ABCA1/ApoE/HDL Signaling Pathway Facilitates Myelination and Oligodendrogenesis after Stroke
Abstract
1. Introduction
2. Results
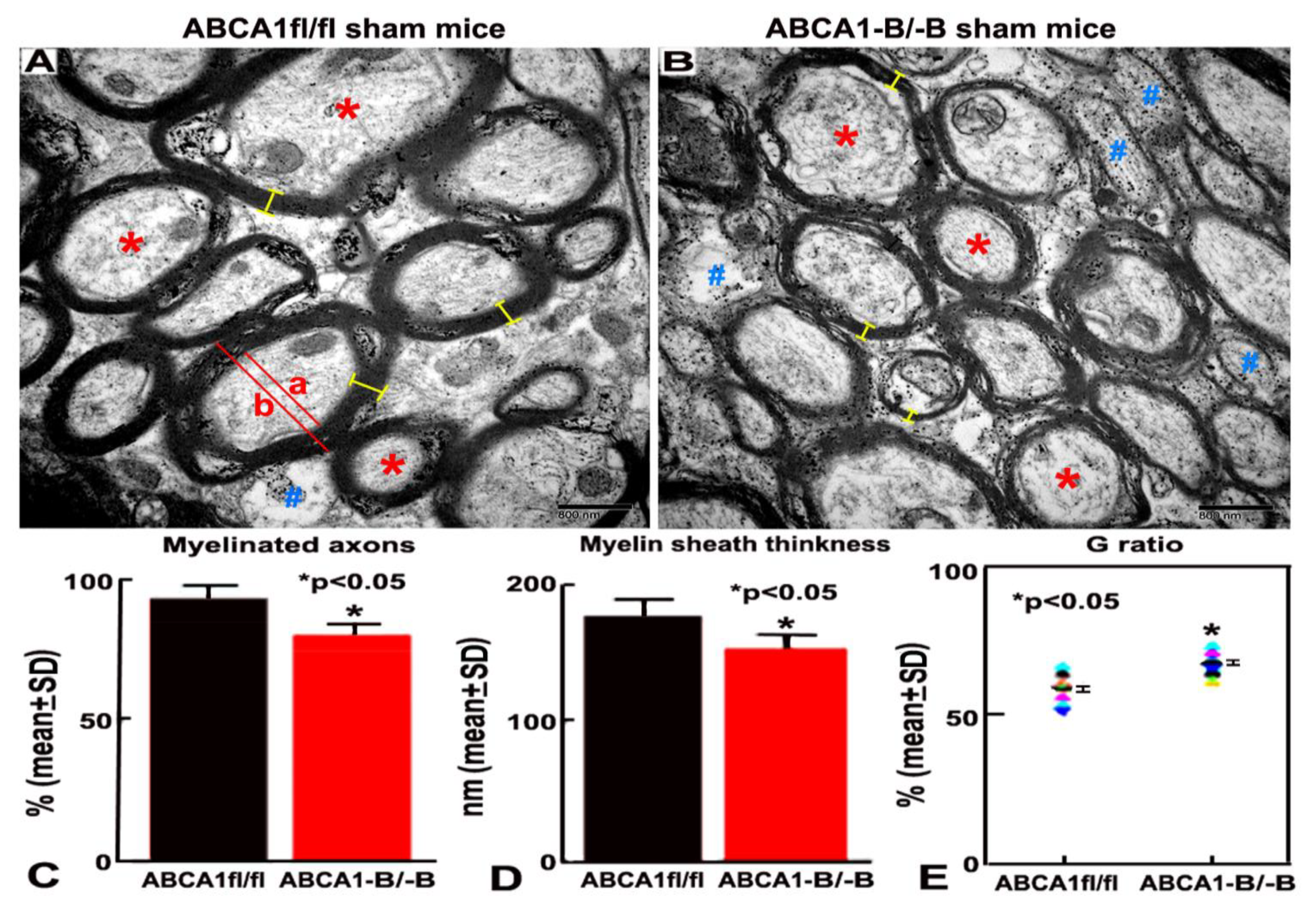
2.1. ABCA1-B/-B Mice Exhibit Reduced Myelination in the WM Area
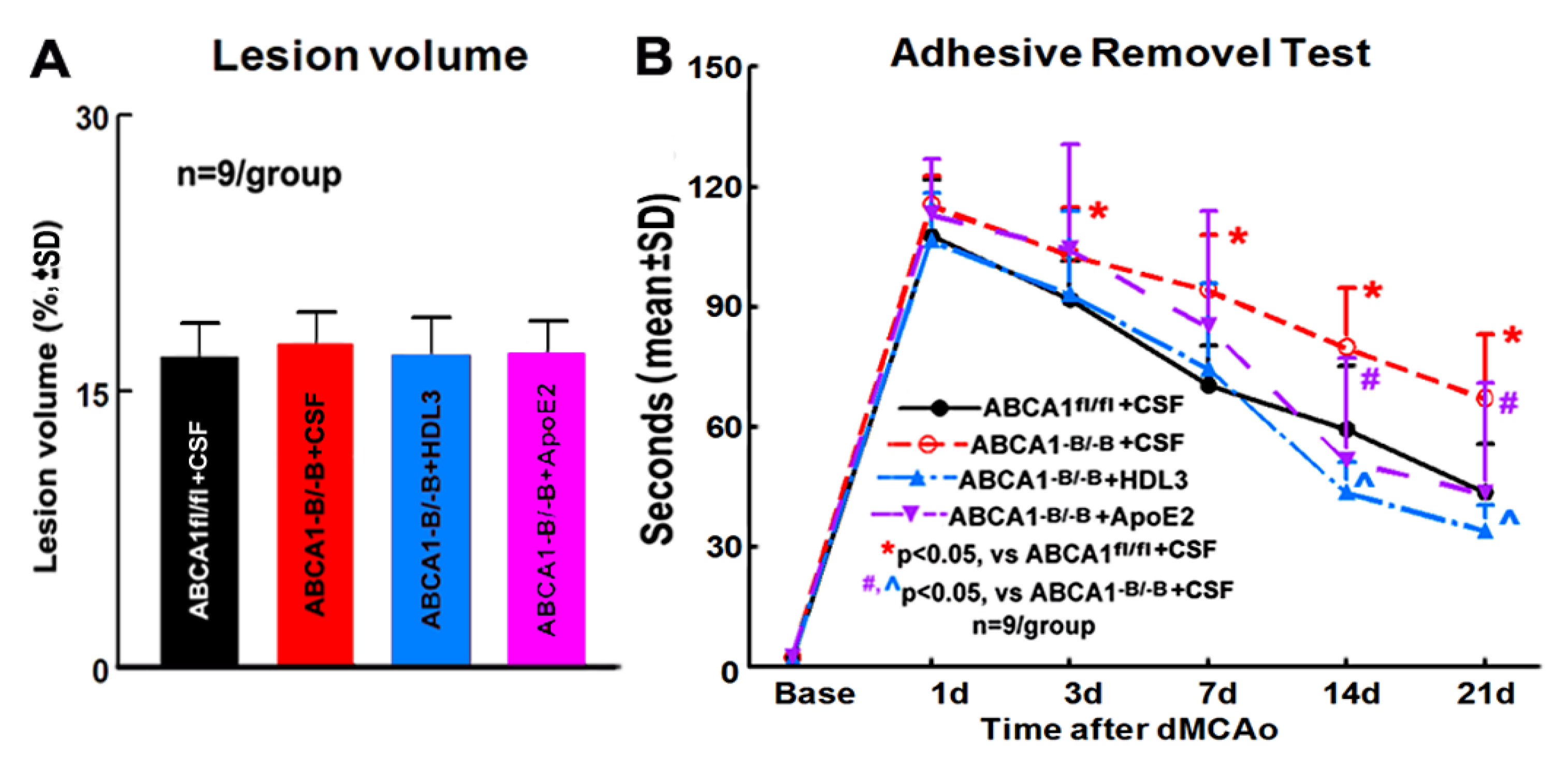
2.2. ABCA1-B/-B Stroke Mice did not Exhibit Change in the Ischemic Lesion Volume but Show Decreased Functional Outcome Compared with ABCA1fl/f Stroke Mice; Administration of HDL3 or ApoE2 in ABCA1-B/-B Stroke Mice Attenuated ABCA1-B/-B-Induced Functional Deficits 14 and 21 Days after Stroke
2.3. ABCA1-B/-B Decreased Myelination in the CC of Ischemic Boundary Zone (IBZ) after Stroke; Administration of HDL3 or ApoE2 Attenuated the Deficits in the Myelination in the CC of IBZ in ABCA1-B/-B Stroke Mice
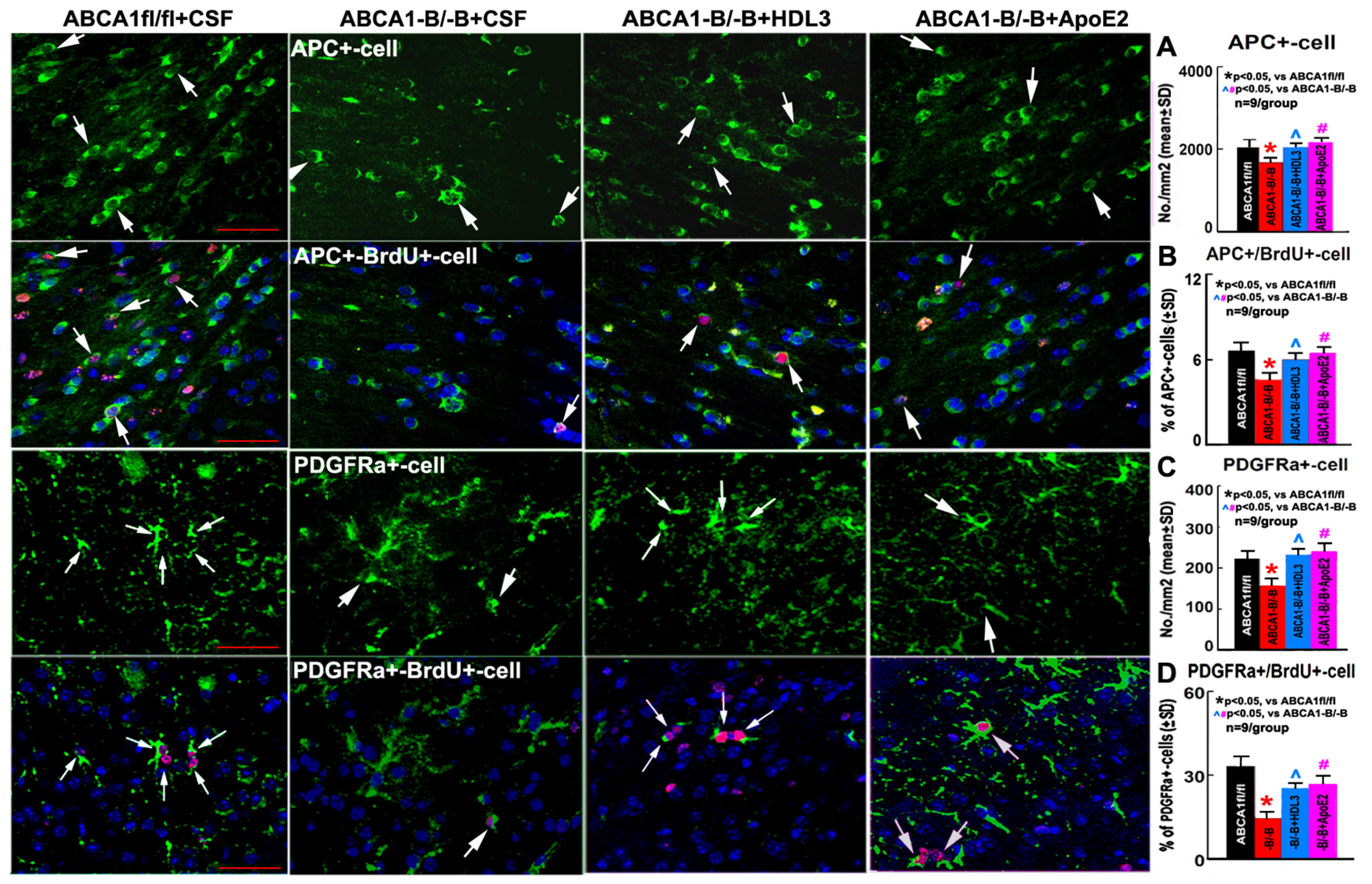
2.4. ABCA1-B/-B Stroke Mice Exhibit Decreased Oligodendrogenesis Compared to ABCA1fl/fl Stroke Mice; Administration of HDL3 or ApoE2 Significantly Attenuated the Reduced Oligodendrogenesis in the IBZ of ABCA1-B/-B After Stroke
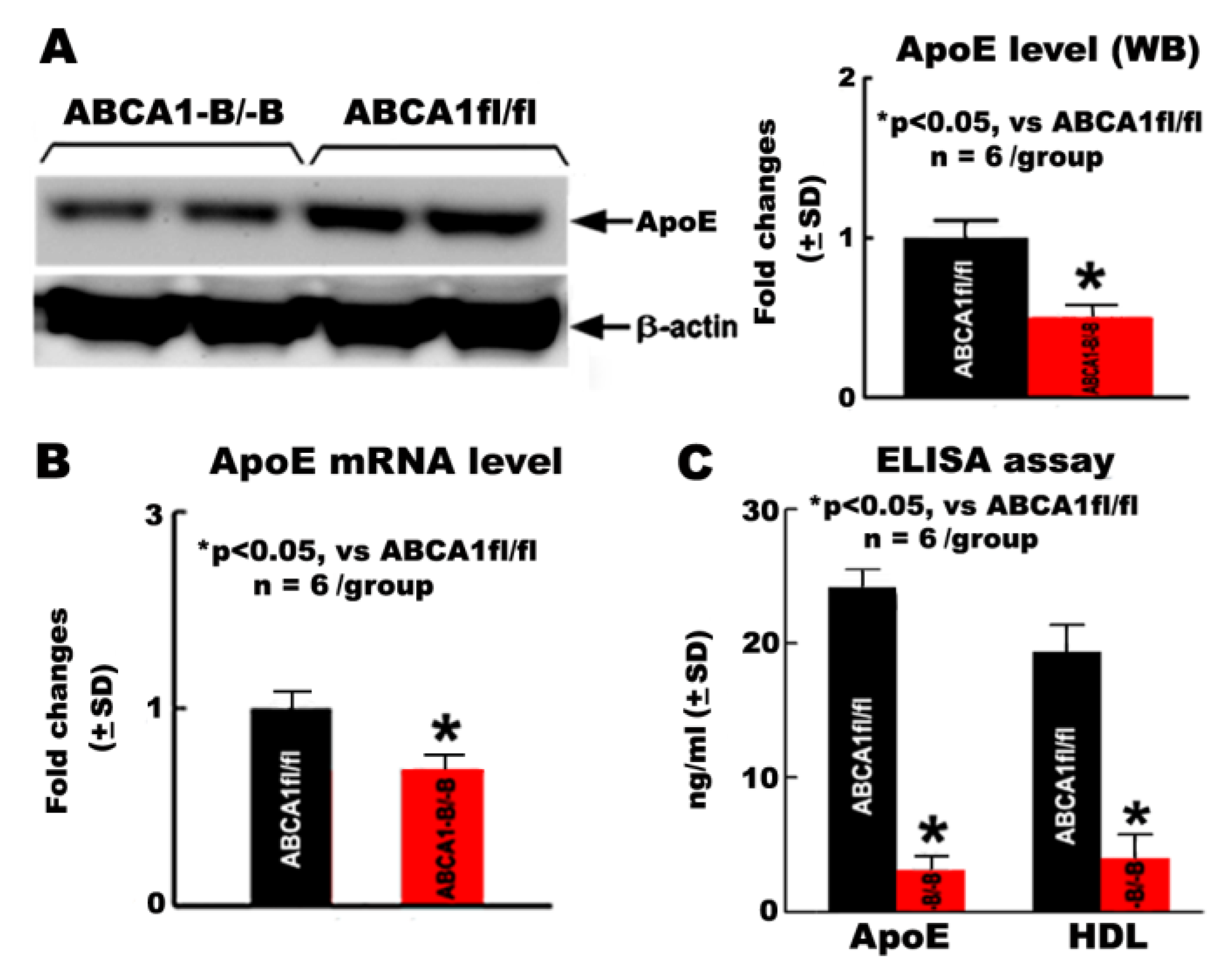
2.5. ABCA1-B/-B Reduced ApoE and HDL Levels in OPCs
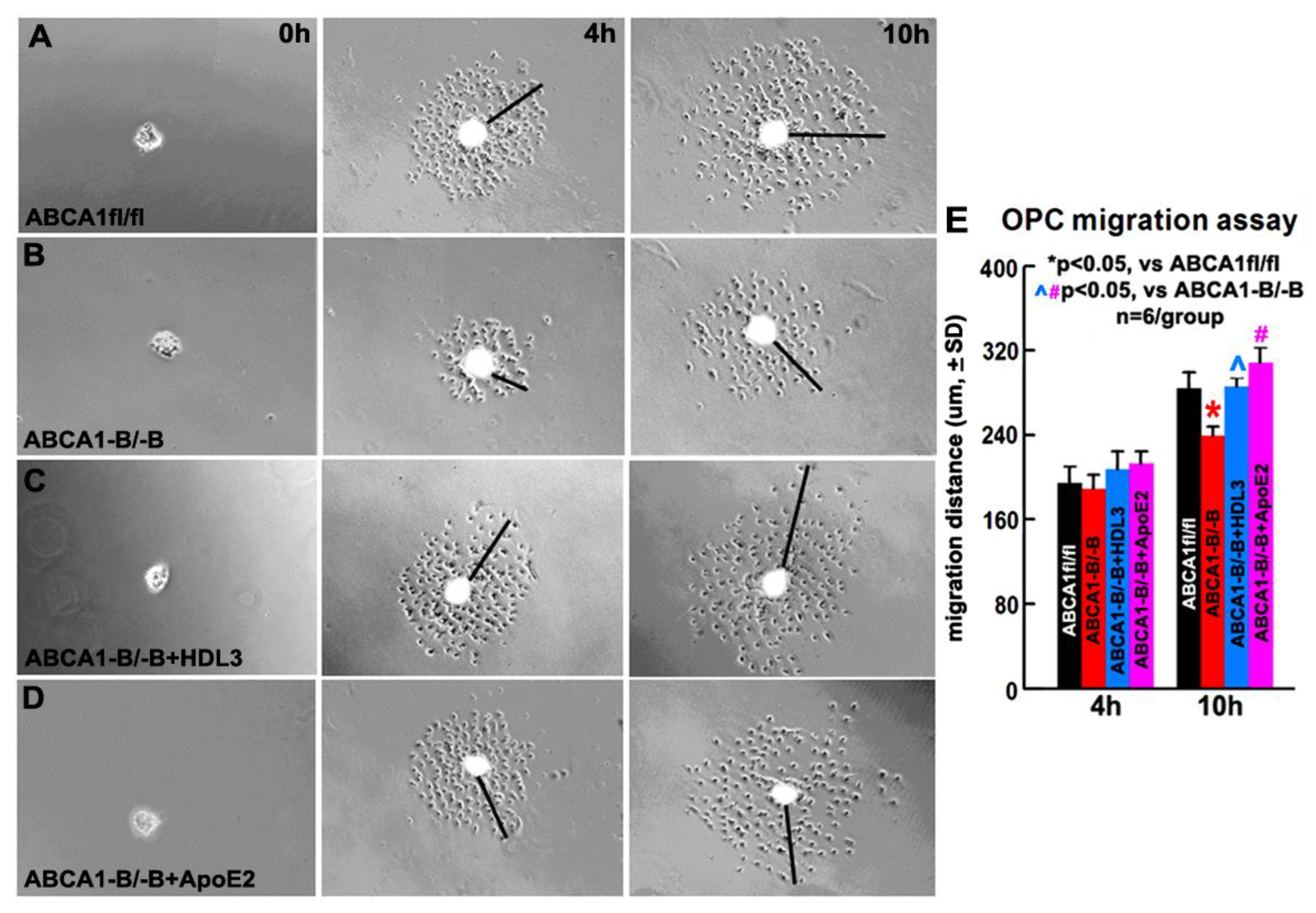
2.6. OPCs Derived from ABCA1-B/-B Mice Exhibit Decreased Migration Compared with OPCs Derived from ABCA1fl/fl Mice; HDL3 and ApoE2 Treatment Significantly Increase OPC Migration of OPCs Derived from ABCA1-B/-B Mice
2.7. ABCA1-B/-B Reduced Maturation of OLs; HDL3 and ApoE2 Treatment Significantly Increase Maturation of OLs
3. Discussion
4. Materials and Methods
4.1. Experimental Groups and dMCAo Model
4.2. Behavioal Testing
4.3. Lesion Volume Measurement
4.4. Quantification of Myelination on EM Images
4.5. Immunohistostaining
4.6. Preparation of OPCs
4.7. OPC Migration Measurement
4.8. OL Maturation Measurement
4.9. RT-PCR
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Lo, E.H. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp. Transl. Stroke Med. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Maki, T.; Lok, J.; Arai, K. Mechanisms of cell-cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015, 1623, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Alix, J.J.; Domingues, A.M. White matter synapses: Form, function, and dysfunction. Neurology 2011, 76, 397–404. [Google Scholar] [CrossRef]
- Bakiri, Y.; Karadottir, R.; Cossell, L.; Attwell, D. Morphological and electrical properties of oligodendrocytes in the white matter of the corpus callosum and cerebellum. J. Physiol. 2011, 589, 559–573. [Google Scholar] [CrossRef]
- Marner, L.; Nyengaard, J.R.; Tang, Y.; Pakkenberg, B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003, 462, 144–152. [Google Scholar] [CrossRef]
- Shi, X.; Kang, Y.; Hu, Q.; Chen, C.; Yang, L.; Wang, K.; Chen, L.; Huang, H.; Zhou, C. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 2010, 1317, 257–267. [Google Scholar] [CrossRef]
- Franklin, R.J. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002, 3, 705–714. [Google Scholar] [CrossRef]
- Fancy, S.P.; Chan, J.R.; Baranzini, S.E.; Franklin, R.J.; Rowitch, D.H. Myelin regeneration: A recapitulation of development? An. Rev. Neurosci. 2011, 34, 21–43. [Google Scholar] [CrossRef]
- Fancy, S.P.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef]
- Li, L.; Harms, K.M.; Ventura, P.B.; Lagace, D.C.; Eisch, A.J.; Cunningham, L.A. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 2010, 58, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. Cns-resident glial progenitor/stem cells produce schwann cells as well as oligodendrocytes during repair of cns demyelination. Cell Stem Cell. 2010, 6, 578–590. [Google Scholar] [CrossRef]
- Zhang, R.L.; Chopp, M.; Roberts, C.; Jia, L.; Wei, M.; Lu, M.; Wang, X.; Pourabdollah, S.; Zhang, Z.G. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J. Cereb. Blood Flow. Metab. 2011, 31, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Chopp, M.; Roberts, C.; Wei, M.; Wang, X.; Liu, X.; Lu, M.; Zhang, Z.G. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS ONE 2012, 7, e48141. [Google Scholar] [CrossRef] [PubMed]
- Rafalski, V.A.; Ho, P.P.; Brett, J.O.; Ucar, D.; Dugas, J.C.; Pollina, E.A.; Chow, L.M.; Ibrahim, A.; Baker, S.J.; Barres, B.A.; et al. Expansion of oligodendrocyte progenitor cells following sirt1 inactivation in the adult brain. Nat. Cell Biol. 2013, 15, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chopp, M.; Zhang, Z.G. Oligodendrogenesis after cerebral ischemia. Front. Cell Neurosci. 2013, 7, 201. [Google Scholar] [CrossRef]
- Gregersen, R.; Christensen, T.; Lehrmann, E.; Diemer, N.H.; Finsen, B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp. Brain Res. 2001, 138, 384–392. [Google Scholar] [CrossRef]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef]
- Franklin, R.J.; Ffrench-Constant, C. Remyelination in the cns: From biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- McTigue, D.M.; Tripathi, R.B. The life, death, and replacement of oligodendrocytes in the adult cns. J. Neurochem. 2008, 107, 1–19. [Google Scholar] [CrossRef]
- Fancy, S.P.; Zhao, C.; Franklin, R.J. Increased expression of nkx2.2 and olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult cns. Mol. Cell Neurosci. 2004, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.A.; Carmichael, S.T. Stroke in cns white matter: Models and mechanisms. Neurosci. Lett. 2018, 684, 193–199. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Gerndt, N.; Winchenbach, J.; Stumpf, S.K.; Hosang, L.; Odoardi, F.; Ruhwedel, T.; Bohler, C.; Barrette, B.; Stassart, R.; et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017, 8, 14241. [Google Scholar] [CrossRef]
- Hirsch-Reinshagen, V.; Zhou, S.; Burgess, B.L.; Bernier, L.; McIsaac, S.A.; Chan, J.Y.; Tansley, G.H.; Cohn, J.S.; Hayden, M.R.; Wellington, C.L. Deficiency of abca1 impairs apolipoprotein e metabolism in brain. J. Biol. Chem. 2004, 279, 41197–41207. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.; Kragol, M.; Aglow, E.; Showe, L.; Heber-Katz, E. Apolipoprotein e expression after spinal cord injury in the mouse. J. Neurosci. Res. 2003, 71, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, M.; Crespo, P.; Berciano, M.T.; Andres, M.A.; Leon, J. Apolipoprotein e expression in the cerebellum of normal and hypercholesterolemic rabbits. Brain Res. Mol. Brain Res. 1994, 21, 115–123. [Google Scholar] [CrossRef]
- Whitney, K.D.; Watson, M.A.; Collins, J.L.; Benson, W.G.; Stone, T.M.; Numerick, M.J.; Tippin, T.K.; Wilson, J.G.; Winegar, D.A.; Kliewer, S.A.; et al. Regulation of cholesterol homeostasis by the liver x receptors in the central nervous system. Mol. Endocrinol. 2002, 16, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Karasinska, J.M.; Rinninger, F.; Lutjohann, D.; Ruddle, P.; Franciosi, S.; Kruit, J.K.; Singaraja, R.R.; Hirsch-Reinshagen, V.; Fan, J.; Brunham, L.R.; et al. Specific loss of brain abca1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 2009, 29, 3579–3589. [Google Scholar] [CrossRef]
- Cui, X.; Chopp, M.; Zhang, Z.; Li, R.; Zacharek, A.; Landschoot-Ward, J.; Venkat, P.; Chen, J. Abca1/apoe/hdl pathway mediates gw3965-induced neurorestoration after stroke. Stroke 2017, 48, 459–467. [Google Scholar] [CrossRef]
- Cui, X.; Chopp, M.; Zacharek, A.; Karasinska, J.M.; Cui, Y.; Ning, R.; Zhang, Y.; Wang, Y.; Chen, J. Deficiency of brain atp-binding cassette transporter a-1 exacerbates blood-brain barrier and white matter damage after stroke. Stroke 2015, 46, 827–834. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.; Zacharek, A.; Landschoot-Ward, J.; Wang, F.; Wu, K.H.; Chopp, M.; Chen, J.; Cui, X. Administration of downstream apoe attenuates the adverse effect of brain abca1 deficiency on stroke. Int. J. Mol. Sci. 2018, 19, 3368. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni-Sajja, A.; Christensen, S.; Straka, M.; Inoue, M.; Lansberg, M.G.; Mlynash, M.; Bammer, R.; Parsons, M.W.; Donnan, G.A.; Davis, S.M.; et al. Prediction of final infarct volume on subacute mri by quantifying cerebral edema in ischemic stroke. J. Cereb. Blood Flow. Metab. 2017, 37, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, R.W.; Cummings, S.E.; Michalski, J.P.; Kothary, R. A new in vitro mouse oligodendrocyte precursor cell migration assay reveals a role for integrin-linked kinase in cell motility. BMC Neurosci. 2016, 17, 7. [Google Scholar] [CrossRef]
- O’Meara, R.W.; Ryan, S.D.; Colognato, H.; Kothary, R. Derivation of enriched oligodendrocyte cultures and oligodendrocyte/neuron myelinating co-cultures from post-natal murine tissues. J. Vis. Exp. 2011, 21, 3324. [Google Scholar] [CrossRef]
- Lourenco, T.; Paes de Faria, J.; Bippes, C.A.; Maia, J.; Lopes-da-Silva, J.A.; Relvas, J.B.; Graos, M. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Sci. Rep. 2016, 6, 21563. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Sc1/hevin identifies early white matter injury after ischemia and intracerebral hemorrhage in young and aged rats. J. Neuropathol. Exp. Neurol. 2012, 71, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Gutierrez-Fernandez, M.; Ramos-Cejudo, J.; Rodriguez-Frutos, B.; Fuentes, B.; Sobrino, T.; Hermanz, Z.N.; Campos, F.; Lopez, J.A.; Cerdan, S.; et al. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Stem Cell Res. Ther. 2015, 6, 121. [Google Scholar] [CrossRef]
- Pham, L.D.; Hayakawa, K.; Seo, J.H.; Nguyen, M.N.; Som, A.T.; Lee, B.J.; Guo, S.; Kim, K.W.; Lo, E.H.; Arai, K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia 2012, 60, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sozmen, E.G.; Hinman, J.D.; Carmichael, S.T. Models that matter: White matter stroke models. Neurotherapeutics 2012, 9, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Satriotomo, I.; Fazal, J.A.; Nadeau, S.E.; Dore, S. Optimization of a clinically relevant model of white matter stroke in mice: Histological and functional evidences. J. Neurol. Neurosurg. 2015, 2, 114. [Google Scholar] [CrossRef] [PubMed]
- Riddle, A.; Maire, J.; Gong, X.; Chen, K.X.; Kroenke, C.D.; Hohimer, A.R.; Back, S.A. Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke 2012, 43, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Vincze, A.; Mazlo, M.; Seress, L.; Komoly, S.; Abraham, H. A correlative light and electron microscopic study of postnatal myelination in the murine corpus callosum. Int. J. Dev. Neurosci. Offic. J. Int. Soc. Dev. Neurosci. 2008, 26, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Karasinska, J.M.; de Haan, W.; Franciosi, S.; Ruddle, P.; Fan, J.; Kruit, J.K.; Stukas, S.; Lutjohann, D.; Gutmann, D.H.; Wellington, C.L.; et al. Abca1 influences neuroinflammation and neuronal death. Neurobiol. Dis. 2013, 54, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.D.; Leonardo, C.C.; Hall, A.A.; Shahaduzzaman, M.D.; Collier, L.A.; Willing, A.E.; Pennypacker, K.R. Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Res. 2010, 1366, 172–188. [Google Scholar] [CrossRef]
- Grinspan, J.B. Bone morphogenetic proteins: Inhibitors of myelination in development and disease. Vitam. Horm. 2015, 99, 195–222. [Google Scholar]
- Sozmen, E.G.; Rosenzweig, S.; Llorente, I.L.; DiTullio, D.J.; Machnicki, M.; Vinters, H.V.; Havton, L.A.; Giger, R.J.; Hinman, J.D.; Carmichael, S.T. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8453–E8462. [Google Scholar] [CrossRef]
- From, R.; Eilam, R.; Bar-Lev, D.D.; Levin-Zaidman, S.; Tsoory, M.; LoPresti, P.; Sela, M.; Arono, R.; Aharoni, R. Oligodendrogenesis and myelinogenesis during postnatal development effect of glatiramer acetate. Glia 2014, 62, 649–665. [Google Scholar] [CrossRef]
- Tanaka, K.; Nogawa, S.; Suzuki, S.; Dembo, T.; Kosakai, A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003, 989, 172–179. [Google Scholar] [CrossRef]
- Ueno, Y.; Chopp, M.; Zhang, L.; Buller, B.; Liu, Z.; Lehman, N.L.; Liu, X.S.; Zhang, Y.; Roberts, C.; Zhang, Z.G. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke 2012, 43, 2221–2228. [Google Scholar] [CrossRef]
- Karadottir, R.; Hamilton, N.B.; Bakiri, Y.; Attwell, D. Spiking and nonspiking classes of oligodendrocyte precursor glia in cns white matter. Nat. Neurosci. 2008, 11, 450–456. [Google Scholar] [CrossRef]
- Moyon, S.; Dubessy, A.L.; Aigrot, M.S.; Trotter, M.; Huang, J.K.; Dauphinot, L.; Potier, M.C.; Kerninon, C.; Melik Parsadaniantz, S.; Franklin, R.J.; et al. Demyelination causes adult cns progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci. 2015, 35, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Miron, V.; Cui, Q.; Wegner, C.; Antel, J.; Bruck, W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, M.; Zhang, X.; Miao, Z.; Chua, B.H.; Hamdy, R.C.; Zhang, Q.-G.; Liu, C.-F.; Xu, X. Increased oligodendrogenesis by humanin promotes axonal remyelination and neurological recovery in hypoxic/ischemic brains. Hippocampus 2015, 25, 62–71. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, H.; Yao, E.; Tian, Y.; Zhang, H.; Xu, L.; Yu, Z.; Fang, Y.; Wang, W.; Du, P.; et al. Soluble epoxide hydrolase inhibition promotes white matter integrity and long-term functional recovery after chronic hypoperfusion in mice. Sci. Rep. 2017, 7, 7758. [Google Scholar] [CrossRef] [PubMed]
- Tontsch, U.; Archer, D.R.; Dubois-Dalcq, M.; Duncan, I.D. Transplantation of an oligodendrocyte cell line leading to extensive myelination. Proc. Natl. Acad. Sci. USA 1994, 91, 11616–11620. [Google Scholar] [CrossRef]
- Chari, D.M.; Blakemore, W.F. New insights into remyelination failure in multiple sclerosis: Implications for glial cell transplantation. Mult. Scler. 2002, 8, 271–277. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Levine, J.M.; Blakemore, W.F. Response of the oligodendrocyte progenitor cell population (defined by ng2 labelling) to demyelination of the adult spinal cord. Glia 1998, 22, 161–170. [Google Scholar] [CrossRef]
- Schonberg, D.L.; Goldstein, E.Z.; Sahinkaya, F.R.; Wei, P.; Popovich, P.G.; McTigue, D.M. Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to ng2 cells in vivo. J. Neurosci. 2012, 32, 5374–5384. [Google Scholar] [CrossRef]
- Shibahara, T.; Ago, T.; Nakamura, K.; Tachibana, M.; Yoshikawa, Y.; Komori, M.; Yamanaka, K.; Wakisaka, Y.; Kitazono, T. Pericyte-mediated tissue repair through pdgfrbeta promotes peri-infarct astrogliosis, oligodendrogenesis, and functional recovery after acute ischemic stroke. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Yang, H.C.; Zhang, M.; Wu, R.; Zheng, H.Q.; Zhang, L.Y.; Luo, J.; Li, L.L.; Hu, X.Q. C-c chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage m2 polarization. World J. Stem Cells 2020, 12, 152–167. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Gutierrez-Fernandez, M.; Otero-Ortega, L.; Rodriguez-Frutos, B.; Fuentes, B.; Vallejo-Cremades, M.T.; Hernanz, T.N.; Cerdan, S.; Diez-Tejedor, E. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke 2015, 46, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Komitova, M.; Perfilieva, E.; Mattsson, B.; Eriksson, P.S.; Johansson, B.B. Enriched environment after focal cortical ischemia enhances the generation of astroglia and ng2 positive polydendrocytes in adult rat neocortex. Exp. Neurol. 2006, 199, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Itoh, N.; Tassoni, A.; Matsukawa, M.A.; Ren, E.; Tse, V.; Jang, E.; Suen, T.T.; Itoh, Y. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, K.; Mulder, M.; Smets, I.; Timmermans, S.; Smeets, K.; Ameloot, M.; Hendriks, J.J. Liver × receptors regulate cholesterol homeostasis in oligodendrocytes. J. Neurosci. Res. 2012, 90, 60–71. [Google Scholar] [CrossRef]
- Saher, G.; Brugger, B.; Lappe-Siefke, C.; Mobius, W.; Tozawa, R.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef]
- Ballerini, P.; Ciccarelli, R.; Di Iorio, P.; Buccella, S.; D’Alimonte, I.; Giuliani, P.; Masciulli, A.; Nargi, E.; Beraudi, A.; Rathbone, M.P. Guanosine effect on cholesterol efflux and apolipoprotein e expression in astrocytes. Purinergic Signal 2006, 2, 637–649. [Google Scholar] [CrossRef][Green Version]
- Lund-Katz, S.; Phillips, M.C. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell. Biochem. 2010, 51, 183–227. [Google Scholar]
- Panzenboeck, U.; Kratzer, I.; Sovic, A.; Wintersperger, A.; Bernhart, E.; Hammer, A.; Malle, E.; Sattler, W. Regulatory effects of synthetic liver x receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int. J. Biochem. Cell Biol. 2006, 38, 1314–1329. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J.D.; Kowalewski, T.; Holtzman, D.M. Abca1 is required for normal central nervous system apoe levels and for lipidation of astrocyte-secreted apoe. J. Biol. Chem. 2004, 279, 40987–40993. [Google Scholar] [CrossRef]
- Danik, M.; Champagne, D.; Petit-Turcotte, C.; Beffert, U.; Poirier, J. Brain lipoprotein metabolism and its relation to neurodegenerative disease. Crit. Rev. Neurobiol. 1999, 13, 357–407. [Google Scholar] [CrossRef]
- Bjorkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Okuhira, K.; Tsujita, M.; Yamauchi, Y.; Abe-Dohmae, S.; Kato, K.; Handa, T.; Yokoyama, S. Potential involvement of dissociated apoa-i in the abca1-dependent cellular lipid release by hdl. J. Lipid Res. 2004, 45, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Bernardo, A.; Walker, D.; Kanegawa, T.; Mahley, R.W.; Huang, Y. Profile and regulation of apolipoprotein e (apoe) expression in the cns in mice with targeting of green fluorescent protein gene to the apoe locus. J. Neurosci. 2006, 26, 4985–4994. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Han, W.; Hu, F.; Qian, Y.; Chen, Q. Tro19622 promotes myelin repair in a rat model of demyelination. Int. J. Neurosci. 2013, 123, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Fowler, K.A.; Neil, J.E.; Colton, C.A.; Vitek, M.P. An apolipoprotein e-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. J. Pharmacol. Exp. Ther. 2010, 334, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Makoukji, J.; Shackleford, G.; Meffre, D.; Grenier, J.; Liere, P.; Lobaccaro, J.M.; Schumacher, M.; Massaad, C. Interplay between lxr and wnt/beta-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J. Neurosci. 2011, 31, 9620–9629. [Google Scholar] [CrossRef]
- Jan, A.; Karasinska, J.M.; Kang, M.H.; de Haan, W.; Ruddle, P.; Kaur, A.; Connolly, C.; Leavitt, B.R.; Sorensen, P.H.; Hayden, M.R. Direct intracerebral delivery of a mir-33 antisense oligonucleotide into mouse brain increases brain abca1 expression. [corrected]. Neurosci. Lett. 2015, 598, 66–72. [Google Scholar] [CrossRef]
- Freret, T.; Bouet, V.; Leconte, C.; Roussel, S.; Chazalviel, L.; Divoux, D.; Schumann-Bard, P.; Boulouard, M. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behav. Neurosci. 2009, 123, 224–230. [Google Scholar] [CrossRef]
- Swanson, R.A.; Morton, M.T.; Tsao-Wu, G.; Savalos, R.A.; Davidson, C.; Sharp, F.R. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow. Metab. 1990, 10, 290–293. [Google Scholar] [CrossRef]
- Kim, E.; Woo, M.S.; Qin, L.; Ma, T.; Beltran, C.D.; Bao, Y.; Bailey, J.A.; Corbett, D.; Ratan, R.R.; Lahiri, D.K. Daidzein augments cholesterol homeostasis via apoe to promote functional recovery in chronic stroke. J. Neurosci. 2015, 35, 15113–15126. [Google Scholar] [CrossRef]
- Mack, C.M.; Boehm, G.W.; Berrebi, A.S.; Denenberg, V.H. Sex differences in the distribution of axon types within the genu of the rat corpus callosum. Brain Res. 1995, 697, 152–160. [Google Scholar] [CrossRef]
- Sandell, J.H.; Peters, A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. J. Comp. Neurol. 2003, 466, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Sethares, C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002, 442, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Harper, C.G.; Evans, S.F.; Newnham, J.P.; Dunlop, S.A. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int. J. Dev. Neurosci. Offic. J. Int. Soc. Dev. Neurosci. 2001, 19, 415–425. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Kempermann, G.; Kuhn, H.G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997, 386, 493–495. [Google Scholar] [CrossRef]
- Chen, Y.; Balasubramaniyan, V.; Peng, J.; Hurlock, E.C.; Tallquist, M.; Li, J.; Lu, Q.R. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat. Protoc. 2007, 2, 1044–1051. [Google Scholar] [CrossRef]
- Huang, W.; Bai, X.; Stopper, L.; Catalin, B.; Cartarozzi, L.P.; Scheller, A.; Kirchhoff, F. During development ng2 glial cells of the spinal cord are restricted to the oligodendrocyte lineage, but generate astrocytes upon acute injury. Neuroscience 2018, 385, 154–165. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, R.; Zacharek, A.; Wang, F.; Landschoot-Ward, J.; Chopp, M.; Chen, J.; Cui, X. ABCA1/ApoE/HDL Signaling Pathway Facilitates Myelination and Oligodendrogenesis after Stroke. Int. J. Mol. Sci. 2020, 21, 4369. https://doi.org/10.3390/ijms21124369
Li L, Li R, Zacharek A, Wang F, Landschoot-Ward J, Chopp M, Chen J, Cui X. ABCA1/ApoE/HDL Signaling Pathway Facilitates Myelination and Oligodendrogenesis after Stroke. International Journal of Molecular Sciences. 2020; 21(12):4369. https://doi.org/10.3390/ijms21124369
Chicago/Turabian StyleLi, Li, Rongwen Li, Alex Zacharek, Fengjie Wang, Julie Landschoot-Ward, Michael Chopp, Jieli Chen, and Xu Cui. 2020. "ABCA1/ApoE/HDL Signaling Pathway Facilitates Myelination and Oligodendrogenesis after Stroke" International Journal of Molecular Sciences 21, no. 12: 4369. https://doi.org/10.3390/ijms21124369
APA StyleLi, L., Li, R., Zacharek, A., Wang, F., Landschoot-Ward, J., Chopp, M., Chen, J., & Cui, X. (2020). ABCA1/ApoE/HDL Signaling Pathway Facilitates Myelination and Oligodendrogenesis after Stroke. International Journal of Molecular Sciences, 21(12), 4369. https://doi.org/10.3390/ijms21124369




