Scholar] [CrossRef]
- Miedaner, T.; Voss, H.-H. Effect of dwarfing Rht genes on Fusarium head blight resistance in two sets of near-isogenic lines of wheat and check cultivars. Crop. Sci. 2008, 48, 2115–2122. [Google Scholar] [CrossRef]
- Voss, H.H.; Holzapfel, J.; Hartl, L.; Korzun, V.; Rabenstein, F.; Ebmeyer, E.; Coester, H.; Kempf, H.; Miedaner, T. Effect of the Rht-D1 dwarfing locus on Fusarium head blight rating in three segregating populations of winter wheat. Plant. Breed. 2008, 127, 333–339. [Google Scholar] [CrossRef]
- McCartney, C.A.; Somers, D.J.; Fedak, G.; DePauw, R.M.; Thomas, J.; Fox, S.L.; Humphreys, D.G.; Lukow, O.; Savard, M.E.; McCallum, B.D.; et al. The evaluation of FHB resistance QTLs introgressed into elite Canadian spring wheat germplasm. Mol. Breed. 2007, 20, 209–221. [Google Scholar] [CrossRef]
- Gervais, L.; Dedryver, F.; Morlais, J.Y.; Bodusseau, V.; Negre, S.; Bilous, M.; Groos, C.; Trottet, M. Mapping of quantitative trait loci for field resistance to Fusarium head blight in an European winter wheat. Theor. Appl. Genet. 2003, 106, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.J.; Fedak, G.; Savard, M. Molecular mapping of novel genes controlling Fusarium head blight resistance and deoxynivalenol accumulation in spring wheat. Genome 2003, 46, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Snijders, C.H.A.; Perkowski, J. Effects of head blight caused by Fusarium culmorum on toxin content and weight of wheat kernels. Phytopathology 1990, 80, 566–570. [Google Scholar] [CrossRef]
- Ban, T.; Suenaga, K. Genetic analysis of resistance to Fusarium head blight caused by Fusarium graminearum in Chinese wheat cultivar Sumai 3 and the Japanese cultivar Saikai 165. Euphytica 2000, 113, 87–99. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant. Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Holzapfel, J.; Voss, H.H.; Miedaner, T.; Korzun, V.; Haberle, J.; Schweizer, G.; Mohler, V.; Zimmermann, G.; Hartl, L. Inheritance of resistance to Fusarium head blight in three European winter wheat populations. Theor. Appl. Genet. 2008, 117, 1119–1128. [Google Scholar] [CrossRef]
- Venske, E.; dos Santos, R.S.; da Rosa, F.D.; Rother, V.; da Maia, L.C.; Pegoraro, C.; Costa de Oliveira, A. Meta-analysis of the QTLome of Fusarium head blight resistance in bread wheat: Refining the current puzzle. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef]
- Waldron, B.L.; Moreno-Sevilla, B.; Anderson, J.A.; Stack, R.W.; Frohberg, R.C. RFLP mapping of QTL for Fusarium head blight resistance in wheat. Crop. Sci. 1999, 39, 805–811. [Google Scholar] [CrossRef]
- Anderson, J.A.; Stack, R.W.; Liu, S.; Waldron, B.L.; Fjeld, A.D.; Coyne, C.; Moreno-Sevilla, B.; Fetch, J.M.; Song, Q.J.; Cregan, P.B.; et al. DNA markers for Fusarium head blight resistance QTLs in two wheat populations. Theor. Appl. Genet. 2001, 102, 1164–1168. [Google Scholar] [CrossRef]
- Yang, Z.P.; Gilbert, J.; Somers, D.J.; Fedak, G.; Procunier, J.D.; McKenzie, I.H. Marker assisted selection of Fusarium head blight resistance genes in two doubled haploid populations of wheat. Mol. Breed. 2003, 12, 309–317. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Steiner, B.; Hartl, L.; Griesser, M.; Angerer, N.; Lengauer, D.; Miedaner, T.; Schneider, B.; Lemmens, M. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. II. Resistance to fungal penetration and spread. Theor. Appl. Genet. 2003, 107, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, P.A.; Somers, D.J.; Thomas, J.; Cloutier, S.; Brule-Babel, A. Fine mapping Fhb1, a major gene controlling Fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 112, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Pumphrey, M.O.; Stack, R.W.; Gill, B.S.; Anderson, J.A. Complex microcolinearity among wheat, rice, and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat. Funct. Integr. Genom. 2006, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xu, F.; Tang, M.; Zhou, Y.; Li, G.; An, X.; Lin, F.; Xu, H.; Jia, H.; Zhang, L.; et al. Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 123, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, M.; Steiner, B.; Wagner, C.; Schwarz, P.; Brugger, K.; Barabaschi, D.; Volante, A.; Vale, G.; Cattivelli, L.; Buerstmayr, H. High-resolution mapping of the pericentromeric region on wheat chromosome arm 5AS harbouring the Fusarium head blight resistance QTL Qfhs.ifa-5a. Plant. Biotechnol. J. 2018, 16, 1046–1056. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef]
- Cainong, J.C.; Bockus, W.W.; Feng, Y.; Chen, P.; Qi, L.; Sehgal, S.K.; Danilova, T.V.; Koo, D.-H.; Friebe, B.; Gill, B.S. Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor. Appl. Genet. 2015, 128, 1019–1027. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Hou, Y.; Cai, J.; Shen, X.; Zhou, T.; Xu, H.; Ohm, H.W.; Wang, H.; Li, A.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Buerstmayr, M.; Schweiger, W.; Steiner, B. Genomics-assisted breeding for Fusarium head blight resistance in wheat. In Translational Genomics for Crop Breeding: Biotic Stress; Varshney, R.K., Tuberosa, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 1, pp. 45–61. [Google Scholar]
- Yao, J.; Ge, Y.; Wang, S.; Yao, G.; Zhou, C.; Qian, C. Chromosomal location of genes for scab resistance in wheat cultivar Sumai 3. Zuo Wu Xue Bao 1997, 23, 450–453. [Google Scholar]
- Zhou, W.-C.; Kolb, F.L.; Bai, G.-H.; Domier, L.L.; Yao, J.-B. Effect of individual Sumai 3 chromosomes on resistance to scab spread within spikes and deoxynivalenol accumulation within kernels in wheat. Hereditas 2002, 137, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Basnet, B.R.; Glover, K.D.; Ibrahim, A.M.H.; Yen, Y.; Chao, S. A QTL on chromosome 2DS of ‘Sumai 3’ increases susceptibility to Fusarium head blight in wheat. Euphytica 2012, 186, 91–101. [Google Scholar] [CrossRef]
- Ma, H.-X.; Bai, G.-H.; Gill, B.S.; Hart, L.P. Deletion of a chromosome arm altered wheat resistance to Fusarium head blight and deoxynivalenol accumulation in Chinese Spring. Plant. Dis. 2006, 90, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Pumphrey, M.O.; Liu, S.; Zhang, X.; Tiwari, V.K.; Ando, K.; Trick, H.N.; Bockus, W.W.; Akhunov, E.; Anderson, J.A.; et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 2016, 48, 1576. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef]
- Gadaleta, A.; Colasuonno, P.; Giove, S.L.; Blanco, A.; Giancaspro, A. Map-based cloning of Qfhb.mgb-2A identifies a WAK2 gene responsible for Fusarium head blight resistance in wheat. Sci. Rep. 2019, 9, 6929. [Google Scholar] [CrossRef]
- Handa, H.; Namiki, N.; Xu, D.; Ban, T. Dissecting of the FHB resistance QTL on the short arm of wheat chromosome 2D using a comparative genomic approach: From QTL to candidate gene. Mol. Breed. 2008, 22, 71–84. [Google Scholar] [CrossRef]
- Kage, U.; Karre, S.; Kushalappa, A.C.; McCartney, C. Identification and characterization of a Fusarium head blight resistance gene TaACT in wheat QTL-2DL. Plant. Biotechnol. J. 2017, 15, 447–457. [Google Scholar] [CrossRef]
- Kage, U.; Yogendra, K.N.; Kushalappa, A.C. TaWRKY70 transcription factor in wheat QTL-2DL regulates downstream metabolite biosynthetic genes to resist Fusarium graminearum infection spread within spike. Sci. Rep. 2017, 7, 42596. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Z.; Gao, C.; Cheng, S.; Xia, X.; He, C. The complexity of Fhb1, is it a gene or a gene cluster? In Proceedings of the 9th Canadian Workshop on Fusarium Head Blight 4th Canadian Wheat Symposium, Winnipeg, Manitoba, ON, Canada, 19–22 November 2018; p. 28. [Google Scholar]
- Lagudah, E.S.; Krattinger, S.G. A new player contributing to durable Fusarium resistance. Nat. Genet. 2019, 51, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.A.; Brûlé-Babel, A.L.; Fedak, G.; Martin, R.A.; McCallum, B.D.; Gilbert, J.; Hiebert, C.W.; Pozniak, C.J. Fusarium head blight resistance QTL in the spring wheat cross Kenyon/86ISMN 2137. Front. Microbiol. 2016, 7, 1542. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Randhawa, H.S.; Mitchell Fetch, J.; Meiklejohn, M.; Fox, S.L.; Humphreys, D.G.; Green, D.; Wise, I.; Fetch, T.; Gilbert, J.; et al. AAC Tenacious red spring wheat. Can. J. Plant. Sci. 2015, 95, 805–810. [Google Scholar] [CrossRef]
- Tekauz, A.; Gilbert, J.; Abramson, D. Reaction to Fusarium head blight of spring wheats based on symptoms, Fusarium spp., and mycotoxins. Cereal Res. Commun. 1997, 25, 821–822. [Google Scholar] [CrossRef]
- Campbell, A.B. Neepawa hard red spring wheat. Can. J. Plant. Sci. 1970, 50, 752–753. [Google Scholar] [CrossRef]
- Randhawa, H.S.; Graf, R.J.; Sadasivaiah, R.S. AAC Innova general purpose spring wheat. Can. J. Plant. Sci. 2015, 95, 787–791. [Google Scholar] [CrossRef]
- Dhariwal, R.; Fedak, G.; Dion, Y.; Pozniak, C.; Laroche, A.; Eudes, F.; Randhawa, H. High density single nucleotide polymorphism (SNP) mapping and quantitative trait loci (QTL) analysis in a biparental spring triticale population localized major and minor effect Fusarium head blight resistance and associated traits QTL. Genes 2018, 9, 19. [Google Scholar] [CrossRef]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A.; et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar] [CrossRef]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90000 single nucleotide polymorphism array. Plant. Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef]
- Yu, L.-X.; Barbier, H.; Rouse, M.N.; Singh, S.; Singh, R.P.; Bhavani, S.; Huerta-Espino, J.; Sorrells, M.E. A consensus map for UG99 stem rust resistance loci in wheat. Theor. Appl. Genet. 2014, 127, 1561–1581. [Google Scholar] [CrossRef]
- Li, C.; Bai, G.; Chao, S.; Wang, Z. A high-density SNP and SSR consensus map reveals segregation distortion regions in wheat. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; He, Z.; Gao, F.; Liu, J.; Jin, H.; Zhai, S.; Qu, Y.; Xia, X. A high-density consensus map of common wheat integrating four mapping populations scanned by the 90k snp array. Front. Plant. Sci 2017, 8, 1389. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.; Kruglyak, L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat. Genet. 1995, 11, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Christopher, M.D.; Griffey, C.A.; Hall, M.D.; Gundrum, P.G.; Brooks, W.S. Molecular characterization of resistance to Fusarium head blight in U.S. soft red winter wheat breeding line VA00W-38. Crop. Sci. 2012, 52, 2283–2292. [Google Scholar] [CrossRef]
- Liu, S.; Griffey, C.A.; Hall, M.D.; McKendry, A.L.; Chen, J.; Brooks, W.S.; Brown-Guedira, G.; Van Sanford, D.; Schmale, D.G. Molecular characterization of field resistance to Fusarium head blight in two US soft red winter wheat cultivars. Theor. Appl. Genet. 2013, 126, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhang, X.; Zhang, W.; Zhang, N.; Song, L.; Liu, L.; Xue, X.; Liu, G.; Liu, J.; Meng, D.; et al. QTL detection for kernel size and weight in bread wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map. Front. Plant. Sci. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.L.; Echalier, B.; Chao, S.; Lazo, G.R.; Butler, G.E.; Anderson, O.D.; Akhunov, E.D.; Dvorák, J.; Linkiewicz, A.M.; Ratnasiri, A.; et al. A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 2004, 168, 701–712. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, M.; Lu, W.; Ohm, H. Detection of Fusarium head blight resistance QTL in a wheat population using bulked segregant analysis. Theor. Appl. Genet. 2003, 106, 1041–1047. [Google Scholar] [CrossRef]
- Yi, X.; Cheng, J.; Jiang, Z.; Hu, W.; Bie, T.; Gao, D.; Li, D.; Wu, R.; Li, Y.; Chen, S.; et al. Genetic analysis of Fusarium head blight resistance in CIMMYT bread wheat line C615 using traditional and conditional QTL mapping. Front. Plant. Sci. 2018, 9, 573. [Google Scholar] [CrossRef]
- Yang, Z.; Gilbert, J.; Fedak, G.; Somers, D.J. Genetic characterization of QTL associated with resistance to Fusarium head blight in a doubled-haploid spring wheat population. Genome 2005, 48, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, M.; Lemmens, M.; Steiner, B.; Buerstmayr, H. Advanced backcross QTL mapping of resistance to Fusarium head blight and plant morphological traits in a Triticum macha × T. aestivum population. Theor. Appl. Genet. 2011, 123, 293. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.H.; Juan, H.F.; Nohda, M.; Ban, T. QTLs mapping of type I and type II resistance to FHB in wheat. In 2001 National Fusarium Head Blight Forum Proceedings Erlanger, KY, USA; Canty, S.M., Lewis, J., Siler, L., Ward, R.W., Eds.; Kinko’s, FEdex: Okemos, MI, USA, 2001; pp. 40–42. [Google Scholar]
- Cai, J.; Bai, G. Quantitative trait loci for Fusarium head blight resistance in Huangcandou × ‘Jagger’ wheat population. Crop. Sci. 2014, 54, 2520–2528. [Google Scholar] [CrossRef]
- He, X.; Lillemo, M.; Shi, J.; Wu, J.; Bjørnstad, Å.; Belova, T.; Dreisigacker, S.; Duveiller, E.; Singh, P. QTL characterization of Fusarium head blight resistance in CIMMYT bread wheat line Soru#1. PLoS ONE 2016, 11, e0158052. [Google Scholar]
- Jia, G.; Chen, P.; Qin, G.; Bai, G.; Wang, X.; Wang, S.; Zhou, B.; Zhang, S.; Liu, D. QTLs for Fusarium head blight response in a wheat DH population of Wangshuibai/Alondra‘s’. Euphytica 2005, 146, 183–191. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Yang, J.; Luo, P. Identification of a new QTL for Fusarium head blight resistance in the wheat genotype “Wang shui-bai”. Mol. Biol. Rep. 2010, 37, 1031–1035. [Google Scholar] [CrossRef]
- Lv, C.; Song, Y.; Gao, L.; Yao, Q.; Zhou, R.; Xu, R.; Jia, J. Integration of QTL detection and marker assisted selection for improving resistance to Fusarium head blight and important agronomic traits in wheat. Crop. J. 2014, 2, 70–78. [Google Scholar] [CrossRef]
- Korzun, V.; Röder, M.S.; Ganal, M.W.; Worland, A.J.; Law, C.N. Genetic analysis of the dwarfing gene (Rht8) in wheat. Part, I. Molecular mapping of Rht8 on the short arm of chromosome 2D of bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 1998, 96, 1104–1109. [Google Scholar] [CrossRef]
- Dhariwal, R.; Graf, J.R.; Randhawa, H.S. Validation and origin of FHB resistance QTL identified from Canadian wheat cv AAC Tenacious. (unpublished).
- Gasperini, D.; Greenland, A.; Hedden, P.; Dreos, R.; Harwood, W.; Griffiths, S. Genetic and physiological analysis of Rht8 in bread wheat: An alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. J. Exp. Bot. 2012, 63, 4419–4436. [Google Scholar]
- Law, C.N.; Sutka, J.; Worland, A.J. A genetic study of day-length response in wheat. Heredity 1978, 41, 185–191. [Google Scholar] [CrossRef]
- Worland, A.J.; Law, C.N. Genetic analysis of chromosome 2D of wheat. I. The location of genes affecting height, daylength insensitivity, hybrid dwarfism and yellow-rust resistance. Zeitschrift für Pflanzenzüchtung 1986, 96, 331–345. [Google Scholar]
- Jiang, G.-L.; Shi, J.; Ward, R.W. QTL analysis of resistance to Fusarium head blight in the novel wheat germplasm CJ 9306. I. Resistance to fungal spread. Theor. Appl. Genet. 2007, 116, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bonnett, D.; Ellis, M.; He, X.; Heslot, N.; Dreisigacker, S.; Gao, C.; Singh, P. Characterization of Fusarium head blight resistance in a CIMMYT synthetic-derived bread wheat line. Euphytica 2016, 208, 367–375. [Google Scholar] [CrossRef]
- Lin, F.; Xue, S.L.; Zhang, Z.Z.; Zhang, C.Q.; Kong, Z.X.; Yao, G.Q.; Tian, D.G.; Zhu, H.L.; Li, C.J.; Cao, Y.; et al. Mapping QTL associated with resistance to Fusarium head blight in the Nanda2419 × Wangshuibai population. II: Type I resistance. Theor. Appl. Genet. 2006, 112, 528–535. [Google Scholar] [CrossRef]
- Lewis, J.M.; Suenaga, K.; Ginkel, M.V.; Gilchrist, L.; Shi, J.R.; Jiang, G.L.; Kravchenko, S.; Mujeeb-Kazi, A.; Ward, R.W. Identification and mapping of a QTL for type II resistance to Fusarium head blight on chromosome arm 2DL of wheat. In Proceedings of the 2nd International Symposium on Fusarium Head Blight Incorporating the 8th European Fusarium Seminar; Canty, S.M., Boring, T., Wardwell, J., Ward, R.W., Eds.; Michigan State University: Orlando, FL, USA, 2004; Volume 1, pp. 89–92. [Google Scholar]
- Paillard, S.; Schnurbusch, T.; Tiwari, R.; Messmer, M.; Winzeler, M.; Keller, B.; Schachermayr, G. QTL analysis of resistance to Fusarium head blight in Swiss winter wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 323–332. [Google Scholar] [CrossRef]
- Mardi, M.; Buerstmayr, H.; Ghareyazie, B.; Lemmens, M.; Mohammadi, S.A.; Nolz, R.; Ruckenbauer, P. QTL analysis of resistance to Fusarium head blight in wheat using a ‘Wangshuibai’-derived population. Plant. Breed. 2005, 124, 329–333. [Google Scholar] [CrossRef]
- Ma, H.-X.; Bai, G.-H.; Zhang, X.; Lu, W.-Z. Main effects, epistasis, and environmental interactions of quantitative trait loci for Fusarium head blight resistance in a recombinant inbred population. Phytopathology 2006, 96, 534–541. [Google Scholar] [CrossRef]
- He, X.; Singh, P.K.; Dreisigacker, S.; Singh, S.; Lillemo, M.; Duveiller, E. Dwarfing genes Rht-B1b and Rht-D1b are associated with both type I FHB susceptibility and low anther extrusion in two bread wheat populations. PLoS ONE 2016, 11, e0162499. [Google Scholar] [CrossRef]
- Buhrow, L.M.; Cram, D.; Tulpan, D.; Foroud, N.A.; Loewen, M.C. Exogenous abscisic acid and gibberellic acid elicit opposing effects on Fusarium graminearum infection in wheat. Phytopathology 2016, 106, 986–996. [Google Scholar] [CrossRef]
- Herter, C.P.; Ebmeyer, E.; Kollers, S.; Korzun, V.; Leiser, W.L.; Würschum, T.; Miedaner, T. Rht24 reduces height in the winter wheat population ‘Solitär × Bussard’ without adverse effects on Fusarium head blight infection. Theor. Appl. Genet. 2018, 131, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, D.; Roeder, M.; Ganal, M.; Schnurbusch, T. Genetic dissection of pre-anthesis sub-phase durations during the reproductive spike development of wheat. Plant. J. 2018, 95, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Eckard, J.T.; Gonzalez-Hernandez, J.L.; Caffe, M.; Berzonsky, W.; Bockus, W.W.; Marais, G.F.; Baenziger, P.S. Native Fusarium head blight resistance from winter wheat cultivars ‘Lyman,’ ‘Overland,’ ‘Ernie,’ and ‘Freedom’ mapped and pyramided onto ‘Wesley’-Fhb1 backgrounds. Mol. Breed. 2015, 35, 6. [Google Scholar] [CrossRef]
- Kumar, A.; Mantovani, E.E.; Simsek, S.; Jain, S.; Elias, E.M.; Mergoum, M. Genome wide genetic dissection of wheat quality and yield related traits and their relationship with grain shape and size traits in an elite × non-adapted bread wheat cross. PLoS ONE 2019, 14, e0221826. [Google Scholar] [CrossRef]
- Suenaga, K.; Nakajima, K. Efficient production of haploid wheat (Triticum aestivum) through crosses between Japanese wheat and maize (Zea mays). Plant Cell Rep. 1989, 8, 263–266. [Google Scholar] [CrossRef]
- Gilbert, J.; Morgan, K. Field-based rating of spring wheat infected with Fusarium graminearum, cause of Fusarium head blight. In Proceedings of the 6th European Seminar on Fusarium—Mycotoxins, Taxonomy and Pathogenicity, Berlin, Germany, 11–16 September 2000; p. 73. [Google Scholar]
- Sinha, R.C.; Savard, M.E. Comparison of immunoassay and gas chromatography methods for the detection of the mycotoxin deoxynivalenol in grain samples. Can. J. Plant Pathol. 1996, 18, 233–236. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Schloerke, B.; Crowley, J.; Cook, D.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Larmarange, J. Ggally: Extension to ‘Ggplot2’, R package version 1.4.0.; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wu, Y.; Bhat, P.R.; Close, T.J.; Lonardi, S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet. 2008, 4, e1000212. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Jansen, J.; de Jong, A.G.; van Ooijen, J.W. Constructing dense genetic linkage maps. Theor. Appl. Genet. 2001, 102, 1113–1122. [Google Scholar] [CrossRef]
- Lorieux, M. Mapdisto: Fast and efficient computation of genetic linkage maps. Mol. Breed. 2012, 30, 1231–1235. [Google Scholar] [CrossRef]
- Zeng, Z.B. Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc. Natl Acad. Sci. USA 1993, 90, 10972–10976. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.B. Precision mapping of quantitative trait loci. Genetics 1994, 136, 1457–1468. [Google Scholar] [PubMed]
- Yang, J.; Hu, C.; Hu, H.; Yu, R.; Xia, Z.; Ye, X.; Zhu, J. QTLNetwork: Mapping and visualizing genetic architecture of complex traits in experimental populations. Bioinformatics 2008, 24, 721–723. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C. Omiccircos: High-Quality Circular Visualization of Omics Data, R Package Version 1.16.0.; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
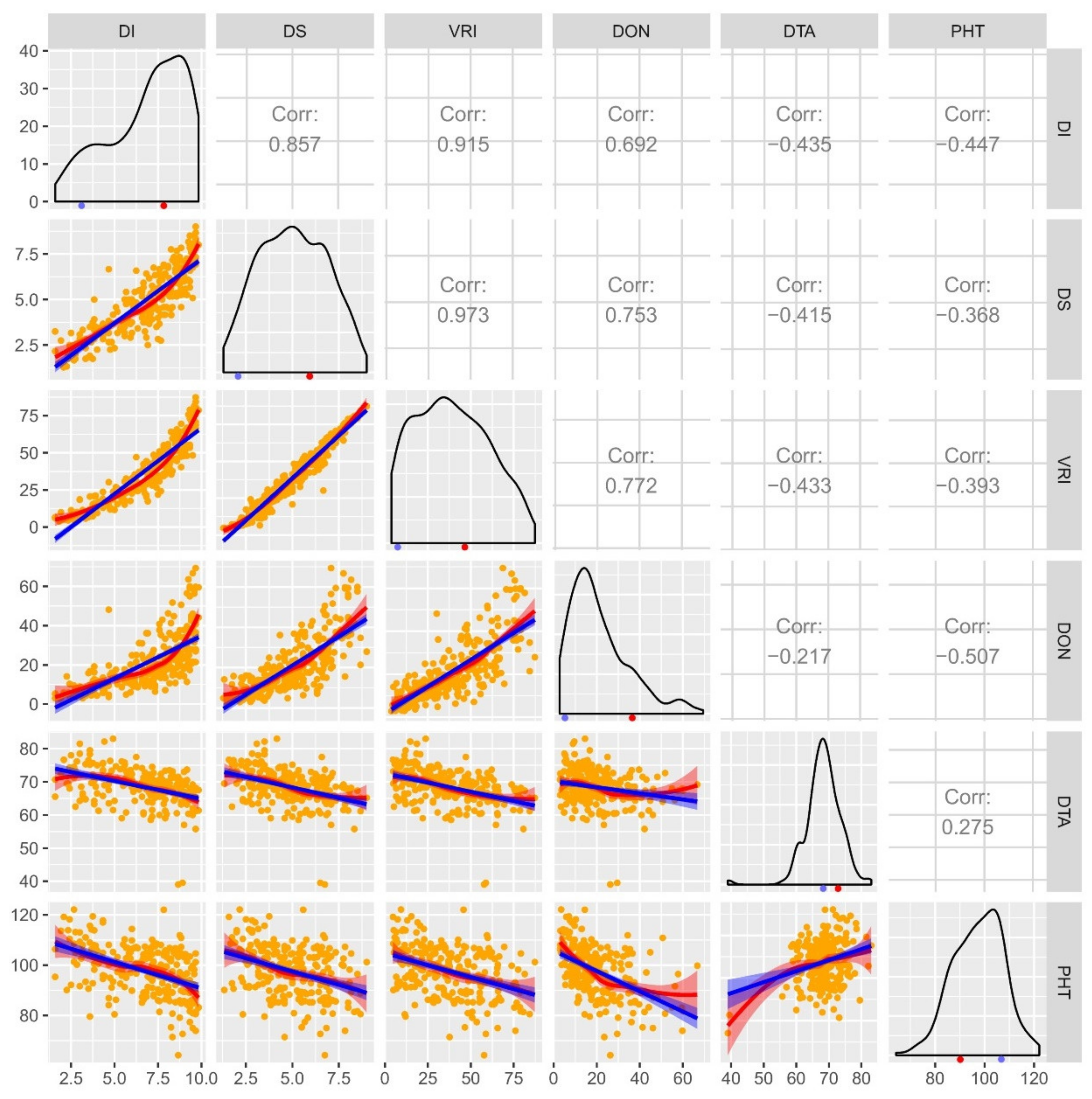

| Source | d.f. | DI | DS | VRI | DON |
|---|---|---|---|---|---|
| Environment (E) | 1 | 20.25 ** | 175.80 ** | 967.00 * | 8135.00 ** |
| Block (within E) | 1 | 9.91 | 0.40 | 172.00 | 94.00 |
| Genotype (G) | 236 | 31.19 ** | 20.60 ** | 2864.00 ** | 1094.00 ** |
| (G х E) | 236 | 3.22 | 2.70 | 237.00 | 130.00 ** |
| Error | 875 | 2.93 | 2.30 | 250.00 | 83.00 |
| CV, % | 24.00 | 31.00 | 40.00 | 41.00 |
| Genome | LG/ Chromosome | No. of Markers | No. of Linkage Bins | Map Length (cM) | Marker Density | Map Density | ||
|---|---|---|---|---|---|---|---|---|
| Markers/cM | Linkage Bins/cM | cM/ Marker | cM/Linkage Bin | |||||
| A | 1 | 737.00 | 124.00 | 187.48 | 3.93 | 0.66 | 0.25 | 1.51 |
| 2 | 626.00 | 107.00 | 178.64 | 3.50 | 0.60 | 0.29 | 1.67 | |
| 3 | 430.00 | 87.00 | 237.23 | 1.81 | 0.37 | 0.55 | 2.73 | |
| 4 | 406.00 | 72.00 | 176.58 | 2.30 | 0.41 | 0.43 | 2.45 | |
| 5 | 760.00 | 116.00 | 232.93 | 3.26 | 0.50 | 0.31 | 2.01 | |
| 6 | 432.00 | 69.00 | 187.11 | 2.31 | 0.37 | 0.43 | 2.71 | |
| 7 | 721.00 | 138.00 | 297.25 | 2.43 | 0.46 | 0.41 | 2.15 | |
| Total | 4112.00 | 713.00 | 1497.23 | 2.75 | 0.48 | 0.36 | 2.10 | |
| % | 39.81 | 46.21 | 41.44 | |||||
| B | 1 | 1721.00 | 88.00 | 140.42 | 12.26 | 0.63 | 0.08 | 1.60 |
| 2 | 817.00 | 109.00 | 202.65 | 4.03 | 0.54 | 0.25 | 1.86 | |
| 3.1 | 89.00 | 28.00 | 38.94 | 2.29 | 0.72 | 0.44 | 1.39 | |
| 3.2 | 385.00 | 68.00 | 113.39 | 3.40 | 0.60 | 0.29 | 1.67 | |
| 4 | 302.00 | 60.00 | 119.83 | 2.52 | 0.50 | 0.40 | 2.00 | |
| 5 | 576.00 | 103.00 | 264.67 | 2.18 | 0.39 | 0.46 | 2.57 | |
| 6 | 693.00 | 87.00 | 206.49 | 3.36 | 0.42 | 0.30 | 2.37 | |
| 7 | 494.00 | 95.00 | 194.88 | 2.53 | 0.49 | 0.39 | 2.05 | |
| Total | 5077.00 | 638.00 | 1281.26 | 3.96 | 0.50 | 0.25 | 2.01 | |
| % | 49.16 | 41.35 | 35.46 | |||||
| D | 1 | 107.00 | 33.00 | 79.61 | 1.34 | 0.41 | 0.74 | 2.41 |
| 2 | 552.00 | 45.00 | 166.82 | 3.31 | 0.27 | 0.30 | 3.71 | |
| 3.1 | 25.00 | 6.00 | 18.14 | 1.38 | 0.33 | 0.73 | 3.02 | |
| 3.2 | 19.00 | 10.00 | 46.52 | 0.41 | 0.21 | 2.45 | 4.65 | |
| 3.3 | 87.00 | 7.00 | 26.64 | 3.27 | 0.26 | 0.31 | 3.81 | |
| 4 | 52.00 | 26.00 | 145.98 | 0.36 | 0.18 | 2.81 | 5.61 | |
| 5 | 156.00 | 30.00 | 181.74 | 0.86 | 0.17 | 1.16 | 6.06 | |
| 6 | 66.00 | 12.00 | 31.22 | 2.11 | 0.38 | 0.47 | 2.60 | |
| 7.1 | 45.00 | 9.00 | 23.12 | 1.95 | 0.39 | 0.51 | 2.57 | |
| 7.2 | 30.00 | 14.00 | 115.09 | 0.26 | 0.12 | 3.84 | 8.22 | |
| Total | 1139.00 | 192.00 | 834.87 | 1.36 | 0.23 | 0.73 | 4.35 | |
| % | 11.03 | 12.44 | 23.10 | |||||
| A+B+D | 1 | 2565.00 | 245.00 | 407.50 | 6.29 | 0.60 | 0.16 | 1.66 |
| 2 | 1995.00 | 261.00 | 548.11 | 3.64 | 0.48 | 0.27 | 2.10 | |
| 3 | 1035.00 | 206.00 | 480.86 | 2.15 | 0.43 | 0.46 | 2.33 | |
| 4 | 760.00 | 158.00 | 442.39 | 1.72 | 0.36 | 0.58 | 2.80 | |
| 5 | 1492.00 | 249.00 | 679.34 | 2.20 | 0.37 | 0.46 | 2.73 | |
| 6 | 1191.00 | 168.00 | 424.82 | 2.80 | 0.40 | 0.36 | 2.53 | |
| 7 | 1290.00 | 256.00 | 630.34 | 2.05 | 0.41 | 0.49 | 2.46 | |
| Total | 10328.00 | 1543.00 | 3613.36 | 2.86 | 0.43 | 0.35 | 2.34 | |
| Response Variable/ Trait | Chr. (arm) | QTL Name a | QTL Position b | QTL Interval | LOD | Absolute Additive Effect | R2 | Closest Marker | Individual Environment(s) | Favourable Donor Allele for Respective Trait | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker Name | Linkage Map Position | Physical Position (IWGSC RefSeq v2.0) c Start End | |||||||||||
| DI | 2B | QFhi.lrdc-2B | 68.8 | 64.2–71.8 | 3.2 | 0.5 | 4.4 | BS00012078_51 | 66.73 | 265366661 | 265366634 | Mrd16, Mrd17 | T |
| 2DS | QFhi.lrdc-2D* | 40.9 | 40.2–63.7 | 15.5 | 1.0 | 25.0 | Ppd-D1 | 40.76 | 36205433 | 36204772 | Mrd15, Mrd16, Mrd17 | T | |
| 4A | QFhi.lrdc-4A | 118.0 | 114.8–121.6 | 8.0 | 0.7 | 11.9 | BobWhite_c10610_1096 | 117.82 | 688409042 | 688408942 | Mrd16, Mrd17 | I | |
| 4D | QFhi.lrdc-4D | 146.0 | 145.1–146.0 | 2.8 | 0.4 | 4.0 | BobWhite_c28101_376 | 145.98 | 515801764 | 515801671 | Mrd16, Mrd17 | I | |
| 5D | QFhi.lrdc-5D | 0.1 | 0.0–0.6 | 2.6 | 0.4 | 3.9 | RAC875_c92929_92 | 0.00 | 279491136 | 279491036 | Mrd17 | T | |
| 7A | QFhi.lrdc-7A | 206.4 | 205.9–206.8 | 2.6 | 0.4 | 2.1 | IACX2471 | 205.99 | 673715328 | 673715447 | -- | T | |
| DS | 2DS | QFhs.lrdc-2D.1* | 40.9 | 40.2–63.4 | 12.7 | 0.8 | 19.0 | Ppd-D1 | 40.76 | 36205433 | 36204772 | Mrd15, Mrd16, Mrd17 | T |
| 2DL | QFhs.lrdc-2D.2* | 115.6 | 105.2–126.4 | 5.4 | 0.5 | 15.2 | BobWhite_c17782_194 | 115.38 | 555098707 | 555098805 | Mrd15, Mrd16 | T | |
| 4A | QFhs.lrdc-4A | 118.0 | 114.8–121.3 | 5.4 | 0.5 | 10.1 | BobWhite_c10610_1096 | 117.82 | 688409042 | 688408942 | Mrd15, Mrd16, Mrd17 | I | |
| 7BS | QFhs.lrdc-7B | 32.3 | 24.3–35.5 | 3.6 | 0.4 | 6.3 | wsnp_CAP7_c44_26549 | 32.15 | 17091249 | 17091049 | Mrd16 | I | |
| VRI | 2DS | QFhb.lrdc-2D.1* | 40.9 | 40.2–63.4 | 12.4 | 9.4 | 18.0 | Ppd-D1 | 40.76 | 36205433 | 36204772 | Mrd15, Mrd16, Mrd17 | T |
| 2DL | QFhb.lrdc-2D.2* | 114.6 | 106.4–120.8 | 3.6 | 4.8 | 12.2 | BobWhite_c17782_194 | 115.38 | 555098707 | 555098805 | Mrd15 | T | |
| 4A | QFhb.lrdc-4A | 118.0 | 114.7–121.3 | 7.1 | 6.6 | 11.5 | BobWhite_c10610_1096 | 117.82 | 688409042 | 688408942 | Mrd15, Mrd16, Mrd17 | I | |
| 5D | QFhb.lrdc-5D | 0.2 | 0.0–0.4 | 2.5 | 3.4 | 3.0 | RAC875_c92929_92 | 0.00 | 279491136 | 279491036 | Mrd17 | T | |
| 7BS | QFhb.lrdc-7B | 31.6 | 24.1–35.2 | 3.9 | 4.9 | 7.3 | wsnp_CAP7_c44_26549 | 32.15 | 17091249 | 17091049 | Mrd16 | I | |
| DON | 2DS | QDon.lrdc-2D.1* | 40.6 | 37.9–41.9 | 3.9 | 3.0 | 12.3 | Ppd-D1 | 40.76 | 36205433 | 36204772 | Mrd16, Mrd17 | T |
| 2DL | QDon.lrdc-2D.2* | 112.6 | 105. 1–126.6 | 14.4 | 6.0 | 34.5 | BobWhite_c17782_194 | 115.38 | 555098707 | 555098805 | Mrd15, Mrd16, Mrd17 | T | |
| 3B | QDon.lrdc-3B.1* | 132.8 | 132.2–133.2 | 2.7 | 2.1 | 9.3 | Kukri_c13345_481 | 132.75 | 753508932 | 753508832 | Mrd15 | T | |
| 3B | QDon.lrdc-3B.2 | 147.3 | 144.5–150.3 | 2.7 | 2.3 | 4.0 | Excalibur_rep_c97324_623 | 151.49 | 771943609 | 771943709 | -- | T | |
| 4B | QDon.lrdc-4B* | 49.5 | 46.4–52.4 | 6.6 | 3.6 | 8.0 | Ex_c101685_705 | 49.48 | 31725418 | 31725518 | Mrd17 | T | |
| 5A | QDon.lrdc-5A.1 | 164.8 | 156.4–169.0 | 3.1 | 2.3 | 3.0 | IACX2540 | 164.62 | 621553818 | 621553699 | Mrd16, Mrd17 | T | |
| 7BS | QDon.lrdc-7B* | 52.9 | 52.2–57.7 | 3.4 | 2.7 | 5.0 | Kukri_c19823_491 | 52.69 | 60918162 | 60918262 | Mrd15 | I | |
| DTA | 2B | QDta.lrdc-2B | 65.9 | 64.9–66.7 | 2.6 | 0.6 | 3.0 | BS00065276_51 | 66.14 | 57526894 | 57526994 | Mrd17, Let18 | I |
| 2DS | QDta.lrdc-2D.1 | 41.9 | 40.2–63.5 | 22.9 | 2.1 | 30.0 | RAC875_c7319_195 | 41.83 | 36481727 | 36481627 | Mrd17, Let17, Let18, Let19 | I | |
| 4A | QDta.lrdc-4A.2 | 120.0 | 117.4–123.8 | 6.8 | 1.1 | 7.0 | Excalibur_c4325_1440 | 121.65 | 686650643 | 686650543 | Mrd17, Let18, Let19 | T | |
| 7BS | QDta.lrdc-7B | 27.3 | 18.1–31.9 | 6.6 | 1.1 | 8.0 | IACX198 | 24.26 | 10548520 | 10548633 | Mrd17, Let17, Let18, Let19 | T | |
| 7D | QDta.lrdc-7D.1 | 79.9 | 77.8–91.5 | 2.78 | 1.2 | 5.8 | D_GCE8AKX02ILA1U_88 | 79.8186 | 56638676 | 56638464 | Let17, Let18, Let19 | T | |
| 7D | QDta.lrdc-7D.2 | 117.7 | 113.0–118.0 | 3.4 | 0.8 | 3.5 | wsnp_Ex_c10430_17064001 | 118.58 | 114113970 | 114114170 | Mrd17 | T | |
| PHT | 1B | QPht.lrdc-1B.1 | 36.0 | 33.0–38.5 | 7.1 | 2.4 | 6.0 | IAAV4702 | 34.94 | 544295939 | 544296139 | Mrd17, LetPGH17 | I |
| 2DS | QPht.lrdc-2D.1 | 41.9 | 40.2–63.5 | 11.1 | 3.2 | 10.0 | RAC875_c7319_195 | 41.83 | 36481727 | 36481627 | Mrd17, Let17, Let18, Let19 | I | |
| 4A | QPht.lrdc-4A.1 | 22.6 | 17.8–26.8 | 2.6 | 1.5 | 2.0 | BS00092244_51 | 26.38 | 11756178 | 11756078 | Mrd17 | T | |
| 4B | QPht.lrdc-4B | 49.6 | 46.0–52.6 | 42.0 | 7.3 | 53.0 | Ex_c101685_705 | 49.48 | 31725418 | 31725518 | Mrd17, Let17, LetPGH17, Let18, Let19 | I | |
| 5A | QPht.lrdc-5A.2 | 97.8 | 91.9–103.0 | 3.7 | 1.7 | 3.0 | RAC875_c25072_389 | 97.75 | 524944276 | 524944376 | Let17, Let18 | T | |
| 5D | QPht.lrdc-5D | 26.5 | 24.7–42.5 | 2.9 | 1.7 | 3.0 | wsnp_JD_c3690_4731341 | 26.59 | 401527745 | 401527907 | -- | T | |
| 7D | QPht.lrdc-7D.1 | 79.9 | 67.9–95.2 | 5.0 | 2.0 | 4.0 | D_GCE8AKX02ILA1U_88 | 79.82 | 56638676 | 56638464 | Mrd17, Let17, Let18, Let19 | T | |
| Trait | QTL_i a | Chr | Interval_i b | Position_i c | Range_i d | QTL_j a | Chr | Interval_jb | Position_j c | Range_j d | AA e | SE f |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DI | 1–12 | 1A | BS00103478_51-BS00081682_51 | 17.7 | 12.7–23.2 | 2–86 | 1B | RAC875_c102886_73-Tdurum_contig9144_222 | 138.0 | 133.7–139.9 | 0.44 | 0.08 ** |
| 5–82 | 2B | IAAV1743-Jagger_c3435_145 | 118.4 | 115.5–126.3 | 13–83 | 5A | RAC875_rep_c109969_119-RFL_contig316_572 | 151.3 | 148.0–161.4 | −0.41 | 0.10 ** | |
| 6–11 # | 2D | Ppd-D1-wsnp_CAP12_c1503_764765 | 38.9 | 36–44.4 | 5–51 # | 2B | Tdurum_contig53156_111-Tdurum_contig1653_190 | 81.3 | 72.6–82.5 | −0.38 | 0.08 ** | |
| 6–42 | 2D | RAC875_c5998_1056-D_F5MV3MU01EDEO3_100 | 145.5 | 142.6–155.6 | 4–6 | 2A | Excalibur_rep_c106338_424-RAC875_c54668_102 | 36.5 | 25.5–49.0 | −0.51 | 0.09 ** | |
| DS | 6–10 # | 2D | wsnp_CAP12_c812_428290-RAC875_c7319_195 | 38.1 | 34.5–42.4 | 6–22 # | 2D | BobWhite_c17782_194-TA002913-0806 | 115.4 | 106.2–120.4 | −0.20 | 0.08 ** |
| VRI | 6–10 # | 2D | wsnp_CAP12_c812_428290-RAC875_c7319_195 | 38.1 | 36.1–39.4 | 6–22 # | 2D | BobWhite_c17782_194-TA002913-0806 | 112.4 | 108.2–119.4 | −2.90 | 0.98 ** |
| 10–41 | 4A | RAC875_c6075_214-BobWhite_c35402_66 | 84.8 | 84.2–87.8 | 14–7 | 5B | RAC875_c39204_91-TA015732-1144 | 20.6 | 12.6–27.6 | 6.40 | 0.89 ** | |
| DON | 6–10 # | 2D | wsnp_CAP12_c812_428290-RAC875_c7319_195 | 38.1 | 28.5–48.4 | 11–17 # | 4B | Ex_c101685_705-Tdurum_contig42229_113 | 49.5 | 47.7–50.0 | 2.02 | 0.41 ** |
| 6–21 # | 2D | wsnp_ku_c8712_14751858- BobWhite_c17782_194 | 107.2 | 104.2–110.2 | 8–69 # | 3B | Kukri_c13345_481-wsnp_Ex_c19778_28779907 | 132.7 | 130.1–135.3 | 1.52 | 0.57 ** | |
| 8–12 | 3B | RAC875_rep_C107068_182-RAC875_rep_C69171_241 | 6.7 | 0.0–18.4 | 17–12 | 6B | IAAV5385-Tdurum_contig61178_618 | 28.5 | 25.8–34.5 | 1.92 | 0.53 ** | |
| 8–69 # | 3B | Kukri_c13345_481-wsnp_Ex_c19778_28779907 | 132.7 | 130.1–135.3 | 20–16 # | 7B | Kukri_c19823_491-CAP11_rep_c8279_82 | 53.7 | 52.1–58.3 | −1.65 | 0.54 ** | |
| 13–9 | 5A | Ex_C95453_1499-Excalibur_c13536_202 | 20.3 | 16.3–33.3 | 13–98 # | 5A | Kukri_c108256_381-BS00090847_51 | 195.7 | 184.7–196.9 | −1.93 | 0.43 ** | |
| 13–52 | 5A | wsnp_Ku_c15816_24541162-GENE-3314_78 | 88.4 | 87.9–91.1 | 21–12 | 7D | D_GCE8AKX02ILA1U_88-D_CONTIG12156_209 | 103.8 | 94.8–111.9 | −3.62 | 0.46 ** | |
| DTA | 6–10 # | 2D | wsnp_CAP12_c812_428290-RAC875_c7319_195 | 38.1 | 36.1–40.4 | 19–123 | 7A | Wsnp_Ku_c8437_14341371-Excalibur_c3188_1352 | 234.8 | 228.3–238.5 | 0.55 | 0.20 ** |
| PHT | 6–12 # | 2D | wsnp_CAP12_c1503_764765-wsnp_Ku_c12022_19520410 | 39.4 | 36.1–45.4 | 7–35 # | 3A | Excalibur_c19671_139-wsnp_Ex_c9458_15679797 | 88.9 | 85.0–92.2 | −0.67 | 0.31 * |
| 11–17 # | 4B | Ex_c101685_705-Tdurum_contig42229_113 | 49.5 | 48.7–50.0 | 21–12 # | 7D | D_GCE8AKX02ILA1U_88-D_CONTIG12156_209 | 79.8 | 74.0–85.8 | −1.08 | 0.31 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhariwal, R.; Henriquez, M.A.; Hiebert, C.; McCartney, C.A.; Randhawa, H.S. Mapping of Major Fusarium Head Blight Resistance from Canadian Wheat cv. AAC Tenacious. Int. J. Mol. Sci. 2020, 21, 4497. https://doi.org/10.3390/ijms21124497
Dhariwal R, Henriquez MA, Hiebert C, McCartney CA, Randhawa HS. Mapping of Major Fusarium Head Blight Resistance from Canadian Wheat cv. AAC Tenacious. International Journal of Molecular Sciences. 2020; 21(12):4497. https://doi.org/10.3390/ijms21124497
Chicago/Turabian StyleDhariwal, Raman, Maria A. Henriquez, Colin Hiebert, Curt A. McCartney, and Harpinder S. Randhawa. 2020. "Mapping of Major Fusarium Head Blight Resistance from Canadian Wheat cv. AAC Tenacious" International Journal of Molecular Sciences 21, no. 12: 4497. https://doi.org/10.3390/ijms21124497
APA StyleDhariwal, R., Henriquez, M. A., Hiebert, C., McCartney, C. A., & Randhawa, H. S. (2020). Mapping of Major Fusarium Head Blight Resistance from Canadian Wheat cv. AAC Tenacious. International Journal of Molecular Sciences, 21(12), 4497. https://doi.org/10.3390/ijms21124497








