Influence of Thermal Pollution on the Physiological Conditions and Bioaccumulation of Metals, Metalloids, and Trace Metals in Whitefish (Coregonus lavaretus L.)
Abstract
1. Introduction
2. Results
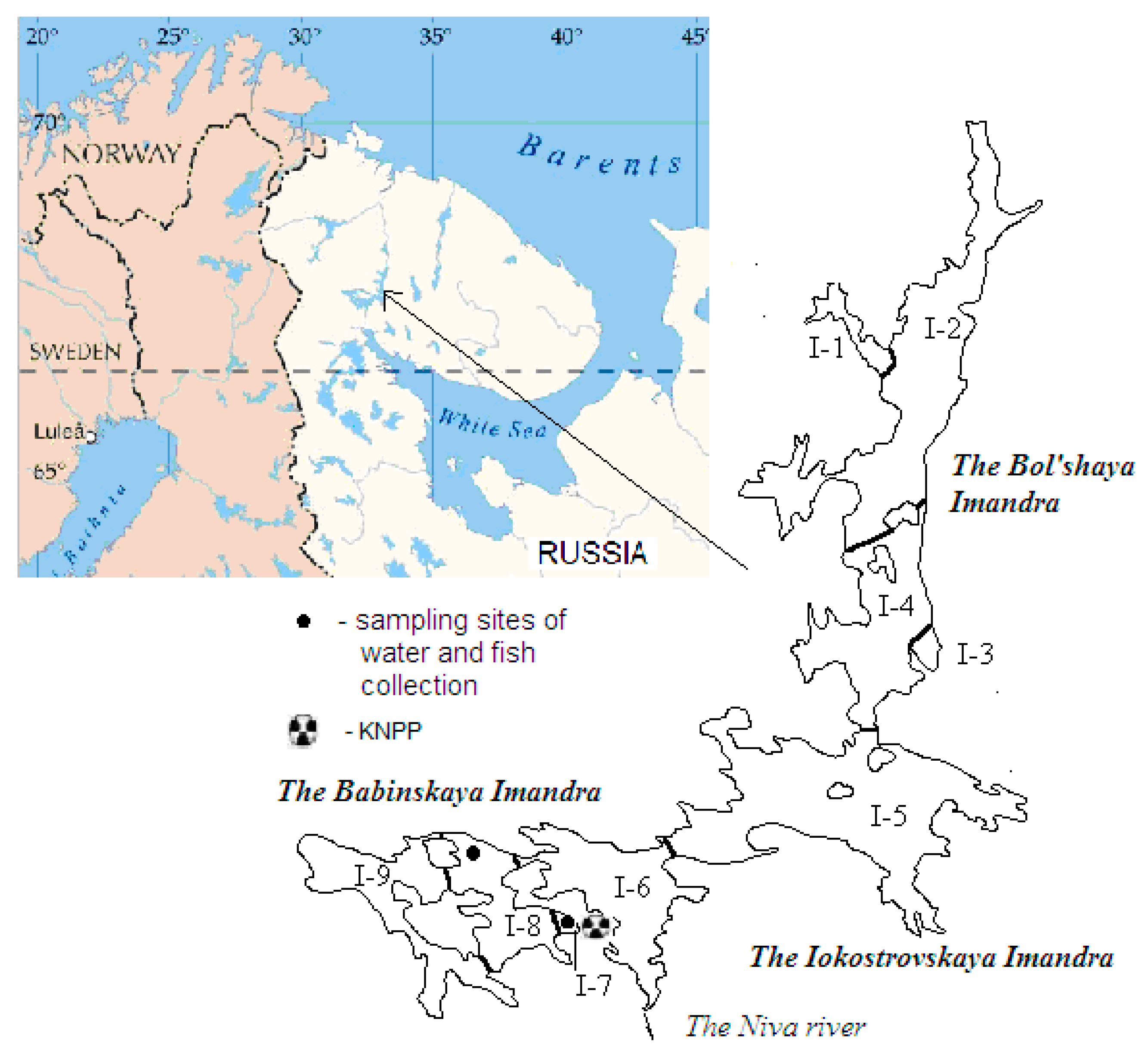
2.1. Habitat Conditions
2.2. Physiological Condition
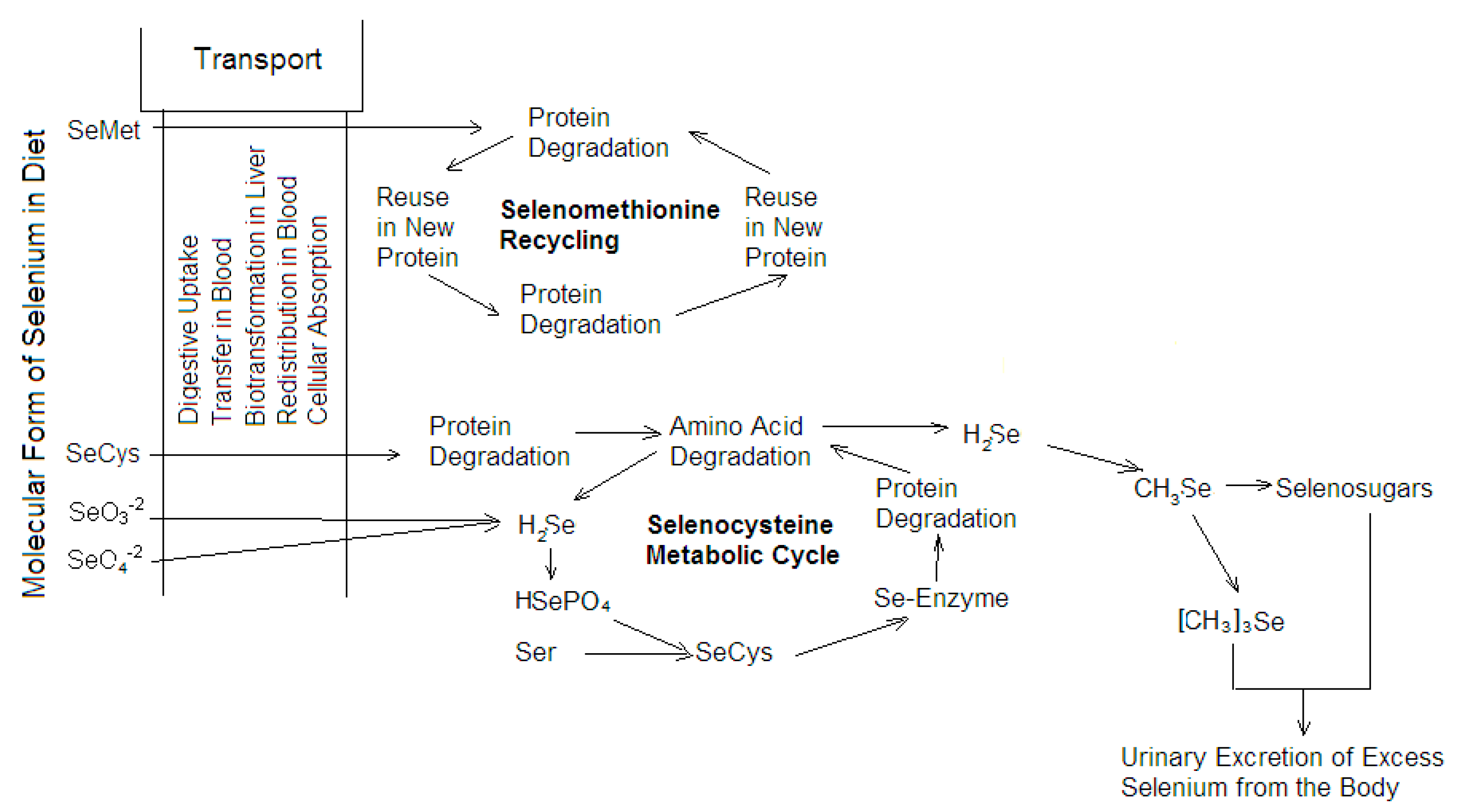
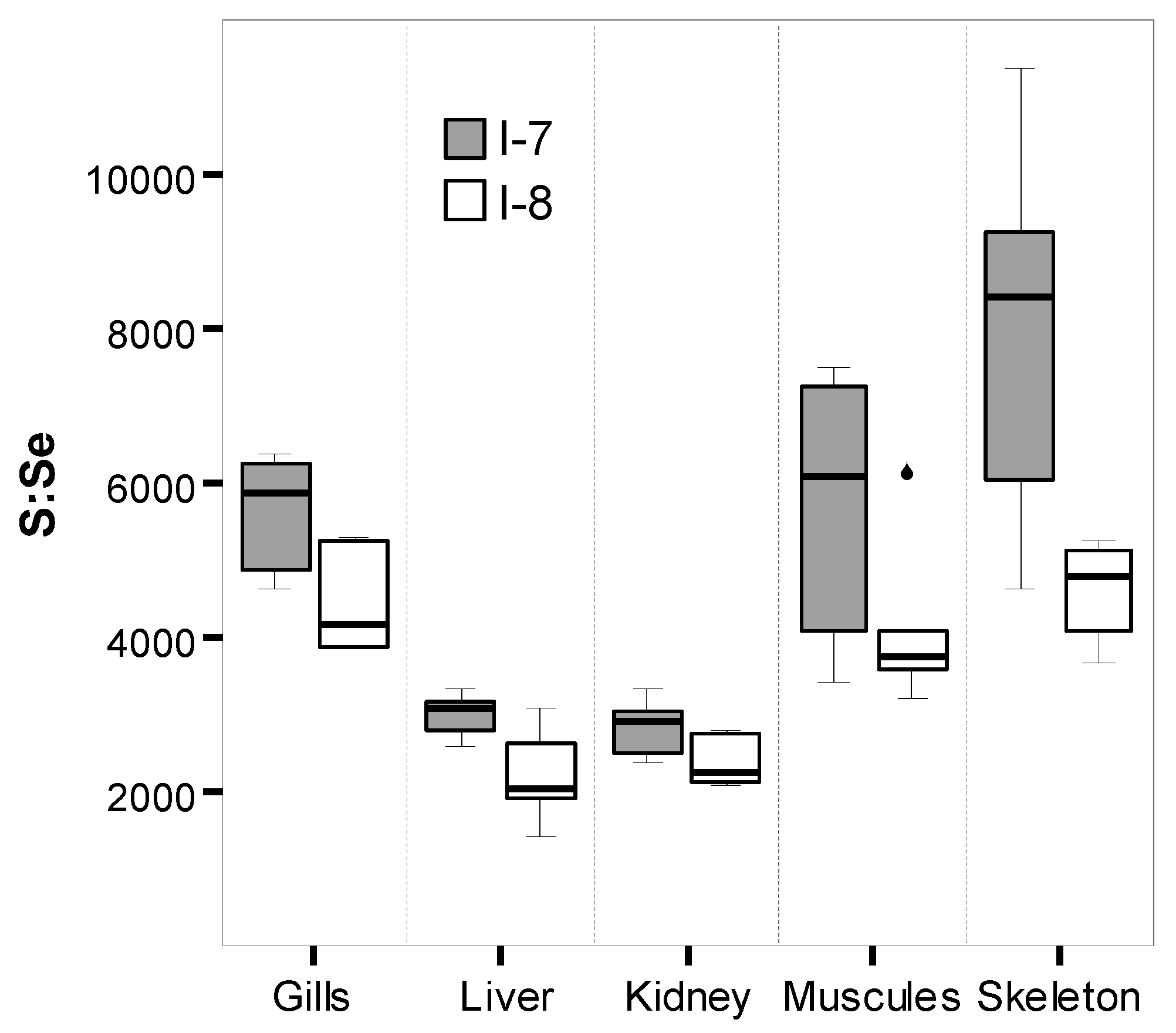
2.3. Bioaccumulation
3. Materials and Methods
3.1. Water Sample Collection
3.2. Concentrations of Metals, Metalloids, and Trace Metals in Fish Organs and Tissues
3.3. Physiological Condition
3.4. Statistical Analysis and Data Presentation
4. Discussion
4.1. Permeation, Metabolism, and Bioaccumulation of Metals, Metalloids, and Trace Metals Associated with Respiratory Activity and Ion Regulation
4.2. Metabolism and Bioaccumulation of Metals, Metalloids, and Trace Metals Associated with Somatic Growth
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Environmental Safety Report 2018; Kola Nuclear Plant Branch of Rosenergoatom Concern: Polyarnie Zory, Russia, 2018; p. 44. Available online: https://rosatom.ru/upload/iblock/d68/d68934ec26c9a5d00078147b911cf8ba.pdf (accessed on 17 June 2020).
- Moiseenko, T.I.; Dauvalter, V.A.; Lukin, A.A.; Kudryavtseva, L.P.; Il’yashchuk, B.P.; Sandimirov, S.S.; Kagan, L.Y.; Vandysh, O.I.; Sharov, A.N.; Sharova, Y.N.; et al. Antropogenic Modifications of the Imandra Lake Ecosystems; Nauka: Moscow, Russia, 2002; p. 403. [Google Scholar]
- Moe, J.; De Schamphelaert, K.; Clements, W.H.; Sorensen, M.T.; Van den Brink, P.J.; Liess, M. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ. Toxicol. Chem. 2013, 32, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Armitage, J.M.; Cousins, I.T.; Muir, D.C.G.; Ng, C.A.; Reid, L.; Tao, S. Influence of global climate change on chemical fate and bioaccumulation: The role of multimedia models. Environ. Toxicol. Chem. 2013, 32, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ingleton, T.; McMinn, A. Thermal plume effects: A multi-disciplinary approach for assessing effects of thermal pollution on estuaries using benthic diatoms and satellite imagery. Estuar. Coast. Shelf Sci. 2012, 99, 132–144. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Lukin, A.A. Fish pathology in polluted water bodies of the Subarctic and its diagnosis. J. Ichthyol. 1999, 39, 532–543. [Google Scholar]
- Hooper, M.J.; Ankley, G.T.; Cristol, D.A.; Maryoung, L.A.; Noyes, P.D.; Pinkerton, K.E. Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ. Toxicol. Chem. 2013, 32, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.I. Hematological indices of fishes in the evaluation of their toxicosis with reference to Coregonus lavaretus. J. Ichthyol. 1998, 38, 315–324. [Google Scholar]
- Falfushynska, H.; Gnatyshyna, L.; Yurchak, I.; Ivanina, A.; Stoliar, O.; Sokolova, I. Habitat pollution and thermal regime modify molecular stress responses to elevated temperature in freshwater mussels (Anodonta anatina: Unionidae). Sci. Total Environ. 2014, 500–501, 339–350. [Google Scholar] [CrossRef]
- Intergovernmental Panel for Global Climate Change (IPCC). The Physical Science Basis. Climate Change. 2013. Available online: http://www.ipcc.ch/report/ar5/wg1 (accessed on 17 June 2020).
- Dietz, R.; Letcher, R.J.; Sonne, C.; Eulaers, I. AMAP Assessment 2018: Biological Effects of Contaminants on Arctic Wildlife and Fish; Arctic Monitoring and Assessment Programme (AMAP): Tromsø, Norway, 2018. [Google Scholar]
- Sokolova, S.A. Water Quality Standards for Fishery Water Bodies, Including Norms of Maximum Permissible Concentrations of Toxic Matters in Fishery Basins; VNIRO: Moscow, Russia, 2011; p. 257. [Google Scholar]
- Moiseenko, T.I.; Morgunov, B.A.; Gashkina, N.A.; Megorskiy, V.V.; Pesiakova, A.A. Ecosystem and human health assessment in relation to aquatic environment pollution by heavy metals: Case study of northwest of the Russian arctic, Kola peninsula. Environ. Res. Lett. 2018, 13, 065005. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Lan, W.-R.; Huang, X.-G.; Lin, L.-X.; Li, S.-X.; Liu, F.-J. Thermal discharge influences the bioaccumulation and bioavailability of metals in oysters: Implications of ocean warming. Environ. Pollut. 2020, 259, 113821. [Google Scholar] [CrossRef]
- Yokel, R.A. Blood–brain barrier flux of aluminum, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J. Alzheimers Dis. 2006, 10, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Tylenda, C.A.; Sullivan, D.W., Jr.; Fowler, B.A. Antimony. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: London, UK, 2015; Volume II, pp. 565–579. [Google Scholar] [CrossRef]
- Griffith, M.B. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: Teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 2017, 36, 576–600. [Google Scholar] [CrossRef] [PubMed]
- Jilkina, O.; Kuzio, B.; Kupriyanov, V.V. Hyposmotic shock: Effects on rubidium/potassium efflux in normal and ischemic rat hearts, assessed by 87Rb and 31P NMR. Biochim. Biophys. Acta 2003, 1637, 20–30. [Google Scholar] [CrossRef]
- Orban, E.; Masci, M.; Nevigato, T.; Di Lena, G.; Casini, I.; Caproni, R.; Gambelli, L.; De Angelis, P.; Rampacci, M. Nutritional quality and safety of whitefish (Coregonus lavaretus) from Italian lakes. J. Food Composit. Anal. 2006, 19, 737–746. [Google Scholar] [CrossRef]
- Lee, J.; Peña, M.M.; Nose, Y.; Thiele, D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Gashkina, N.A. Essential elements in the organs and tissues of fish depending on the freshwater toxicity and physiological state. Geochem. Int. 2017, 55, 927–934. [Google Scholar] [CrossRef]
- Gashkina, N.A.; Moiseenko, T.I.; Kudryavtseva, L.P. Fish response of metal bioaccumulation to reduced toxic load on long-term contaminated Lake Imandra. Ecotoxicol. Environ. Saf. 2020, 191, 110205. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M. Copper. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrel, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2012; Volume 31A, pp. 53–133. [Google Scholar] [CrossRef]
- Cameron, J.N. Unusual aspects of calcium metabolism in aquatic animals. Annu. Rev. Physiol. 1990, 52, 77–95. [Google Scholar] [CrossRef]
- Ransberry, V.E.; Morash, A.J.; Blewett, T.A.; Wood, C.M.; McClelland, G.B. Oxidative stress and metabolic responses to copper in freshwater-and seawater-acclimated killifish, Fundulus heteroclitus. Aquat. Toxicol. 2015, 161, 242–252. [Google Scholar] [CrossRef]
- Reist, J.D.; Wrona, F.J.; Prowse, T.D.; Power, M.; Dempson, J.B.; King, J.R.; Beamish, R.J. An overview of effects of climate change on selected Arctic freshwater and anadromous fishes. Ambio 2006, 35, 381–387. [Google Scholar] [CrossRef]
- Mariotti, M.; Ridge, P.G.; Zhang, Y.; Lobanov, A.V.; Pringle, T.H.; Guigo, R.; Hatfield, D.L.; Gladyshev, V.N. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS ONE 2012, 7, e33066. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.M. Selenium. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrel, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2012; Volume 31A, pp. 327–374. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Carlson, B.A.; Xu, X.; Mix, H.; Gladyshev, V.N. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog. Nucleic. Acid. Res. Mol. Biol. 2006, 81, 7–142. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H. Effects ofdifferent dietary selenium sources (sodium selenite, selenomethionine and nanose-lenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquacult. Nutr. 2017, 23, 611–617. [Google Scholar] [CrossRef]
- McKinley, A.C.; Taylor, M.D.; Johnston, E.L. Relationships between body burdens of trace metals (As, Cu, Fe, Hg, Mn, Se, and Zn) and the relative body size of small tooth flounder (Pseudorhombus jenynsii). Sci. Total Environ. 2012, 423, 84–94. [Google Scholar] [CrossRef] [PubMed]
- De Riu, N.; Lee, J.-W.; Huang, S.S.Y.; Moniello, G.; Hung, S.S.O. Effect of dietary selenomethionine on growth performance, tissue burden, and histopathology in green and white sturgeon. Aquat. Toxicol. 2014, 148, 65–73. [Google Scholar] [CrossRef]
- Phibbs, J.; Franz, E.; Hauck, D.; Gallego, M.; Tse, J.J.; Pickering, I.J.; Liber, K.; Janz, D.M. Evaluating the trophic transfer of selenium in aquatic ecosystems using caged fish, X-ray absorption spectroscopy and stable isotope analysis. Ecotoxicol. Environ. Saf. 2011, 74, 1855–1863. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Ralston, C.R.; Blackwell, J.L., III; Raymond, L.J. Dietary and tissue selenium in relation to methylmercury toxicity. NeuroToxicology 2008, 29, 802–811. [Google Scholar] [CrossRef]
- Reid, S.D. Molybdenum and chromium. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrel, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2012; Volume 31A, pp. 375–415. [Google Scholar] [CrossRef]
- Leffler, P.E.; Kazantzic, G. Tungsten. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: London, UK, 2015; Volume II, pp. 1297–1306. [Google Scholar] [CrossRef]
- Gashkina, N.A.; Tatsii, Y.G.; Udachin, V.N.; Aminov, P.G. Biogeochemical indication of environmental contamination: A case study of a large copper smelter. Geochem. Int. 2015, 53, 253–264. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Bioaccumulation of mercury in fish as indicator of water pollution. Geochem. Int. 2016, 54, 485–493. [Google Scholar] [CrossRef]
- Madenjian, C.; Ebener, M.; Krabbenhoft, D. Mercury accumulation, and the mercury-PCB-sex interaction, in lake whitefish (Coregonus clupeaformis). Environments 2016, 3, 7. [Google Scholar] [CrossRef]
- Ahonen, S.A.; Hayden, B.; Leppänen, J.J.; Kahilainen, K.K. Climate and productivity affect total mercury concentration and bioaccumulation rate of fish along a spatial gradient of subarctic lakes. Sci. Total Environ. 2018, 637–638, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, D.O.; Linton, T.K. Arsenic. In Homeostasis and Toxicology of Non-Essential Metals; Wood, C.M., Farrel, A.P., Brauner, C.J., Eds.; Academic Press: San Diego, CA, USA, 2012; Volume 31B, pp. 297–349. [Google Scholar] [CrossRef]
- Couture, P.; Pyle, G. Live fast and die young: Metal effects on condition and physiology of wild yellow perch from along two metal contamination gradients. Hum. Ecol. Risk. Assess. 2008, 14, 73–96. [Google Scholar] [CrossRef]
- Martin, K.R. Chapter 14 Silicon: The Health Benefits of a Metalloid. In Interrelations between Essential Metal Ions and Human Diseases, Metal Ions in Life Sciences; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2013; Volume 13, pp. 451–473. [Google Scholar] [CrossRef]

| Metal | Water | Gills | Liver | Kidney | Muscles | Skeleton |
|---|---|---|---|---|---|---|
| μg/L | μg/g Dry Weight | |||||
| Ca | 3740 | 30,991 ± 2232 | 654 ± 180 | 880 ± 147 | 2369 ± 891 | 155,291 ± 1500 |
| 3590 | 31,909 ± 1840 | 627 ± 161 | 1318 ± 283 | 1006 ± 310 | 148,031 ± 3047 | |
| Mg | 1120 | 1083 ± 73 | 713 ± 73 | 883 ± 91 | 1367 ± 76 | 2033 ± 30 |
| 1090 | 1163 ± 34 | 878 ± 37 | 1307 ± 131 | 1233 ± 65 | 2120 ± 37 | |
| Na | 7270 | 6418 ± 449 | 3558 ± 387 | 6536 ± 556 | 815 ± 96 | 7055 ± 163 |
| 6250 | 5745 ± 347 | 5316 ± 431 | 8979 ± 469 | 834 ± 59 | 6577 ± 106 | |
| K | 1470 | 9941 ± 458 | 10,276 ± 562 | 10,583 ± 1235 | 18,264 ± 1111 | 3086 ± 220 |
| 1300 | 10,080 ± 534 | 12,105 ± 835 | 12,088 ± 826 | 17,646 ± 1216 | 3850 ± 188 | |
| S | 5300 | 10,857 ± 340 | 10,684 ± 621 | 10,924 ± 912 | 10,479 ± 85 | 4613 ± 140 |
| 4245 | 10,712 ± 225 | 11,946 ± 390 | 11,096 ± 514 | 9679 ± 339 | 4632 ± 220 | |
| P | 0.8 | 26,150 ± 1601 | 14,099 ± 985 | 14,031 ± 1248 | 12,724 ± 964 | 83,475 ± 960 |
| 0.8 | 26,587 ± 660 | 17,878 ± 679 | 16,413 ± 514 | 11,675 ± 865 | 78,618 ± 1495 | |
| Fe | 6.1 | 168 ± 14 | 155 ± 19 | 289 ± 39 | 13.9 ± 2.6 | 13.3 ± 4.3 |
| 13.9 | 385 ± 57 | 172 ± 23 | 310 ± 24 | 13.0 ± 2.8 | 12.6 ± 3.3 | |
| Zn | 0.44 | 504 ± 122 | 290 ± 82 | 231 ± 29 | 19.3 ± 1.3 | 120 ± 12 |
| 2.65 | 713 ± 113 | 334 ± 78 | 274 ± 43 | 17.6 ± 1.3 | 141 ± 16 | |
| Cu | 2.3 | 3.0 ± 0.4 | 68.1 ± 11.3 | 27.6 ± 16.5 | 1.2 ± 0.2 | 0.7 ± 0.2 |
| 2.8 | 2.2 ± 0.1 | 32.9 ± 7.4 | 10.2 ± 0.9 | 1.3 ± 0.1 | 0.6 ± 0.2 | |
| Mn | 1.3 | 19.6 ± 4.4 | 7.21 ± 0.53 | 3.98 ± 0.82 | 2.23 ± 1.32 | 49.7 ± 13.8 |
| 1.4 | 25.0 ± 5.3 | 8.62 ± 0.38 | 4.27 ± 0.40 | 0.84 ± 0.07 | 36.9 ± 3.8 | |
| Co | <0.1 | <0.04 (0.10) | 0.10 ± 0.02 | 1.09 ± 0.22 | <0.04 | <0.04 |
| <0.1 | 0.13 ± 0.03 | 0.12 ± 0.02 | 0.74 ± 0.16 | <0.04 | <0.04 | |
| Se | <0.3 | 4.8 ± 0.4 | 8.7 ± 0.3 | 9.4 ± 0.7 | 4.9 ± 0.7 | 1.6 ± 0.3 |
| <0.3 | 6.0 ± 0.4 | 14.2 ± 1.8 | 11.6 ± 0.7 | 6.1 ± 0.5 | 2.5 ± 0.2 | |
| Mo | 1.03 | 0.11 ± 0.02 | 0.67 ± 0.07 | 0.59 ± 0.08 | 0.02 ± 0.02 | 0.18 ± 0.03 |
| 0.74 | 0.21 ± 0.03 | 0.73 ± 0.07 | 0.94 ± 0.13 | 0.02 ± 0.01 | 0.34 ± 0.07 | |
| B | <8 | <0.3 (3.7) | <0.3 | 1.0 ± 0.4 | <0.3 (0.5) | <0.3 |
| <8 | 2.2 ± 0.4 | 1.0 ± 0.4 | 1.6 ± 0.5 | <0.3 | 2.4 ± 0.7 | |
| Br | 12.3 | 65.5 ± 18.7 | 46.0 ± 7.1 | 50.8 ± 5.7 | 38.0 ± 8.6 | 32.8 ± 16.1 |
| 8.8 | 72.9 ± 13.3 | 54.2 ± 7.5 | 110 ± 22.6 | 37.6 ± 5.9 | 80.2 ± 15.1 | |
| Cr | <0.5 | <0.3 (3.7) | <0.3 | <0.3 | <0.3 | <0.3 (0.6) |
| <0.5 | 0.9 ± 0.1 | <0.3 | <0.3 | <0.3 | <0.3 (0.7) | |
| Ni | 1.5 | 1.1 ± 0.1 | 0.6 ± 0.2 | 5.4 ± 1.3 | 0.4 ± 0.2 | <0.1 |
| 1.3 | 1.5 ± 0.4 | 1.1 ± 0.3 | 7.6 ± 1.5 | 0.1 ± 0.1 | 0.2 ± 0.1 | |
| Si | 1.27 | 54.8 ± 20.5 | 18.5 ± 1.1 | 40.7 ± 7.6 | 18.4 ± 1.2 | 12.5 ± 1.2 |
| 1.38 | 272 ± 38.4 | 13.1 ± 0.8 | 68.9 ± 7.2 | 15.0 ± 3.2 | 13.1 ± 4.6 | |
| Li | 0.54 | 0.017 ± 0.005 | 0.002 ± 0.002 | 0.006 ± 0.003 | <0.003 (0.008) | 0.026 ± 0.003 |
| 0.58 | 0.095 ± 0.016 | 0.003 ± 0.001 | 0.016 ± 0.003 | <0.003 | 0.030 ± 0.005 | |
| Metal | Water | Gills | Liver | Kidney | Muscles | Skeleton |
|---|---|---|---|---|---|---|
| μg/L | μg/g Dry Weight | |||||
| Hg | <0.01 | 0.064 ± 0.006 | 0.162 ± 0.021 | 0.200 ± 0.022 | 0.098 ± 0.021 | 0.018 ± 0.006 |
| <0.01 | 0.069 ± 0.009 | 0.120 ± 0.007 | 0.193 ± 0.025 | 0.099 ± 0.025 | 0.033 ± 0.003 | |
| Tl | 0.001 | 0.042 ± 0.003 | 0.473 ± 0.054 | 0.231 ± 0.111 | 0.031 ± 0.004 | 0.020 ± 0.002 |
| 0.002 | 0.045 ± 0.006 | 0.564 ± 0.119 | 0.100 ± 0.025 | 0.032 ± 0.006 | 0.025 ± 0.004 | |
| Be | <0.006 | <0.002 | <0.002 | 0.023 ± 0.023 | <0.002 | <0.002 |
| <0.006 | 0.004 ± 0.001 | <0.002 | <0.002 | <0.002 | <0.002 | |
| Cd | <0.004 | 0.076 ± 0.014 | 0.172 ± 0.034 | 2.01 ± 0.49 | <0.004 | 0.013 ± 0.003 |
| <0.004 | 0.057 ± 0.007 | 0.250 ± 0.041 | 3.46 ± 0.35 | <0.004 | 0.008 ± 0.002 | |
| Pb | 1.43 | 0.24 ± 0.10 | 0.05 ± 0.02 | 0.07 ± 0.03 | 0.01 ± 0.01 | 0.06 ± 0.01 |
| 0.2 | 0.27 ± 0.04 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.004 | 0.10 ± 0.02 | |
| Ag | <0.005 | 0.003 ± 0.001 | 0.100 ± 0.024 | 0.039 ± 0.029 | <0.002 | <0.002 |
| <0.005 | 0.012 ± 0.007 | 0.068 ± 0.011 | 0.013 ± 0.004 | 0.002 ± 0.002 | 0.016 ± 0.011 | |
| W | 0.044 | 0.034 ± 0.006 | 0.003 ± 0.001 | 0.040 ± 0.012 | 0.002 ± 0.001 | 0.158 ± 0.029 |
| 0.031 | 0.040 ± 0.009 | 0.007 ± 0.001 | 0.077 ± 0.012 | <0.002 | 0.155 ± 0.029 | |
| V | 0.13 | <0.2 (0.4) | <0.2 | 0.3 ± 0.2 | <0.2 | 0.3 ± 0.2 |
| 0.09 | 0.7 ± 0.3 | 0.2 ± 0.1 | 0.6 ± 0.2 | <0.2 | 0.4 ± 0.4 | |
| Te | <0.006 | <0.004 | 0.012 ± 0.003 | 0.031 ± 0.007 | <0.004 | <0.004 |
| <0.006 | 0.008 ± 0.001 | 0.016 ± 0.002 | 0.034 ± 0.005 | <0.004 | <0.004 | |
| Sb | 0.042 | <0.002 | <0.002 | <0.002 | <0.002 | 0.016 ± 0.006 |
| 0.037 | 0.004 ± 0.0004 | 0.003 ± 0.001 | 0.005 ± 0.0005 | <0.002 (0.007) | 0.017 ± 0.007 | |
| Bi | <0.004 | 0.0021 ± 0.0005 | 0.0022 ± 0.0010 | 0.0109 ± 0.0082 | 0.0005 ± 0.0002 | 0.0009 ± 0.0003 |
| <0.004 | 0.0026 ± 0.0004 | 0.0012 ± 0.0002 | 0.0037 ± 0.0006 | 0.0010 ± 0.0002 | 0.0017 ± 0.0006 | |
| As | <0.007 | 0.15 ± 0.09 | 0.31 ± 0.26 | 0.21 ± 0.11 | <0.04 | 0.21 ± 0.13 |
| <0.007 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | |
| Sr | 44.2 | 225 ± 23 | 5.1 ± 1.4 | 5.9 ± 0.9 | 16.5 ± 6.5 | 1083 ± 127 |
| 36.7 | 182 ± 11 | 5.2 ± 1.7 | 9.2 ± 2.1 | 4.1 ± 1.5 | 812 ± 46 | |
| Al | 7.6 | 16.5 ± 7.9 | 7.3 ± 1.3 | 10.8 ± 2.3 | 1.7 ± 1.3 | 5.0 ± 0.7 |
| 12.3 | 195 ± 36 | 14.2 ± 3.2 | 22.0 ± 3.7 | 7.0 ± 4.7 | 10.9 ± 3.2 | |
| Ti | <0.5 | 1.3 ± 1.0 | <0.5 (1.2) | <0.5 (2.0) | <0.5 | 1.7 ± 0.4 |
| <0.5 | 16.7 ± 2.8 | <0.5 | 0.5 ± 0.5 | <0.5 | 3.0±0.9 | |
| Zr | 0.004 | 0.10 ± 0.04 | 0.08 ± 0.02 | 0.12 ± 0.04 | 0.04 ± 0.01 | 0.06 ± 0.01 |
| 0.012 | 0.08 ± 0.02 | 0.02 ± 0.01 | 0.08 ± 0.01 | <0.02 | 0.02 ± 0.01 | |
| Rb | 2.03 | 35.0 ± 1.7 | 41.0 ± 3.7 | 40.4 ± 4.8 | 50.3 ± 3.3 | 9.5 ± 0.7 |
| 1.66 | 23.0 ± 2.0 | 31.6 ± 2.2 | 27.1 ± 2.0 | 31.1 ± 4.1 | 7.5 ± 0.5 | |
| Sn | <0.012 | <0.005 | 0.005 ± 0.005 | <0.005 | <0.005 | <0.005 |
| 0.045 | 0.022 ± 0.017 | 0.008 ± 0.007 | 0.014 ± 0.007 | <0.005 (0.017) | 0.019 ± 0.007 | |
| Ba | 4.47 | 3.16 ± 0.51 | 0.47 ± 0.09 | 0.90 ± 1.86 | 0.37 ± 0.12 | 8.17 ± 0.93 |
| 6.13 | 5.37 ± 0.94 | 0.41 ± 0.08 | 1.55 ± 0.22 | 0.43 ± 0.12 | 11.4 ± 1.62 | |
| U | 0.036 | 0.017 ± 0.002 | 0.006 ± 0.001 | 0.028 ± 0.010 | 0.007 ± 0.003 | 0.034 ± 0.004 |
| 0.039 | 0.028 ± 0.004 | 0.009 ± 0.002 | 0.029 ± 0.006 | 0.004 ± 0.002 | 0.064 ± 0.011 | |
| Cs | 0.012 | 0.156 ± 0.009 | 0.150 ± 0.028 | 0.232 ± 0.038 | 0.231 ± 0.021 | 0.059 ± 0.010 |
| 0.046 | 0.174 ± 0.026 | 0.123 ± 0.034 | 0.304 ± 0.115 | 0.139 ± 0.024 | 0.042 ± 0.004 | |
| Element | Gills | Liver | Kidney | Muscles | Skeleton |
|---|---|---|---|---|---|
| Hb | |||||
| Na | – | – | −0.683 ** | – | – |
| P | – | −0.590 * | – | – | – |
| Cu | – | – | 0.627 * | – | – |
| Tl | – | – | 0.600 * | – | – |
| Te | −0.649 * | – | – | – | – |
| Sb | −0.630 * | −0.627 * | −0.659 ** | – | – |
| Sr | 0.743 *** | – | – | – | 0.838 **** |
| Zr | – | 0.655 ** | – | – | 0.703 *** |
| Rb | 0.759 *** | – | 0.675 ** | 0.776 *** | – |
| FCF | |||||
| Na | – | – | −0.676 ** | – | – |
| Fe | −0.635 * | – | – | – | – |
| Cu | – | 0.640 * | – | – | – |
| Co | −0.681 ** | – | – | – | – |
| Se | – | −0.738 *** | −0.596 * | – | – |
| Mo | −0.683 ** | – | −0.635 * | −0.602 * | −0.607 * |
| B | −0.674 ** | – | – | – | −0.692 ** |
| Cr | −0.659 * | – | – | – | – |
| Ni | – | – | −0.575 * | – | −0.905 **** |
| Si | – | 0.638 * | −0.659 ** | – | – |
| Li | −0.694 ** | – | – | – | – |
| Be | −0.637 * | – | – | – | – |
| Ag | −0.669 ** | – | – | – | −0.697 ** |
| W | – | −0.626 * | – | – | – |
| Sb | −0.627 * | −0.641 * | – | – | – |
| Al | −0.651 ** | – | – | – | – |
| Ti | −0.603 * | – | – | – | – |
| Zr | – | 0.619 * | – | – | 0.728 *** |
| Rb | −0.600 * | – | – | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashkina, N.A.; Moiseenko, T.I. Influence of Thermal Pollution on the Physiological Conditions and Bioaccumulation of Metals, Metalloids, and Trace Metals in Whitefish (Coregonus lavaretus L.). Int. J. Mol. Sci. 2020, 21, 4343. https://doi.org/10.3390/ijms21124343
Gashkina NA, Moiseenko TI. Influence of Thermal Pollution on the Physiological Conditions and Bioaccumulation of Metals, Metalloids, and Trace Metals in Whitefish (Coregonus lavaretus L.). International Journal of Molecular Sciences. 2020; 21(12):4343. https://doi.org/10.3390/ijms21124343
Chicago/Turabian StyleGashkina, Natalia A., and Tatyana I. Moiseenko. 2020. "Influence of Thermal Pollution on the Physiological Conditions and Bioaccumulation of Metals, Metalloids, and Trace Metals in Whitefish (Coregonus lavaretus L.)" International Journal of Molecular Sciences 21, no. 12: 4343. https://doi.org/10.3390/ijms21124343
APA StyleGashkina, N. A., & Moiseenko, T. I. (2020). Influence of Thermal Pollution on the Physiological Conditions and Bioaccumulation of Metals, Metalloids, and Trace Metals in Whitefish (Coregonus lavaretus L.). International Journal of Molecular Sciences, 21(12), 4343. https://doi.org/10.3390/ijms21124343






