Decreased Excretion of Urinary Exosomal Aquaporin-2 in a Puromycin Aminonucleoside-Induced Nephrotic Syndrome Model
Abstract
1. Introduction
2. Results
2.1. Blood and Urine Parameters after PAN Treatment
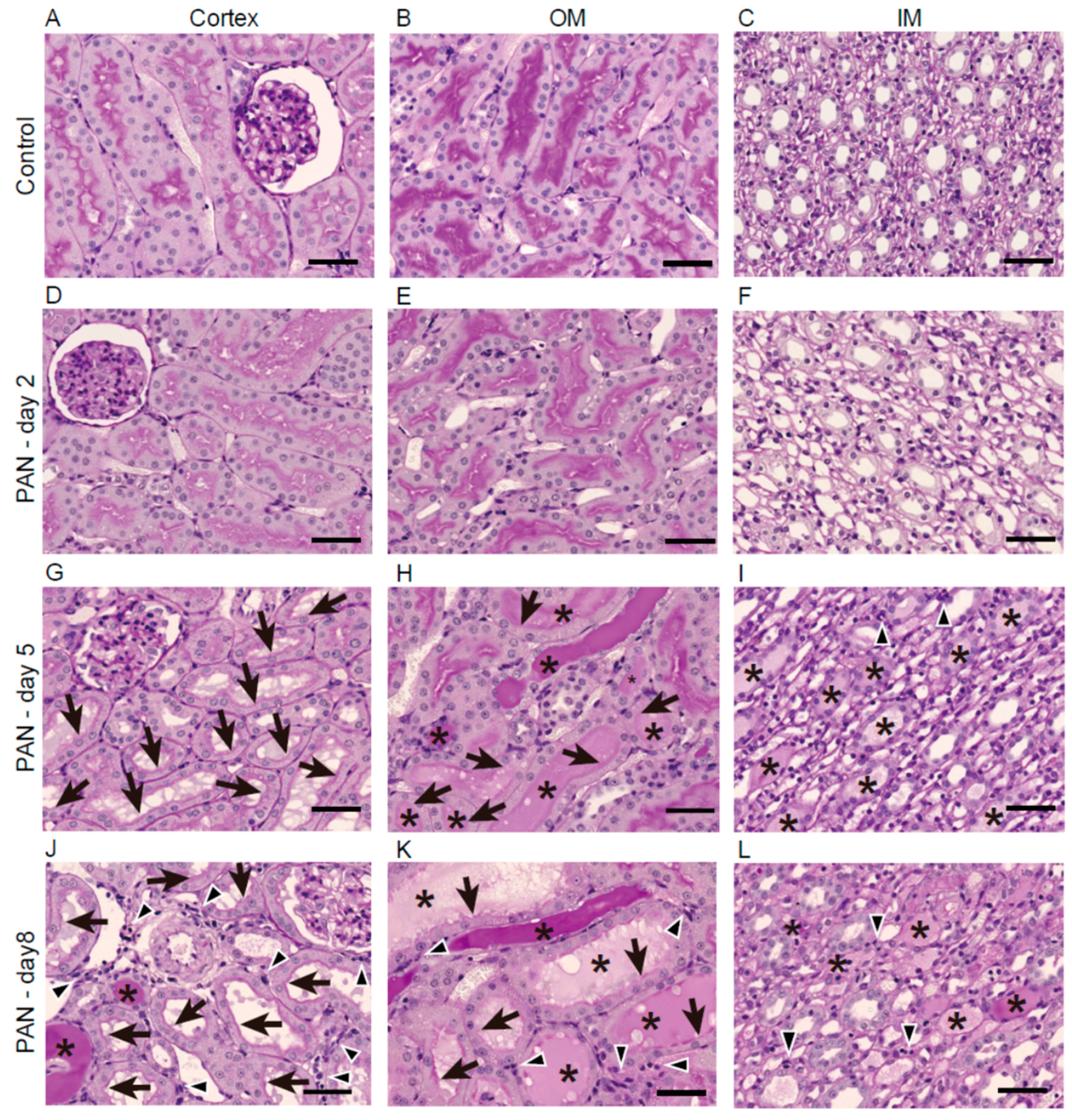
2.2. Kidney Histology after PAN Treatment
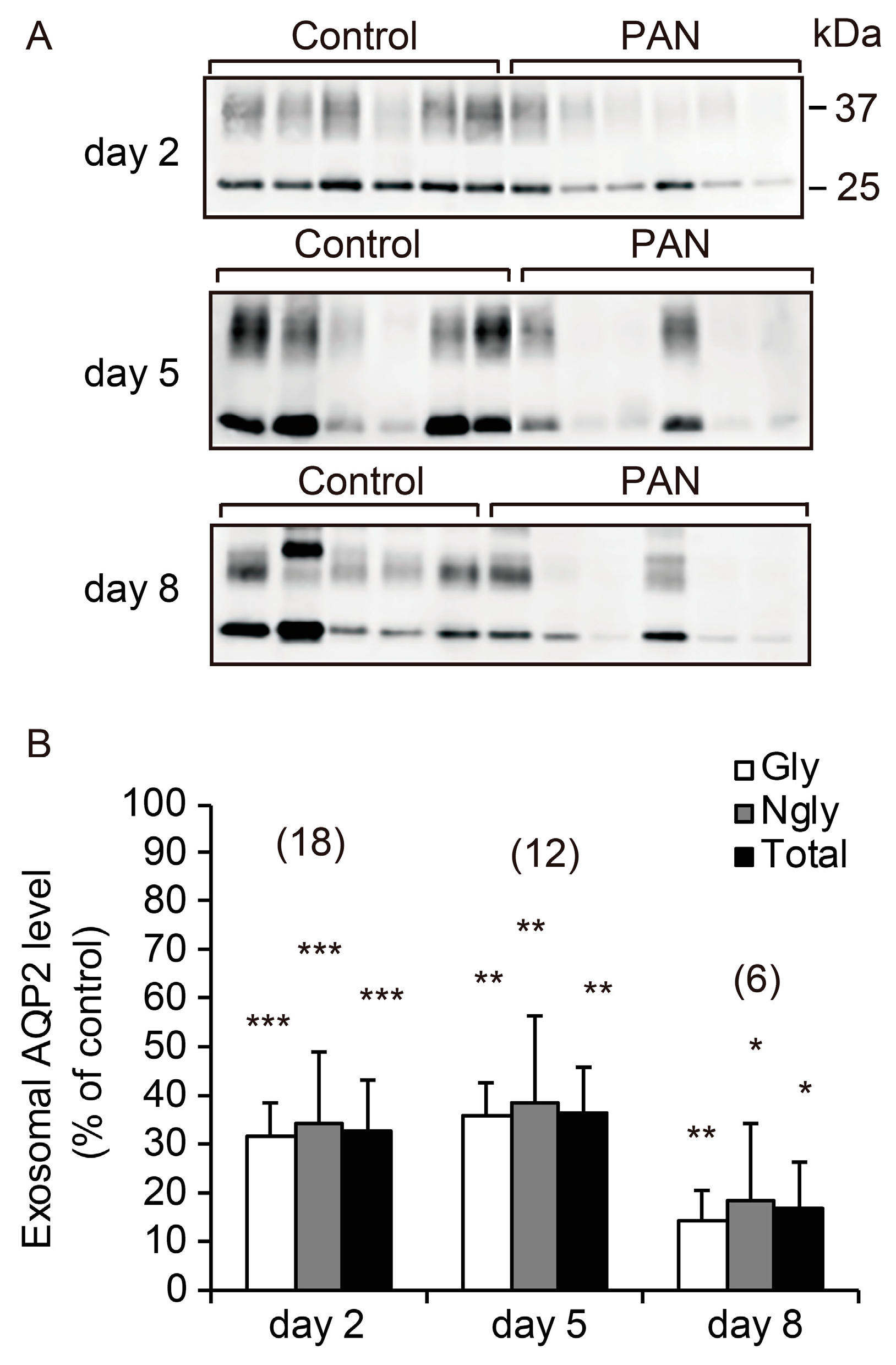
2.3. Excretion of UE-AQP2 after PAN Treatment
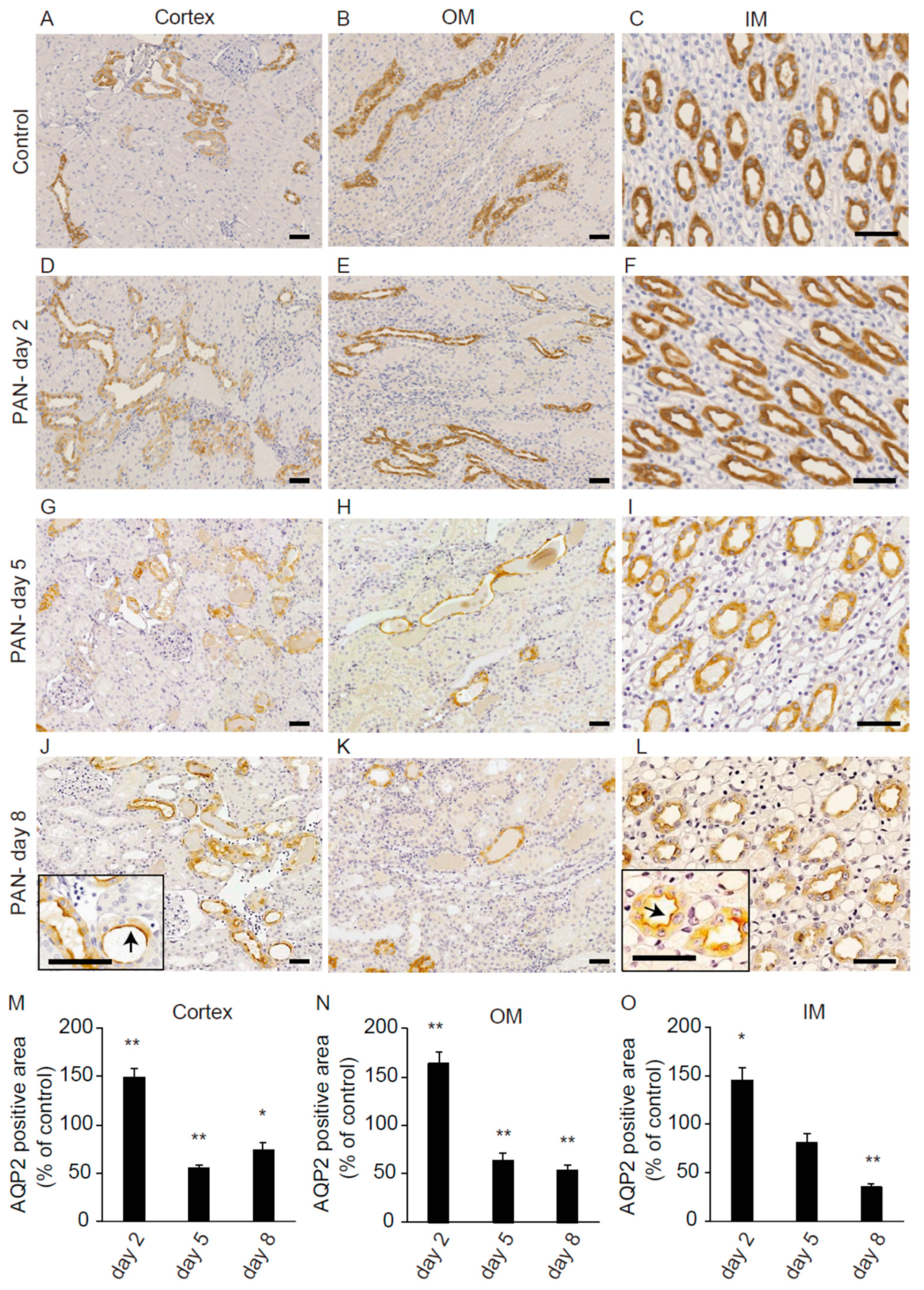
2.4. Renal AQP2 Expression after PAN Treatment
2.5. Excretion of Urinary Exosomal Marker Proteins after PAN Treatment
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Animals and Experimental Protocol
4.3. Measurement of Blood and Urine Parameters
4.4. Isolation of Urinary Exosomes
4.5. Western Blotting Analyses
4.6. Histopathology and Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALIX | ALG-2 interacting protein X |
| AQP | aquaporin |
| ESCRT | endosomal sorting complex required for transport |
| MVB | multivesicular body |
| PAN | puromycin aminonucleoside |
| TSG101 | tumor susceptibility gene 101 protein |
| UE | urinary exosome |
References
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Berrondo, C.; Flax, J.; Kucherov, V.; Siebert, A.; Osinski, T.; Rosenberg, A.; Fucile, C.; Richheimer, S.; Beckham, C.J. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-Scale Proteomics and Phosphoproteomics of Urinary Exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef]
- Sonoda, H.; Lee, B.R.; Park, K.H.; Nihalani, D.; Yoon, J.H.; Ikeda, M.; Kwon, S.H. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Svenningsen, P.; Sabaratnam, R.; Jensen, B.L. Urinary extracellular vesicles: Origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Physiol. 2020, 228, e13346. [Google Scholar] [CrossRef]
- Oshikawa, S.; Sonoda, H.; Ikeda, M. Aquaporins in urinary extracellular vesicles (Exosomes). Int. J. Mol. Sci. 2016, 17, 957. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Liu, R.; Chen, B.; Liu, C.; Xing, J. Long noncoding RNA (MEG3) in urinal exosomes functions as a biomarker for the diagnosis of Hunner-type interstitial cystitis (HIC). J. Cell. Biochem. 2020, 121, 1227–1237. [Google Scholar] [CrossRef]
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsuzaki, T. Regulation of aquaporins by vasopressin in the kidney. Vitam. Horm. 2015, 98, 307–337. [Google Scholar] [PubMed]
- Miyazawa, Y.; Mikami, S.; Yamamoto, K.; Sakai, M.; Saito, T.; Yamamoto, T.; Ishibashi, K.; Sasaki, S. AQP2 in human urine is predominantly localized to exosomes with preserved water channel activities. Clin. Exp. Nephrol. 2018, 22, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Sonoda, H.; El-Shawarby, R.; Takahashi, S.; Ikeda, M. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am. J. Physiol. Renal Physiol. 2014, 307, F1227–F1237. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Oshikawa-Hori, S.; Ikeda, M. An early decrease of aquaporin-2 in urinary extracellular vesicles after cisplatin treatment in rats. Cells 2019, 3, 139. [Google Scholar] [CrossRef]
- Oshikawa-Hori, S.; Yokota-Ikeda, N.; Sonoda, H.; Ikeda, M. Urinary extracellular vesicular release of aquaporins in patients with renal transplantation. BMC Nephrol. 2019, 20, 1–7. [Google Scholar] [CrossRef]
- Asvapromtada, S.; Sonoda, H.; Kinouchi, M.; Oshikawa, S.; Takahashi, S.; Hoshino, Y.; Sinlapadeelerdkul, T.; Yokota-Ikeda, N.; Matsuzaki, T.; Ikeda, M. Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am. J. Physiol. Renal Physiol. 2018, 314, F584–F601. [Google Scholar] [CrossRef]
- Bohlin, A.B.; Berg, U. Renal water handling in minimal change nephrotic syndrome. Int. J. Pdeiatr. Nephrol. 1984, 5, 93–98. [Google Scholar]
- Apostol, E.; Ecelbarger, C.A.; Terris, J.; Bradford, A.D.; Andrews, P.; Knepper, M.A. Reduced renal medullary water channel expression in puromycin aminonucleoside--induced nephrotic syndrome. J. Am. Soc. Nephrol. 1997, 8, 15–24. [Google Scholar]
- Fernández-Llama, P.; Andrews, P.; Nielsen, S.; Ecelbarger, C.A.; Knepper, M.A. Impaired aquaporin and urea transporter expression in rats with adriamycin-induced nephrotic syndrome. Kidney Int. 1998, 53, 1244–1253. [Google Scholar] [CrossRef]
- Koritzinsky, E.H.; Street, J.M.; Chari, R.R.; Glispie, D.M.; Bellomo, T.R.; Aponte, A.M.; Star, R.A.; Yuen, P.S.T. Circadian variation in the release of small extracellular vesicles can be normalized by vesicle number or TSG101. Am. J. Physiol. Renal Physiol. 2019, 317, F1098–F1110. [Google Scholar] [CrossRef]
- Nielsen, S.; Frøkiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef] [PubMed]
- Ecelbarger, C.A.; Chou, C.L.; Lee, A.J.; Digiovanni, S.R.; Verbalis, J.G.; Knepper, M.A. Escape from vasopressin-induced antidiuresis: Role of vasopressin resistance of the collecting duct. Am. J. Physiol. 1998, 274, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Brovko, M.; Kozlovskaya, L.; Pulin, A.; Moiseev, S.; Sholomova, V.; Shchekochikhin, D.; Gognieva, D.; Milovanova, L.; Fomin, V. Low aquaporin-2 excretion in the nephrotic syndrome: An escape from the vasopressin regulating effect. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, Y.; Sonoda, H.; Takahashi, S.; Kondo, H.; Shigemura, K.; Ikeda, M. Excretion of urinary exosomal AQP2 in rats is regulated by vasopressin and urinary pH. Am. J. Physiol. Renal Physiol. 2013, 305, F1412–F1421. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Tamma, G.; Valenti, G. Vasopressin-aquaporin-2 pathway: Recent advances in understanding water balance disorders. F1000Res. 2019, 8. F1000 Faculty Rev-149. [Google Scholar] [CrossRef] [PubMed]
- Ando, F.; Mori, S.; Yui, N.; Morimoto, T.; Nomura, N.; Sohara, E.; Rai, T.; Sasaki, S.; Kondo, Y.; Kagechika, H.; et al. AKAPs-PKA disruptors increase AQP2 activity independently of vasopressin in a model of nephrogenic diabetes insipidus. Nat. Commun. 2018, 9, 1411. [Google Scholar] [CrossRef]
- Zhang, Y.; Hansson, K.M.; Liu, T.; Magnell, K.; Huang, Y.; Carlson, N.G.; Kishore, B.K. Genetic deletion of ADP-activated P2Y12 receptor ameliorates lithium-induced nephrogenic diabetes insipidus in mice. Acta Physiol. 2019, 225, e13191. [Google Scholar] [CrossRef]
- Vukićević, T.; Hinze, C.; Baltzer, S.; Himmerkus, N.; Quintanova, C.; Zühlke, K.; Compton, F.; Ahlborn, R.; Dema, A.; Eichhorst, J.; et al. Fluconazole increases osmotic water transport in renal collecting duct through effects on aquaporin-2 trafficking. J. Am. Soc. Nephrol. 2019, 30, 795–810. [Google Scholar] [CrossRef]
- Sinlapadeelerdkul, T.; Sonoda, H.; Uchida, K.; Kitahara, G.; Ikeda, M. Release of urinary aquaporin-2-bearing extracellular vesicles is decreased in pregnant Japanese Black cattle. J. Vet. Med. Sci. 2019, 81, 1609–1615. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Group | Day 2 | Day 5 | Day 8 |
|---|---|---|---|---|
| Plasma Creatinine (mg/dL) | Control | 0.18 ± 0.02 (n = 6) | 0.15 ± 0.02 (n = 6) | 0.16 ± 0.02 (n = 5) |
| PAN | 0.20 ± 0.00 (n = 6) | 0.48 ± 0.10 * (n = 6) | 0.45 ± 0.10 * (n = 6) | |
| Blood pH | Control | 7.35 ± 0.01 (n = 6) | 7.35 ± 0.01 (n = 6) | 7.34 ± 0.01 (n = 5) |
| PAN | 7.34 ± 0.01 (n = 6) | 7.36 ± 0.01 (n = 6) | 7.39 ± 0.01 ** (n = 6) | |
| Blood Na+ (mmol/L) | Control | 140.83 ± 0.31 (n = 6) | 141.67 ± 0.21 (n = 6) | 141.00 ± 0.45 (n = 5) |
| PAN | 140.17 ± 0.70 (n = 6) | 137.00 ± 1.15 ** (n = 6) | 139.00 ± 0.68 * (n = 6) | |
| Blood K+ (mmol/L) | Control | 4.48 ± 0.07 (n = 6) | 4.67 ± 0.13 (n = 6) | 4.58 ± 0.12 (n = 5) |
| PAN | 4.43 ± 0.11 (n = 6) | 5.25 ± 0.19 * (n = 6) | 4.52 ± 0.14 (n = 6) | |
| Blood Cl− (mmol/L) | Control | 102.00 ± 0.58 (n = 6) | 101.50 ± 0.22 (n = 6) | 100.40 ± 0.68 (n = 5) |
| PAN | 101.00 ± 1.06 (n = 6) | 105.33 ± 0.92 ** (n = 6) | 101.33 ± 1.28 (n = 6) |
| Parameters | Group | Day 2 | Day 5 | Day 8 |
|---|---|---|---|---|
| Urinary creatinine (mg/6 h) | Control | 6.37 ± 0.29 (n = 18) | 5.14 ± 0.43 (n = 12) | 6.93 ± 0.48 (n = 5) |
| PAN | 6.54 ± 0.59 (n = 18) | 3.65 ± 0.48 ** (n = 12) | 4.46 ± 0.77 * (n = 6) | |
| Urine volume (ml/6 h) | Control | 9.28 ± 0.49 (n = 18) | 9.60 ± 0.89 (n = 12) | 15.01 ± 1.32 (n = 5) |
| PAN | 9.93 ± 0.97 (n = 18) | 4.53 ± 0.70 *** (n = 12) | 8.68 ± 1.21 ** (n = 6) | |
| Urinary protein (mg/6 h) | Control | 4.48 ± 0.29 (n = 18) | 4.39 ± 0.37 (n = 12) | 5.56 ± 0.70 (n = 5) |
| PAN | 5.95 ± 0.81 (n = 18) | 88.46 ± 19.99 *** (n = 12) | 184.09 ± 62.48 * (n = 6) | |
| Urine pH | Control | 7.23 ± 0.07 (n = 18) | 7.39 ± 0.06 (n = 12) | 7.20 ± 0.19 (n = 5) |
| PAN | 6.15 ± 0.11 *** (n = 18) | 6.44 ± 0.24 *** (n = 12) | 6.30 ± 0.27 * (n = 6) | |
| Urinary Na+ (mEq/6 h) | Control | 0.95 ± 0.07 (n = 18) | 0.66 ± 0.09 (n = 12) | 0.89 ± 0.06 (n = 5) |
| PAN | 0.35 ± 0.06 *** (n = 18) | 0.23 ± 0.09 ** (n = 12) | 0.53 ± 0.13 * (n = 6) | |
| Urinary K+ (mEq/6 h) | Control | 1.00 ± 0.06 (n = 18) | 0.77 ± 0.06 (n = 12) | 0.90 ± 0.11 (n = 5) |
| PAN | 0.56 ± 0.06 *** (n = 18) | 0.33 ± 0.08 *** (n = 12) | 0.57 ± 0.10 * (n = 6) | |
| Urinary Cl− (mEq/6 h) | Control | 0.80 ± 0.05 (n = 18) | 0.56 ± 0.07 (n = 12) | 0.81 ± 0.11 (n = 5) |
| PAN | 0.34 ± 0.05 *** (n = 18) | 0.17 ± 0.08 *** (n = 12) | 0.38 ± 0.06 ** (n = 6) | |
| Urinary osmolality (mOsm/kg H2O) | Control | 828.8 ± 54.8 (n = 18) | 698.4 ± 103.1 (n = 12) | 843.0 ± 245.5 (n = 5) |
| PAN | 629.6 ± 66.6 * (n = 18) | 725.7 ± 58.4 (n = 12) | 508.5 ± 59.6 (n = 6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeen, A.; Sonoda, H.; Kaito, A.; Oshikawa-Hori, S.; Fujimoto, N.; Ikeda, M. Decreased Excretion of Urinary Exosomal Aquaporin-2 in a Puromycin Aminonucleoside-Induced Nephrotic Syndrome Model. Int. J. Mol. Sci. 2020, 21, 4288. https://doi.org/10.3390/ijms21124288
Abdeen A, Sonoda H, Kaito A, Oshikawa-Hori S, Fujimoto N, Ikeda M. Decreased Excretion of Urinary Exosomal Aquaporin-2 in a Puromycin Aminonucleoside-Induced Nephrotic Syndrome Model. International Journal of Molecular Sciences. 2020; 21(12):4288. https://doi.org/10.3390/ijms21124288
Chicago/Turabian StyleAbdeen, Ahmed, Hiroko Sonoda, Ayaha Kaito, Sayaka Oshikawa-Hori, Naruki Fujimoto, and Masahiro Ikeda. 2020. "Decreased Excretion of Urinary Exosomal Aquaporin-2 in a Puromycin Aminonucleoside-Induced Nephrotic Syndrome Model" International Journal of Molecular Sciences 21, no. 12: 4288. https://doi.org/10.3390/ijms21124288
APA StyleAbdeen, A., Sonoda, H., Kaito, A., Oshikawa-Hori, S., Fujimoto, N., & Ikeda, M. (2020). Decreased Excretion of Urinary Exosomal Aquaporin-2 in a Puromycin Aminonucleoside-Induced Nephrotic Syndrome Model. International Journal of Molecular Sciences, 21(12), 4288. https://doi.org/10.3390/ijms21124288





