Impact of the Renin–Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives
Abstract
1. Introduction
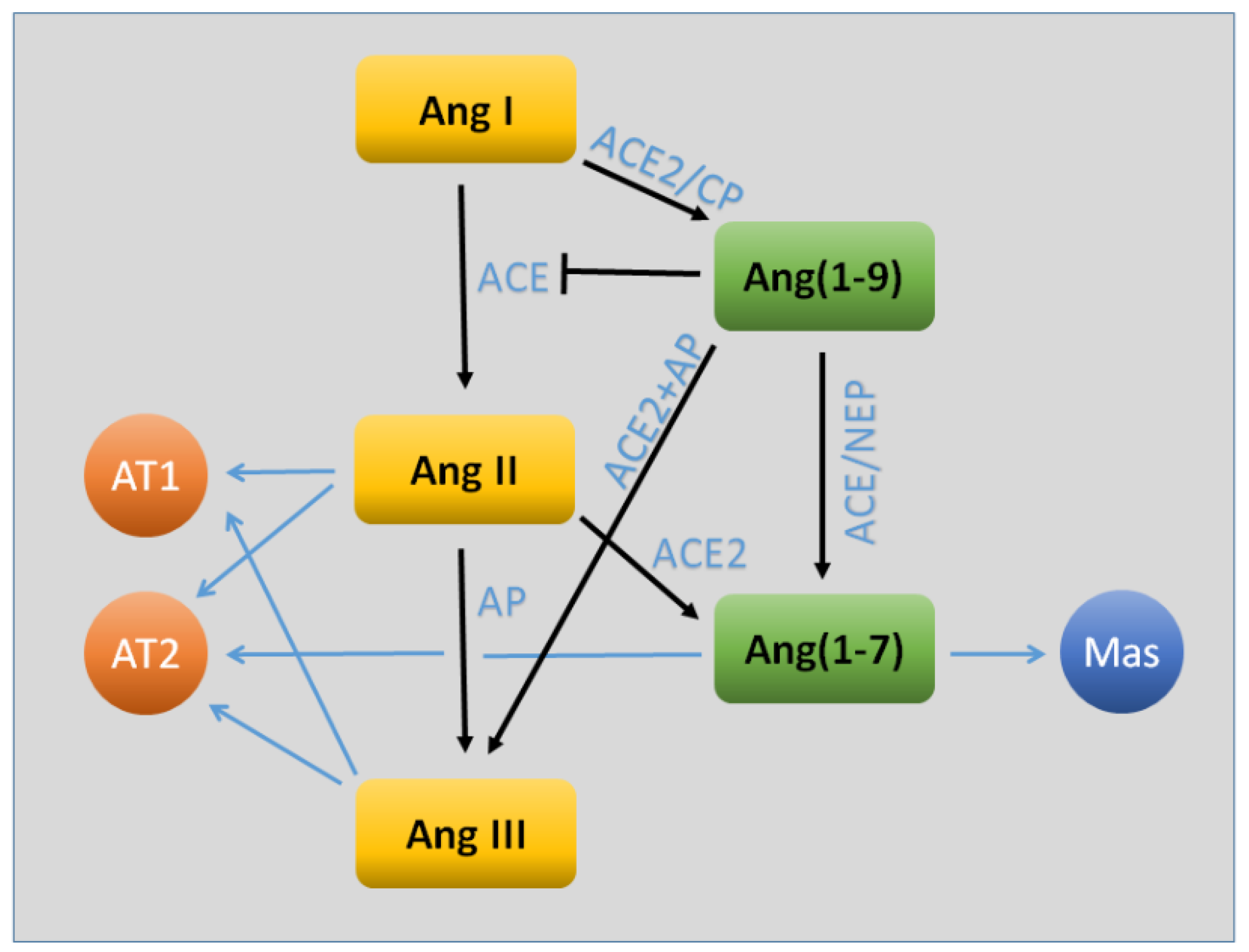
2. Role of the Renin–Angiotensin System
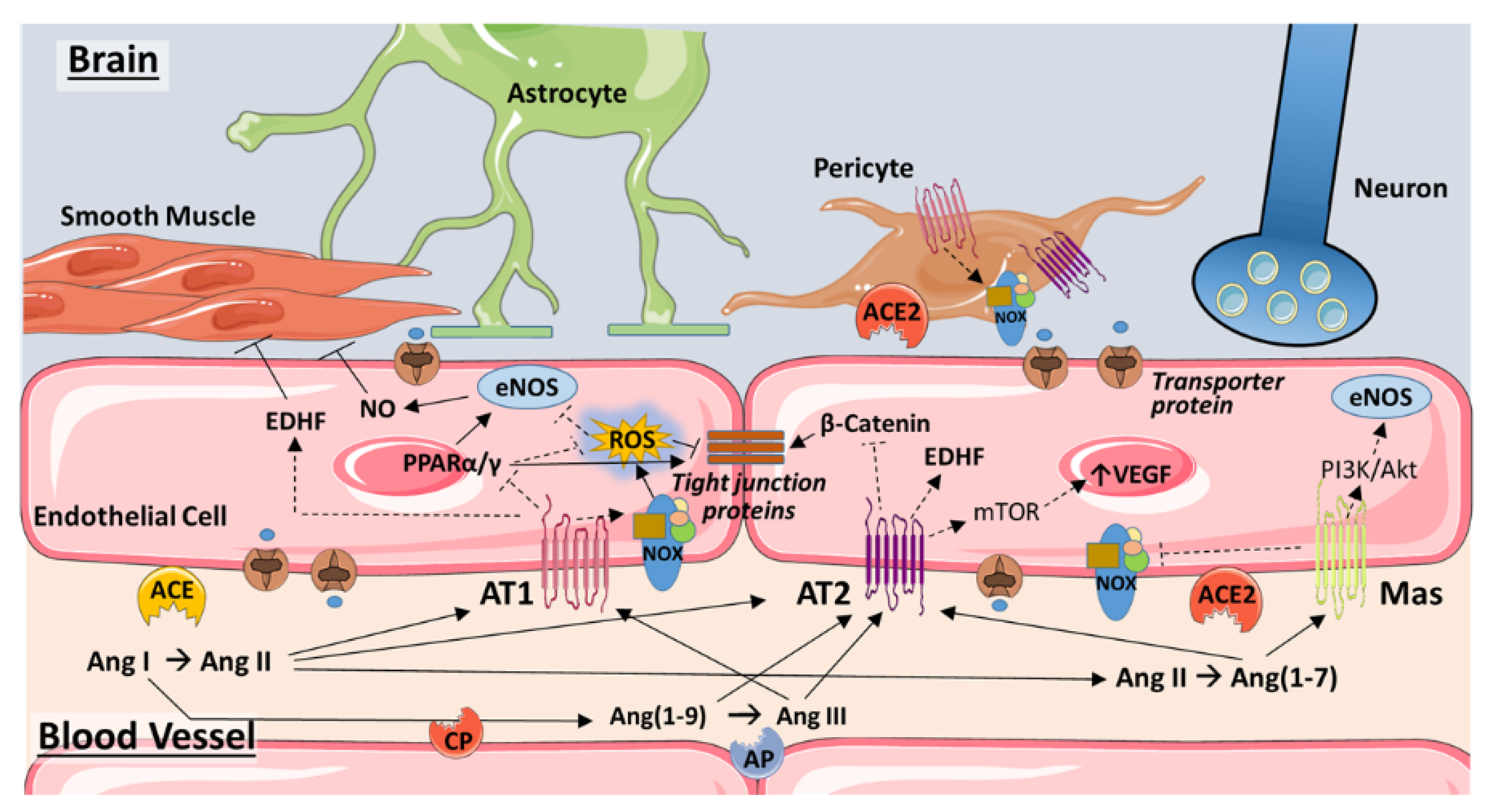
3. Endothelium
3.1. Angiotensin and AT1R
3.2. ACE2
3.3. Ang(1–7) and the Mas Receptor
3.4. Other Novel RAS Components: AT2 and AT4 Receptors
3.5. Role of Pericytes
4. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- O’Brien, T.J.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Scheltens, P. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, v2–v7. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Tran, C.H.T.; Gordon, G.R.; Ungvari, Z.; Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 2017, 94, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Tarantini, S.; Fülöp, G.A.; Kiss, T.; Valcarcel-Ares, M.N.; Galvan, V.; Ungvari, Z.; Yabluchanskiy, A. Hypertension impairs neurovascular coupling and promotes microvascular injury: Role in exacerbation of Alzheimer’s disease. Geroscience 2017, 39, 359–372. [Google Scholar] [CrossRef]
- Pires, P.W.; Ramos, C.M.D.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Fulop, G.A.; Ramirez-Perez, F.I.; Kiss, T.; Tarantini, S.; Ares, M.N.V.; Toth, P.; Yabluchanskiy, A.; Conley, S.M.; Ballabh, P.; Martinez-Lemus, L.A.; et al. IGF-1 Deficiency Promotes Pathological Remodeling of Cerebral Arteries: A Potential Mechanism Contributing to the Pathogenesis of Intracerebral Hemorrhages in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 446–454. [Google Scholar] [CrossRef]
- Wiesmann, M.; Capone, C.; Zerbi, V.; Mellendijk, L.; Heerschap, A.; Claassen, J.A.H.R.; Kiliaan, A.J. Hypertension impairs cerebral blood flow in a mouse model for Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 914–922. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H. Blood Pressure Control and Dementia Risk in Midlife Patients with Atrial Fibrillation. Hypertension 2020, 75, 1296–1304. [Google Scholar] [CrossRef]
- Cifuentes, D.; Poittevin, M.; Dere, E.; Broquères-You, D.; Bonnin, P.; Benessiano, J.; Pocard, M.; Mariani, J.; Kubis, N.; Merkulova-Rainon, T. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 2015, 65, 218–224. [Google Scholar] [CrossRef]
- Abrahamson, E.E.; Ikonomovic, M.D. Brain injury-induced dysfunction of the blood brain barrier as a risk for dementia. Exp. Neurol. 2020, 328, 113257. [Google Scholar] [CrossRef]
- Kaplan, A.; Yabluchanskiy, A.; Ghali, R.; Altara, R.; Booz, G.W.; Zouein, F.A. Cerebral blood flow alteration following acute myocardial infarction in mice. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Cunningham, M.W.; Pabbidi, M.R.; Wang, S.; Booz, G.W.; Fan, F. Targeting vascular inflammation in ischemic stroke: Recent developments on novel immunomodulatory approaches. Eur. J. Pharmacol. 2018, 833, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.; Thomas, W.G. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimulis. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Wackenfors, A.; Vikman, P.; Nilsson, E.; Edvinsson, L.; Malmsjo, M. Angiotensin II-induced vasodilatation in cerebral arteries is mediated by endothelium-derived hyperpolarising factor. Eur. J. Pharmacol. 2006, 531, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Haberl, R.L.; Anneser, F.; Villringer, A.; Einhaupl, K.M. Angiotensin II induces endothelium-dependent vasodilation of rat cerebral arterioles. Am. J. Physiol. 1990, 258, H1840–H1846. [Google Scholar] [CrossRef]
- Walker, A.E.; Kronquist, E.K.; Chinen, K.T.; Reihl, K.D.; Li, D.Y.; Lesniewski, L.A.; Donato, A.J. Cerebral and skeletal muscle feed artery vasoconstrictor responses in a mouse model with greater large elastic artery stiffness. Exp. Physiol. 2019, 104, 434–442. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of cerebrovascular endothelial cells: Prelude to vascular dementia. Front. Aging Neurosci. 2018, 10, 376. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef]
- Adamski, M.G.; Sternak, M.; Mohaissen, T.; Kaczor, D.; Wierońska, J.M.; Malinowska, M.; Czaban, I.; Byk, K.; Lyngsø, K.S.; Przyborowski, K. Vascular cognitive impairment linked to brain endothelium inflammation in early stages of heart failure in mice. J. Am. Heart Assoc. 2018, 7, e007694. [Google Scholar] [CrossRef]
- Yin, S.; Bai, W.; Li, P.; Jian, X.; Shan, T.; Tang, Z.; Jing, X.; Ping, S.; Li, Q.; Miao, Z. Berberine suppresses the ectopic expression of miR-133a in endothelial cells to improve vascular dementia in diabetic rats. Clin. Exp. Hypertens. 2019, 41, 708–716. [Google Scholar] [CrossRef]
- De Silva, T.M.; Faraci, F. Effects of angiotensin II on the cerebral circulation: Role of oxidative stress. Front. Physiol. 2013, 3, 484. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Xiao-Ping, G.; Hui, L. Atorvastatin prevents angiotensin II-induced high permeability of human arterial endothelial cell monolayers via ROCK signaling pathway. Biochem. Biophys. Res. Commun. 2015, 459, 94–99. [Google Scholar] [CrossRef]
- Wosik, K.; Cayrol, R.; Dodelet-Devillers, A.; Berthelet, F.; Bernard, M.; Moumdjian, R.; Bouthillier, A.; Reudelhuber, T.L.; Prat, A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: Relevance to multiple sclerosis. J. Neurosci. 2007, 27, 9032–9042. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Kawaguchi, H.; Miura, N.; Miyoshi, N.; Yamazaki-Himeno, E.; Shiraishi, M.; Miyamoto, A.; Tanimoto, A. Hypertension alters the endothelial-dependent biphasic response of bradykinin in isolated Microminipig basilar artery. Microvasc. Res. 2017, 114, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chrissobolis, S.; Banfi, B.; Sobey, C.G.; Faraci, F.M. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J. Appl. Physiol. 2012, 113, 184–191. [Google Scholar] [CrossRef]
- Girouard, H.; Park, L.; Anrather, J.; Zhou, P.; Iadecola, C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2–derived radicals. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 826–832. [Google Scholar] [CrossRef]
- Masi, S.; Uliana, M.; Virdis, A. Angiotensin II and vascular damage in hypertension: Role of oxidative stress and sympathetic activation. Vasc. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.M.; Geng, L.; Cahill-Smith, S.; Liu, F.; Douglas, G.; Mckenzie, C.-A.; Smith, C.; Brooks, G.; Channon, K.M.; Li, J.-M. Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J. Clin. Investig. 2019, 129, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, H.; Liu, S.; Wang, H.; Hu, M.; Song, L. MAPK/AP-1 pathway activation mediates AT1R upregulation and vascular endothelial cells dysfunction under PM2. 5 exposure. Ecotoxicol. Environ. Saf. 2019, 170, 188–194. [Google Scholar] [CrossRef]
- Ma, M.-M.; Gao, M.; Guo, K.-M.; Wang, M.; Li, X.-Y.; Zeng, X.-L.; Sun, L.; Lv, X.-F.; Du, Y.-H.; Wang, G.-L. TMEM16A Contributes to Endothelial Dysfunction by Facilitating Nox2 NADPH Oxidase–Derived Reactive Oxygen Species Generation in Hypertension. Hypertension 2017, 69, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Nyul-Toth, A.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Som, A.T.; Arai, K.; Lo, E.H. Effects of angiotensin-II on brain endothelial cell permeability via PPARalpha regulation of para-and trans-cellular pathways. Brain Res. 2019, 1722, 146353. [Google Scholar] [CrossRef]
- Hu, C.; Lu, K.-T.; Mukohda, M.; Davis, D.R.; Faraci, F.M.; Sigmund, C.D. Interference with PPARγ in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol. Genom. 2016, 48, 124–134. [Google Scholar] [CrossRef]
- Yakubu, M.A.; Nsaif, R.H.; Oyekan, A.O. Peroxisome proliferator-activated receptor α activation-mediated regulation of endothelin-1 production via nitric oxide and protein kinase C signaling pathways in piglet cerebral microvascular endothelial cell culture. J. Pharmacol. Exp. Ther. 2007, 320, 774–781. [Google Scholar] [CrossRef]
- Chi, L.; Hu, X.; Zhang, W.; Bai, T.; Zhang, L.; Zeng, H.; Guo, R.; Zhang, Y.; Tian, H. Adipokine CTRP6 improves PPARγ activation to alleviate angiotensin II-induced hypertension and vascular endothelial dysfunction in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2017, 482, 727–734. [Google Scholar] [CrossRef]
- Beyer, A.M.; de Lange, W.J.; Halabi, C.M.; Modrick, M.L.; Keen, H.L.; Faraci, F.M.; Sigmund, C.D. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ. Res. 2008, 103, 654–661. [Google Scholar] [CrossRef]
- Nair, A.R.; Agbor, L.N.; Mukohda, M.; Liu, X.; Hu, C.; Wu, J.; Sigmund, C.D. Interference With Endothelial PPAR (Peroxisome Proliferator–Activated Receptor)-γ Causes Accelerated Cerebral Vascular Dysfunction in Response to Endogenous Renin-Angiotensin System Activation. Hypertension 2018, 72, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Saavedra, J.M. Neuroprotective effects of angiotensin receptor blockers. Am. J. Hypertens. 2015, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mao, P.; Wang, J.; Wang, T.; Xie, C.-H. Azilsartan, an angiotensin II type 1 receptor blocker, attenuates tert-butyl hydroperoxide-induced endothelial cell injury through inhibition of mitochondrial dysfunction and anti-inflammatory activity. Neurochem. Int. 2016, 94, 48–56. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Jaffe, I.Z. The role of the mineralocorticoid receptor in the vasculature. J. Endocrinol. 2017, 234, T67. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, I.Z.; Mendelsohn, M.E. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ. Res. 2005, 96, 643–650. [Google Scholar] [CrossRef]
- Keidar, S.; Gamliel-Lazarovich, A.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Hayek, T.; Karry, R.; Abassi, Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ. Res. 2005, 97, 946–953. [Google Scholar] [CrossRef]
- Diaz-Otero, J.M.; Yen, T.-C.; Fisher, C.; Bota, D.; Jackson, W.F.; Dorrance, A.M. Mineralocorticoid receptor antagonism improves parenchymal arteriole dilation via a TRPV4-dependent mechanism and prevents cognitive dysfunction in hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1304–H1315. [Google Scholar] [CrossRef]
- McClain, J.L.; Dorrance, A.M. Temporary mineralocorticoid receptor antagonism during the development of hypertension improves cerebral artery dilation. Exp. Biol. Med. 2014, 239, 619–627. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Ma, X.; Wang, W.; Zhao, B.; Chen, Y.; Chen, C.; Bihl, J.C. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J. Cell. Mol. Med. 2018, 22, 1873–1882. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Bihl, J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxidative Med. Cell. Longev. 2020, 2020, 4213541. [Google Scholar] [CrossRef]
- Silva, R.A.P.; Chu, Y.; Miller, J.D.; Mitchell, I.J.; Penninger, J.M.; Faraci, F.M.; Heistad, D.D. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 2012, 43, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, X.; Chen, S.; Zhang, C.; Chen, J.; Yi, D.; Shenoy, V.; Raizada, M.K.; Zhao, B.; Chen, Y. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension 2013, 61, 681–689. [Google Scholar] [CrossRef][Green Version]
- Sriramula, S.; Xia, H.; Xu, P.; Lazartigues, E. Brain-targeted ACE2 overexpression attenuates neurogenic hypertension by inhibiting COX mediated inflammation. Hypertension 2015, 65, 577. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Y.; Chen, S.; Wang, J.; Xiao, X.; Ma, X.; Penchikala, M.; Xia, H.; Lazartigues, E.; Zhao, B. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology 2014, 79, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Polt, R.; Heien, M.L.; Vanderah, T.W.; Largent-Milnes, T.M.; Rodgers, K.; Falk, T.; Bartlett, M.J.; Doyle, K.P.; Konhilas, J.P. A Novel Angiotensin-(1-7) Glycosylated Mas Receptor Agonist for Treating Vascular Cognitive Impairment and Inflammation-Related Memory Dysfunction. J. Pharmacol. Exp. Ther. 2019, 369, 9–25. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, C.; Ma, X.; Miao, H.; Wang, J.; Liu, L.; Chen, S.; Zeng, R.; Chen, Y.; Bihl, J.C. Angiotensin-(1–7) counteracts angiotensin II-induced dysfunction in cerebral endothelial cells via modulating Nox2/ROS and PI3K/NO pathways. Exp. Cell Res. 2015, 336, 58–65. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Zhang, Q.Q.; Tan, M.S.; Cao, L.; Wang, H.F.; Lu, J.; Gao, Q.; Zhang, Y.D. Angiotensin-(1–7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br. J. Pharmacol. 2014, 171, 4222–4232. [Google Scholar] [CrossRef]
- Levine, D.A.; Galecki, A.T.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Wadley, V.G. Trajectory of cognitive decline after incident stroke. JAMA 2015, 314, 41–51. [Google Scholar] [CrossRef]
- Wiesmann, M.; Kiliaan, A.J.; Claassen, J.A. Vascular aspects of cognitive impairment and dementia. J. Cereb. Blood Flow Metab. 2013, 33, 1696–1706. [Google Scholar] [CrossRef]
- Fukuoka, T.; Hayashi, T.; Hirayama, M.; Maruyama, H.; Mogi, M.; Horiuchi, M.; Takao, M.; Tanahashi, N. Platelet–endothelial cell interaction in brain microvessels of angiotensin II type-2 receptor knockout mice following transient bilateral common carotid artery occlusion. J. Thromb. Thrombolysis 2015, 40, 401–405. [Google Scholar] [CrossRef]
- Gallego-Delgado, J.; Basu-Roy, U.; Ty, M.; Alique, M.; Fernandez-Arias, C.; Movila, A.; Gomes, P.; Weinstock, A.; Xu, W.; Edagha, I. Angiotensin receptors and β-catenin regulate brain endothelial integrity in malaria. J. Clin. Investig. 2016, 126, 4016–4029. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Ishrat, T.; Pillai, B.; Fouda, A.Y.; Sayed, M.A.; Eldahshan, W.; Waller, J.L.; Ergul, A.; Fagan, S.C. RAS modulation prevents progressive cognitive impairment after experimental stroke: A randomized, blinded preclinical trial. J. Neuroinflamm. 2018, 15, 1–16. [Google Scholar] [CrossRef]
- Eldahshan, W.; Ishrat, T.; Pillai, B.; Sayed, M.A.; Alwhaibi, A.; Fouda, A.Y.; Ergul, A.; Fagan, S.C. Angiotensin II type 2 receptor stimulation with compound 21 improves neurological function after stroke in female rats: A pilot study. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1192–H1201. [Google Scholar] [CrossRef] [PubMed]
- Mateos, L.; Perez-Alvarez, M.J.; Wandosell, F. Angiotensin II type-2 receptor stimulation induces neuronal VEGF synthesis after cerebral ischemia. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; He, T.; Pan, Y.; Katusic, Z.S. Effects of senescence and angiotensin II on expression and processing of amyloid precursor protein in human cerebral microvascular endothelial cells. Aging (Albany NY) 2018, 10, 100. [Google Scholar] [CrossRef]
- Dao, V.T.-V.; Medini, S.; Bisha, M.; Balz, V.; Suvorava, T.; Bas, M.; Kojda, G. Nitric oxide up-regulates endothelial expression of angiotensin II type 2 receptors. Biochem. Pharmacol. 2016, 112, 24–36. [Google Scholar] [CrossRef]
- Hafko, R.; Villapol, S.; Nostramo, R.; Symes, A.; Sabban, E.L.; Inagami, T.; Saavedra, J.M. Commercially available angiotensin II At2 receptor antibodies are nonspecific. PLoS ONE 2013, 8, e69234. [Google Scholar] [CrossRef]
- Singh, K.D.; Karnik, S.S. Angiotensin receptors: Structure, function, signaling and clinical applications. J. Cell Signal. 2016, 1, 111. [Google Scholar] [CrossRef]
- Royea, J.; Martinot, P.; Hamel, E. Memory and cerebrovascular deficits recovered following angiotensin IV intervention in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2020, 134, 104644. [Google Scholar] [CrossRef]
- Hirunpattarasilp, C.; Attwell, D.; Freitas, F. The role of pericytes in brain disorders: From the periphery to the brain. J. Neurochem. 2019, 150, 648–665. [Google Scholar] [CrossRef]
- Kuroda, J.; Ago, T.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kamouchi, M.; Kitazono, T. Nox4 is a major source of superoxide production in human brain pericytes. J. Vasc. Res. 2014, 51, 429–438. [Google Scholar] [CrossRef]
- García-Quintans, N.; Sánchez-Ramos, C.; Prieto, I.; Tierrez, A.; Arza, E.; Alfranca, A.; Redondo, J.M.; Monsalve, M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 2016, 19, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.T.; Maki, T.; Ihara, M.; Lee, V.M.; Trojanowski, J.Q. Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front. Aging Neurosci. 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 358. [Google Scholar]
- Beltramo, E.; Berrone, E.; Giunti, S.; Gruden, G.; Perin, P.C.; Porta, M. Effects of mechanical stress and high glucose on pericyte proliferation, apoptosis and contractile phenotype. Exp. Eye Res. 2006, 83, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Sano, Y.; Tominaga, O.; Maeda, T.; Abe, M.A.; Kanda, T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-beta by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol. Aging 2013, 34, 1902–1912. [Google Scholar] [CrossRef]
- Kawamura, H.; Kobayashi, M.; Li, Q.; Yamanishi, S.; Katsumura, K.; Minami, M.; Wu, D.M.; Puro, D.G. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J. Physiol. 2004, 561, 671–683. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noureddine, F.Y.; Altara, R.; Fan, F.; Yabluchanskiy, A.; Booz, G.W.; Zouein, F.A. Impact of the Renin–Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4268. https://doi.org/10.3390/ijms21124268
Noureddine FY, Altara R, Fan F, Yabluchanskiy A, Booz GW, Zouein FA. Impact of the Renin–Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives. International Journal of Molecular Sciences. 2020; 21(12):4268. https://doi.org/10.3390/ijms21124268
Chicago/Turabian StyleNoureddine, Fatima Y., Raffaele Altara, Fan Fan, Andriy Yabluchanskiy, George W. Booz, and Fouad A. Zouein. 2020. "Impact of the Renin–Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives" International Journal of Molecular Sciences 21, no. 12: 4268. https://doi.org/10.3390/ijms21124268
APA StyleNoureddine, F. Y., Altara, R., Fan, F., Yabluchanskiy, A., Booz, G. W., & Zouein, F. A. (2020). Impact of the Renin–Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives. International Journal of Molecular Sciences, 21(12), 4268. https://doi.org/10.3390/ijms21124268




