Extracellular Adenine Nucleotides and Adenosine Modulate the Growth and Survival of THP-1 Leukemia Cells
Abstract
1. Introduction
2. Results
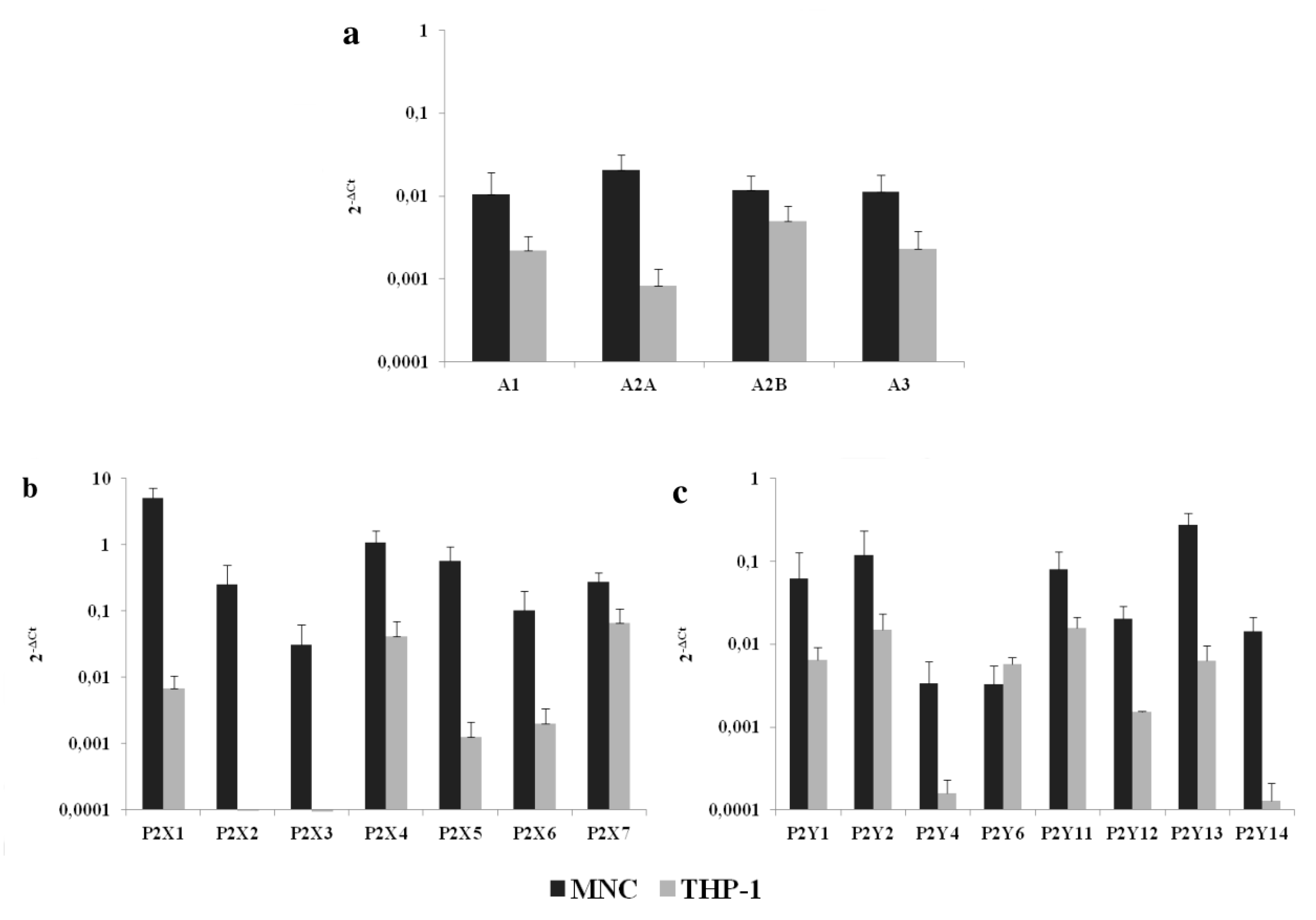
2.1. P1 and P2 Receptor mRNA Are Present in THP-1 Cells
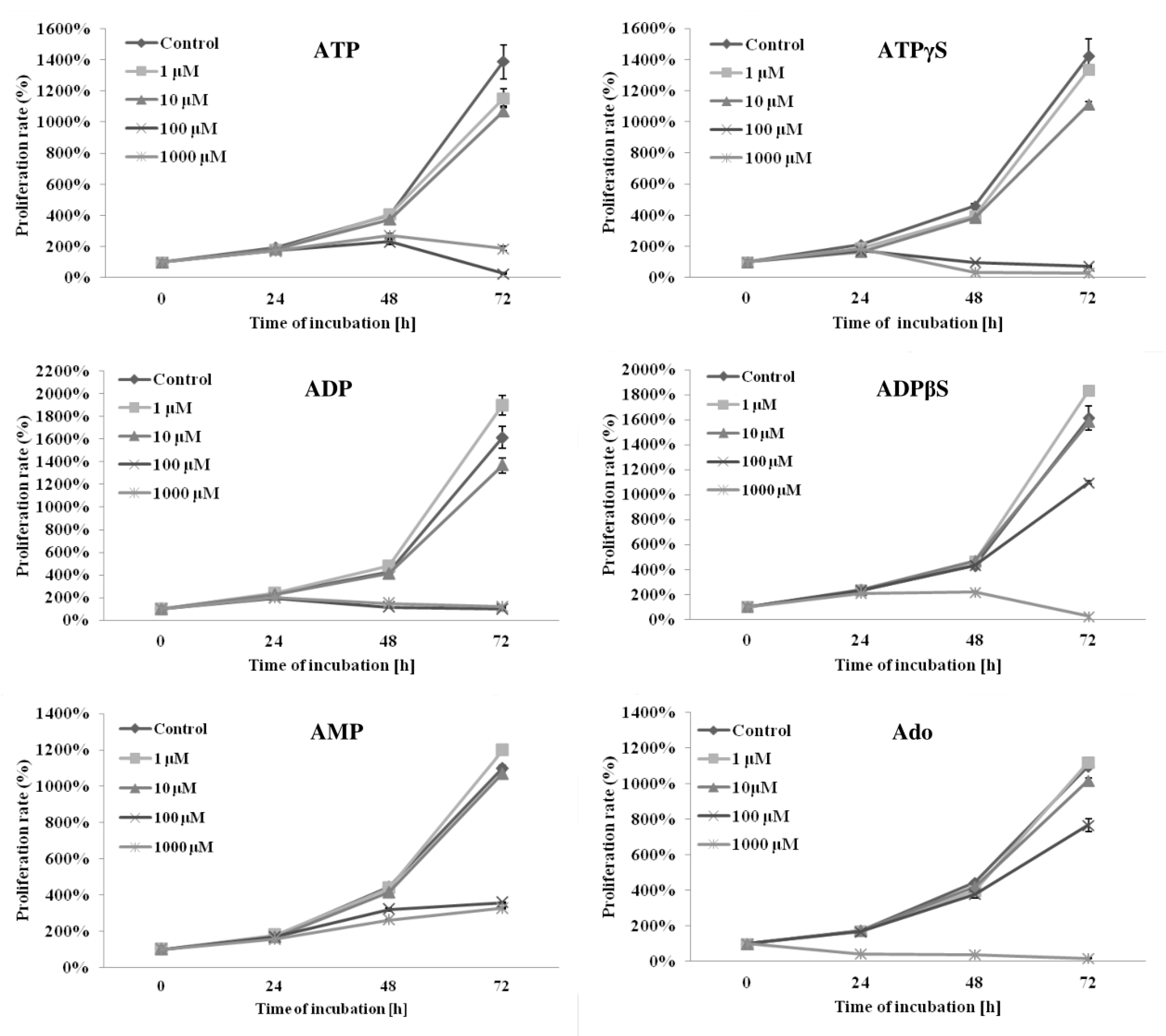
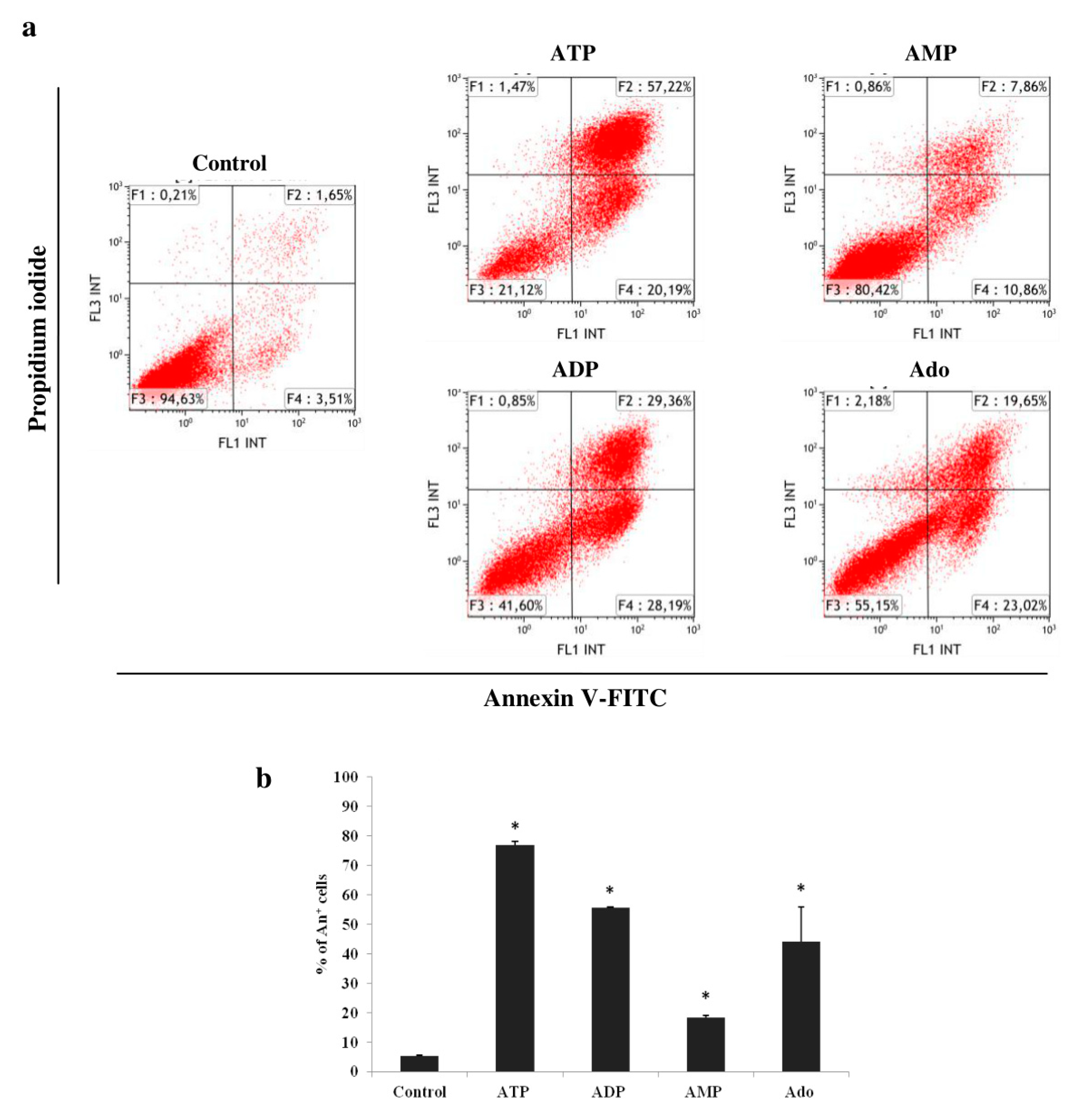
2.2. Adenine Nucleotides and Adenosine Inhibit the Growth of THP-1 Cells by Induction of Apoptosis and Cell Cycle Arrest
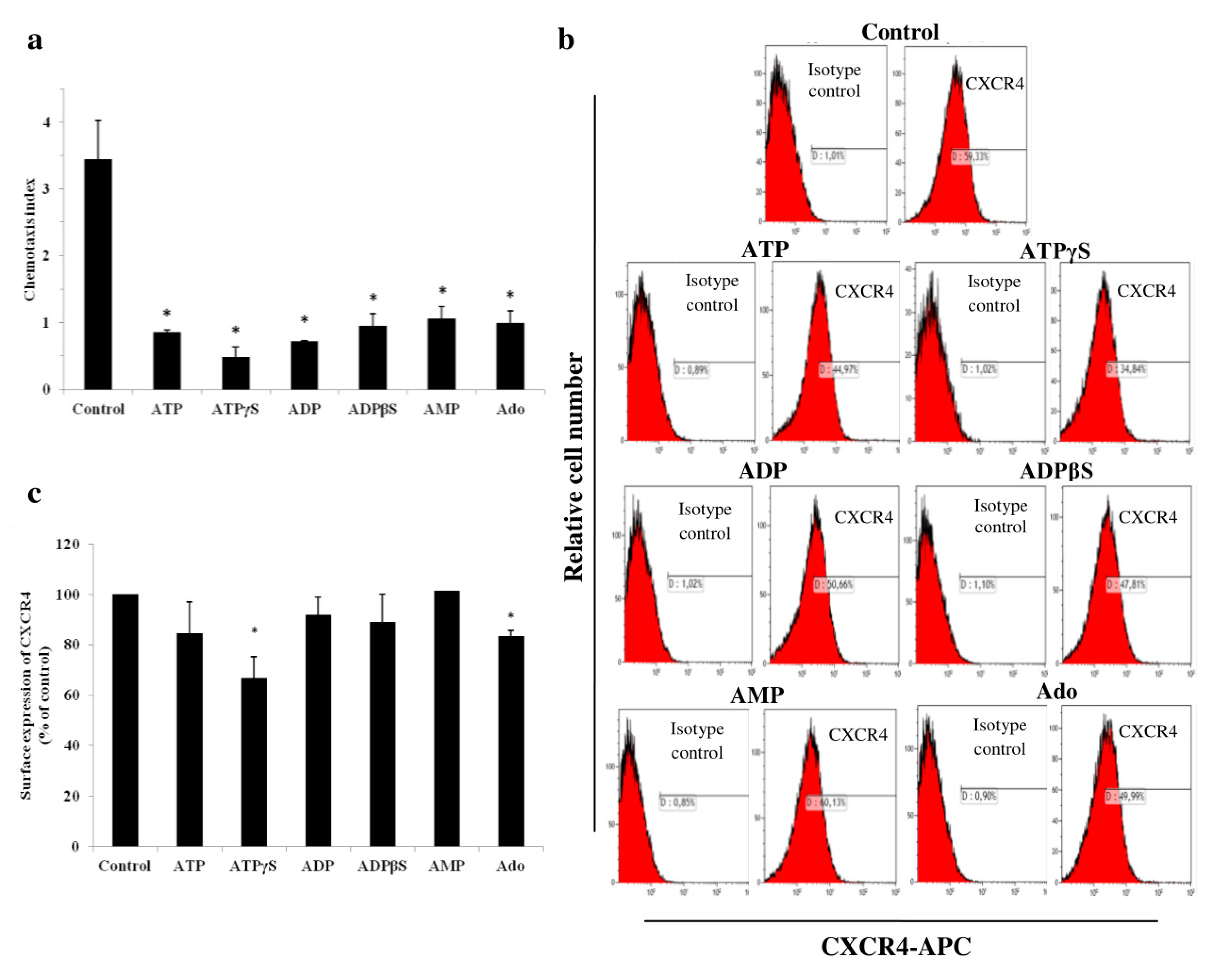
2.3. Adenine Nucleotides and Adenosine Inhibit THP-1 Cell Chemotaxis towards SDF-1
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Real-Time PCR
4.4. Proliferation
4.5. Apoptosis
4.6. Cell Cycle Analysis
4.7. Chemotaxis Assay
4.8. CXCR4 Receptor Expression Analysis by Flow Cytometry
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| CD73 | ecto-5′-nucleotidase |
| CXCR4 | chemokine receptor type 4 |
| E-NTPDase1/CD39 | ecto-nucleoside triphosphate diphosphohydrolase 1 |
| HSC | hematopoietic stem cells |
| MRD | minimal residual disease |
| LSC | leukemic stem cells |
| SDF-1 | stromal cell-derived factor-1 |
References
- Saultz, J.N.; Garzon, R. Acute Myeloid Leukemia: A Concise Review. J. Clin. Med. 2016, 5, 33. [Google Scholar] [CrossRef]
- World Health Organization. Review of Cancer Medicines on the WHO List of Essential Medicines. Available online: http://www.who.int/selection_medicines/committees/expert/20/applications/AML_APL.pdf (accessed on 26 January 2020).
- National Cancer Institute. Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 26 January 2020).
- Fey, M.F.; Buske, C.; ESMO Guidelines Working Group. Acute myeloblastic leukaemias in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi138–vi143. [Google Scholar] [CrossRef] [PubMed]
- Fiegl, M. Epidemiology, pathogenesis, and etiology of acute leukemia. In Handbook of Acute Leukemia; Hiddemann, W., Ed.; Adis: Cham, Switzerland, 2016; pp. 3–13. ISBN 978-3-319-26770-8. [Google Scholar]
- Prada-Arismendy, J.; Arroyave, J.C.; Röthlisberger, S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017, 31, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Tavor, S. Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics 2013, 3, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Hawkins, E.D.; Lo Celso, C. The interplay of leukemia cells and the bone marrow microenvironment. Blood 2008, 131, 1507–1511. [Google Scholar] [CrossRef]
- Kumar, R.; Godavarthy, P.S.; Krause, D.S. The bone marrow microenvironment in health and disease at a glance. J. Cell Sci. 2018, 131, jcs201707. [Google Scholar] [CrossRef]
- Rossi, L.; Salvestrini, V.; Ferrari, D.; Di Virgilio, F.; Lemoli, R.M. The sixth sense: Hematopoietic stem cells detect danger through purinergic signaling. Blood 2012, 120, 2365–2375. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef]
- Di Virgilio, F. Purines, purinergic receptors, and cancer. Cancer Res. 2012, 72, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Adamiak, M.; Plonka, M.; Abdel-Latif, A.; Ratajczak, J. Mobilization of hematopoietic stem cells as a result of innate immunity-mediated sterile inflammation in the bone marrow microenvironment-the involvement of extracellular nucleotides and purinergic signaling. Leukemia 2018, 32, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Filippin, K.J.; de Souza, K.F.S.; de Araujo Júnior, R.T.; Torquato, H.F.V.; Dias, D.A.; Parisotto, E.B.; Ferreira, A.T.; Paredes-Gamero, E.J. Involvement of P2 receptors in hematopoiesis and hematopoietic disorders, and as pharmacological targets. Purinergic Signal. 2020, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Bujko, K.; Brzezniakiewicz-Janus, K.; Kucia, M.; Ratajczak, J.; Ratajczak, M.Z. The inhibition of CD39 and CD73 cell surface ectonucleotidases by small molecular inhibitors enhances the mobilization of bone marrow residing stem cells by decreasing the extracellular level of adenosine. Stem Cell Rev. Rep. 2019, 15, 892–899. [Google Scholar] [CrossRef]
- Salvestrini, V.; Orecchioni, S.; Talarico, G.; Reggiani, F.; Mazzetti, C.; Bertolini, F.; Orioli, E.; Adinolfi, E.; Di Virgilio, F.; Pezzi, A.; et al. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget 2017, 8, 5895–5908. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, G.; Ma, X.; Yang, Y.; Li, G.; Rao, Q.; Nie, K.; Wu, K. Expression of P2X7 in human hematopoietic cell lines and leukemia patients. Leuk. Res. 2004, 28, 1313–1322. [Google Scholar] [CrossRef]
- Chong, J.H.; Zheng, G.G.; Zhu, X.F.; Guo, Y.; Wang, L.; Ma, C.H.; Liu, S.Y.; Xu, L.L.; Lin, Y.M.; Wu, K.F. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem. Biophys. Res. Commun. 2010, 391, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, V.; Zini, R.; Rossi, L.; Gulinelli, S.; Manfredini, R.; Bianchi, E.; Piacibello, W.; Caione, L.; Migliardi, G.; Ricciardi, M.R.; et al. Purinergic signaling inhibits human acute myeloblastic leukemia cell proliferation, migration, and engraftment in immunodeficient mice. Blood 2012, 119, 217–226. [Google Scholar] [CrossRef]
- Dulphy, N.; Henry, G.; Hemon, P.; Khaznadar, Z.; Dombret, H.; Boissel, N.; Bensussan, A.; Toubert, A. Contribution of CD39 to the immunosuppressive microenvironment of acute myeloid leukaemia at diagnosis. Br. J. Haematol. 2014, 165, 722–725. [Google Scholar] [CrossRef]
- Lemoli, R.M.; Ferrari, D.; Fogli, M.; Rossi, L.; Pizzirani, C.; Forchap, S.; Chiozzi, P.; Vaselli, D.; Bertolini, F.; Foutz, T.; et al. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood 2004, 104, 1662–1670. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmerman, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Di Virgilio, F. Purinergic signalling and cancer. Purinergic Signal. 2013, 9, 491–540. [Google Scholar] [CrossRef]
- Seetulsingh-Goorah, S.P.; Stewart, B.W. Growth inhibition of HL-60 cells by extracellular ATP: Concentration-dependent involvement of a P2 receptor and adenosine generation. Biochem. Biophys. Res. Commun. 1998, 250, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Conigrave, A.D.; van der Weyden, L.; Holt, L.; Jiang, L.; Wilson, P.; Christopherson, R.I.; Morris, M.B. Extracellular ATP-dependent suppression of proliferation and induction of differentiation of human HL-60 leukemia cells by distinct mechanisms. Biochem. Pharmacol. 2000, 60, 1585–1591. [Google Scholar] [CrossRef]
- Yoon, M.J.; Lee, H.J.; Kim, J.H.; Kim, D.K. Extracellular ATP induces apoptotic signaling in human monocyte leukemic cells, HL-60 and F-36P. Arch. Pharm. Res. 2006, 29, 1032–1041. [Google Scholar] [CrossRef]
- Lertsuwan, K.; Peters, W.; Johnson, L.; Lertsuwan, J.; Marwa, I.; Sikes, R.A. Purinergic receptor expression and cellular responses to purinergic agonists in human prostate cancer cells. Anticancer Res. 2017, 37, 529–537. [Google Scholar] [CrossRef]
- Joshaghani, H.R.; Jafari, S.M.; Aghaei, M.; Panjehpour, M.; Abedi, H. A3 adenosine receptor agonist induce G1 cell cycle arrest via Cyclin D and cyclin-dependent kinase 4 pathways in OVCAR-3 and Caov-4 cell lines. J. Cancer Res. Ther. 2017, 13, 107–112. [Google Scholar] [CrossRef]
- Lertsuwan, J.; Ruchirawat, M. Inhibitory effects of ATP and adenosine on cholangiocarcinoma cell proliferation and motility. Anticancer Res. 2017, 37, 3553–3561. [Google Scholar] [CrossRef]
- Zimmermann, H. 5′-Nucleotidase: Molecular structure and functional aspects. Biochem. J. 1992, 285, 345–365. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; He, B.; Chen, J.; Liu, P.; Zhao, J.; Zhang, Y.; Li, M.; An, D. The role of P2X7 receptor in ATP-mediated human leukemia cell death: Calcium influx-independent. Acta Biochim. Biophys. Sin. (Shanghai) 2009, 41, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Rittiner, J.E.; Korboukh, I.; Hull-Ryde, E.A.; Jin, J.; Janzen, W.P.; Frye, S.V.; Zylka, M.J. AMP is an adenosine A1 receptor agonist. J. Biol. Chem. 2012, 287, 5301–5309. [Google Scholar] [CrossRef] [PubMed]
- Holien, J.K.; Seibt, B.; Roberts, V.; Salvaris, E.; Parker, M.W.; Cowan, P.J.; Dwyer, K.M. AMP and adenosine are both ligands for adenosine 2B receptor signaling. Bioorg. Med. Chem. Lett. 2018, 28, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.J.; Bowler, W.B.; Fleetwood, P.; Ginty, A.F.; Gallagher, J.A.; Carron, J.A. Extracellular nucleotides stimulate proliferation in MCF-7 breast cancer cells via P2-purinoceptors. Br. J. Cancer 1997, 75, 34–39. [Google Scholar] [CrossRef]
- Batra, S.; Fadeel, I. Release of intracellular calcium and stimulation of cell growth by ATP and histamine in human ovarian cancer cells (SKOV-3). Cancer Lett. 1994, 77, 57–63. [Google Scholar] [CrossRef]
- Jacobson, K.A. P2X and P2Y receptors. Tocris Sci. Rev. Ser. 2010, 33, 1–15. [Google Scholar]
- Rapaport, E. Treatment of human tumor cells with ADP or ATP yields arrest of growth in the S phase of the cell cycle. J. Cell Physiol. 1983, 114, 279–283. [Google Scholar] [CrossRef]
- Lee, E.J.; Min, H.Y.; Chung, H.J.; Park, E.J.; Shin, D.H.; Jeong, L.S.; Lee, S.K. A novel adenosine analog, thio-Cl-IB-MECA, induces G0/G1 cell cycle arrest and apoptosis in human promyelocytic leukemia HL-60 cells. Biochem. Pharmacol. 2005, 70, 918–924. [Google Scholar] [CrossRef]
- Schnerch, D.; Yalcintepe, J.; Schmidts, A.; Becker, H.; Follo, M.; Engelhardt, M.; Wäsch, R. Cell cycle control in acute myeloid leukemia. Am. J. Cancer Res. 2012, 2, 508–528. [Google Scholar]
- Cho, B.S.; Kim, H.J.; Konopleva, M. Targeting the CXCL12/CXCR4 axis in acute myeloid leukemia: From bench to bedside. Korean J. Intern. Med. 2017, 32, 248–257. [Google Scholar] [CrossRef]
- Richard, C.L.; Tan, E.Y.; Blay, J. Adenosine upregulates CXCR4 and enhances the proliferative and migratory responses of human carcinoma cells to CXCL12/SDF-1α. Int. J. Cancer 2006, 119, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, C.; Miele, L.; Porta, A.; Pinto, A.; Morello, S. Activation of the A2B adenosine receptor in B16 melanomas induces CXCL12 expression in FAP-positive tumor stromal cells, enhancing tumor progression. Oncotarget 2016, 7, 64274–64288. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Adamiak, M. Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking. Leukemia 2015, 29, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Tabe, Y.; Jin, L.; Iwabuchi, K.; Wang, R.Y.; Ichikawa, N.; Miida, T.; Cortes, J.; Andreeff, M.; Konopleva, M. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia 2012, 26, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, H.; Yang, Y.; Meng, J.; Liu, J.; Wang, C.; Xu, H. A designed peptide targeting CXCR4 displays anti-acute myelocytic leukemia activity in vitro and in vivo. Sci. Rep. 2014, 4, 6610. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, D.; Genova, T.; Fiorio Pla, A.; Bernardini, M.; Bianco, S.; Bussolati, B.; Mancardi, D.; Giraudo, E.; Maione, F.; Cassoni, P.; et al. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016, 6, 32602. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse |
|---|---|---|
| B2M | 5′-AATGCGGCATCTTCAAACCT-3′ | 5′-TGACTTTGTCACAGCCCAAGATA-3′ |
| ADORA1 | 5′-TGCGAGTTCGAGAAGGTCATC-3′ | 5′-GAGCTGCTTGCGGATTAGGTA-3′ |
| ADORA2A | 5′-CGAGGGCTAAGGGCATCATTG-3′ | 5′-CTCCTTTGGCTGACCGCAGTT-3′ |
| ADORA2B | 5′-CTCTTCCTCGCCTGCTTCGTG-3′ | 5′-TTATACCTGAGCGGGACACAG-3′ |
| ADORA3 | 5′-TACATCATTCGGAACAAACTC-3′ | 5′-GTCTTGAACTCCCGTCCATAA-3′ |
| P2RX1 | 5′-CGCCTTCCTCTTCGAGTATGA-3′ | 5′AGATAACGCCCACCTTCTTATTACG-3′ |
| P2RX2 | 5′-GCCTACGGGATCCGCATT-3′ | 5′-TGGTGGGAATCAGGCTGAAC-3′ |
| P2RX3 | 5′-GCTGGACCATCGGGATCA-3′ | 5′-GAAAACCCACCCTACAAAGTAGGA-3′ |
| P2RX4 | 5′-CCTCTGCTTGCCCAGGTACTC-3′ | 5′-CCAGGAGATACGTTGTGCTCAA-3′ |
| P2RX5 | 5′-CTGCCTGTCGCTGTTCGA-3′ | 5′-GCAGGCCCACCTTCTTGTT-3′ |
| P2RX6 | 5′-AGGCCAGTGTGTGGTGTTCA-3′ | 5′-TCTCCACGGGGCACCAACTC-3′ |
| P2RX7 | 5′-TCTTCGTGATGACAAACTTTCTCAA-3′ | 5′-GTCCTGCGGGTGGGATACT-3′ |
| P2RY1 | 5′-CGTGCTGGTGTGGCTCATT-3′ | 5′-GGACCCCGGTACCTGAGTAGA-3′ |
| P2RY2 | 5′-CCAGGTCCAGGCGTGTGCAT-3′ | 5′-CATCAGGGTTGTGGCCGGAGC-3′ |
| P2RY4 | 5′-TCGCCTCGCAGGCCTTCTCT-3′ | 5′-CAGGCAGGGCACGCCAAAGA-3′ |
| P2RY6 | 5′-GGTGCGGTCCTCAGTGAGCC-3′ | 5′-CGCCAGCACCGCCGAATACA-3′ |
| P2RY11 | 5′-GGCTGAGGATCGGCACGGGA-3′ | 5′-ATGGGCCACAGGAAGTCCCCC-3′ |
| P2RY12 | 5′-AGGTCCTCTTCCCACTGCTCTA-3′ | 5′-CATCGCCAGGCCATTTGT-3′ |
| P2RY13 | 5′-GAGACACTCGGATAGTACAGCTGGTA-3′ | 5′-GCAGGATGCCGGTCAAGA-3′ |
| P2RY14 | 5′-TTCCTTTCAAGATCCTTGGTGACT-3′ | 5′-ACGGAGACCCTGCACACAAA-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchałowicz, K.; Tarnowski, M.; Tkacz, M.; Chlubek, D.; Kłos, P.; Dziedziejko, V. Extracellular Adenine Nucleotides and Adenosine Modulate the Growth and Survival of THP-1 Leukemia Cells. Int. J. Mol. Sci. 2020, 21, 4425. https://doi.org/10.3390/ijms21124425
Puchałowicz K, Tarnowski M, Tkacz M, Chlubek D, Kłos P, Dziedziejko V. Extracellular Adenine Nucleotides and Adenosine Modulate the Growth and Survival of THP-1 Leukemia Cells. International Journal of Molecular Sciences. 2020; 21(12):4425. https://doi.org/10.3390/ijms21124425
Chicago/Turabian StylePuchałowicz, Kamila, Maciej Tarnowski, Marta Tkacz, Dariusz Chlubek, Patrycja Kłos, and Violetta Dziedziejko. 2020. "Extracellular Adenine Nucleotides and Adenosine Modulate the Growth and Survival of THP-1 Leukemia Cells" International Journal of Molecular Sciences 21, no. 12: 4425. https://doi.org/10.3390/ijms21124425
APA StylePuchałowicz, K., Tarnowski, M., Tkacz, M., Chlubek, D., Kłos, P., & Dziedziejko, V. (2020). Extracellular Adenine Nucleotides and Adenosine Modulate the Growth and Survival of THP-1 Leukemia Cells. International Journal of Molecular Sciences, 21(12), 4425. https://doi.org/10.3390/ijms21124425





