Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone
Abstract
1. Introduction
2. Results and Discussion
2.1. Isoplumbagin Suppresses Proliferation and Invasion of Cancer Cells
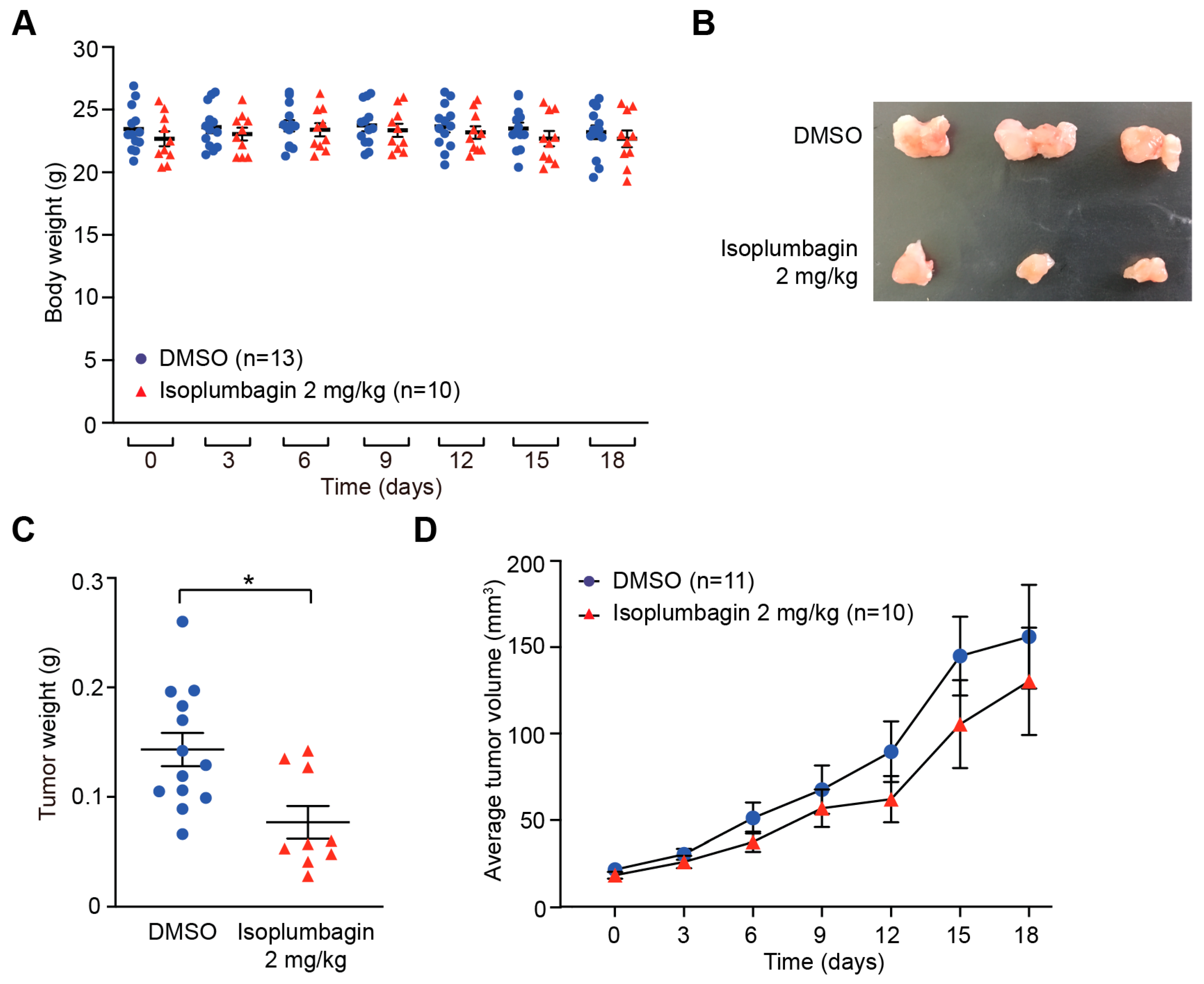
2.2. Isoplumbagin Inhibits Tumor Growth in an OSCC Xenograft Model
2.3. Isoplumbagin Is a Substrate of NAD(P)H Dehydrogenase Quinone 1 (NQO1)
2.4. NQO1 Levels Vary in Different Types of Cancer
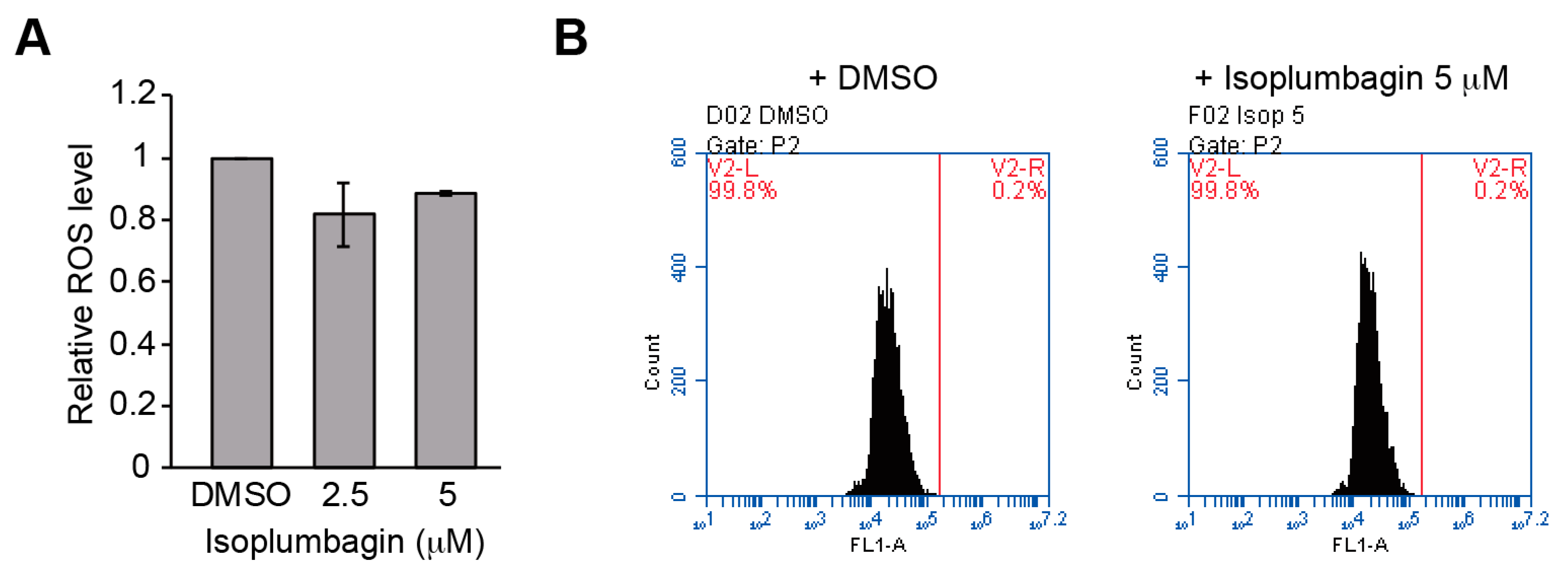
2.5. Isoplumbagin Does Not Increase Oxidative Stress or DNA Fragmentation in the Highly Invasive Oral Cancer Cells
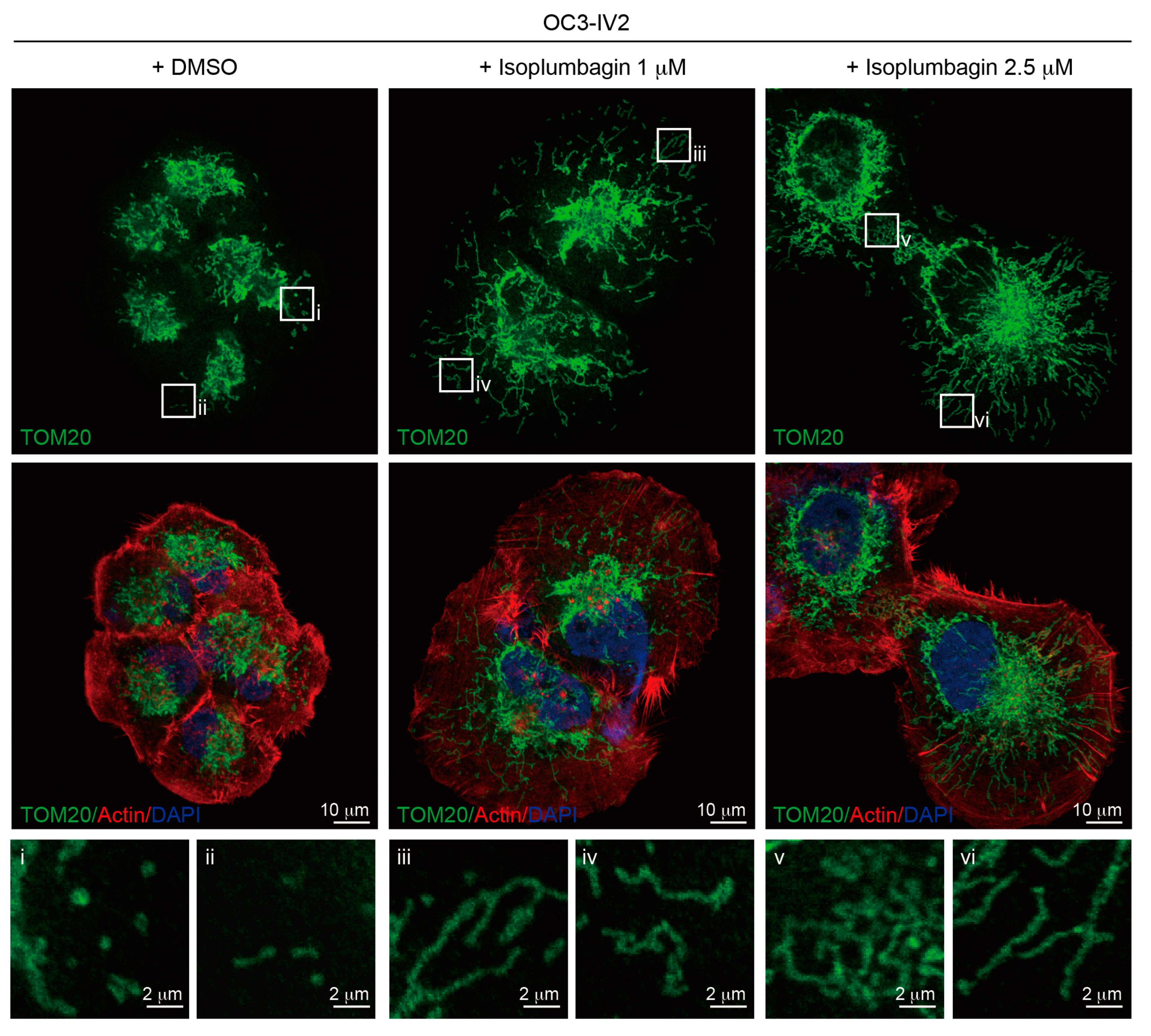
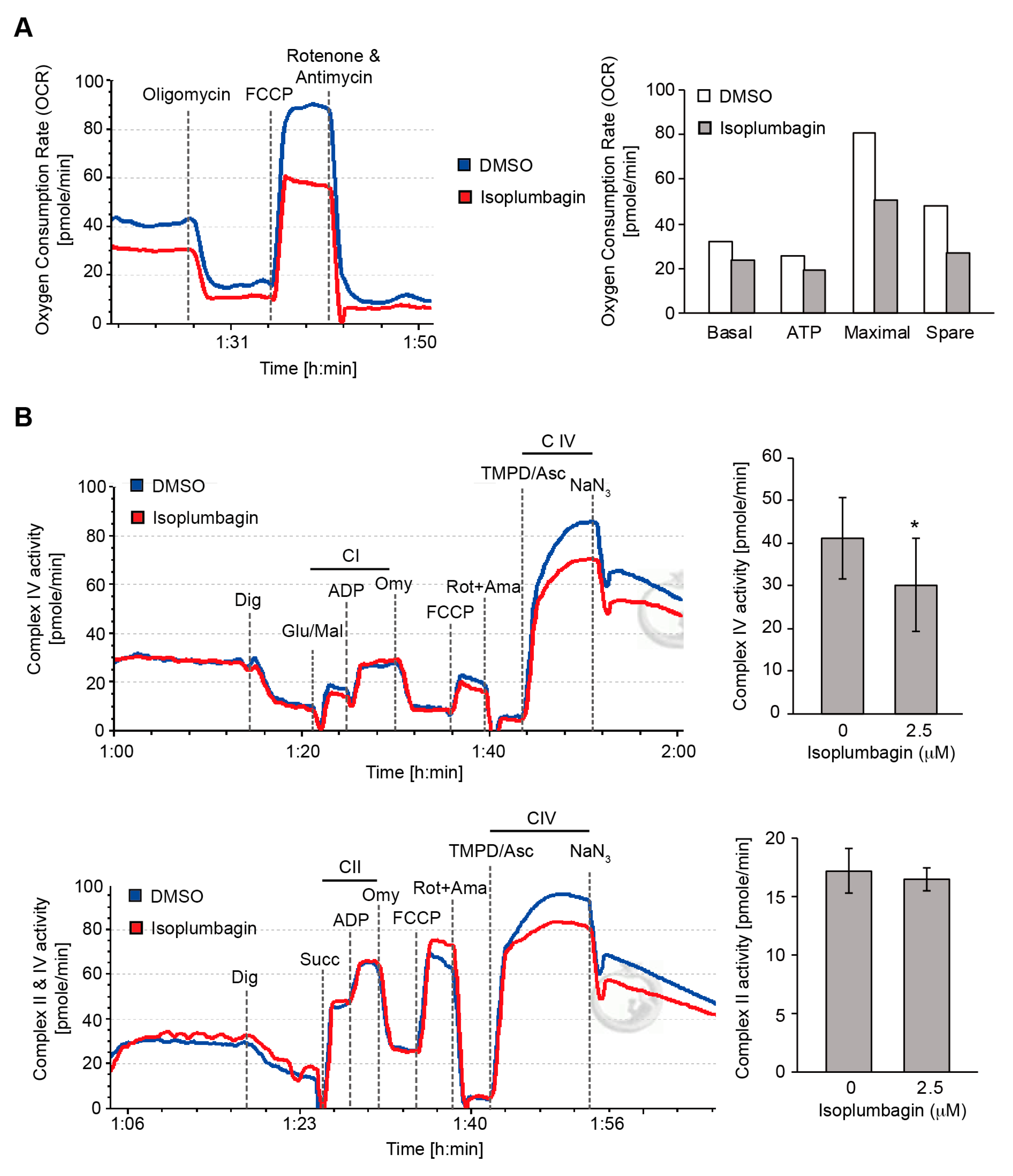
2.6. Isoplumbagin Regulates Mitochondrial Morphogenesis and Respiration in Highly Invasive OC3-IV2 Cells
3. Materials and Methods
3.1. Antibodies and Reagents
3.2. Cell Culture
3.3. MTT Assays and Boyden Chamber Assays
3.4. In Vivo Xenograft Mice Model
3.5. Molecular Docking
3.6. NQO1 Activity Assays
3.7. Immunobloting
3.8. Reactive Oxygen Species Assays
3.9. TUNEL Assays
3.10. Immunofluorescence Staining
3.11. High.-Resolution Respirometry
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OSCC | Oral squamous cell carcinoma |
| NQO1 | NAD(P)H dehydrogenase quinone 1 |
| OCR | Oxygen consumption rate |
| OXPHOS | Mitochondrial respiration |
References
- Majolo, F.; Delwing, L.K.D.B.; Marmitt, D.J.; Bustamante, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Bao, J.L.; Wu, G.S.; Xu, W.S.; Huang, M.Q.; Chen, X.P.; Wang, Y.T. Quinones derived from plant secondary metabolites as anti-cancer agents. Anticancer Agents Med. Chem. 2013, 13, 456–463. [Google Scholar] [PubMed]
- Klopcic, I.; Dolenc, M.S. Chemicals and Drugs Forming Reactive Quinone and Quinone Imine Metabolites. Chem. Res. Toxicol. 2019, 32, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ali, M.; Alam, M.S. A Naphthoquinone from Lawsonia-Inermis Stem Bark. Phytochemistry 1993, 33, 723–724. [Google Scholar] [CrossRef]
- Sobhani, M.; Abbas-Mohammadi, M.; Ebrahimi, S.N.; Aliahmadi, A. Tracking leading anti-Candida compounds in plant samples; Plumbago europaea. Iran J. Microbiol. 2018, 10, 187–193. [Google Scholar]
- Gupta, S.A.M.; Pillai, K.K.; Alam, M.S. Evaluation of anti-inflammatory activity of some constituents of Lawsonia inermis. Fitoterapia 1993, 64, 365–366. [Google Scholar]
- Munn, L.L. Cancer and inflammation. Wiley Int. Rev. Syst. Biol. Med. 2017, 9. [Google Scholar] [CrossRef]
- Shih, C.H.; Chang, Y.J.; Huang, W.C.; Jang, T.H.; Kung, H.J.; Wang, W.C.; Yang, M.H.; Lin, M.C.; Huang, S.F.; Chou, S.W.; et al. EZH2-mediated upregulation of ROS1 oncogene promotes oral cancer metastasis. Oncogene 2017, 36, 6542–6554. [Google Scholar] [CrossRef]
- Lewis, A.M.; Ough, M.; Hinkhouse, M.M.; Tsao, M.S.; Oberley, L.W.; Cullen, J.J. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol. Carcinog. 2005, 43, 215–224. [Google Scholar] [CrossRef]
- Marin, A.; Lopez de Cerain, A.; Hamilton, E.; Lewis, A.D.; Martinez-Penuela, J.M.; Idoate, M.A.; Bello, J. DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. Br. J. Cancer 1997, 76, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, D.; Ma, K.; Wu, X.; Hao, H.; Jiang, S. NAD(P)H:Quinone Oxidoreductase 1 (NQO1) as a Therapeutic and Diagnostic Target in Cancer. J. Med. Chem. 2018, 61, 6983–7003. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Thapa, D.; Huang, S.B.; Munoz, A.R.; Yang, X.; Bedolla, R.G.; Hung, C.N.; Chen, C.L.; Huang, T.H.; Liss, M.A.; Reddick, R.L.; et al. Attenuation of NAD[P]H:quinone oxidoreductase 1 aggravates prostate cancer and tumor cell plasticity through enhanced TGFbeta signaling. Commun. Biol. 2020, 3, 12. [Google Scholar] [CrossRef]
- Pink, J.J.; Planchon, S.M.; Tagliarino, C.; Varnes, M.E.; Siegel, D.; Boothman, D.A. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J. Biol. Chem. 2000, 275, 5416–5424. [Google Scholar] [CrossRef]
- Reinicke, K.E.; Bey, E.A.; Bentle, M.S.; Pink, J.J.; Ingalls, S.T.; Hoppel, C.L.; Misico, R.I.; Arzac, G.M.; Burton, G.; Bornmann, W.G.; et al. Development of beta-lapachone prodrugs for therapy against human cancer cells with elevated NAD(P)H:quinone oxidoreductase 1 levels. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 3055–3064. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhang, A.; Han, C.; Shen, A.; Jiang, L.; Boothman, D.A.; Qiao, J.; Wang, Y.; Huang, X.; et al. NQO1 targeting prodrug triggers innate sensing to overcome checkpoint blockade resistance. Nat. Commun. 2019, 10, 3251. [Google Scholar] [CrossRef]
- Yu-Jung Chang, K.-W.C.; Chen, L. Mitochondrial ROS1 increases mitochondrial fission and respiration in oral squamous. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 2013, 32, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Peiris-Pages, M.; Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Mitochondrial fission as a driver of stemness in tumor cells: mDIVI1 inhibits mitochondrial function, cell migration and cancer stem cell (CSC) signalling. Oncotarget 2018, 9, 13254–13275. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, W.; Yin, W.; Kang, Y.J. Feature Article: The involvement of mitochondrial fission in maintenance of the stemness of bone marrow mesenchymal stem cells. Exp. Biol. Med. (Maywood) 2019, 244, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Makrecka-Kuka, M.; Krumschnabel, G.; Gnaiger, E. High-Resolution Respirometry for Simultaneous Measurement of Oxygen and Hydrogen Peroxide Fluxes in Permeabilized Cells, Tissue Homogenate and Isolated Mitochondria. Biomolecules 2015, 5, 1319–1338. [Google Scholar] [CrossRef]
- Lin, S.C.; Liu, C.J.; Chiu, C.P.; Chang, S.M.; Lu, S.Y.; Chen, Y.J. Establishment of OC3 oral carcinoma cell line and identification of NF-kappa B activation responses to areca nut extract. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2004, 33, 79–86. [Google Scholar] [CrossRef]
- Huang, W.C.; Chan, S.H.; Jang, T.H.; Chang, J.W.; Ko, Y.C.; Yen, T.C.; Chiang, S.L.; Chiang, W.F.; Shieh, T.Y.; Liao, C.T.; et al. miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res. 2014, 74, 751–764. [Google Scholar] [CrossRef]
- Gajdacs, M.; Spengler, G.; Sanmartin, C.; Marc, M.A.; Handzlik, J.; Dominguez-Alvarez, E. Selenoesters and selenoanhydrides as novel multidrug resistance reversing agents: A confirmation study in a colon cancer MDR cell line. Bioorg. Med. Chem. Lett. 2017, 27, 797–802. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model 2011, 51, 2778–2786. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, Y.-C.; Chang, Y.-J.; Wang, C.-H.; Chen, L. Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone. Int. J. Mol. Sci. 2020, 21, 4378. https://doi.org/10.3390/ijms21124378
Tsao Y-C, Chang Y-J, Wang C-H, Chen L. Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone. International Journal of Molecular Sciences. 2020; 21(12):4378. https://doi.org/10.3390/ijms21124378
Chicago/Turabian StyleTsao, Yen-Chi, Yu-Jung Chang, Chun-Hsien Wang, and Linyi Chen. 2020. "Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone" International Journal of Molecular Sciences 21, no. 12: 4378. https://doi.org/10.3390/ijms21124378
APA StyleTsao, Y.-C., Chang, Y.-J., Wang, C.-H., & Chen, L. (2020). Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone. International Journal of Molecular Sciences, 21(12), 4378. https://doi.org/10.3390/ijms21124378




