The Circulating Nucleic Acid Characteristics of Non-Metastatic Soft Tissue Sarcoma Patients
Abstract
1. Introduction
2. Results
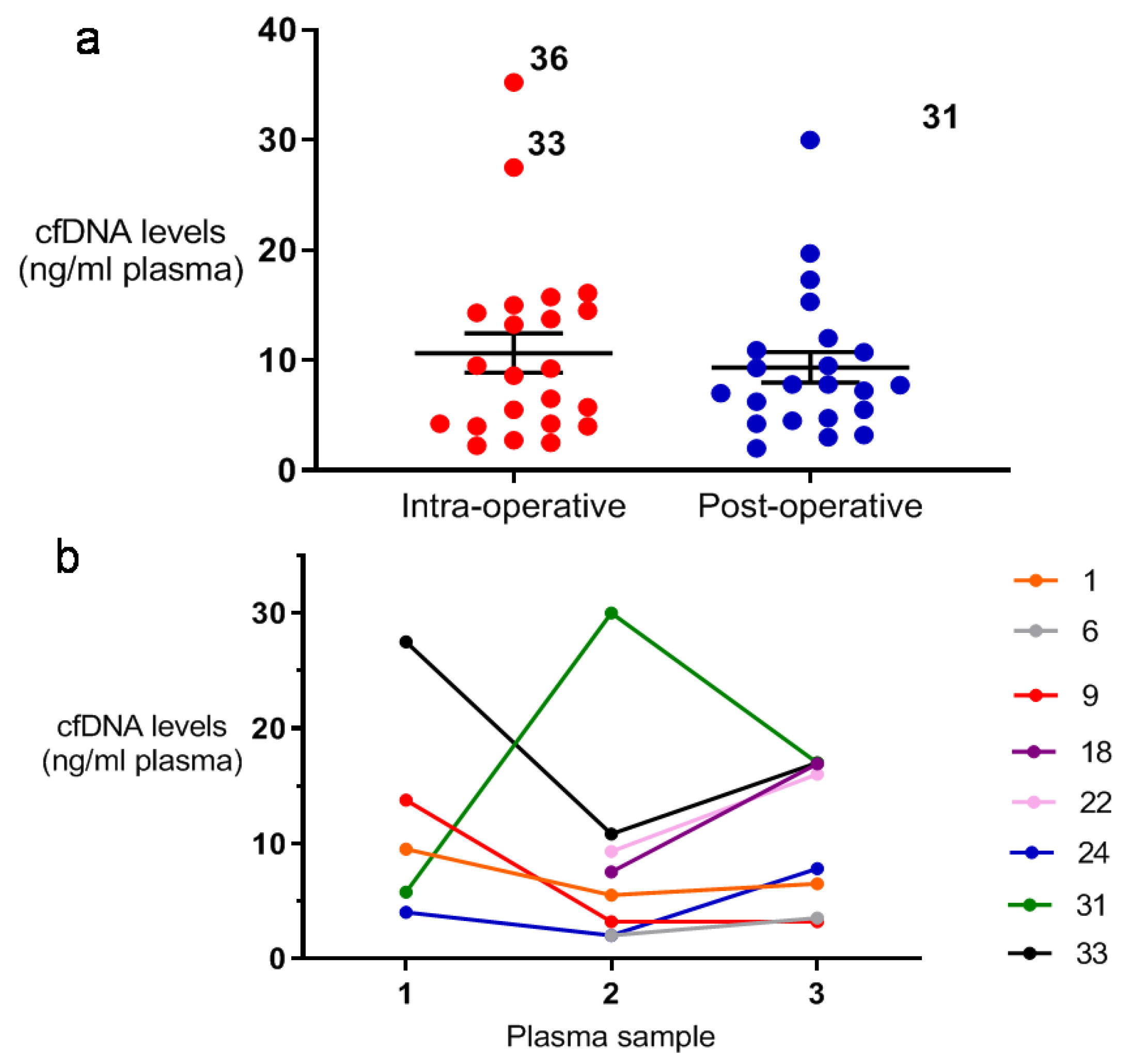
2.1. Comparison of Peri- and Post-Operative cfDNA Levels Among STS Patients
2.2. Targeted NGS of Patients’ Tumour and ctDNA
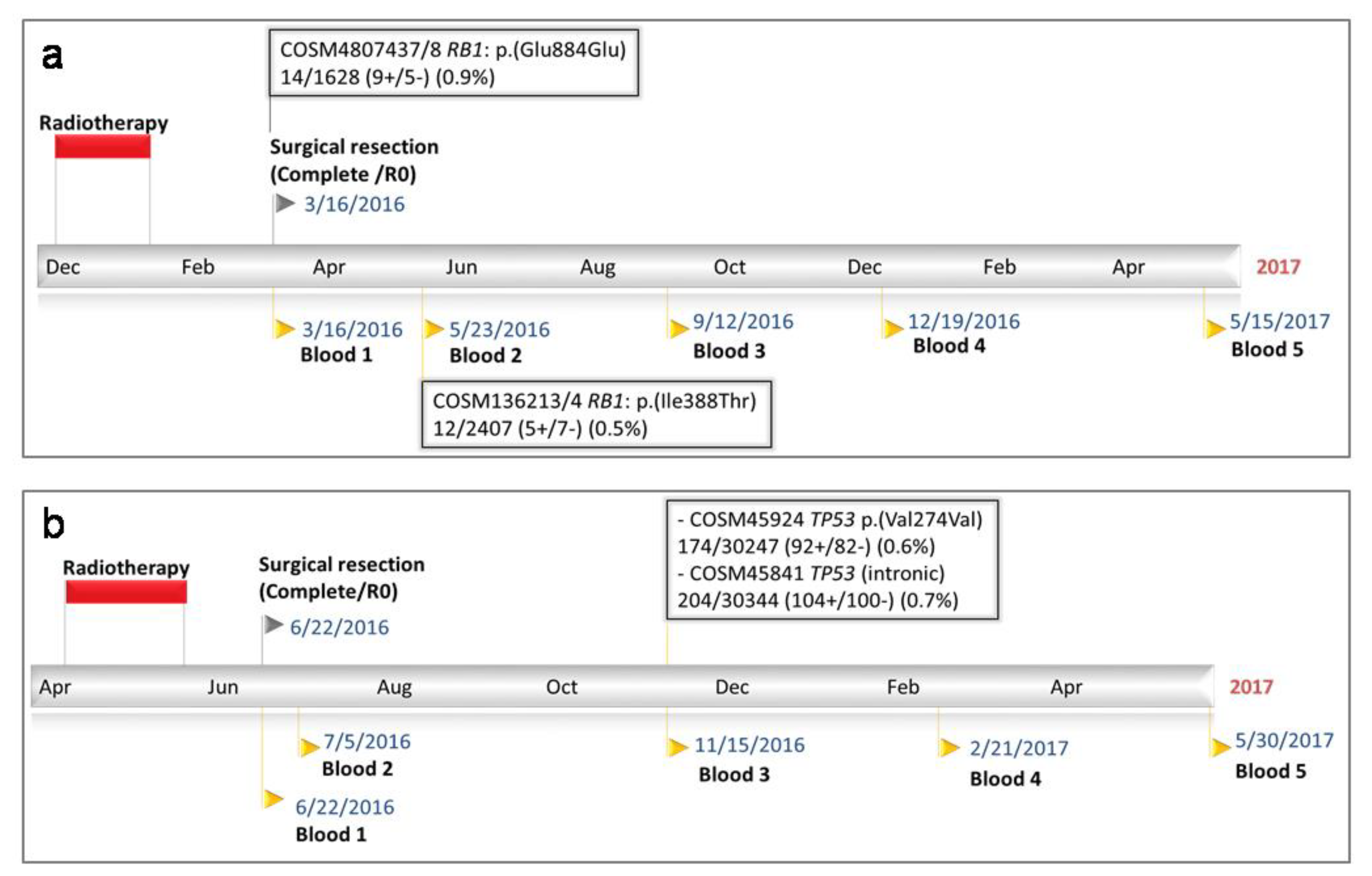
2.2.1. Patient 21
2.2.2. Patient 23
2.3. Patient-Specific ddPCR Analysis of Tumour and ctDNA
2.3.1. Patient 6
2.3.2. Patient 22
2.4. Patient Specific tNGS Analysis of Intra Operative Plasma Samples
2.4.1. Tumour and Plasma Analysis
2.4.2. Patient 43
3. Discussion
4. Methods
4.1. Ethics and Registration
4.2. Patient Enrolment
4.3. Patient Assessment
4.4. Tissue Collection
4.5. Blood Collection
4.6. DNA Extraction and Quantification
4.7. IonTorrent SNV Panel Design
4.8. Semiconductor tNGS and Somatic Variant Calling
4.9. Droplet Digital PCR Assay Development for Tumour Derived SNVs
4.10. SNV ddPCR Reaction Conditions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beckingsale, T.B.; Shaw, C. Epidemiology of bone & soft tissue sarcomas. Orthop. Trauma 2015, 29, 182–188. [Google Scholar]
- Weitz, J.; Antonescu, C.R.; Brennan, M.F. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J. Clin. Oncol 2003, 21, 2719–2725. [Google Scholar] [CrossRef]
- Cormier, J.N.; Pollock, R.E. Soft tissue sarcomas. CA Cancer J. Clin. 2004, 54, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Barretina, J.; Maki, R.G.; Antonescu, C.R.; Singer, S.; Ladany, M. Advances in sarcoma genomics and new therapeutic targets. Nat. Rev. Cancer 2011, 11, 541–557. [Google Scholar] [CrossRef]
- Trovik, C.S.; Scanadinavian Sarcoma Group P. Local recurrence of soft tissue sarcoma. A Scandinavian Sarcoma Group Project. Acta Orthop. Scand. Suppl. 2001, 72, 1–31. [Google Scholar] [PubMed]
- Sabolch, A.; Feng, M.; Griffith, K.; Rzasa, C.; Gadzala, L.; Feng, F.; Biermann, J.S.; Chugh, R.; Ray, M.; Ben-Josef, E. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity. Am. J. Clin. Oncol. 2012, 35, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Zaher, E.R.; Anwar, M.M.; Kohail, H.M.; El-Zoghby, S.M.; Abo-El-Eneen, M.S. Cell-free DNA concentration and integrity as a screening tool for cancer. Indian J. Cancer 2013, 50, 175–183. [Google Scholar] [CrossRef]
- Spindler, K.L.; Pallisgaard, N.; Andersen, R.F.; Brandslund, I.; Jakobsen, A. Circulating free DNA as biomarker and source for mutation detection in metastatic colorectal cancer. PLoS ONE 2015, 10, e0108247. [Google Scholar] [CrossRef]
- Shao, Z.M.; Wu, J.; Shen, Z.Z.; Nguyen, M. p53 mutation in plasma DNA and its prognostic value in breast cancer patients. Clin. Cancer Res. 2001, 7, 2222–2227. [Google Scholar]
- Fournie, G.J.; Courtin, J.P.; Laval, F.; Chalé, J.J.; Pourrat, J.P.; Pujazon, M.C.; Lauque, D.; Carles, P. Plasma DNA as a marker of cancerous cell death. Investigations in patients suffering from lung cancer and in nude mice bearing human tumours. Cancer Lett. 1995, 91, 221–227. [Google Scholar] [CrossRef]
- Kopreski, M.S.; Benko, F.A.; Kwee, C.; Leitzel, K.E.; Eskander, E.; Lipton, A.; Gocke, C.D. Detection of mutant K-ras DNA in plasma or serum of patients with colorectal cancer. Br. J. Cancer 1997, 76, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Kopreski, M.S.; Benko, F.A.; Borys, D.J.; Khan, A.; McGarrity, T.J.; Gocke, C.D. Somatic mutation screening: Identification of individuals harboring K-ras mutations with the use of plasma DNA. J. Natl. Cancer Ins. 2000, 92, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, C.; Kagara, N.; Shimazu, N.; Shimomura, A.; Naoi, Y.; Shimoda, M.; Jin Kim, S.; Noguchi, S. Detection of TP53/PIK3CA Mutations in Cell-Free Plasma DNA From Metastatic Breast Cancer Patients Using Next Generation Sequencing. Clin. Breast Cancer 2016, 16, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Przybyl, J.; Chabon, J.J.; Spans, L.; Ganjoo, K.N.; Vennam, S.; Newman, A.M.; Forgó, E.; Varma, S.; Zhu, S.; Debiec-Rychter, M.; et al. Combination Approach for Detecting Different Types of Alterations in Circulating Tumor DNA in Leiomyosarcoma. Clin. Cancer Res. 2018, 24, 2688–2699. [Google Scholar] [CrossRef]
- Hemming, M.L.; Klega, K.S.; Rhoades, J.; Ha, G.; Acker, K.E.; Andersen, J.L.; Thai, E.; Nag, A.; Thorner, A.R.; Raut, C.P.; et al. Detection of Circulating Tumor DNA in Patients With Leiomyosarcoma With Progressive Disease. JCO Precis. Oncol 2019. [Google Scholar] [CrossRef]
- Eastley, N.C.; Ottolini, B.; Neumann, R.; Luo, J.L.; Hastings, R.K.; Khan, I.; Moore, D.A.; Esler, C.P.; Shaw, J.A.; Royle, N.J.; et al. Circulating tumour-derived DNA in metastatic soft tissue sarcoma. Oncotarget 2018, 9, 10549–10560. [Google Scholar] [CrossRef][Green Version]
- Butler, T.M.; Johnson-Camacho, K.; Peto, M.; Wang, N.J.; Macey, T.A.; Korkola, J.E.; Koppie, T.M.; Corless, C.L.; Gray, J.W.; Spellman, P.T. Exome Sequencing of Cell-Free DNA from Metastatic Cancer Patients Identifies Clinically Actionable Mutations Distinct from Primary Disease. PLoS ONE 2015, 10, e0136407. [Google Scholar] [CrossRef]
- Namlos, H.M.; Zaikova, O.; Bjerkehagen, B.; Vodák, D.; Hovig, E.; Myklebost, O.; Boye, K.; Meza-Zepeda, L.A. Use of liquid biopsies to monitor disease progression in a sarcoma patient: A case report. BMC Cancer 2017, 17, 29. [Google Scholar] [CrossRef]
- Demoret, B.; Gregg, J.; Liebner, D.A.; Tinoco, G.; Lenobel, S.; Chen, J.L. Prospective Evaluation of the Concordance of Commercial Circulating Tumor DNA Alterations with Tumor-Based Sequencing across Multiple Soft Tissue Sarcoma Subtypes. Cancers 2019, 11, 1829. [Google Scholar] [CrossRef]
- Braig, D.; Becherer, C.; Bickert, C.; Braig, M.; Claus, R.; Eisenhardt, A.E.; Heinz, J.; Scholber, J.; Herget, G.W.; Bronsert, P.; et al. Genotyping of circulating cell-free DNA enables noninvasive tumor detection in myxoid liposarcomas. Int. J. Cancer 2019, 145, 1148–1161. [Google Scholar] [CrossRef]
- Szpechcinski, A.; Chorostowska-Wynimko, J.; Struniawski, R.; Kupis, W.; Rudzinski, P.; Langfort, R.; Puscinska, E.; Bielen, P.; Sliwinski, P.; Orlowski, T. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br. J. Cancer 2015, 113, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, O.; Jaiwang, W.; Tangvarasittichai, S. The plasma DNA concentration as a potential breast cancer screening marker. Indian J. Clin. Biochem. 2015, 30, 55–58. [Google Scholar] [CrossRef]
- Mead, R.; Duku, M.; Bhandari, P.; Cree, I.A. Circulating tumour markers can define patients with normal colons, benign polyps, and cancers. Br. J. Cancer 2011, 105, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gupta, S.; Pandey., R.M.; Chauhan, S.S.; Saraya, A. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement, metastasis and poor survival. Cancer Invest. 2015, 33, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.; Massie, C.; Garcia-Corbacho, J.; Mouliere, K.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Destouni, A.; Michailidis, J.; Vrettou, C.; Douroudos, I.I.; Avloniti, A.; Chatzinikolaou, A.; Taxildaris, K.; et al. Time of sampling is crucial for measurement of cell-free plasma DNA following acute aseptic inflammation induced by exercise. Clin. Biochem 2010, 43, 1368–1370. [Google Scholar] [CrossRef]
- Lo, Y.M.; Zhang, J.; Leung, T.N.; Lau, T.K.; Chang, A.M.; Hjelm, N.M. Rapid clearance of fetal DNA from maternal plasma. Am. J. Hum. Genet. 1999, 64, 218–224. [Google Scholar] [CrossRef]
- Cassinotti, E.; Boni, L.; Segato, S.; Rausei, S.; Marzorati, A.; Rovera, F.; Dionigi, G.; David, G.; Mangano, A.; Sambucci, D.; et al. Free circulating DNA as a biomarker of colorectal cancer. Int. J. Surg. 2013, 11 (Suppl. 1), S54–S57. [Google Scholar] [CrossRef]
- Madsen, A.T.; Hojbjerg, J.A.; Sorensen, B.S.; Winther-Larsen, A. Day-to-day and within-day biological variation of cell-free DNA. EBioMedicine 2019, 49, 284–290. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Page, K.; Guttery, D.S.; Zahra, N.; Primrose, L.; Elshaw, S.R.; Pringle, J.H.; Blighe, K.; Marchese, S.D.; Hills, A.; Woodley, L.; et al. Influence of plasma processing on recovery and analysis of circulating nucleic acids. PLoS ONE 2013, 8, e77963. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Page, K.; Salari, R.; Hastings, R.K.; Armstrong, A.; Ahmed, S.; Ali, S.; Cleator, S.; Kenny, L.; Stebbing, J.; et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin. Cancer Res. 2019, 25, 4255–4263. [Google Scholar] [CrossRef] [PubMed]

| Pt | Age (yrs)/Gender | STS Subtype. (+Trojani Tumour Grade) | Tumour Volume (cm3) | Radiotherapy/Chemotherapy (Nil/Neo/Adj) | STS Recurrence | Oncology Outcome | WES | No. Samples Screened for ctDNA: Intra-op (Post-op) | Mode of Analysis. (No. SNVs Screened). | ctDNA Detected. (No. SNVs Detected). | ctDNA Predictive |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76.1/M | MFS (3) | 663 | Rad (Neo) | Metastatic | AWD | Yes | 1 (5). | tNGS-V2. | / | / |
| 3 | 63.1/M | ExMC (unknown) | 588 | Rad (Adj) | No | NED | Yes | 1 (4). | ddPCR (1) | No (0) | / |
| 6 | 55.3/M | UPS (2) | 8 | Rad (Neo) | Metastatic | AWD | Yes | 1 (4). | ddPCR (2) | Yes (1) | No |
| 9 | 62.3/F | LMS (3) | Unknown | Rad (Neo) | Metastatic | AWD | Yes | 1 (1). | ddPCR (2) | No (0) | No |
| 10 | 59.7/F | SS (2) | 9 | Nil | No | NED | No | 1 (6). | tNGS-V2 | / | / |
| 17 | 27.6/M | MFS (1) | 539 | Nil | No | NED | No | 1 (4). | tNGS-V2 | / | / |
| 18 | 80.0/F | HS (unknown) | Unknown | Nil | Metastatic | AWD | Yes | 1 (2). | ddPCR (1) | No (0) | No |
| 21 | 76.5/F | MLS (2) | 198 | Rad (Neo) | No | NED | No | 1 (4). | tNGS-V2 | Yes (1) Uncertain a | / |
| 22 | 65.4/F | UPS (3) | 364 | Chemo (Neo) & Rad(Adj) | Metastatic | AWD | Yes | 1 (4). | ddPCR (2) | Yes (1) | Yes |
| 23 | 53.2/M | UPS (2) | 117 | Rad (Neo) | No | NED | No | 1 (4). | tNGS-V2 | Uncertain b | / |
| 24 | 68.9/M | MFS (2) | 144 | Rad (Adj) | Metastatic | DOD | Yes | 1 (2). | ddPCR (1) | No | No |
| 25 | 36.7/F | UPS (3) | 630 | Nil | unknown. | Lost to FU | Yes | 1 (2). | ddPCR (2) | No | / |
| 26 | 62.8/M | DDLS (2) | 759 | Rad (Neo) | No | NED | Yes | 1 (3). | ddPCR (1) | No | / |
| 27 | 67.0/F | UPS (2) | 129 | Rad (Neo) | No | NED | Yes | / | / | / | / |
| 28 | 70.6/F | MFS (2) | 113 | Nil | No | NED | No | 1 (2). | tNGS-V2. | / | / |
| 29 | 74.0/M | LMS (3) | 525 | Rad (Neo) | No | NED | Yes | / | / | / | / |
| 30 | 22.2/M | ES (3) | 151 | Chemo (Neo) & Rad(Adj) | unknown. | Lost to FU | Yes | / | / | / | / |
| 31 | 45.8/M | UPS (3) | 2947 | Rad (Neo) | Metastatic | DOD | Yes | 1 (3) | tNGS-V2 & V345(3) | No | / |
| 32 | 64.0/F | MFS (3) | 4 | Nil | No | NED | No | 1 (1) | tNGS-V2 (1) | No | / |
| 33 | 79.7 /M | LMS (3) | 3289 | Rad (Neo/Adj) | Metastatic | AWD | Yes | 1 (2) | tNGS-V2 | / | / |
| 34 | 69.0 /M | UPS (2) | 27 | Nil | Local. | NED | Yes. | 1 | tNGS-V345 (3) | No | / |
| 35 | 87.2/F | MFS (3) | 38 | Nil | No | NED | Yes | 1 | tNGS-V345 (2) | No | / |
| 36 | 74.2/M | DDLS (2) | 576 | Rad (Neo). | No | NED | Yes | 1 | tNGS-V345 (4) | No | / |
| 37 | 74.4/F | MFS (2) | 9 | Nil | No | NED | Yes | 1 | tNGS-V345 (1) | No | / |
| 38 | 48.7/F | LMS (2) | 61 | Rad (Adj) | No | NED | Yes | 1 | tNGS-V345 (3) | No | / |
| 40 | 70.3/M | MFS (3) | 68 | Rad (Adj) | No | NED | Yes | 1 | tNGS-V345 (5) | No | / |
| 41 | 81.2/F | MFS (3) | 70 | Rad (Adj) | No | NED | Yes | 1 | tNGS-V345 (3) | No | / |
| 43 | 77.0/M | MFS (2) | 2160 | Rad (Neo) | No | NED | Yes | 1 | tNGS-V345 (5) | Yes (4) | / |
| 44 | 74.0/M | LMS (3) | 506 | Rad (Neo) | No | NED | Yes | 1 | tNGS-V345 (1) | No | / |
| STS Tissue DNA. | cfDNA DNA. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Chr. | Location of SNV | Gene | Coding Strand | Base Chang | Cosmic ID | Predicted Effect | Depth (Reads) | Variant Reads | % | Depth (Reads) | Variant Reads (%) | Total cfDNA (ng/mL) | Plasma Sample. (Weeks Post-op) | |
| 17 | X | 76814213 | ATRX | - | T > C. | 4971451/2 | p.(Asp2106Gly) | 5477 | 23+/28− | 1% | Not detected. | ||||
| 21 | 13 | 49050968 | RB1 | + | A > G | 4807437/8 | p.(Glu884Glu) | Not available | 1628 | 9+/5− (0.9%) | 13.3. | IO | |||
| 13 | 48947576 | RB1 | + | T > C | 136213/4 | p.(Ile388Thr) | 2407 | 5+/7− (0.5%) | 5.8 | PO (10) | |||||
| 23 | 17 | 7577116 | TP53 | - | T > C | 1386598/45924 | p.(Val274Val) | Not detected | 30247 | 92+/82− (0.6%) | 5.0 | PO (21) | |||
| 17 | 7578346 | TP53 | - | G > A | 45841 | Intronic | Not detected | 30344 | 104+/100− (0.7%) | ||||||
| 32 | 17 | 7578526 | TP53 | - | G > T | 303849-52 | p.(Cys135Phe) | 25409 | 2003+/2534− | 18% | Not detected | ||||
| Patient Number | Gene | SNV Position (Chr:Loci) | Coding Strand (+/−) | Base Change | Predicted Effect | Mutation Frequency in STS a | SIFT Prediction b | SNV Detected in Matched Plasma |
|---|---|---|---|---|---|---|---|---|
| 3 | VWDE | 7:12384078 | - | T>C | Cys1302Arg | 42% | D | N |
| 6 | TP53 | 17:7577022 | - | C>T | Arg306Ter | 56% | - | Y |
| BRIP1 | 17:59761496 | - | C>G | Pro971Ala | 20% | T | N | |
| 9 | PTCH1 | 9: 98239884 | - | C>A | Ala332Glu | 23% | D | N |
| LPP | 3:188327063 | + | C>A | Pro182Thr | 46% | D | N | |
| 18 | FLT4 | 5: 180046092 | - | G>A | Val927Met | 18% | D | N |
| 22 | DACH1 | 13: 72053389 | - | A>C | Glu594Asp | 21% | - | N |
| EPHB6 | 7: 142563798 | + | G>A | Gly397Arg | 44% | - | Y | |
| 24 | MMS22L | 6: 97634424 | - | C>T | Gln728Ter | 25% | - | N |
| 25 | ITIH2 | 10: 7769692 | + | C>T | Arg394Trp | 37% | D | N |
| KDM5B | 1: 202777369 | - | C>T | Pro22Leu | 88% | D | N | |
| 26 | PTPRB | 12:70970320 | - | C>T | Thr677Ile | 73% | T | N |
| STS tissue DNA | cfDNA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Chr | Location of SNV | Gene | Coding Strand | Base Change | Predicted Effect | Depth (Reads) | Variant reads | % | Depth (Reads) | Variant Reads (%) | Total cfDNA (ng/mL) |
| 43 | 3 | 183681223 | ABCC5 | - | G > A | p.(R729W) | 2889 | 629+/1053− | 58.22% | 3939 | 11+/36− (1.19%) | 43.6 |
| 5 | 14367062 | TRIO | + | C > T | p.(H950Y) | 17322 | 4071+/2949− | 40.53% | 5670 | 23+/14− (0.65%) | ||
| 8 | 57078921 | PLAG1 | - | C > A | p.(D380Y) p.(D462Y) | 5230 | 864+/1488− | 44.21% | 2259 | 12+/9− (0.93%) | ||
| 5 | 137895674 | HSPA9 | - | C > T | p.(G430D) | 25278 | 6605+/5019− | 45.98% | 5936 | 23+/26− (0.83%) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eastley, N.; Sommer, A.; Ottolini, B.; Neumann, R.; Luo, J.-L.; Hastings, R.K.; McCulloch, T.; Esler, C.P.; Shaw, J.A.; Ashford, R.U.; et al. The Circulating Nucleic Acid Characteristics of Non-Metastatic Soft Tissue Sarcoma Patients. Int. J. Mol. Sci. 2020, 21, 4483. https://doi.org/10.3390/ijms21124483
Eastley N, Sommer A, Ottolini B, Neumann R, Luo J-L, Hastings RK, McCulloch T, Esler CP, Shaw JA, Ashford RU, et al. The Circulating Nucleic Acid Characteristics of Non-Metastatic Soft Tissue Sarcoma Patients. International Journal of Molecular Sciences. 2020; 21(12):4483. https://doi.org/10.3390/ijms21124483
Chicago/Turabian StyleEastley, Nicholas, Aurore Sommer, Barbara Ottolini, Rita Neumann, Jin-Li Luo, Robert K. Hastings, Thomas McCulloch, Claire P. Esler, Jacqueline A. Shaw, Robert U. Ashford, and et al. 2020. "The Circulating Nucleic Acid Characteristics of Non-Metastatic Soft Tissue Sarcoma Patients" International Journal of Molecular Sciences 21, no. 12: 4483. https://doi.org/10.3390/ijms21124483
APA StyleEastley, N., Sommer, A., Ottolini, B., Neumann, R., Luo, J.-L., Hastings, R. K., McCulloch, T., Esler, C. P., Shaw, J. A., Ashford, R. U., & Royle, N. J. (2020). The Circulating Nucleic Acid Characteristics of Non-Metastatic Soft Tissue Sarcoma Patients. International Journal of Molecular Sciences, 21(12), 4483. https://doi.org/10.3390/ijms21124483





