Abstract
Tacrolimus has been used to induce remission in patients with steroid-refractory ulcerative colitis. It poses a problem of large individual differences in dosage necessary to attain target blood concentration and, often, this leads to drug inefficacy. We examined the difference in mRNA expression levels of ATP binding cassette transporter B1 (ABCB1) between inflamed and non-inflamed tissues, and the influence of CYP3A5 genotype on tacrolimus therapy. The mRNA expression of CYP3A4 in colonic mucosa and that of cytochrome p450 3A5 (CYP3A5) and ABCB1 in inflamed and non-inflamed areas were examined in 14 subjects. The mRNA expression levels of CYP3A5 were higher than that of CYP3A4. The mRNA expression of ABCB1 was lower in the inflamed than in the non-inflamed mucosa, despite that of CYP3A5 mRNA level being not significantly changed. Hence, the deterioration of the disease is related to the reduction of the barrier in the inflamed mucosa. The relationship between CYP3A5 genotype and blood concentration, dose, and concentration/dose (C/D) ratio of tacrolimus in 15 subjects was studied. The tacrolimus dose to maintain equivalent blood concentrations was lower in CYP3A5*3/*3 than in CYP3A5*1 carriers, and the C/D ratio was significantly higher in the latter. Thus, CYP3A5 polymorphism information played a role in determining the initial dose of tacrolimus. Furthermore, since the effect of tacrolimus appears earlier in CYP3A5*3/*3 than in CYP3A5*1/*1 and *1/*3, it seems necessary to change the evaluation time of therapeutic effect by CYP3A5 genotype. Additionally, the relationship between CYP3A5 genotype and C/D ratio of tacrolimus in colonic mucosa was investigated in 10 subjects. Tacrolimus concentration in the mucosa was two-fold higher in CYP3A5*3/*3 than in CYP3A5*1 carriers, although no significant difference in tacrolimus-blood levels was observed. Therefore, the local concentration of tacrolimus affected by CYP3A5 polymorphism might be related to its therapeutic effect.
1. Introduction
Conventionally, 5-aminosalicylic acid (5-ASA or mesalazine), steroids, calcineurin inhibitors, etc., have been used to treat ulcerative colitis (UC) [1,2]. However, in recent years, numerous biological products have been developed, and treatment options for UC are expanding [3]. Tacrolimus, which was first used as an immunosuppressant following an organ transplantation, is now being used in the management of various autoimmune diseases in a number of patients [4,5]. Therefore, the appropriate knowledge of an existing drug, including its merits and demerits in comparison to new drugs, can help make better therapeutic choices.
Tacrolimus is used to induce remission in patients with steroid-refractory UC. The drug binds to the FK506-binding protein 12 (FKBP12) and inhibits calcineurin. As a result, the activation of nuclear factor of activated T-cells (NFAT), a substrate of calcineurin, is inhibited. The NFAT-induced transcription of interleukin 2 and interferon γ in the nucleus is also inhibited. These cytokines promote the proliferation of helper T-cells; tacrolimus suppresses T-cell proliferation and thus exerts its immunosuppressive activity [6,7].
Tacrolimus is effective in UC at a target trough blood concentration of 10–15 ng/mL [4,8]. However, the side effects of tacrolimus including nephropathy, headache, and tremors related to the trough blood levels deter its use [9,10,11]. Furthermore, since individual differences in the pharmacokinetics of tacrolimus are large, it is difficult to adjust the dosage [12,13].
It has been reported that individual differences in pharmacokinetics and efficacy are due to the varying expression levels and activities of metabolic enzymes and transporters, which depend on genetic polymorphism [14].
Tacrolimus is metabolized by cytochrome p450 3A4 (CYP3A4) and CYP3A5 in the gastrointestinal mucosa and liver, and then excreted into the gastrointestinal lumen and bile by P-glycoprotein (Pgp) encoded by the ATP-binding cassette transporter B1 (ABCB1, other name: Multidrug resistance 1 (MDR1)) gene [15,16,17]. It is reported that the influence of CYP3A5 is greater than that of CYP3A4 on the metabolism of tacrolimus [18,19,20]. However, in the CYP3A5 genotype, the efficacy of tacrolimus is affected and, therefore, a dose adjustment based on this genotype is required after organ transplantation [21,22,23]. Therefore, in the treatment of UC, the pharmacokinetics and effects of tacrolimus may be altered in the CYP3A5 genotype [24]. We examined the relationship between the CYP3A5 genotype (*1 allele and *3 allele (s776746)) and clinical activity index (CAI), a disease activity index in UC, including the measurement of tissue concentration of tacrolimus in colon mucosa.
P-glycoprotein, a gene product of ABCB1 (or other name is MDR1) is expressed in several organs including the liver and kidney and plays a role in pumping out drugs and exogenous compounds from the cells [25]. Therefore, a decrease in Pgp is considered as to be not only responsible for altering the pharmacokinetics of tacrolimus, but is also attributed to the worsening of the disease condition of UC [26,27]. Based on these backgrounds, the relationship between the degree of inflammation and ABCB1 mRNA-expression level was used as a positive control for the sampling of biopsy specimens for examining the mRNA expression levels of CYP3A4 and CYP3A5 in mucosal tissues.
2. Results
2.1. The mRNA Expression of CYP3A4, CYP3A5, and ABCB1
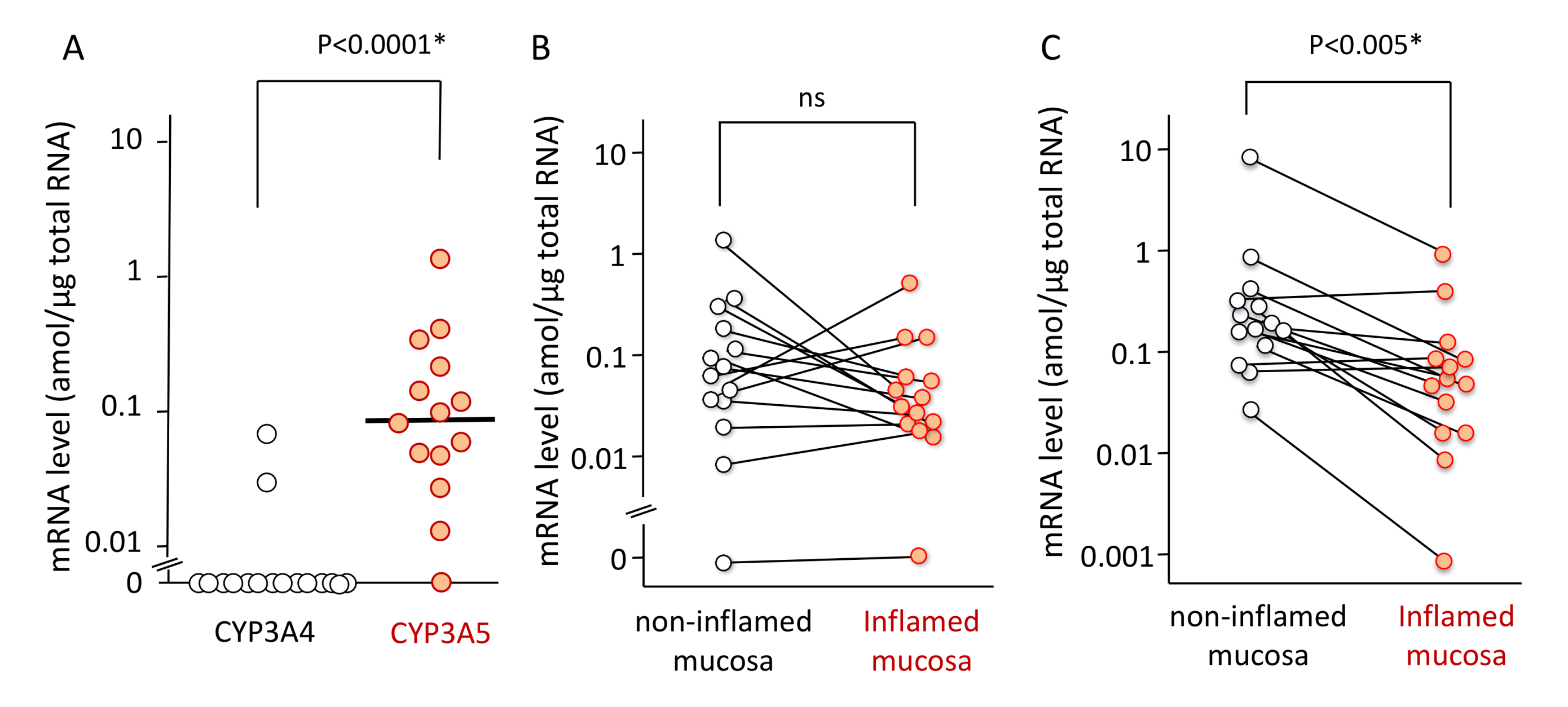
Patient characteristics including age, sex, and extent of UC are listed in Table 1. The mRNA expression level of CYP3A5 was higher than that of CYP3A4 in colonic or rectal mucosa. The expression levels of CYP3A4 ranged from <0.01 (not detected) to 0.07 amol/µg total RNA (median < 0.01 amol/µg), while these values for CYP3A5 ranged from < 0.01 to 1.37 amol/µg total RNA (median 0.09 amol/µg). There was significant difference (p < 0.0001) (Figure 1A).

Table 1.
Patient demographics for mRNA expression level measurement.

Figure 1.
The mRNA expression levels of CYP3A4 and CYP3A5 in colonic or rectal mucosa (A), the mRNA expression levels of CYP3A5 in non-inflamed and inflamed mucosa (B), and the mRNA expression levels of ABCB1 in non-inflamed and inflamed mucosa (C). (A) Among 14 mucosal biopsy specimens, the mRNA expression level of CYP3A4 and CYP3A5 in 12 specimens and 1 specimen, respectively, were below the detection limit (0.01 amol/µg total RNA). The bar shows the median for all mRNA expression levels including data below the detection limitation whose value was set as zero. Statistical analysis was performed using the Mann–Whitney’s U-test. (B) The mRNA expression level of CYP3A5 in inflamed colorectal mucosal was compared to non-inflamed mucosa in 14 UC patients. Among them, the measurement was below the detection limit (0.01 amol/µg total RNA) in a specimen. Each line represents the non-inflamed and inflamed sample derived from the same patient. (C) The mRNA expression level of ABCB1 in inflamed colorectal mucosal was compared to non-inflamed mucosa in 14 UC patients. Each line represents the non-inflamed and inflamed sample derived from the same patient. Statistical analysis was performed using the paired t-test with logarithmical values.
The mRNA expression of CYP3A5 was not significantly changed between non-inflamed and inflamed mucosa (Figure 1B). In contrast, the mRNA expression of ABCB1 was reduced in the inflamed regions as opposed to the non-inflamed regions in patients (median 0.23 [0.03–9.62], 0.07 [0.001–1.08] amol/µg, respectively). There was a significant difference (p < 0.005) (Figure 1C). Because the mRNA expression levels of CYP3A4 was almost negative in the non-inflamed biopsy specimens, those in the inflamed mucosa were not examined.
2.2. Blood Concentration, Dose, and Concentration/Dose (C/D) Ratio of Tacrolimus
Patient characteristics based on CYP3A5 genotypes reflecting CYP3A5 protein expression are summarized in Table 2. We examined the influence of CYP3A5 genotype on tacrolimus therapy comparing two groups between CYP3A5 expressor (CYP3A5 *1/*3) and nonfunctional (CYP3A5 *3/*3). The chi-squared test was used to verify that the CYP3A5 genotype frequency distribution for the present patients was consistent with the Hardy–Weinberg equilibrium (p > 0.05).

Table 2.
Patients characteristics based on CYP3A5 genotype.
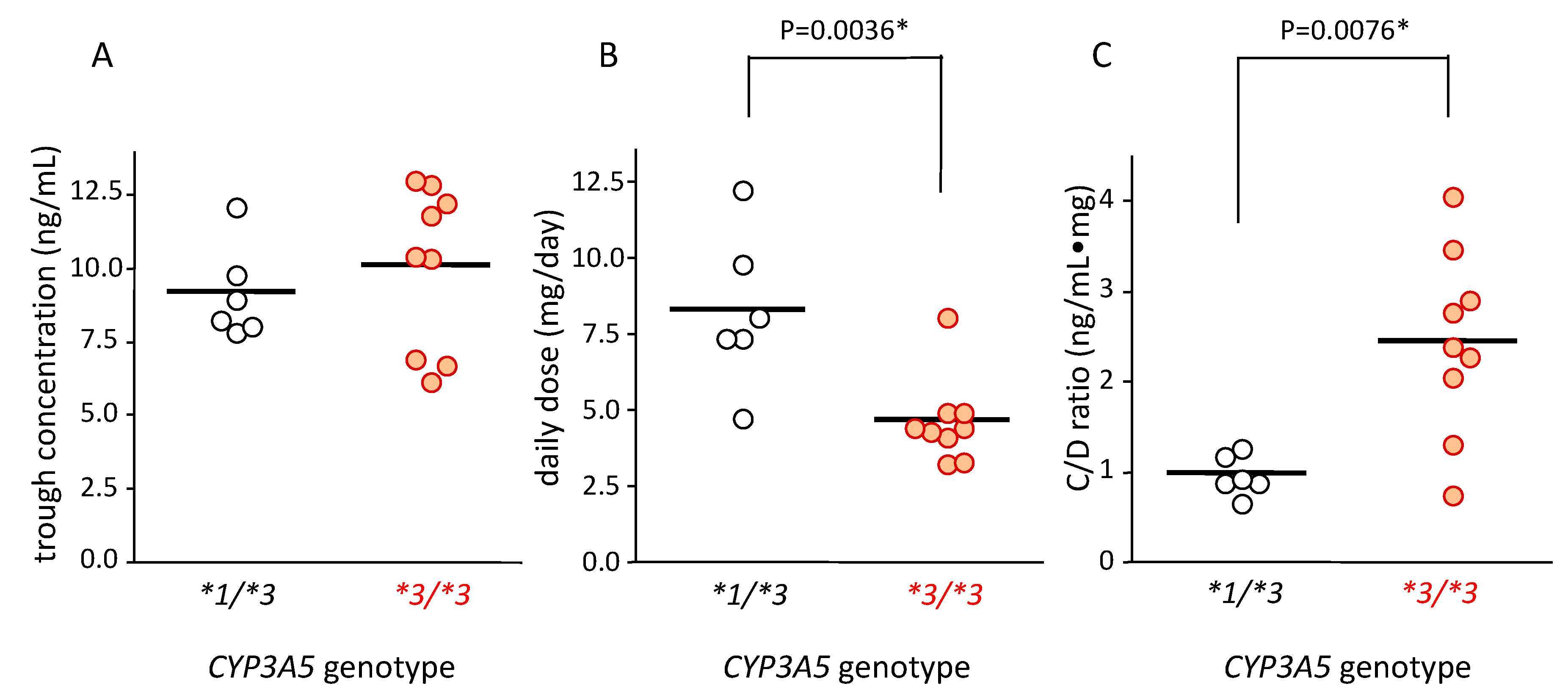
The tacrolimus concentration in blood in patients with CYP3A5*1/*3 and CYP3A5*3/*3 was 9.22 ± 1.61 ng/mL (mean ± standard deviation (SD)) and 10.12 ± 2.77 ng/mL (mean ± SD), respectively; there was no significant difference between these groups (Figure 2A).
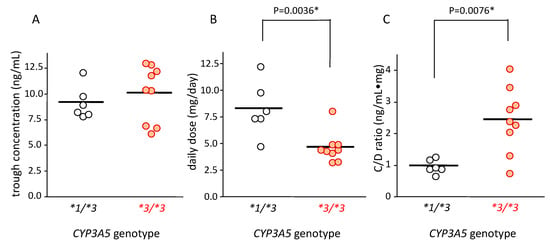
Figure 2.
Relationship between CYP3A5 genotype and blood concentration (A), dose (B), and concentration/dose (C/D) ratio (C) of tacrolimus. The bar shows the mean in each group (A,B) and the median for the C/D ratio of tacrolimus. (A) The average concentration of tacrolimus trough level in each patient was plotted. The mean trough concentration in patients carrying CYP3A5*1/*3 (n = 6) was similar to that of patients carrying CYP3A5*3/*3 (n = 9). (B) The average dose of tacrolimus in each patient was plotted. The mean value of average dose of tacrolimus in patients carrying CYP3A5*1/*3 was higher than that with CYP3A5*3/*3 (p = 0.0036 using Student’s t-test). (C) The average concentration/dose ratio of tacrolimus was plotted for each patient. Each point represents an average of 10 points. Bar shows the median value of all plotted points. Statistical analysis was performed using the Mann–Whitney’s U-test.
The tacrolimus dose in patients carrying the CYP3A5*1/*3 genotype (8.31 ± 2.55 mg/day [mean ± SD]) was 1.7-fold higher than that in patients carrying CYP3A5*3/*3 (4.69 ± 1.42 mg/day (mean ± SD)) (p = 0.0036) (Figure 2B).
The median value of blood C/D ratio of tacrolimus in patients with the CYP3A5*1/*3 and CYP3A5*3/*3 genotypes was 0.93 (0.68–1.28) and 2.40 (0.78–4.07), respectively. This value was approximately 2.5 times higher in patients with the CYP3A5*3/*3 than in those with the CYP3A5*1/*3 genotype, which was significantly different (p = 0.0076) (Figure 2C).
2.3. Association between the Clinical Outcome and CYP3A5 Genotype in Tacrolimus Therapy
Clinical information including the clinical activity index (CAI) values were obtained on days 0, 14, and 30 after the start of tacrolimus therapy. The relationship between the ratio of variation in CAI values between days 0 and 14, and days 14 and 30, and the CYP3A5 genotype was examined. Furthermore, in order to confirm the effectiveness of tacrolimus, the presence or absence of therapy with biologics, anti-tumor necrosis factor (TNF) agents, and surgical intervention for 30 months after the start of tacrolimus therapy was investigated.
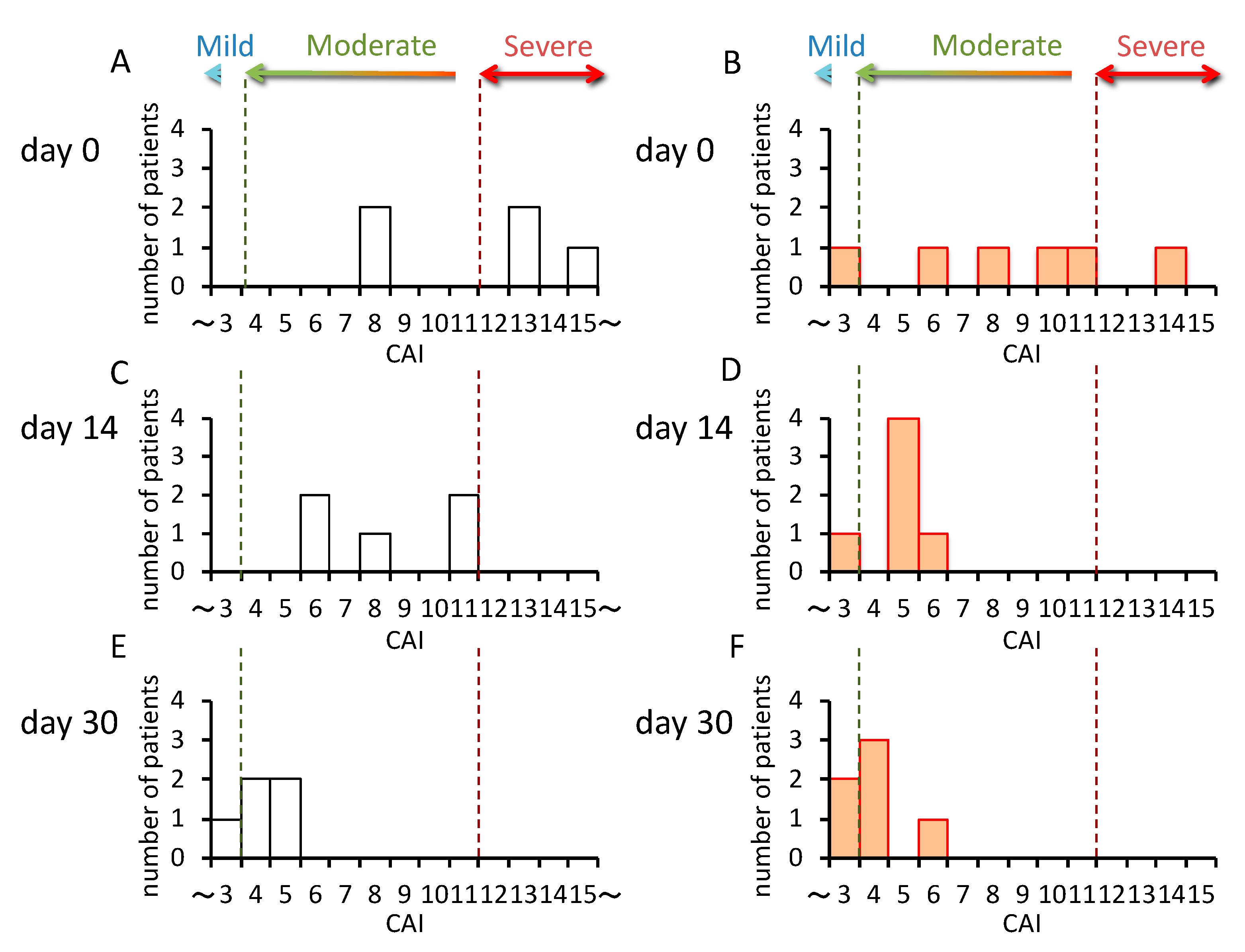
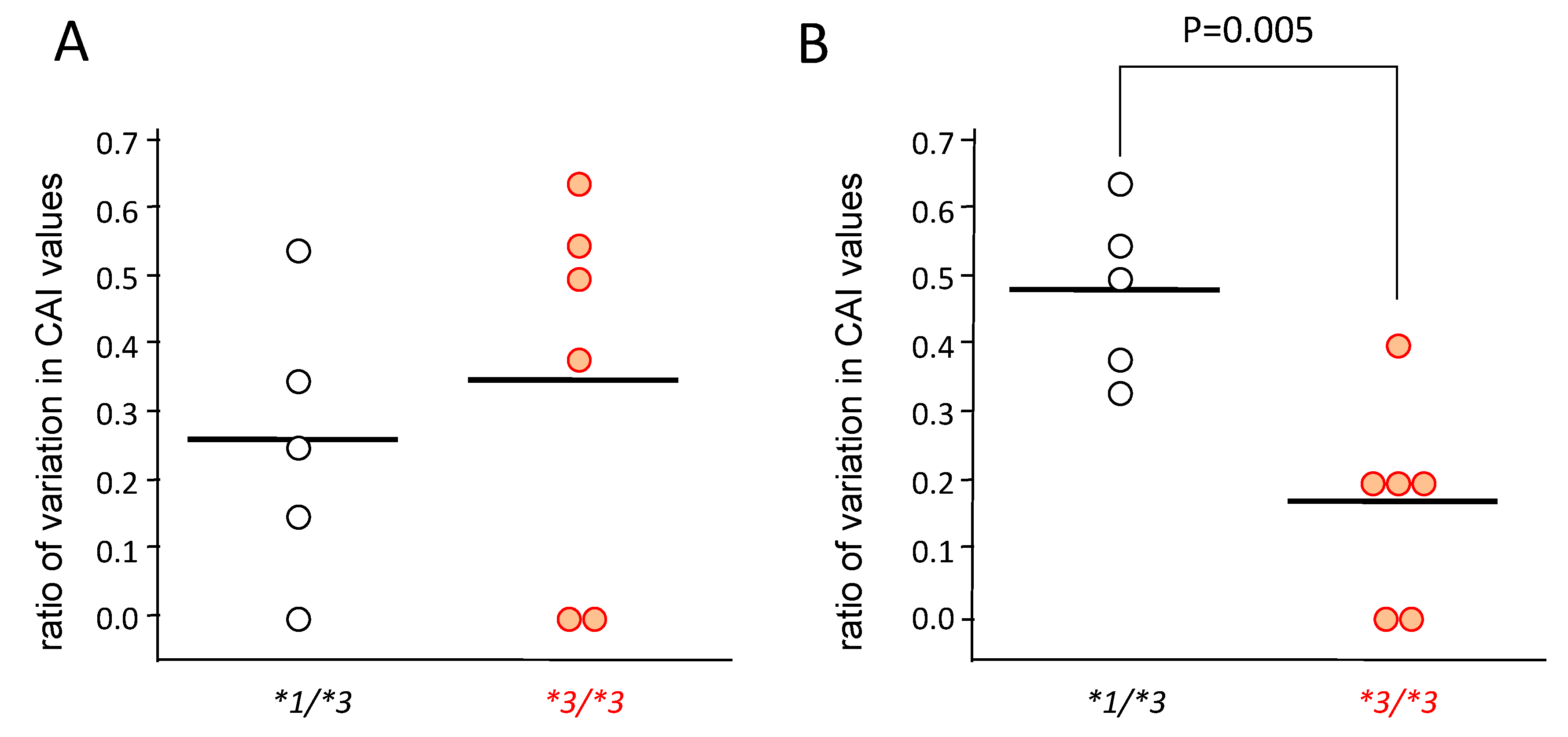
In patients carrying CYP3A5*1/*3 (n = 5), the CAI values decreased gradually from day 0 to day 30 (Figure 3A,C,E), but in patients carrying CYP3A5*3/*3 (n = 6), the CAI values rapidly decreased from day 0–14, and did not change much from day 14–30 (Figure 3B,D,F). Furthermore, although the rate of change was large in CYP3A5*3/*3 from day 0–14, significantly higher rates of change were seen in patients carrying CYP3A5*1/*3 from day 14–30 (p = 0.005) (Figure 4).

Figure 3.
Change in the clinical activity index (CAI) in the CYP3A5 genotype after the start of tacrolimus therapy. The white bars indicate the number of patients carrying the CYP3A5*1/*3 genotypes (CYP3A5 expressor, n = 5) (A,C,E). The orange bars indicate patients carrying the CYP3A5*3/*3 genotype (CYP3A5 nonfunctional, n = 6) (B,D,F).
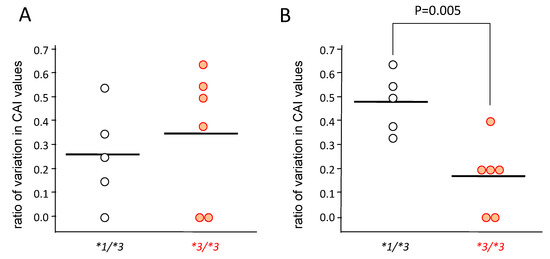
Figure 4.
The rate of change in clinical activity index (CAI) in the CYP3A5 genotype after the start of tacrolimus therapy from day 0 to day 14 (A) and from day 14 to day 30 (B). The value of vertical axis was obtained by dividing the value of difference between the CAI value at day 14 from it at day 0 by the CAI value at day 0 ((CAI at day 0–CAI at day 14)/CAI at day 0) (A), and by dividing the value of difference between the CAI value at day 30 from it at day 14 by that at day 14 ((CAI at day 14–CAI at day 30)/CAI at day 14) (B). Statistical analysis was performed using the Student’s t-test.
2.4. Blood and Mucosal Concentrations and the Mucosal Concentration/Dose of Tacrolimus
We considered the possibility that pharmacological efficacy of tacrolimus was affected by its mucosal concentration dependent on the CYP3A5 genotype. Seven additional subjects provided written informed consent and were added to the data set, after which, it consisted of three cases in the following examination using the data of mucosal concentration of tacrolimus.
The tacrolimus blood concentrations (median (range)) in the CYP3A5*1 (active genotype, sum of *1/*1 and *1/*3, n = 6) and CYP3A5*3 (nonfunctional genotype, *3/*3, n = 4) were 9.3 (3.9–17.6) ng/mL and 9.9 (7.3–11.6) ng/mL, respectively (Figure 5A). The blood tacrolimus C/D ratio (median (range)) in patients carrying the CYP3A5*1 and CYP3A5*3 alleles was 0.96 (0.50–1.1) (ng/mL)/(mg/day) and 1.68 (0.98–2.04) (ng/mL)/(mg/day) (p = 0.0381), respectively (Figure 5B).
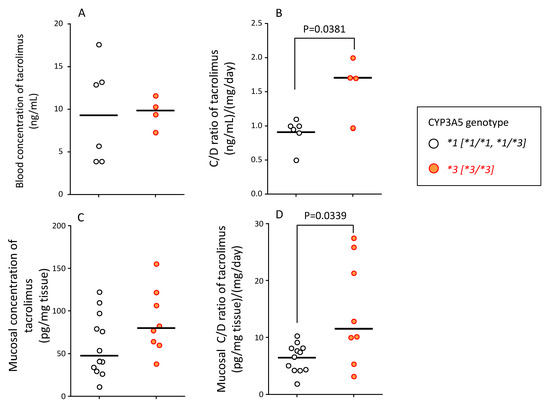
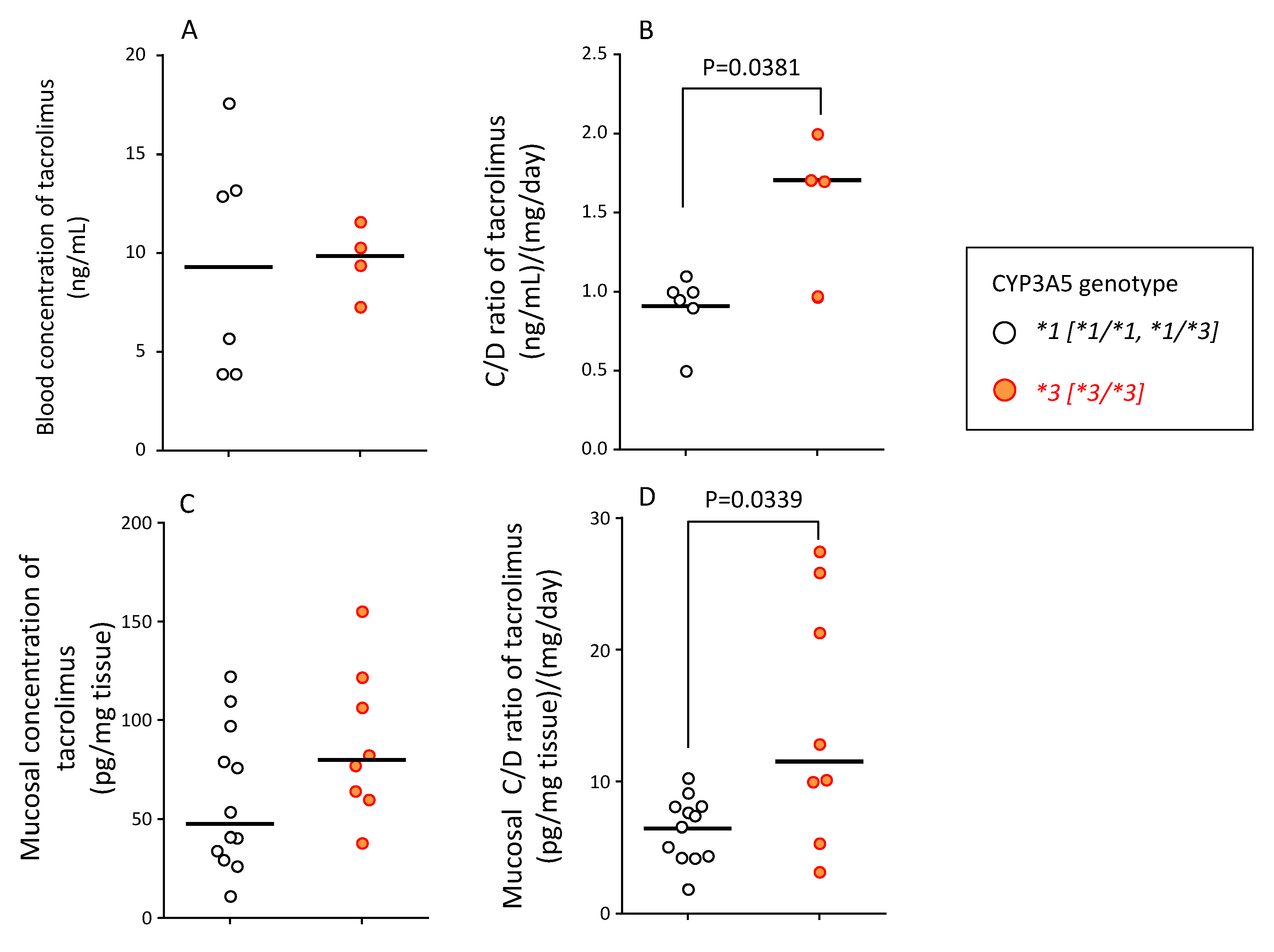
Figure 5.
Relationship between CYP3A5 genotype and blood concentration (A), blood C/D ratio (B), mucosal concentration (C), and mucosal C/D ratio (D) of tacrolimus at endoscopic examination. (A) The blood concentration of tacrolimus in patients carrying the CYP3A5*1 allele and CYP3A5*3/*3 genotype is shown. (B) The blood concentration/dose (C/D) ratio of tacrolimus in patients carrying CYP3A5*1 allele and CYP3A5*3/*3 genotype is shown. (C) The concentration of tacrolimus in the mucosal tissue of biopsy specimens at endoscopic examination is shown. Two biopsy specimens were taken per patient to measure the tissue concentration of tacrolimus to account for intra-individual variation. (D) The mucosal concentration/dose (C/D) ratio of tacrolimus in patients carrying the CYP3A5*1 allele and CYP3A5*3/*3 genotype is shown. Two data points per patient of C/D ratio were used to account for intra-individual variation. The bar shows the median in each group. Statistical analyses were performed using the Mann–Whitney’s U-test.
The mucosal concentration of unchanged tacrolimus (median (range)) in patients with CYP3A5*1 and CYP3A5*3 allele was 47.4 (11.3–122.4) and 79.9 (38.2–155.4) pg/mg tissue, respectively. Although there was no statistical significance, the mucosal concentration of tacrolimus was approximately 1.7-fold higher in patients with CYP3A5*3/*3 than in patients with CYP3A5*1/*1 and CYP3A5*1/*3 genotypes (Figure 5C).
The mucosal C/D ratio of tacrolimus (median (range)) in patients with CYP3A5*1 and CYP3A5*3 allele was 7.0 (1.9–10.3) (ng/mL)/(mg/mL) and 11.5 (3.2–27.5) (ng/mL)/(mg/mL), respectively, with a significant difference between these values (p = 0.0339) (Figure 5D).
3. Discussion
Tacrolimus and azathioprine, among other biological agents, are used in the remission induction of UC [28]. Among these, tacrolimus is one of the few oral therapeutic drugs that can be used in fulminant and steroid-refractory cases, sudden onset of disease conditions, or during deterioration of the existing condition, because a precise dose adjustment is easier for this drug than for others [3,29]. Therefore, the efficacy of tacrolimus in such conditions can be helpful in avoiding surgical treatment. However, a large inter-individual variation in its pharmacokinetics is closely associated with the frequency of its adverse reactions, which often leads to discontinuation of therapy [7,8]. In this study, we investigated the relationship between clinical efficacy of tacrolimus and the CYP3A5 genotype in patients with UC.
The mRNA expression level of ABCB1 in the small-intestinal mucosa shows a negative correlation with C/D ratio of tacrolimus in liver-transplant patients [30]. In addition, downregulated Pgp and CYP3A contribute to cyclosporine blood level in the mice colitis model [31]. Since Pgp is located in the apical membrane and CYP3A5 is in the microsome, the decreased expression of Pgp may affect the absorption process of tacrolimus regarding the phenomenon in liver transplant patients [32]. Therefore, a decrease in mRNA expression level of ABCB1 in the inflammatory areas may influence tacrolimus pharmacokinetics via functional impairment of Pgp. Considering the good correlation between mRNA expression level and protein expression level in the intestinal plasma membrane fraction in the Japanese patients [32], the mRNA expression level of ABCB1 was determined to reflect the activity of Pgp as an efflux transporter in the colon epithelial cells. In the present study, the mRNA expression of ABCB1 (MDR1) was lower in inflamed areas than in the non-inflamed area in patients with UC (Figure 1C), comparable with the previous findings [26,27]. However, the inflamed regions of mucosa were limited in the relatively moderate patients and, therefore, the expressional change in MDR1 mRNA was suggested to influence not so much on tacrolimus pharmacokinetics in the present patients. Interestingly, the mRNA expression level of CYP3A5 was relatively maintained even in the inflamed mucosa (Figure 1B), suggesting mucosal CYP3A5 acts as detoxicating player regardless of inflammatory status in UC patients. Elucidation of difference in the molecular mechanisms of regulation in mRNA expression levels of MDR1 and CYP3A5 may be able to understand the maintained expression level of CYP3A5 in inflamed colon mucosa.
Our study revealed that the mRNA expression level of CYP3A5 was higher than that of CYP3A4 in non-inflamed colonic mucosa not only in healthy subjects, [33] but also in UC patients (Figure 1A). Furthermore, the C/D ratio of tacrolimus was about 2.5-fold higher in the CYP3A5*3/*3 genotype than in CYP3A5*1/*3 (Figure 2C) and was consistent with previous findings [24,34]. These results indicated that CYP3A5, rather than CYP3A4, was largely involved in the metabolism of tacrolimus in the colon, and the patients carrying CYP3A5*3/*3 showed a lower metabolism of tacrolimus compared to those carrying CYP3A5*1/*3. Because of maintained expression level of CYP3A5 mRNA in the inflamed mucosa compared to the non-inflamed mucosa (Figure 1B), colon CYP3A5 may actively metabolize drugs as well as tacrolimus regardless inflammation. In addition, attaining and maintaining blood-tacrolimus levels between 10 and 15 ng/mL at an early stage of administration is an important aspect in the treatment of UC [35]. These results put together suggested CYP3A5 polymorphism as a useful biomarker in determining the initial dose and/or the dose escalation schedule of tacrolimus in the treatment of UC.
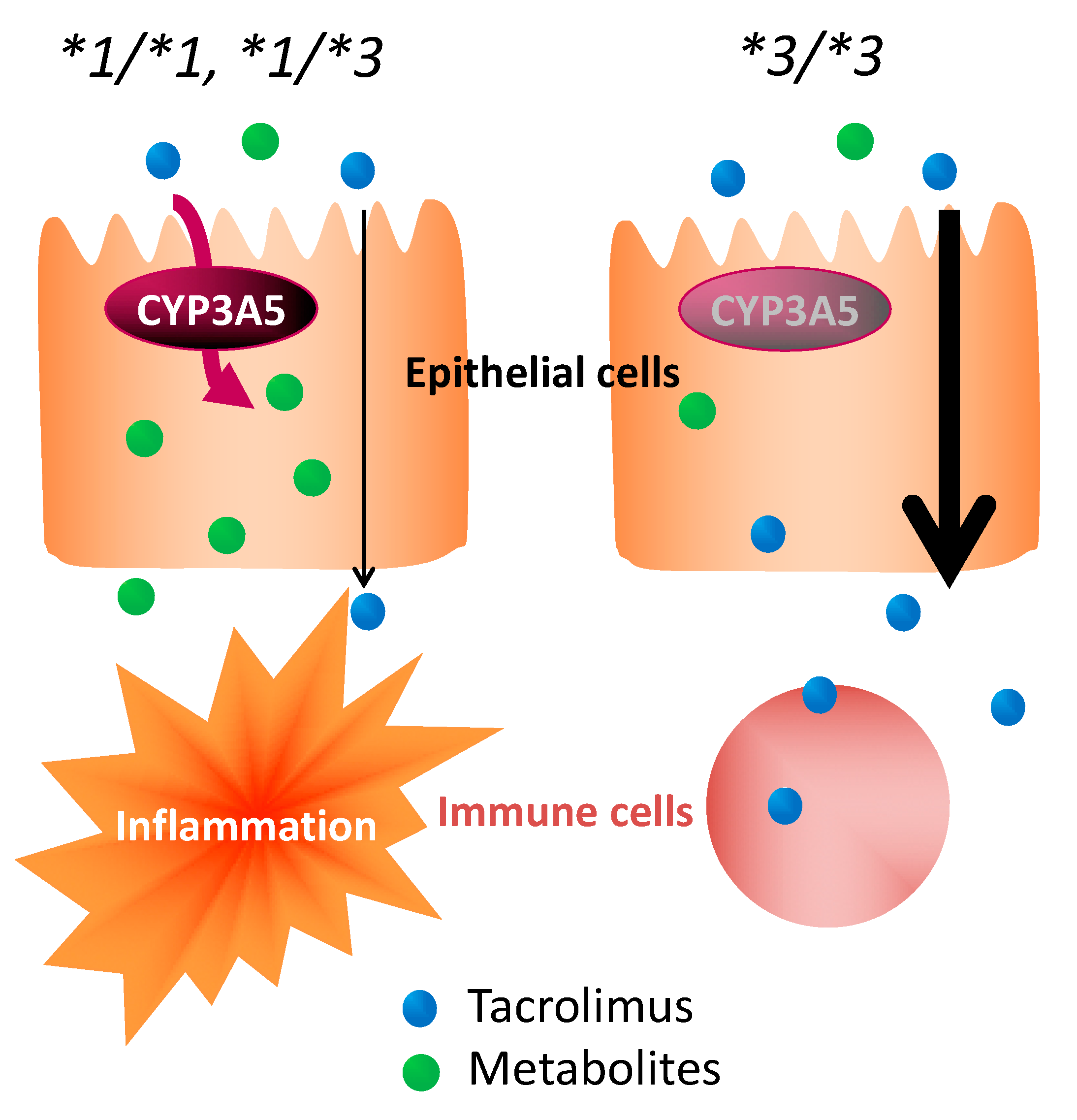
With no concomitant use of drugs potentially showing drug–drug interaction with tacrolimus in the present study, it was suggested that the effect of tacrolimus in the treatment of UC varied, depending on the polymorphism of CYP3A5 (Figure 3 and Figure 4). These results suggested that the time to evaluate tacrolimus treatment for UC should be optimized based on the CYP3A5 genotype. We hypothesized the following to explain these clinical results using molecular biology: The *1 or *3 genotypes reflect the activity or the nonfunctional of CYP3A5, respectively, which is a major enzyme of the CYP3A subfamily expressed in colon epithelial cells. Hence, orally administered tacrolimus would be extensively metabolized in the epithelial cells of the colon in patients carrying the CYP3A5*1 allele and, consequently, the immunocompetent cells would be deprived of the dose of tacrolimus required for optimal therapeutic effect (Figure 6). In contrast, a higher concentration of unmetabolized tacrolimus was found in the immune cells in the CYP3A5*3/*3 genotype. Therefore, the clinical evaluation of tacrolimus therapy in the management of UC requires more time in patients carrying the CYP3A5*1 allele than in those carrying the CYP3A5*3/*3. In fact, in the present study, there was an almost two-fold difference in the mucosal concentration of tacrolimus (Figure 5C) and its mucosal C/D ratio (Figure 5D) despite similar blood levels of tacrolimus among CYP3A5 genotypes (Figure 5). Furthermore, the patients in the CYP3A5*1 group tended to be more frequently prescribed biological products after tacrolimus therapy during the 30 months’ survey after the start of tacrolimus therapy (3 out of 5 in CYP3A5*1/*1, *1/*3 versus 1 out of 6 in CYP3A5*3/*3; odds ratio (OR) = 7.5; 95% CI = 0.5–122.8). These results support our hypothesis (Figure 6). Tacrolimus is also metabolized in the liver, but tacrolimus has a gastrointestinal absorption rate of about 20%. Furthermore, the area under the concentration-time curve (AUC) coefficient of variation for oral administration and continuous IV injection is 72% and 42%, respectively. Therefore, it is considered that about 40% of individual variation in blood concentration after oral administration occur during the absorption process from the digestive tract. The cytochrome P450 (CYP) content of the digestive tract is considered as 1/80 compared to that in liver, but tacrolimus has a high protein-binding rate and, therefore, the metabolic contribution in the digestive tract cannot be important [7]. In liver-transplant patients, the CYP3A5 genotype influences not only the tacrolimus-blood levels, but also the hepatic-drug levels, which may pose a potential risk of acute rejection after transplantation [36]. There are studies that report the efficacy of tacrolimus suppositories and enemas in UC [37]. Based on these backgrounds and present results, the CYP3A5 polymorphisms were suggested to act as an important role in the local mucosal concentration of tacrolimus as well as systemic blood level of tacrolimus mainly affected by hepatic metabolism. Therefore, it was thought that not only the blood concentration but also the concentration at the inflammatory area is important for the therapeutic effect of tacrolimus against UC. Furthermore, CYP3A4 and CYP3A5 have haplotypes, but the most common CYP3A5*3/*3 is in combination with CYP3A4*1 (wild type) [38], and the CYP3A5*1 is in combination with CYP3A4*1G [39]. Considering almost no expression level of CYP3A4 in colon mucosa, it is unlikely that CYP3A4 will affect the mucosal concentration of tacrolimus in patients with CYP3A5*3/*3. Further analysis may be needed to clarify the molecular mechanisms to affect mucosal concentration of tacrolimus in colon in addition to CYP3A5.
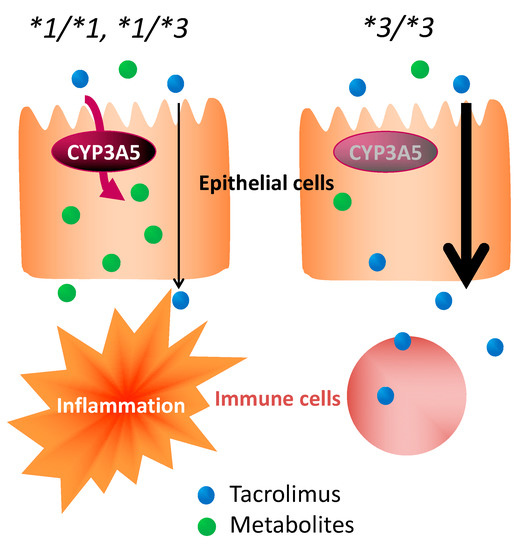
Figure 6.
Impact of CYP3A5 genotype in the pharmacological effect of tacrolimus in colon epithelial cells.
Based on these reports, the local concentration (mucosal concentration) was considered important for the therapeutic effect of tacrolimus. Our findings suggested that the local as well as systemic concentration of tacrolimus was important in the treatment of UC. CYP3A5 polymorphism was closely associated with the mucosal concentration of tacrolimus (Figure 5). Therefore, the CYP3A5*3 genotype could be a useful biomarker in determining the optimal time to determine the clinical efficacy of tacrolimus in UC treatment.
In conclusion, it was suggested that the CYP3A5 genotype not only affected the pharmacokinetics of tacrolimus, but is also associated with its efficacy in the management of UC. In addition, we found that the local concentration of tacrolimus may have played an important role in achieving optimal effects. These results indicated that the CYP3A5 genotype could be a useful biomarker in tacrolimus therapy in association with treatment outcome with tacrolimus.
There were some limitations including the small sample size in this study. The data collection was limited in clinical situation and, therefore, all data sets were not collected from each patient, which is consistent of CYP3A5 genotype, blood concentration of tacrolimus, mucosal concentration of tacrolimus, the change of CAI estimation and outcome such as cure, and surgery or prescription of biologics. In this study, we spent five years investigating four types of research subjects, but each research term was about 1 to 2 years, and there were not many patients who met each research condition. However, the ratio of *1/*1, */*3, and *3/*3 is about the same as the past reports targeting Japanese people [8,39,40]. In addition, the number of measurements of mucosal concentration of tacrolimus was limited in a couple of pieces per patients for ethical considerations. Therefore, further analysis with a larger sample size containing all data sets is required to assess the accuracy of the present results.
4. Materials and Methods
4.1. Materials
Analytical-grade tacrolimus was provided by Astellas Pharma Inc. (Tokyo, Japan). Sirolimus was purchased from LC Laboratories (Woburn, MA, USA) and used as an internal standard. The liquid chromatography-tandem mass spectrometry/mass spectrometry (LC-MS/MS) system was comprised of a high-performance liquid chromatography (HPLC) machine (LC-10AS; Shimadzu Corp., Kyoto, Japan), a column (Inertsil-ODS3, 150 mm × 2.1 mm i.d.; GL Sciences, Inc., Tokyo, Japan), and an MS/MS detector (API4000; Applied Biosystems, Foster City, CA, USA).
4.2. Subjects
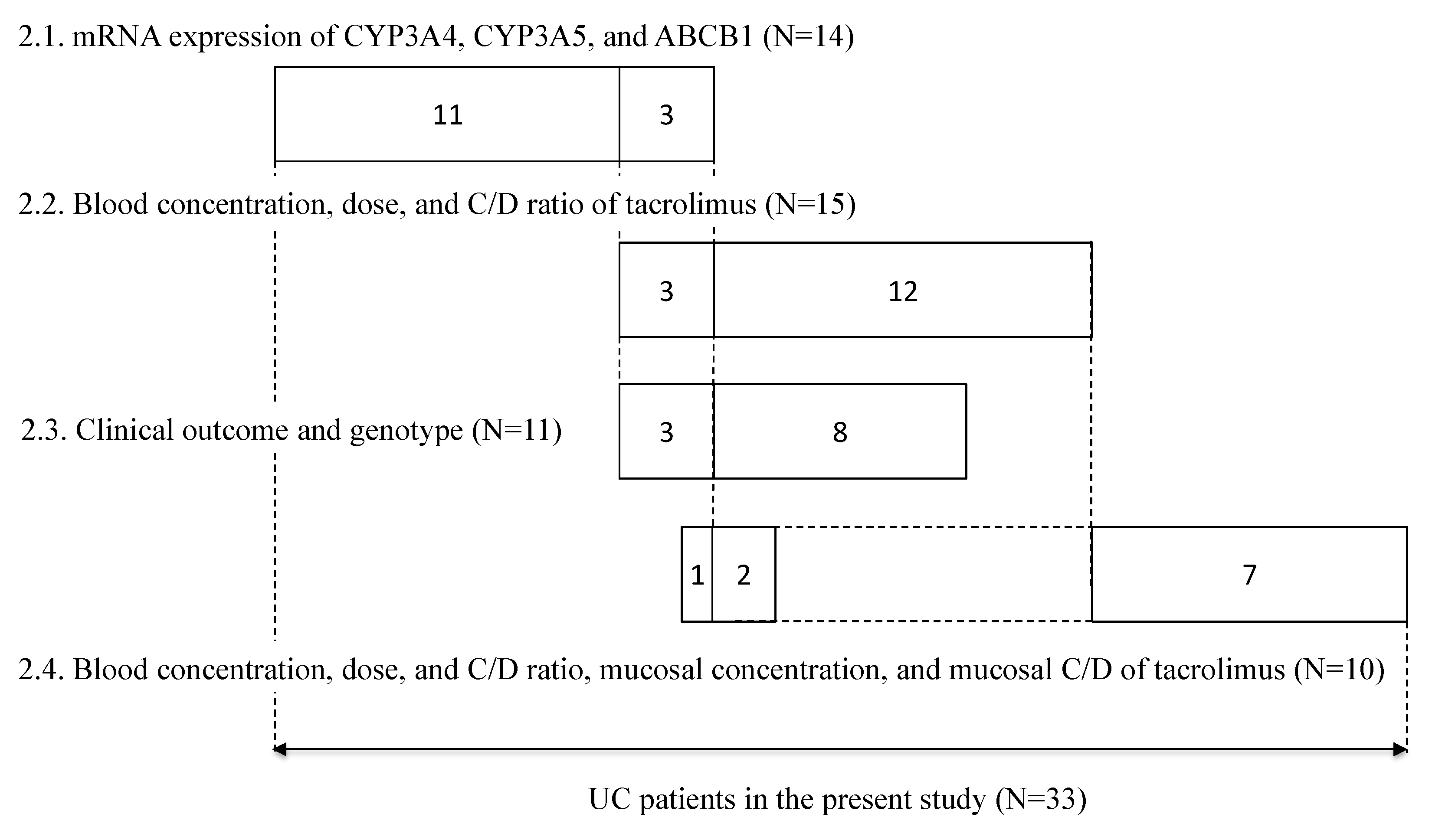
Between May 2010 and May 2015, patients participating in the retrospective study at Kyoto University Hospital (Kyoto, Japan) provided written informed consent. Patients aged 15 years or younger and those treated with topical formulations were excluded from the study; a total of 33 patients were included in the study. Each patient underwent periodic sigmoidoscopy, in which mucosal biopsy samples (about 1-mm-cube each) were collected from a couple of areas in the rectum. The endoscopy was not done just for this study, but for taking biopsy specimens for pathological examination. The samples were put in a microtube, immediately frozen in liquid nitrogen, and stored at −80 °C until analysis to determine the mRNA expression levels of CYP3A4, CYP3A5, and ABCB1, and/or the concentration of tacrolimus. In 14 patients, the mRNA expression levels of CYP3A4 and CYP3A5 in non-inflamed colonic or rectal mucosa and the mRNA expression levels of ABCB1 in non-inflamed and inflamed colonic or rectal mucosa were examined. The relationship between tacrolimus concentration/dose (C/D) ratio and CYP3A5 genotype in 15 patients treated with tacrolimus was examined. The relationship between the ratio of variation in CAI values and CYP3A5 genotype was examined in 11 patients. The relationship of the mucosal concentration/dose ratio of tacrolimus and CYP3A5 genotype was examined in 10 patients, on whom a biopsy was performed during tacrolimus therapy (Figure 7).
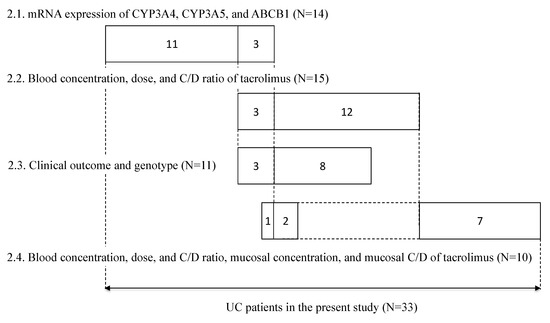
Figure 7.
Summary of subjects enrolled in this study. The numbers in each box reflect the number of subjects. The numbers in each line correspond to each subsection of the results section, respectively.
This study was conducted in accordance with the Declaration of Helsinki and its amendments, and was approved by the Kyoto University Graduate School and Faculty of Medicine, Ethics Committee (Approval number: G-408, 22 March 2011).
4.3. Measurement of mRNA Expression of CYP3A4, CYP3A5, and ABCB1
Mucosal biopsy specimens were homogenized in RLT buffer (QIAGEN, Hilden, Germany). Total RNA was isolated using MagNAPure LC RNA Isolation kit II (Roche, Mannheim, Germany) and reverse transcribed, as described previously [20]. The mRNA expression levels of ABCB1, CYP3A4, and CYP3A5 were evaluated by using real-time polymerase chain reaction (PCR) using an ABI prism 7700 sequence detector (Applied Biosystems, Foster, CA). The primer/probe sets used in this study were those reported by Koch et al. [41]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control, as described previously [42]. The mRNA expression data for each sample were corrected by the amount of GAPDH.
4.4. Isolation of Genomic DNA and Genotyping
Genomic DNA was isolated from a homogenate of mucosal biopsy specimens from patients, using MagNAPure LC DNA isolation kit I (Roche, Mannheim, Germany). The isolated DNA was used for genotyping using the PCR-restriction fragment length polymorphism method [41,42].
Genomic DNA was also isolated from the peripheral blood of patients, using EZ1 DNA Blood 350 µL kit (QIAGEN, Hilden, Germany). PCR and high-resolution melting (HRM) were consecutively performed using a LightCycler Nano (Roche, Mannheim, Germany). Probes and primers used for PCR were the same as those used for analysis of mucosal biopsy specimens. The PCR process included initial denaturation at 95 °C for 10 min, 45 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 10 s, and synthesis at 72 °C for 15 s. The final HRM step was performed from 40 °C to 75 °C with an increase of 0.1 °C per second after denaturation at 95 °C for 30 s.
4.5. Measurement of Tacrolimus Blood Concentration
Routine analysis of the therapeutic drug monitoring of tacrolimus trough levels was conducted. Blood samples were collected before the morning dose into tubes containing ethylenediaminetetraacetic acid. Tacrolimus blood concentrations were determined using semiautomated chemiluminescent immunoassay (CLIA) according to the instruction manual (ARCHITECT, Abbott Japan Co., Ltd., Tokyo, Japan).
4.6. Measurement of Mucosal Concentration of Tacrolimus
Each patient was subjected to periodic sigmoidoscopy; mucosal biopsy tissues, each measuring about 1 mm3 were excised from one to three areas in the rectum. These samples were collected in a micro-tube, immediately frozen in liquid nitrogen, and stored at −80 °C until analysis. The individual weight of samples was measured. After each specimen was individually separated, it was transferred to a protein LoBind tube (Eppendorf Co, Ltd. Tokyo, Japan) and homogenized using ultrasonication in 300 µL of ultrapure water. The homogenates (300 µL) were transferred to glass tubes, spiked with 25 µL of the internal standard sirolimus (100 ng/mL), and vortexed for 10 s. Then, 600 µL of water and 2 mL of extraction solution (methyl-t-butyl ether or cyclohexane, 1:3 v/v) were added to the glass tubes. Each tube was capped securely, mixed on a horizontal shaker for 15 min, and centrifuged at 3000 rpm at room temperature for 10 min. The organic layer was transferred to another tube and evaporated using an Automatic Environmental Speed Vac® System (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 60 °C for 30 min. Each sample was reconstituted with 150 µL of the mobile phase (80% methanol with 1 mM ammonium acetate), vortexed for 1 min, and filtered using a 4 mm Cosmonice Filter W (Nacalai Tesque, Kyoto, Japan). LC-MS/MS protocols reported by Hosohata et al. were used [19].
4.7. Statistical Analyses
All statistical analyses were performed using Prism version 8 (GraphPad Software, Inc., San Diego, CA, USA). If the F distributions were the same, t-test was used. If they were different, nonparametric test was used. Mann–Whitney’s U-test and Kruskal–Wallis test were used to compare the differences between CYP3A5*1 (*1/*1 or *1/*3) and CYP3A5*3/*3 genotypes. A value of p < 0.05 was considered statistically significant.
Author Contributions
Conceptualization, Y.Y., H.N., and S.M. (Satohiro Masuda); data curation, Y.Y. and S.M. (Shihoko Maruyama); funding acquisition, Y.Y. and S.M. (Satohiro Masuda); investigation, Y.Y., M.M., and S.M. (Shihoko Maruyama); supervision, H.N. and S.M. (Satohiro Masuda); writing—original draft, Y.Y. and S.M. (Satohiro Masuda); writing—review and editing, H.N., M.M., and S.M. (Satohiro Masuda). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (MEXT) (grant number: 18H02588 to Satohiro Masuda, 23928016 and 15H00524 to Yuki Yamamoto).
Acknowledgments
We would like to thank Yumeko Nishino and Kazumi Letessier for providing patient information.
Conflicts of Interest
The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| ABCB1 | ATP binding cassette transporter B1 |
| CYP | cytochrome p450 |
| C/D | concentration/dose |
| UC | ulcerative colitis |
| FKBP12 | FK506-binding protein 12 |
| NFAT | nuclear factor of activated T |
| MDR1 | Multidrug resistance 1 |
| CAI | clinical activity index |
| TNF | tumor necrosis factor |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry/mass spectrometry |
| HPLC | high-performance liquid chromatography |
| PCR | polymerase chain reaction |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HRM | high-resolution melting |
| CLIA | chemiluminescent immunoassay |
| ABCB1 | ATP binding cassette transporter B1 |
References
- Yamamoto, Y.; Masuda, S.; Nakase, H.; Matsuura, M.; Maruyama, S.; Hisamatsu, T.; Suzuki, Y.; Matsubara, K. Influence of Pharmaceutical Formulation on the Mucosal Concentration of 5-Aminosalicylic Acid and N-Acetylmesalamine in Japanese Patients with Ulcerative Colitis. Biol. Pharm. Bull. 2019, 42, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Allegretti, J.R.; Siddique, S.M.; Terdiman, J.P. AGA Technical Review on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1465–1496.e17. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tong, J.; Ran, Z. Tacrolimus Therapy in Steroid-Refractory Ulcerative Colitis: A Review. Inflamm. Bowel Dis. 2020, 26, 24–32. [Google Scholar] [CrossRef]
- Rodriguez-Lago, I.; Castro-Poceiro, J.; Fernandez-Clotet, A.; Mesonero, F.; Lopez-Sanroman, A.; Lopez-Garcia, A.; Marquez, L.; Clos-Parals, A.; Canete, F.; Vicuna, M.; et al. Tacrolimus induces short-term but not long-term clinical response in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. (Tokyo) 1987, 40, 1256–1265. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Asberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- Fu, R.; Tajima, S.; Suetsugu, K.; Watanabe, H.; Egashira, N.; Masuda, S. Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharm. Sin. 2019, 40, 151–159. [Google Scholar] [CrossRef]
- Abouljoud, M.S.; Kumar, M.S.; Brayman, K.L.; Emre, S.; Bynon, J.S. Neoral rescue therapy in transplant patients with intolerance to tacrolimus. Clin. Transpl. 2002, 16, 168–172. [Google Scholar] [CrossRef]
- Bechstein, W.O. Neurotoxicity of calcineurin inhibitors: Impact and clinical management. Transpl. Int. 2000, 13, 313–326. [Google Scholar] [CrossRef]
- Eidelman, B.H.; Abu-Elmagd, K.; Wilson, J.; Fung, J.J.; Alessiani, M.; Jain, A.; Takaya, S.; Todo, S.N.; Tzakis, A.; Van Thiel, D.; et al. Neurologic complications of FK 506. Transplant. Proc. 1991, 23, 3175–3178. [Google Scholar] [PubMed]
- Yamada, T.; Zhang, M.; Masuda, S. Significance of ethnic factors in immunosuppressive therapy management after organ transplantation. Ther. Drug Monit. 2020. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Inui, K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol. Ther. 2006, 112, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.E.; Relling, M.V. Moving towards individualized medicine with pharmacogenomics. Nature 2004, 429, 464–468. [Google Scholar] [CrossRef]
- Hebert, M.F. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv. Drug Deliv. Rev. 1997, 27, 201–214. [Google Scholar] [CrossRef]
- Yigitaslan, S.; Erol, K.; Cengelli, C. The Effect of P-Glycoprotein Inhibition and Activation on the Absorption and Serum Levels of Cyclosporine and Tacrolimus in Rats. Adv. Clin. Exp. Med. 2016, 25, 237–242. [Google Scholar] [CrossRef]
- Omae, T.; Goto, M.; Shimomura, M.; Masuda, S.; Ito, K.; Okuda, M.; Inui, K. Transient up-regulation of P-glycoprotein reduces tacrolimus absorption after ischemia-reperfusion injury in rat ileum. Biochem. Pharm. 2005, 69, 561–568. [Google Scholar] [CrossRef]
- Dai, Y.; Hebert, M.F.; Isoherranen, N.; Davis, C.L.; Marsh, C.; Shen, D.D.; Thummel, K.E. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab. Dispos. 2006, 34, 836–847. [Google Scholar] [CrossRef]
- Hosohata, K.; Uesugi, M.; Hashi, S.; Hosokawa, M.; Inui, K.I.; Matsubara, K.; Ogawa, K.; Fujimoto, Y.; Kaido, T.; Uemoto, S.; et al. Association between CYP3A5 genotypes in graft liver and increase in tacrolimus biotransformation by steroid treatment in living-donor liver transplant patients. Drug Metab. Pharmacokinet. 2014, 29, 83–89. [Google Scholar] [CrossRef]
- Uesugi, M.; Masuda, S.; Katsura, T.; Oike, F.; Takada, Y.; Inui, K. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharm. Genom. 2006, 16, 119–127. [Google Scholar] [CrossRef]
- Thervet, E.; Loriot, M.A.; Barbier, S.; Buchler, M.; Ficheux, M.; Choukroun, G.; Toupance, O.; Touchard, G.; Alberti, C.; Le Pogamp, P.; et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin. Pharmacol. Ther. 2010, 87, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Fukuda, M.; Matsukane, R.; Suetsugu, K.; Harada, N.; Yoshizumi, T.; Egashira, N.; Mori, M.; Masuda, S. Influence of POR*28 Polymorphisms on CYP3A5*3-Associated Variations in Tacrolimus Blood Levels at an Early Stage after Liver Transplantation. Int. J. Mol. Sci. 2020, 21, 2287. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, K.; Mori, Y.; Yamamoto, N.; Shigematsu, T.; Miyamoto, T.; Egashira, N.; Akashi, K.; Masuda, S. Impact of CYP3A5, POR, and CYP2C19 Polymorphisms on Trough Concentration to Dose Ratio of Tacrolimus in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2019, 20, 2413. [Google Scholar] [CrossRef]
- Hirai, F.; Takatsu, N.; Yano, Y.; Satou, Y.; Takahashi, H.; Ishikawa, S.; Tsurumi, K.; Hisabe, T.; Matsui, T. Impact of CYP3A5 genetic polymorphisms on the pharmacokinetics and short-term remission in patients with ulcerative colitis treated with tacrolimus. J. Gastroenterol. Hepatol. 2014, 29, 60–66. [Google Scholar] [CrossRef]
- Englund, G.; Jacobson, A.; Rorsman, F.; Artursson, P.; Kindmark, A.; Ronnblom, A. Efflux transporters in ulcerative colitis: Decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm. Bowel Dis. 2007, 13, 291–297. [Google Scholar] [CrossRef]
- Cario, E. P-glycoprotein multidrug transporter in inflammatory bowel diseases: More questions than answers. World J. Gastroenterol. 2017, 23, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M.; Hasler, R.; Jacobs, G.; Haenisch, S.; Lachelt, S.; Faltraco, F.; Sina, C.; Rosenstiel, P.; Nikolaus, S.; Schreiber, S.; et al. Decreased sigmoidal ABCB1 (P-glycoprotein) expression in ulcerative colitis is associated with disease activity. Pharmacogenomics 2009, 10, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Keum, B.; Ye, B.D.; Park, S.J.; Koo, H.S.; Eun, C.S. Treatment of inflammatory bowel disease in Asia: The results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest. Res. 2016, 14, 231–239. [Google Scholar] [CrossRef]
- Nakase, H.; Yamamoto, S.; Matsuura, M.; Chiba, T. Tacrolimus: Rescue therapy or experimental drug for severe ulcerative colitis? Aliment. Pharmacol. Ther. 2011, 33, 413–414. [Google Scholar] [CrossRef]
- Masuda, S.; Uemoto, S.; Hashida, T.; Inomata, Y.; Tanaka, K.; Inui, K. Effect of intestinal P-glycoprotein on daily tacrolimus trough level in a living-donor small bowel recipient. Clin. Pharmacol. Ther. 2000, 68, 98–103. [Google Scholar] [CrossRef]
- Kawauchi, S.; Nakamura, T.; Miki, I.; Inoue, J.; Hamaguchi, T.; Tanahashi, T.; Mizuno, S. Downregulation of CYP3A and P-glycoprotein in the secondary inflammatory response of mice with dextran sulfate sodium-induced colitis and its contribution to cyclosporine A blood concentrations. J. Pharmacol. Sci. 2014, 124, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hashida, T.; Masuda, S.; Uemoto, S.; Saito, H.; Tanaka, K.; Inui, K. Pharmacokinetic and prognostic significance of intestinal MDR1 expression in recipients of living-donor liver transplantation. Clin. Pharmacol. Ther. 2001, 69, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Thorn, M.; Finnstrom, N.; Lundgren, S.; Rane, A.; Loof, L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. Br. J. Clin. Pharmacol. 2005, 60, 54–60. [Google Scholar] [CrossRef]
- Hiraoka, S.; Kato, J.; Moritou, Y.; Takei, D.; Inokuchi, T.; Nakarai, A.; Takahashi, S.; Harada, K.; Okada, H.; Yamamoto, K. The earliest trough concentration predicts the dose of tacrolimus required for remission induction therapy in ulcerative colitis patients. BMC Gastroenterol. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Matsui, T.; Nakamura, M.; Iida, M.; Takazoe, M.; Suzuki, Y.; Hibi, T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006, 55, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, M.; Kikuchi, M.; Shinke, H.; Omura, T.; Yonezawa, A.; Matsubara, K.; Fujimoto, Y.; Okamoto, S.; Kaido, T.; Uemoto, S.; et al. Impact of cytochrome P450 3A5 polymorphism in graft livers on the frequency of acute cellular rejection in living-donor liver transplantation. Pharm. Genom. 2014, 24, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Van Dieren, J.M.; van Bodegraven, A.A.; Kuipers, E.J.; Bakker, E.N.; Poen, A.C.; van Dekken, H.; Nieuwenhuis, E.E.; van der Woude, C.J. Local application of tacrolimus in distal colitis: Feasible and safe. Inflamm. Bowel Dis. 2009, 15, 193–198. [Google Scholar] [CrossRef]
- Fukushima-Uesaka, H.; Saito, Y.; Watanabe, H.; Shiseki, K.; Saeki, M.; Nakamura, T.; Kurose, K.; Sai, K.; Komamura, K.; Ueno, K.; et al. Haplotypes of CYP3A4 and their close linkage with CYP3A5 haplotypes in a Japanese population. Hum. Mutat. 2004, 23, 100. [Google Scholar] [CrossRef]
- Uesugi, M.; Hosokawa, M.; Shinke, H.; Hashimoto, E.; Takahashi, T.; Kawai, T.; Matsubara, K.; Ogawa, K.; Fujimoto, Y.; Okamoto, S.; et al. Influence of Cytochrome P450 (CYP) 3A4*1G Polymorphism on the Pharmacokinetics of Tacrolimus, Probability of Acute Cellular Rejection, and mRNA Expression Level of CYP3A5 Rather than CYP3A4 in Living-Donor Liver Transplant Patients. Biol. Pharm. Bull. 2013, 36, 1814–1821. [Google Scholar] [CrossRef]
- Goto, M.; Masuda, S.; Kiuchi, T.; Ogura, Y.; Oike, F.; Okuda, M.; Tanaka, K.; Inui, K. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics 2004, 14, 471–478. [Google Scholar] [CrossRef]
- Koch, I.; Weil, R.; Wolbold, R.; Brockmoller, J.; Hustert, E.; Burk, O.; Nuessler, A.; Neuhaus, P.; Eichelbaum, M.; Zanger, U.; et al. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mRNA. Drug Metab. Dispos. 2002, 30, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y.; Masuda, S.; Goto, M.; Motohashi, H.; Saito, H.; Okuda, M.; Nakamura, E.; Ito, N.; Ogawa, O.; Inui, K.I. Common single nucleotide polymorphisms of the MDR1 gene have no influence on its mRNA expression level of normal kidney cortex and renal cell carcinoma in Japanese nephrectomized patients. J. Hum. Genet. 2004, 49, 40–45. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).