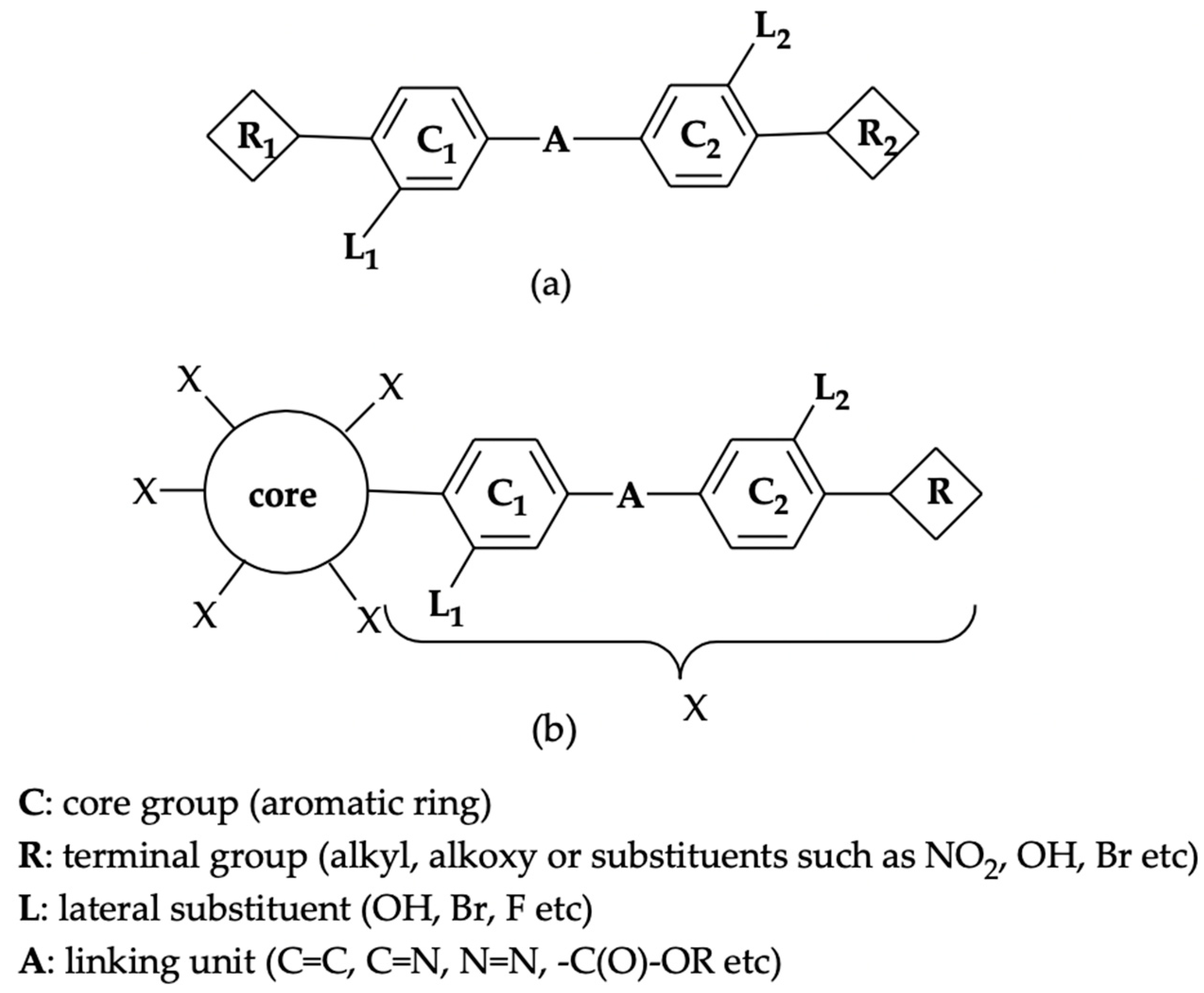
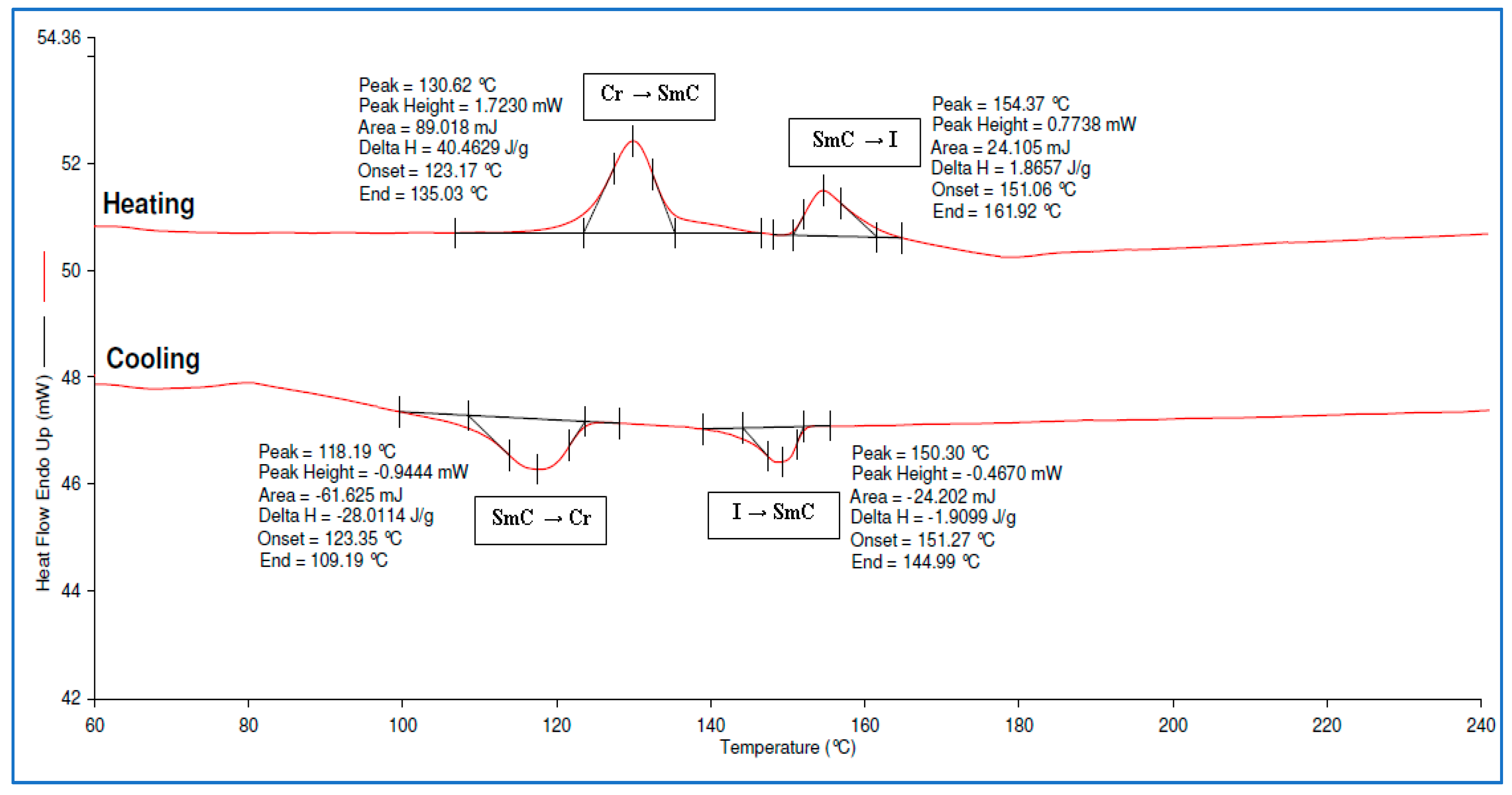
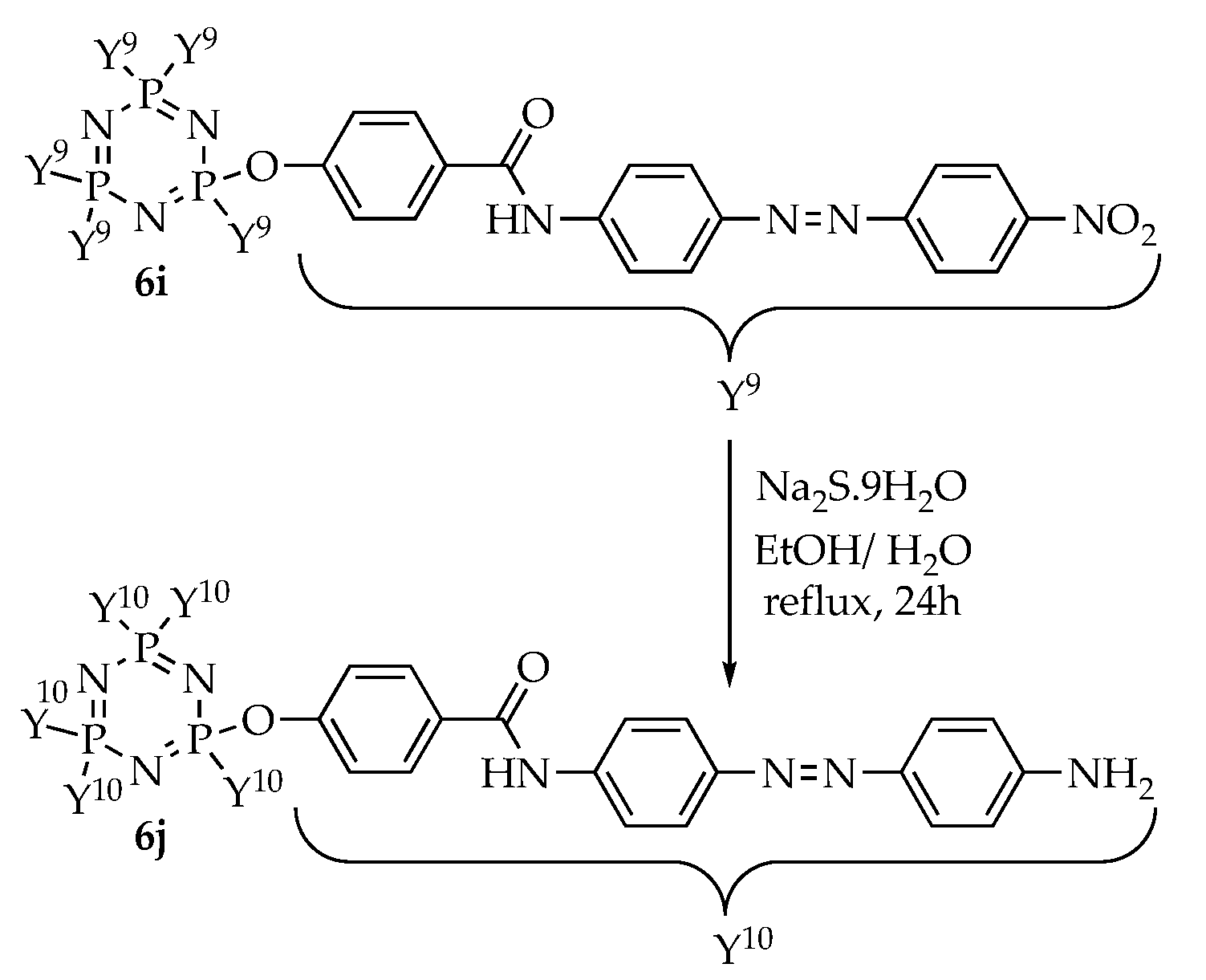
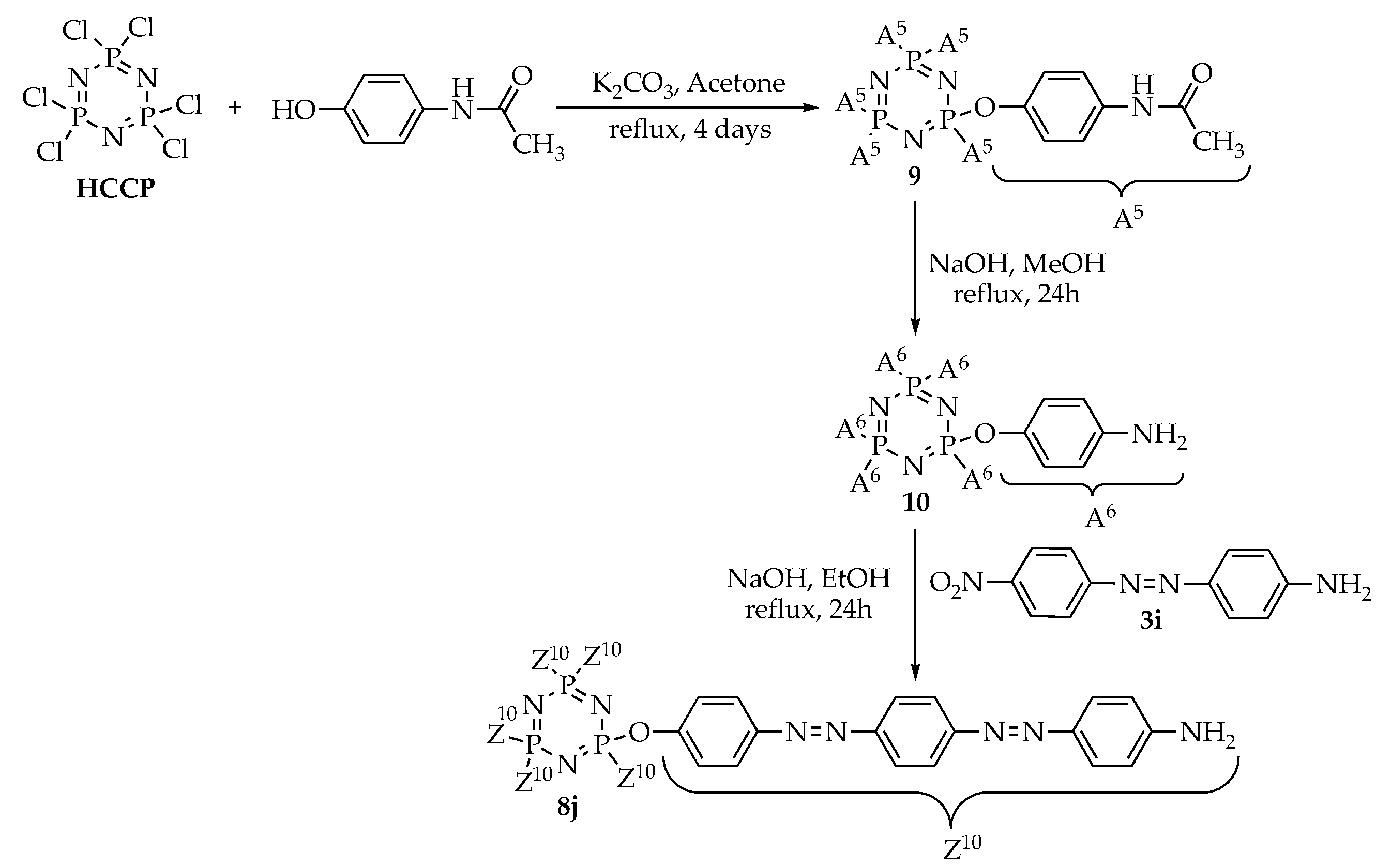
3.3. Syntheses
3.3.1. Synthesis of 4-((4-nitrophenyla)diazenyl)phenol, 1
A solution of 4-nitroaniline (6.91 g, 0.05 mol) in 50 mL methanol was added to 13 mL of 12 M hydrochloric acid, HCl and the mixture was cooled at 0 °C. A solution of sodium nitrite, NaNO2 (3.45 g, 0.05 mol) in 15 mL of water was added dropwise to the mixture, which was left to stir for 30 min. The addition of phenol (4.71 g, 0.05 mol) in 70 mL of 20% sodium acetate hydrate, CH3COONa.3H2O to the mixture led to the formation of an orange precipitate. About 100 mL of water was then added to the solution and the reaction was left for 2 h at 0 °C and another 2 h at room temperature. The reaction progress was monitored by TLC. Upon completion, the precipitate was filtered, washed with water and dried overnight in open air. The product was recrystallised from methanol. Yield: 11.56 g (95.14%), mp: 199.2–203.5 °C, orange powder. FTIR (cm−1): 3250 (O-H stretching), 1504 (aromatic C=C stretching), 1458 (N=N stretching), 1326 (C-O stretching), 1173 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 10.70 (s, 1H), 8.38 (d, J = 10.0 Hz, 2H), 7.97 (d, J = 10.0 Hz, 2H), 7.87 (d, J = 5.0 Hz, 2H), 6.97 (d, J = 10.0 Hz, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.31, 155.54, 147.76, 145.39, 125.77, 125.00, 122.96, 116.21. CHN elemental analysis: Calculated for C12H9N3O3: C: 59.26%, H: 3.73%, N: 17.28%; Found: C: 58.69%, H: 3.71%, N: 16.98%.
3.3.2. Synthesis of (4-alkyloxyphenyl)-(4-nitrophenylazo)diazene, 2a–e
(4-Heptyloxyphenyl)-(4-nitrophenylazo)diazene, 2a: A solution of intermediate 1 (10.00 g, 0.04 mol) in 20 mL DMF and 1-bromoheptane (7.16 g, 0.04 mol) in 20 mL DMF were mixed in a 100 mL round bottom flask. Potassium carbonate (11.06 g, 0.08 mol) and potassium iodide (0.66 g, 4.00 mmol) were added to the mixture, which was refluxed for 12 h. The reaction was monitored using TLC. Upon completion, the mixture was poured into 500 mL cold water and orange-red precipitate began to form. The product was filtered, washed with water and dried in open air. The same method was used to synthesize 2b–e. Yield: 11.32 g (82.33%), mp: 97.4–98.8 °C, orange-red powder. FTIR (cm−1): 2927 and 2857 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1468 (N=N stretching), 1246 (C-O stretching), 1141 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.34 (d, J = 10.0 Hz, 2H), 7.96 (d, J = 10.0 Hz, 2H), 7.94 (d, J = 10.0 Hz, 2H), 7.01 (d, J = 5.0 Hz, 2H), 4.04 (t, J = 7.5 Hz, 2H), 1.79–1.83 (m, 2H), 1.43–1.48 (m, 2H), 1.28–1.38 (m, 6H), 0.89 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.98, 156.11, 148.23, 146.82, 125.62, 124.70, 123.08, 114.95, 68.56, 31.76, 29.15, 29.03, 25.96, 22.60, 14.06. CHN elemental analysis: Calculated for C19H23N3O3: C: 66.84%, H: 6.79%, N: 12.31%; Found: C: 67.18%, H: 6.77%, N: 12.19%.
(4-Nonyloxyphenyl)-(4-nitrophenylazo)diazene, 2b: Yield: 12.11 g (82.05%), mp: 96.7–98.1 °C, light red powder. FTIR (cm−1): 2921 and 2851 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1466 (N=N stretching), 1248 (C-O stretching), 1141 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.34 (d, J = 10.0 Hz, 2H), 7.96 (d, J = 10.0 Hz, 2H), 7.94 (d, J = 10.0 Hz, 2H), 7.01 (d, J = 5.0 Hz, 2H), 4.05 (t, J = 7.5 Hz, 2H), 1.78–1.84 (m, 2H), 1.43–1.49 (m, 2H), 1.26–1.37 (m, 10H), 0.87 (t, J = 5.0 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.99, 156.08, 148.21, 146.80, 125.63, 124.69, 123.08, 114.94, 68.56, 31.88, 29.53, 29.38, 29.26, 29.15, 26.00, 22.68, 14.11. CHN elemental analysis: Calculated for C21H27N3O3: C: 68.27%, H: 7.37%, N: 11.37%; Found: C: 67.98%, H: 7.31%, N: 11.28%.
(4-Decyloxyphenyl)-(4-nitrophenylazo)diazene, 2c: Yield: 13.89 g (90.67%), mp: 95.3–97.9 °C, red powder. FTIR (cm−1): 2921 and 2851 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1468 (N=N stretching), 1248 (C-O stretching), 1141 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.34 (d, J = 5.0 Hz, 2H), 7.96 (d, J = 5.0 Hz, 2H), 7.94 (d, J = 10.0 Hz, 2H), 7.00 (d, J = 10.0 Hz, 2H), 4.04 (t, J = 5.0 Hz, 2H), 1.78–1.84 (m, 2H), 1.43–1.48 (m, 2H), 1.26–1.37 (m, 12H), 0.87 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.99, 156.08, 148.20, 146.80, 125.62, 124.66, 123.07, 114.93, 68.56, 31.90, 29.57, 29.56, 29.38, 29.32, 29.15, 26.00, 22.68, 14.10. CHN elemental analysis: Calculated for C22H29N3O3: C: 68.90%, H: 7.62%, N: 10.96%; Found: C: 68.80%, H: 7.53%, N: 10.88%.
(4-Dodecyloxyphenyl)-(4-nitrophenylazo)diazene, 2d: Yield: 14.98 g (91.11%), mp: 95.1–97.3 °C, red powder. FTIR (cm−1): 2916 and 2849 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1468 (N=N stretching), 1248 (C-O stretching), 1143 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.34 (d, J = 10.0 Hz, 2H), 7.96 (d, J = 10.0 Hz, 2H), 7.94 (d, J = 10.0 Hz, 2H), 7.00 (d, J = 5.0 Hz, 2H), 4.04 (t, J = 7.5 Hz, 2H), 1.78–1.84 (m, 2H), 1.43–1.48 (m, 2H), 1.22–1.37 (m, 16H), 0.86 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.99, 156.08, 148.21, 146.80, 125.62, 124.67, 123.07, 114.93, 68.56, 31.93, 29.67, 29.64, 29.60, 29.57, 29.38, 29.36, 29.15, 26.00, 22.69, 14.11. CHN elemental analysis: Calculated for C24H33N3O3: C: 70.04%, H: 8.08%, N: 10.21%; Found: C: 69.87%, H: 7.99%, N: 10.14%.
(4-Tetradecyloxyphenyl)-(4-nitrophenylazo)diazene, 2e: Yield: 15.44 g (87.93%), mp: 94.3–95.9 °C, dark-red powder. FTIR (cm−1): 2913 and 2849 (Csp3-H stretching), 1603 (aromatic C=C stretching), 1468 (N=N stretching), 1248 (C-O stretching), 1143 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.34 (d, J = 10.0 Hz, 2H), 7.96 (d, J = 10.0 Hz, 2H), 7.94 (d, J = 10.0 Hz, 2H), 7.01 (d, J = 5.0 Hz, 2H), 4.04 (t, J = 7.5 Hz, 2H), 1.78–1.84 (m, 2H), 1.43–1.48 (m, 2H), 1.22–1.37 (m, 20H), 0.86 (t, J = 5.0 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.98, 156.11, 148.24, 146.82, 125.62, 124.70, 123.08, 114.95, 68.56, 31.92, 29.69, 29.67, 29.65, 29.58, 29.55, 29.35, 29.14, 25.99, 22.68, 14.10, two carbon environments not observed due to coincident resonances. CHN elemental analysis: Calculated for C26H37N3O3: C: 71.04%, H: 8.48%, N: 9.56%; Found: C: 71.17%, H: 8.40%, N: 9.45%.
3.3.3. Synthesis of 4-((4-substitutedphenyl)diazenyl)aniline, 3a–f
4-((4-Heptyloxyphenyl)diazenyl)aniline, 3a: In a 250 mL round bottom flask, a solution of sodium sulphide hydrate, Na2S.9H2O (6.24 g, 0.08 mol) in 20 mL ethanol and 20 mL water was mixed with a solution of intermediate 2a (6.82 g, 0.02 mmol) in 40 mL ethanol. The mixture was stirred and refluxed for 12 h. The reaction progress was monitored using TLC. Upon completion, the mixture was cooled in an ice bath. The precipitate formed was filtered, washed with cold ethanol and dried in open air. The same method was used to synthesize 3b–f. Yield: 5.35 g (86.01%), mp: 130.3–132.4 °C, orange powder. FTIR (cm−1): 3470 and 3338 (N-H stretching), 2932 and 2851 (Csp3-H stretching), 1598 (aromatic C=C stretching), 1498 (N=N stretching), 1248 (C-O stretching), 1141 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.71 (d, J = 10.0 Hz, 2H), 7.61 (d, J = 10.0 Hz, 2H), 7.02 (d, J = 10.0 Hz, 2H), 6.66 (d, J = 10.0 Hz, 2H), 5.93 (s, 2H), 4.00 (t, J = 7.5 Hz, 2H), 1.68–1.74 (m, 2H), 1.36–1.42 (m, 2H), 1.22–1.34 (m, 6H), 0.85 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.89, 152.04, 146.40, 142.84, 124.54, 123.35, 114.80, 113.43, 67.80, 31.16, 28.59, 28.40, 21.98, 13.88, one carbon environments not observed due to coincident resonances. CHN elemental analysis: Calculated for C19H25N3O: C: 73.28%, H: 8.09%, N: 13.49%; Found: C: 73.11%, H: 8.07%, N: 13.37%.
4-((4-Nonyloxyphenyl)diazenyl)aniline, 3b: Yield: 5.80 g (85.55%), mp: 128.7–130.5 °C, dark orange powder. FTIR (cm−1): 3465 and 3333 (N-H stretching), 2916 and 2849 (Csp3-H stretching), 1598 (aromatic C=C stretching), 1498 (N=N stretching), 1243 (C-O stretching), 1143 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.71 (d, J = 10.0 Hz, 2H), 7.61 (d, J = 5.0 Hz, 2H), 7.03 (d, J = 10.0 Hz, 2H), 6.66 (d, J = 5.0 Hz, 2H), 5.94 (s, 2H), 4.01 (t, J = 7.5 Hz, 2H), 1.69–1.74 (m, 2H), 1.38–1.44 (m, 2H), 1.22–1.35 (m, 10H), 0.85 (t, J = 5.0 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.88, 152.08, 146.43, 142.84, 124.54, 123.33, 114.77, 113.39, 67.79, 31.23, 28.91, 28.72, 28.60, 28.44, 22.04, 13.90, one carbon environments not observed due to coincident resonances. CHN elemental analysis: Calculated for C21H29N3O: C: 74.30%, H: 8.61%, N: 12.38%; Found: C: 73.91%, H: 8.55%, N: 12.33%.
4-((4-Decyloxyphenyl)diazenyl)aniline, 3c: Yield: 6.23 g (81.33%), mp: 127.9–130.1 °C, orange powder. FTIR (cm−1): 3478 and 3352 (N-H stretching), 2919 and 2849 (Csp3-H stretching), 1595 (aromatic C=C stretching), 1498 (N=N stretching), 1248 (C-O stretching), 1146 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.70 (d, J = 10.0 Hz, 2H), 7.60 (d, J = 5.0 Hz, 2H), 7.01 (d, J = 10.0 Hz, 2H), 6.66 (d, J = 5.0 Hz, 2H), 5.91 (s, 2H), 3.98 (t, J = 7.5 Hz, 2H), 1.66–1.72 (m, 2H), 1.35–1.41 (m, 2H), 1.19–1.30 (m, 12H), 0.82 (t, J = 5.0 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.87, 152.01, 146.37, 142.83, 124.53, 123.35, 114.75, 113.43, 67.76, 31.22, 28.91, 28.86, 28.67, 28.61, 28.55, 25.40, 22.03, 13.88. CHN elemental analysis: Calculated for C22H31N3O: C: 74.75%, H: 8.84%, N: 11.89%; Found: C: 74.66%, H: 8.78%, N: 11.77%.
4-((4-Dodecyloxyphenyl)diazenyl)aniline, 3d: Yield: 6.90 g (90.55%), mp: 125.7–128.3 °C, dark-red powder. FTIR (cm−1): 3473 and 3333 (N-H stretching), 2919 and 2851 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1498 (N=N stretching), 1248 (C-O stretching), 1149 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.66 (d, J = 10.0 Hz, 2H), 7.58 (d, J = 10.0 Hz, 2H), 6.89 (d, J = 10.0 Hz, 2H), 6.64 (d, J = 5.0 Hz, 2H), 5.86 (s, 2H), 3.84 (t, J = 7.5 Hz, 2H), 1.57–1.63 (m, 2H), 1.26–1.32 (m, 2H), 1.10–1.22 (m, 16H), 0.77 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.80, 151.93, 146.39, 142.89, 124.49, 123.27, 114.48, 113.37, 67.64, 31.30, 29.08, 29.05, 29.03, 29.01, 28.80, 28.75, 28.63, 25.46, 22.07, 13.76. CHN elemental analysis: Calculated for C24H35N3O: C: 75.55%, H: 9.25%, N: 11.01%; Found: C: 75.43%, H: 9.19%, N: 10.91%.
4-((4-Tetradecyloxyphenyl)diazenyl)aniline, 3e: Yield: 7.15 g (87.41%), mp: 124.1–126.7 °C, red-brown powder. FTIR (cm−1): 3462 and 3328 (N-H stretching), 2919 and 2849 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1498 (N=N stretching), 1251 (C-O stretching), 1146 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.71 (d, J = 5.0 Hz, 2H), 7.60 (d, J = 10.0 Hz, 2H), 7.03 (d, J = 5.0 Hz, 2H), 6.65 (d, J = 10.0 Hz, 2H), 5.93 (s, 2H), 4.01 (t, J = 7.5 Hz, 2H), 1.69–1.74 (m, 2H), 1.37–1.43 (m, 2H), 1.20–1.33 (m, 20H), 0.84 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.87, 152.06, 146.41, 142.83, 124.53, 123.33, 114.77, 113.40, 67.77, 31.23, 28.99, 28.98, 28.96, 28.95, 28.94, 28.90, 28.88, 28.64, 28.56, 25.39, 22.03, 13.89. CHN elemental analysis: Calculated for C26H39N3O: C: 76.24%, H: 9.60%, N: 10.26%; Found: C: 75.55%, H: 9.57%, N: 10.22%.
4-((4-Aminophenyl)diazenyl)phenol, 3f: Intermediate 1 (9.72 g, 0.04 mol) was used in the reaction. Yield: 7.00 g (81.78%), mp: 208.5–211.1 °C, orange-brown powder. FTIR (cm−1): 3352 and 3298 (NH2 stretching), 3210 (O-H stretching), 1609 (aromatic C=C stretching), 1490 (N=N stretching), 1256 (C-O stretching), 1176 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.64 (d, J = 5.0 Hz, 2H), 7.58 (d, J = 10.0 Hz, 2H), 6.87 (d, J = 10.0 Hz, 2H), 6.66 (d, J = 10.0 Hz, 2H), 5.82 (s, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.81, 152.14, 145.89, 143.42, 124.82, 124.11, 116.26, 114.01. CHN elemental analysis: Calculated for C12H11N3O: C: 67.59%, H: 5.20%, N: 19.71%; Found: C: 67.11%, H: 5.22%, N: 19.55%.
3.3.4. Synthesis of 4-((4-aminophenyl)diazenyl)benzoic acid, 3g
A mixture of 1,4-phenylenediamine (19.44 g, 0.18 mol) and 4-nitrobenzoic acid (16.70 g, 0.1 mol) was placed in 500 mL round bottom flask containing 200 mL of 3% aqueous NaOH. The reaction mixture was stirred and refluxed for 12 h. The reaction progress was monitored by TLC. Upon completion, the mixture was cooled into an ice bath. The precipitate formed was filtered, washed a few times with cold water and dried overnight in open air to give a brown solid. Yield: 17.65 g (73.24%), mp: 220.7–222.4 °C, brown powder. FTIR (cm−1): 3211 (O-H stretching), 3336 and 3207 (N-H stretching), 1700 (C=O stretching), 1601 (aromatic C=C stretching), 1498 (N=N stretching), 1238 (C-O stretching), 1143 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.96 (d, J = 5.0 Hz, 2H), 7.66 (d, J = 10.0 Hz, 2H), 7.64 (d, J = 5.0 Hz, 2H), 6.68 (d, J = 10.0 Hz, 2H), 6.03 (s, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 169.88, 153.19, 153.15, 143.47, 141.25, 130.38, 125.58, 121.15, 113.97. CHN elemental analysis: Calculated for C13H11N3O2: C: 64.72%, H: 4.60%, N: 17.42%; Found: C: 64.33%, H: 4.61%, N: 17.37%.
3.3.5. Synthesis of 4-((4-substitutedphenyl)diazenyl)aniline, 3h–i
4-((4-Chlorophenyl)diazenyl)aniline, 3h: Part 1: A mixture of aniline (4.65 g, 0.05 mol), sodium bisulphite (10.41 g, 0.1 mol), and 10 mL of aqueous formaldehyde were placed in 100 mL round bottom flask and then stirred at room temperature for 2 h. Then, 50 mL of water was added to form a clear solution. Part 2: 4-Chloroaniline (6.42 g, 0.05 mol) was dissolved in 50 mL of methanol, followed by the addition of 13 mL of 12 M hydrochloric acid, HCl. The mixture was stirred and cooled to 0 °C. A solution of sodium nitrite, NaNO2 (3.45 g, 0.05 mol) in 15 mL of water was added dropwise to the mixture and was left for 30 min. Part 3: Sodium bicarbonate (8.40 g) was added to the solution prepared in Part 1. The mixture was cooled at 0 °C and was then poured slowly into the solution prepared in Part 2. The mixture was stirred for 12 h at room temperature and the precipitate formed was filtered. The precipitate was then added in 20 mL of 10% NaOH solution and was refluxed for 2 h. All the reaction progress was monitored by TLC. Upon completion, the precipitate was filtered and dried overnight in open air to form a dark orange solid. The same method was used for synthesis of 3i. Yield: 8.50 g (73.13%), mp: 175.7–178.1 °C, dark orange powder. FTIR (cm−1): 3336 and 3207 (N-H stretching), 1595 (aromatic C=C stretching), 1496 (N=N stretching), 1141 (C-N stretching), 785 (C-Cl bending). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.62 (d, J = 10.0 Hz, 2H), 7.56 (d, J = 10.0 Hz, 2H), 6.86 (d, J = 10.0 Hz, 2H), 6.66 (d, J = 5.0 Hz, 2H), 5.69 (s, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 159.36, 152.00, 145.97, 143.46, 124.85, 124.13, 116.26, 114.21. CHN elemental analysis: Calculated for C12H10N3: C: 62.21%, H: 4.35%, N: 18.14%; Found: C: 62.07%, H: 4.33%, N: 17.98%.
4-((4-Nitrophenyl)diazenyl)aniline, 3i: Yield: 9.00 g (74.07%), mp: 180.1–182.7 °C, brown powder. FTIR (cm−1): 3352 and 3209 (N-H stretching), 1600 (aromatic C=C stretching), 1498 (N=N stretching), 1143 (C-N stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.34 (d, J = 10.0 Hz, 2H), 7.89 (d, J = 10.0 Hz, 2H), 7.73 (d, J = 10.0 Hz, 2H), 6.70 (d, J = 5.0 Hz, 2H), 6.49 (s, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.77, 156.01, 148.22, 145.85, 126.24, 125.46, 123.43, 116.67. CHN elemental analysis: Calculated for C12H10N4O2: C: 59.50%, H: 4.16%, N: 23.13%; Found: C: 58.91%, H: 4.14%, N: 22.99%.
3.3.6. Synthesis of Hexakis(4-benzoate-phenoxy)cyclotriphosphazene, 4
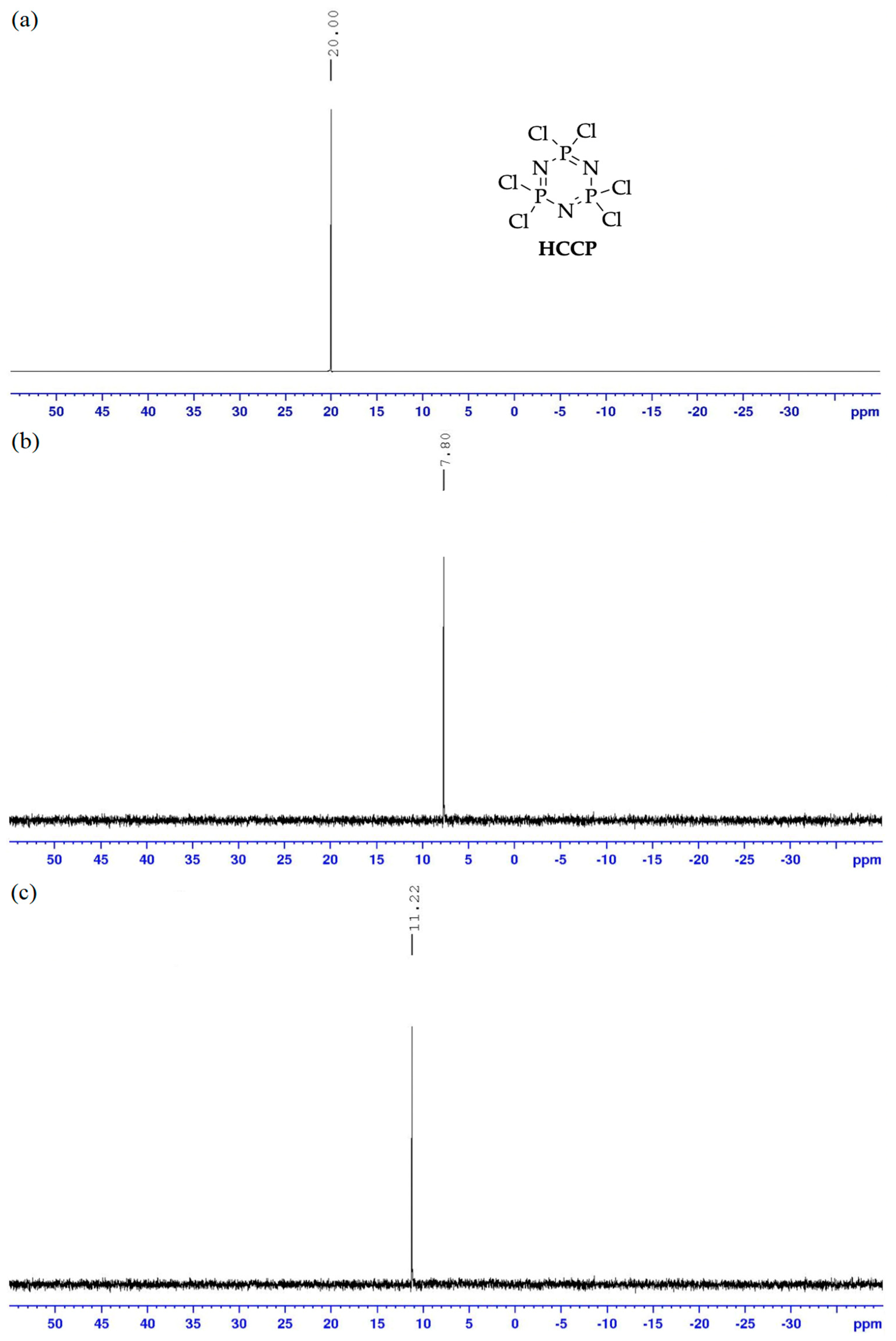
In a 250 mL round bottom flask, a mixture of methyl 4-hydroxybenzoate (10.64 g, 0.07 mol), phosphonitrilic chloride trimer (3.48 g, 0.01 mol), and potassium carbonate, K2CO3 (13.82 g, 0.1 mol) were placed in 150 mL acetone and then refluxed for 4 days. The reaction progress was monitored using TLC. Upon completion, the mixture was poured into 250 mL of cool water. The precipitate formed was filtered, washed with water and dried overnight to give a white solid. Yield: 9.30 g (89.34%), mp: 208.3–210.8 °C, white powder. FTIR (cm−1): 2875 and 2930 (Csp3-H stretching), 1708 (C=O stretching), 1606 (aromatic C=C stretching), 1253 (C-O stretching), 1189 (P=N stretching), 1172 (C-N stretching), 950 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.78 (d, J = 10.0 Hz, 2H), 7.05 (d, J = 5.0 Hz, 2H), 3.87 (s, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 165.13, 152.85, 131.08, 126.91, 120.62, 52.23. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 8.00 (s, 1P). CHN elemental analysis: Calculated for C48H42N3O18P3: C: 55.34%, H: 4.06%, N: 4.03%; Found: C: 55.11%, H: 4.05%, N: 3.99%.
3.3.7. Synthesis of Hexakis(4-carboxy-phenoxy)cyclotriphosphazene, 5
Intermediate 4 (8.50 g, 8.17 mmol) and sodium hydroxide, NaOH (5.88 g, 0.12 mol) in 150 mL of ethanol were mixed in a 250 mL round bottom flask. The mixture was refluxed for 5 h. White solution was formed and the reaction progress was monitored by TLC. Upon completion, the mixture was poured into 250 mL cooled water. A clear solution was observed, which then acidified with HCl until the precipitate was formed. The precipitate was filtered, washed with water and dried to give a white solid. Yield: 6.90 g (88.30%), mp: 217.1–219.5 °C, white powder. FTIR (cm−1): 3250 (O-H stretching), 1695 (C=O stretching), 1601 (aromatic C=C stretching), 1211 (C-O stretching), 1184 (P=N stretching), 1157 (C-N stretching), 951 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.83 (d, J = 10.0 Hz, 2H), 6.99 (d, J = 10.0 Hz, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.25, 152.72, 131.22, 128.24, 120.49. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 8.04 (s, 1P). CHN elemental analysis: Calculated for C42H30N3O18P3: C: 52.68%, H: 3.16%, N: 4.39%; Found: C: 52.47%, H: 3.17%, N: 4.37%.
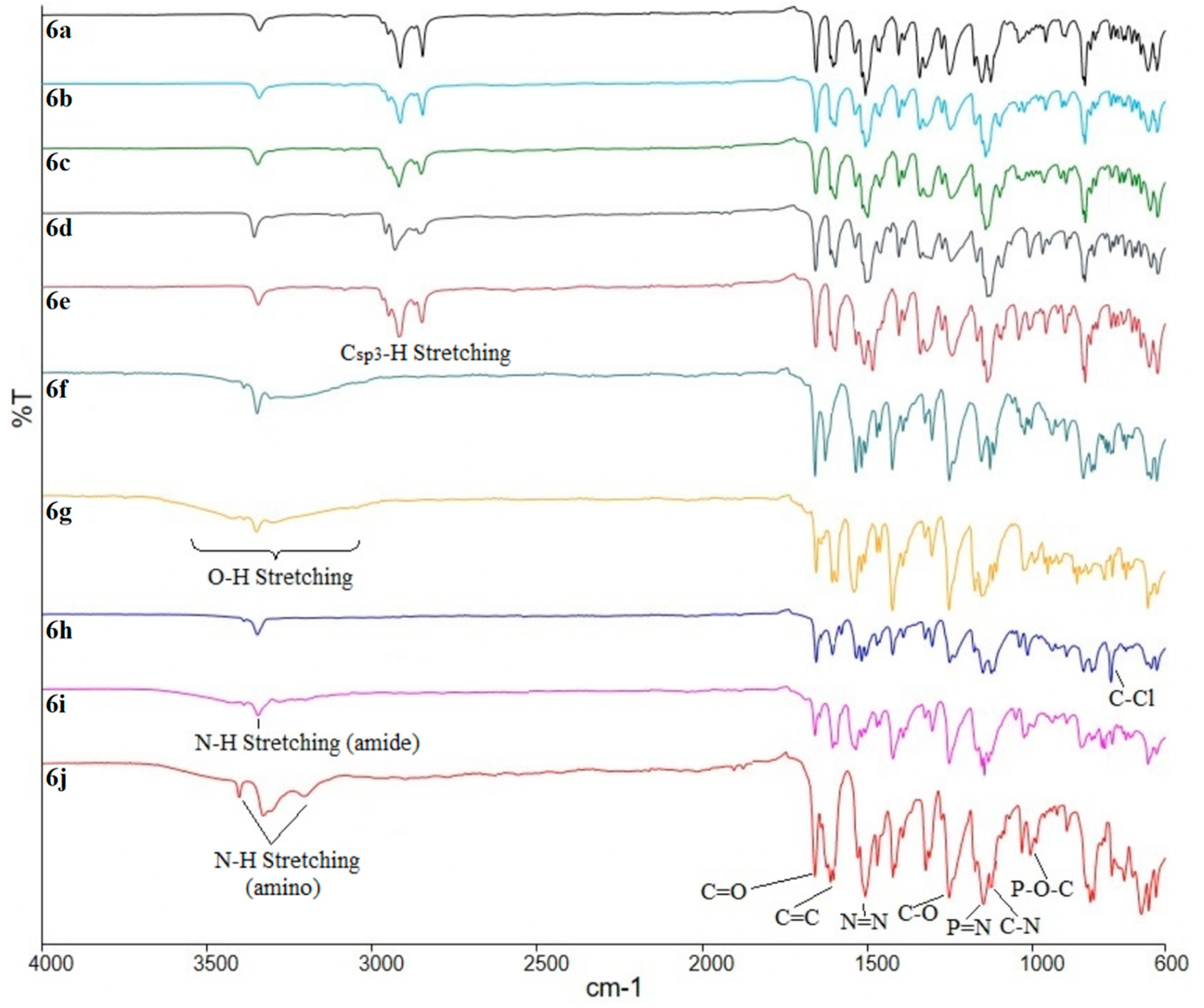
3.3.8. Synthesis of Hexakis{4-((E)-(4-((E)-4-substituted-phenyl)diazenyl)phenyl)benzamide} triazaphosphazene, 6a–i
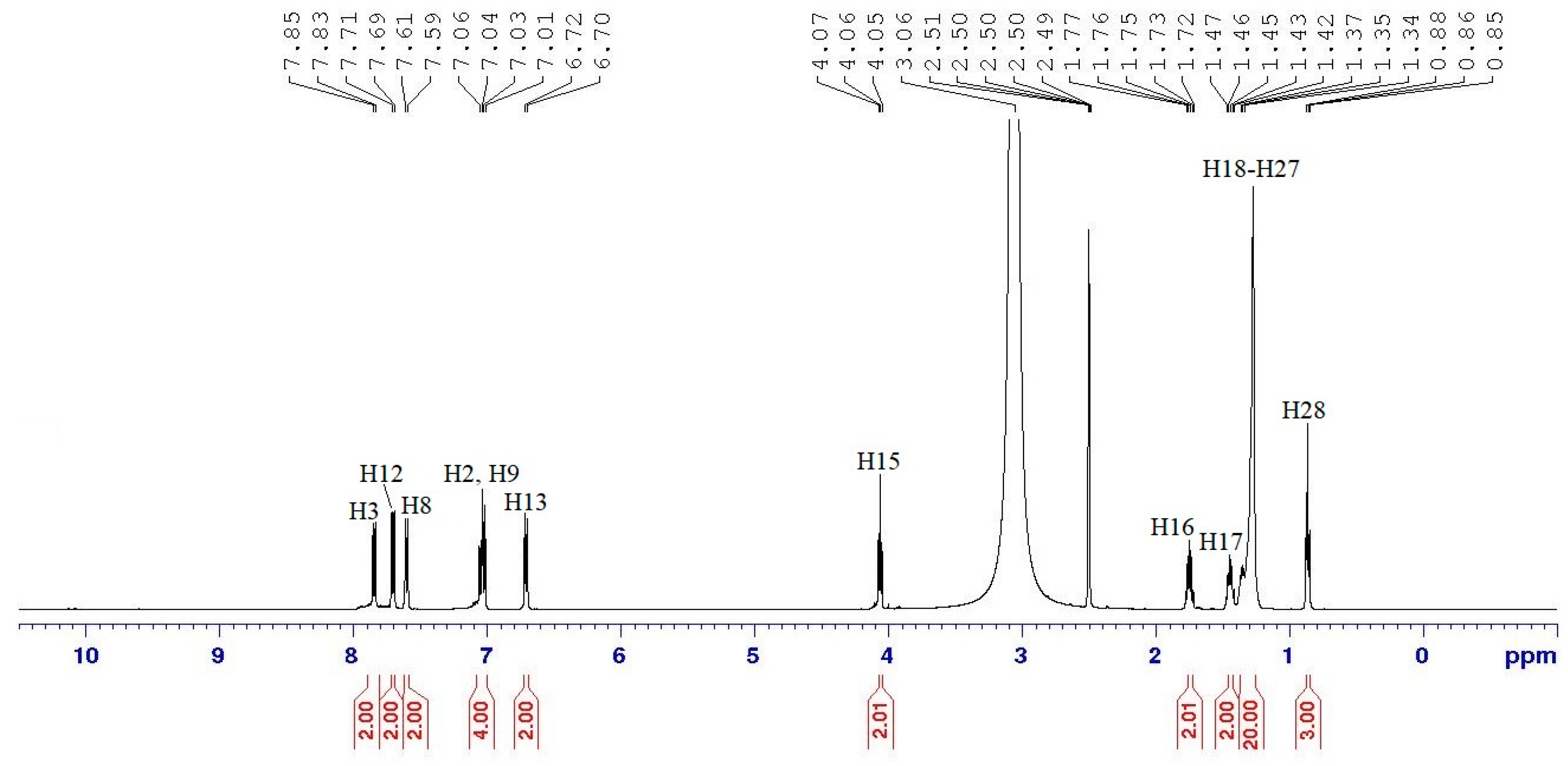
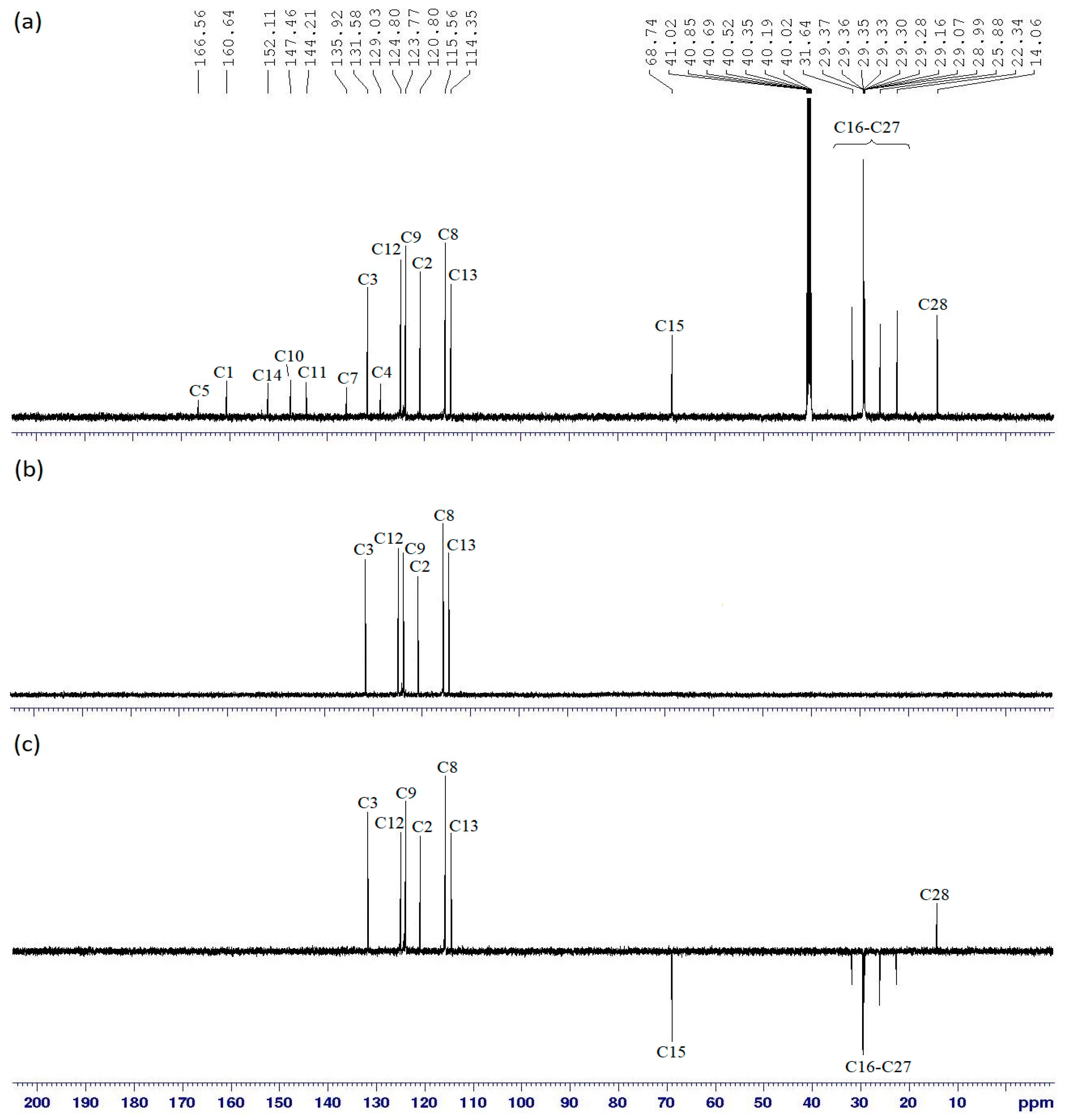
Hexakis{4-((E)-(4-((E)-4-heptyloxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6a: A mixture of intermediate 5 (1.00 g, 1.05 mmol), thionyl chloride (0.87 g, 7.32 mmol) and dichloromethane, DCM (20 mL) was mixed in a 100 mL round bottom flask. The mixture was stirred at room temperature for 6 h and an acid chloride was formed (in-situ reaction) by replacing the carboxyl function of intermediate 5. Without isolation, acid chloride was further reacted with intermediate 3a (2.11 g, 6.79 mmol) in 10 mL tetrahydrofuran, THF. Triethylamine, Et3N (0.32 g, 3.14 mmol) was added dropwise to the mixture which was stirred at room temperature for 4 days. The reaction progress was monitored by TLC. Upon completion, the product was filtered, and the filtrate was evaporated until the precipitate was formed. This precipitate was recrystallised from ethanol to give a yellow solid. The same method was used to synthesize 6b–i. Yield: 2.00 g (70.50%), mp: 155.3–157.7 °C, yellow-orange powder. FTIR (cm−1): 3342 (N-H stretching), 2924 and 2860 (Csp3-H stretching), 1703 (C=O stretching), 1591 (aromatic C=C stretching), 1485 (N=N stretching), 1248 (C-O stretching), 1186 (P=N stretching), 1149 (C-N stretching), 1017 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.82 (d, J = 10.0 Hz, 2H), 7.69 (d, J = 10.0 Hz, 2H), 7.58 (d, J = 10.0 Hz, 2H), 7.03 (d, J = 5.0 Hz, 2H), 7.01 (d, J = 5.0 Hz, 2H), 6.73 (d, J = 5.0 Hz, 2H), 4.04 (t, J = 7.5 Hz, 2H), 1.70–1.75 (m, 2H), 1.39–1.45 (m, 2H), 1.21–1.35 (m, 6H), 0.85 (t, J = 7.5 Hz, 3H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.67, 160.69, 151.73, 147.38, 144.36, 137.03, 131.58, 128.91, 124.77, 123.82, 120.80, 115.63, 114.61, 68.79, 31.55, 29.18, 28.83, 25.82, 22.28, 14.02. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.81 (s, 1P). CHN elemental analysis: Calculated for C156H168N21O18P3: C: 68.93%, H: 6.23%, N: 10.82%; Found: C: 68.77%, H: 6.20%, N: 10.75%.
Hexakis{4-((E)-(4-((E)-4-nonyloxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6b: Yield: 1.93 g (64.07%), mp: 147.6–149.4 °C, orange powder. FTIR (cm−1): 3343 (N-H stretching), 2921 and 2851 (Csp3-H stretching), 1695 (C=O stretching), 1593 (aromatic C=C stretching), 1483 (N=N stretching), 1211 (C-O stretching), 1186 (P=N stretching), 1160 (C-N stretching), 1017 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.82 (d, J = 10.0 Hz, 2H), 7.69 (d, J = 10.0 Hz, 2H), 7.59 (d, J = 10.0 Hz, 2H), 7.03 (d, J = 5.0 Hz, 2H), 7.01 (d, J = 5.0 Hz, 2H), 6.73 (d, J = 5.0 Hz, 2H), 4.04 (t, J = 7.5 Hz, 2H), 1.70–1.75 (m, 2H), 1.39–1.45 (m, 2H), 1.22–1.35 (m, 10H), 0.85 (t, J = 7.5 Hz, 3H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.67, 160.69, 151.73, 147.38, 144.36, 137.03, 131.58, 128.91, 124.77, 123.82, 120.80, 115.63, 114.61, 68.79, 31.55, 29.18, 29.08, 29.02, 28.83, 25.82, 22.28, 14.02. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.82 (s, 1P). CHN elemental analysis: Calculated for C168H192N21O18P3: C: 69.91%, H: 6.71%, N: 10.19%; Found: C: 69.82%, H: 6.73%, N: 10.11%.
Hexakis{4-((E)-(4-((E)-4-decyloxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6c: Yield: 2.12 g (68.38%), mp: 144.1–146.6 °C, yellow powder. FTIR (cm−1): 3343 (N-H stretching), 2919 and 2849 (Csp3-H stretching), 1702 (C=O stretching), 1593 (aromatic C=C stretching), 1488 (N=N stretching), 1216 (C-O stretching), 1184 (P=N stretching), 1149 (C-N stretching), 1019 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.85 (d, J = 5.0 Hz, 2H), 7.70 (d, J = 10.0 Hz, 2H), 7.60 (d, J = 10.0 Hz, 2H), 7.05 (d, J = 10.0 Hz, 2H), 7.02 (d, J = 10.0 Hz, 2H), 6.71 (d, J = 10.0 Hz, 2H), 4.06 (t, J = 7.5 Hz, 2H), 1.72–1.78 (m, 2H), 1.42–1.48 (m, 2H), 1.26–1.38 (m, 12H), 0.87 (t, J = 5.0 Hz, 3H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.54, 160.69, 152.14, 147.54, 144.31, 137.50, 131.57, 129.06, 124.77, 123.77, 120.79, 115.65, 114.37, 68.84, 31.61, 29.28, 29.24, 29.18, 29.07, 28.94, 25.88, 22.31, 14.02. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.77 (s, 1P). CHN elemental analysis: Calculated for C174H204N21O18P3: C: 70.35%, H: 6.92%, N: 9.90%; Found: C: 70.09%, H: 6.88%, N: 9.84%.
Hexakis{4-((E)-(4-((E)-4-dodecyloxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6d: Yield: 2.25 g (68.68%), mp: 140.1–142.8 °C, red powder. FTIR (cm−1): 3344 (N-H stretching), 2921 and 2849 (Csp3-H stretching), 1698 (C=O stretching), 1591 (aromatic C=C stretching), 1485 (N=N stretching), 1211 (C-O stretching), 1186 (P=N stretching), 1154 (C-N stretching), 1017 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.84 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 10.0 Hz, 2H), 7.60 (d, J = 5.0 Hz, 2H), 7.05 (d, J = 10.0 Hz, 2H), 7.02 (d, J = 10.0 Hz, 2H), 6.71 (d, J = 10.0 Hz, 2H), 4.06 (t, J = 7.5 Hz, 2H), 1.72–1.77 (m, 2H), 1.42–1.47 (m, 2H), 1.23–1.38 (m, 16H), 0.86 (t, J = 7.5 Hz, 3H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.58, 160.69, 152.12, 147.52, 145.59, 137.13, 131.57, 129.71, 124.77, 123.77, 120.79, 115.65, 114.37, 68.83, 31.60, 29.31, 29.28, 29.24, 29.20, 29.14, 29.03, 28.94, 25.85, 22.30, 14.00. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.79 (s, 1P). CHN elemental analysis: Calculated for C186H228N21O18P3: C: 71.17%, H: 7.32%, N: 9.37%; Found: C: 70.88%, H: 7.30%, N: 9.33%.
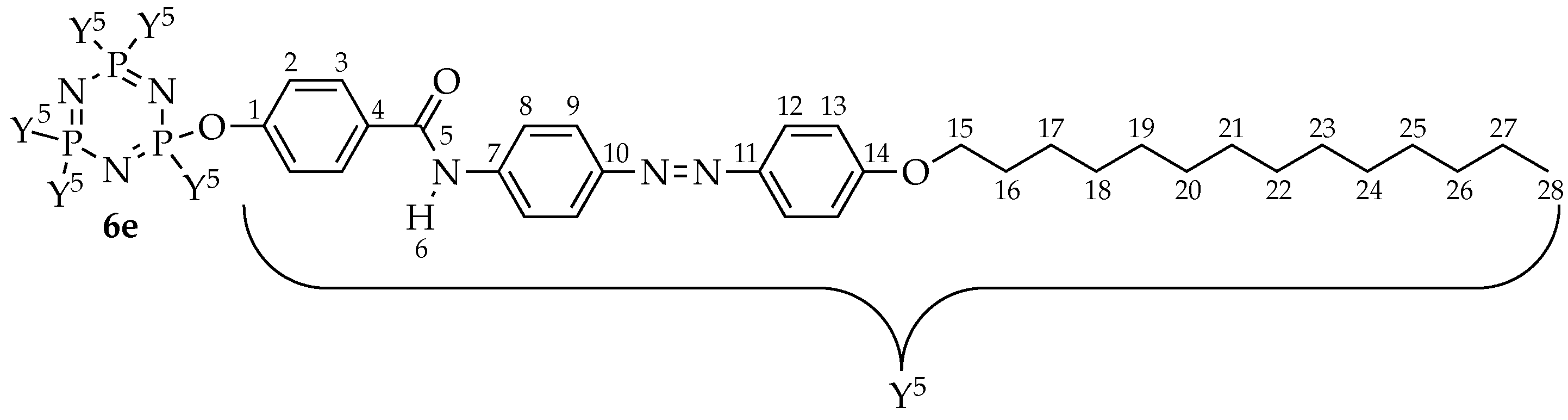
Hexakis{4-((E)-(4-((E)-4-tetradecyloxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6e: Yield: 2.55 g (73.88%), mp: 137.9–140.3 °C, red powder. FTIR (cm−1): 3342 (N-H stretching), 2916 and 2850 (Csp3-H stretching), 1703 (C=O stretching), 1593 (aromatic C=C stretching), 1483 (N=N stretching), 1219 (C-O stretching), 1186 (P=N stretching), 1149 (C-N stretching), 1020 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.84 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 10.0 Hz, 2H), 7.60 (d, J = 10.0 Hz, 2H), 7.05 (d, J = 10.0 Hz, 2H), 7.02 (d, J = 10.0 Hz, 2H), 6.71 (d, J = 10.0 Hz, 2H), 4.06 (t, J = 5.0 Hz, 2H), 1.72–1.77 (m, 2H), 1.42–1.47 (m, 2H), 1.25–1.38 (m, 20H), 0.86 (t, J = 7.5 Hz, 3H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.57, 160.64, 152.11, 147.46, 144.21, 135.92, 131.58, 129.03, 124.80, 123.77, 120.81, 115.56 114.35, 68.74, 31.64, 29.37, 29.36, 29.35, 29.33, 29.30, 29.28, 29.16, 29.07, 28.99, 25.88, 22.34, 14.06. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.80 (s, 1P). CHN elemental analysis: Calculated for C198H252N21O18P3: C: 71.92%, H: 7.68%, N: 8.89%; Found: C: 71.66%, H: 7.63%, N: 8.81%.
Hexakis{4-((E)-(4-((E)-4-hydroxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6f: Yield: 1.43 g (64.34%), mp: 260.4–263.1 °C, orange powder. FTIR (cm−1): 3344 (N-H stretching), 3200 (O-H stretching), 1701 (C=O stretching), 1590 (aromatic C=C stretching), 1470 (N=N stretching), 1245 (C-O stretching), 1186 (P=N stretching), 1149 (C-N stretching), 1013 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 11.13 (s, 1H), 10.45 (s, 1H), 8.09 (d, J = 10.0 Hz, 2H), 7.78 (d, J = 10.0 Hz, 4H), 7.56 (d, J = 5.0 Hz, 2H), 6.94 (d, J = 10.0 Hz, 2H), 6.92 (d, J = 10.0 Hz, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 164.36, 161.71, 151.13, 145.69, 145.58, 140.07, 135.30, 129.82, 126.59, 125.48, 124.17, 116.46, 116.23. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.85 (s, 1P). CHN elemental analysis: Calculated for C114H84N21O18P3: C: 64.32%, H: 3.98%, N: 13.82%; Found: C: 64.29%, H: 4.00%, N: 13.80%.
Hexakis{4-((E)-(4-((E)-4-carboxy-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6g: Yield: 1.45 g (60.46%), mp: 242.7–245.1 °C, dark orange powder. FTIR (cm−1): 3343 (N-H stretching), 3205 (O-H stretching), 1687 (C=O stretching), 1591 (aromatic C=C stretching), 1475 (N=N stretching), 1213 (C-O stretching), 1181 (P=N stretching), 1157 (C-N stretching), 1012 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.81 (d, J = 10.0 Hz, 2H), 7.66 (d, J = 5.0 Hz, 2H), 7.60 (d, J = 10.0 Hz, 2H), 6.89 (d, J = 10.0 Hz, 2H), 6.84 (d, J = 10.0 Hz, 2H), 6.67 (d, J = 10.0 Hz, 2H), two exchangeable protons were not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 167.83, 162.02, 159.63, 152.23, 146.02, 143.42, 132.00, 124.86, 124.28, 124.08, 122.07, 116.18, 115.60, 113.95. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.83 (s, 1P). CHN elemental analysis: Calculated for C120H84N21O24P3: C: 62.75%, H: 3.69%, N: 12.81%; Found: C: 62.67%, H: 3.70%, N: 12.73%.
Hexakis{4-((E)-(4-((E)-4-chloro-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6h: Yield: 1.44 g (61.58%), mp: 233.3–236.1 °C, orange powder. FTIR (cm−1): 3340 (N-H stretching), 1702 (C=O stretching), 1596 (aromatic C=C stretching), 1471 (N=N stretching), 1222 (C-O stretching), 1187 (P=N stretching), 1147 (C-N stretching), 1017 (P-O-C stretching), 811 (C-Cl bending). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.03 (d, J = 10.0 Hz, 2H), 7.69 (d, J = 10.0 Hz, 2H), 7.68 (d, J = 5.0 Hz, 2H), 7.64 (d, J = 10.0 Hz, 2H), 6.70 (d, J = 10.0 Hz, 2H), 6.56 (d, J = 10.0 Hz, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 170.08, 169.01, 153.67, 153.33, 152.94, 143.49, 139.49, 131.62, 130.53, 125.73, 121.36, 114.01, 113.12. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.86 (s, 1P). CHN elemental analysis: Calculated for C114H78Cl6N21O12P3: C: 61.14%, H: 3.51%, N: 13.13%; Found: C: 60.73%, H: 3.49%, N: 13.01%.
Hexakis{4-((E)-(4-((E)-4-nitro-phenyl)diazenyl)phenyl)benzamide}triazaphosphazene, 6i: Yield: 2.84 g (78.75%), mp: 268.7–270.1 °C, brown powder. FTIR (cm−1): 3343 (N-H stretching), 1700 (C=O stretching), 1590 (aromatic C=C stretching), 1475 (N=N stretching), 1213 (C-O stretching), 1184 (P=N stretching), 1160 (C-N stretching), 1017 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.25 (d, J = 5.0 Hz, 2H), 7.88 (d, J = 10.0 Hz, 2H), 7.85 (d, J = 10.0 Hz, 2H), 7.78 (d, J = 10.0 Hz, 2H), 6.95 (d, J = 5.0 Hz, 2H), 6.63 (d, J = 5.0 Hz, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 162.49, 156.35, 155.70, 148.40, 146.32, 140.33, 137.15, 126.43, 125.92, 125.01, 123.16, 116.68, 113.21. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.81 (s, 1P). CHN elemental analysis: Calculated for C114H78N27O24P3: C: 59.46%, H: 3.41%, N: 16.42%; Found: C: 58.98%, H: 3.38%, N: 16.27%.
3.3.9. Synthesis of Hexakis{4-((E)-(4-((E)-4-amino-phenyl)diazenyl)phenyl)benzamide} triazaphosphazene, 6j
A solution of compound 6i (1.60 g, 0.70 mmol) in 20 mL of hot ethanol and a solution of sodium sulphide hydrate, Na2S.9H2O (0.54 g, 6.95 mmol) in 20 mL of ethanol and 20 mL of distilled water were mixed in a 100 mL round bottom flask. The reaction mixture was refluxed for 24 h. The reaction progress was monitored by TLC. Upon completion, the mixture was cooled in ice water and the precipitate formed was filtered, washed with cold ethanol and dried overnight in open air. Yield: 1.11 g (75.26%), mp: 285.5–287.4 °C, brown powder. FTIR (cm−1): 3340 (N-H amide stretching), 3381, and 3218 (N-H amine stretching), 1687 (C=O stretching), 1601 (aromatic C=C stretching), 1490 (N=N stretching), 1211 (C-O stretching), 1186 (P=N stretching), 1158 (C-N stretching), 1013 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 10.46 (s, 1H), 7.80 (d, J = 10.0 Hz, 4H), 7.57 (d, J = 10.0 Hz, 2H), 7.00 (d, J = 10.0 Hz, 2H), 6.96 (d, J = 10.0 Hz, 2H), 6.57 (d, J = 5.0 Hz, 2H), 5.20 (s, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 161.75, 151.21, 151.15, 148.05, 145.59, 135.31, 129.85, 128.95, 125.50, 124.20, 119.33, 116.49, 115.73. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.80 (s, 1P). CHN elemental analysis: Calculated for C114H90N27O12P3: C: 64.49%, H: 4.27%, N: 17.81%; Found: C: 64.11%, H: 4.23%, N: 17.77%.
3.3.10. Synthesis of Hexakis(4-nitro-phenoxy)cyclotriphosphazene, 7
4-Nitrophenol (14.61 g, 0.11 mol), phosphonitrilic chloride trimer (5.22 g, 0.015 mol), and potassium carbonate, K2CO3 (20.73 g, 0.15 mol) were mixed in 150 mL of acetone in a 250 mL round bottom flask. The mixture was refluxed for 4 days. The reaction progress was monitored using TLC. Upon completion, the mixture was poured into 250 mL of cool water. The precipitate formed was filtered and dried overnight in open air. Yield: 12.15 g (84.11%), mp: 182.5–185.1 °C, yellow powder. FTIR (cm−1): 1603 (aromatic C=C stretching), 1256 (C-O stretching), 1182 (P=N stretching), 1160 (C-N stretching), 977 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.13 (d, J = 10.0 Hz, 2H), 7.30 (d, J = 10.0 Hz, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 154.34, 145.68, 126.05, 121.89. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 7.21 (s, 1P). CHN elemental analysis: Calculated for C36H24N9O18P3: C: 44.88%, H: 13.08%, N: 13.08%; Found: C: 44.67%, H: 12.99%, N: 13.03%.
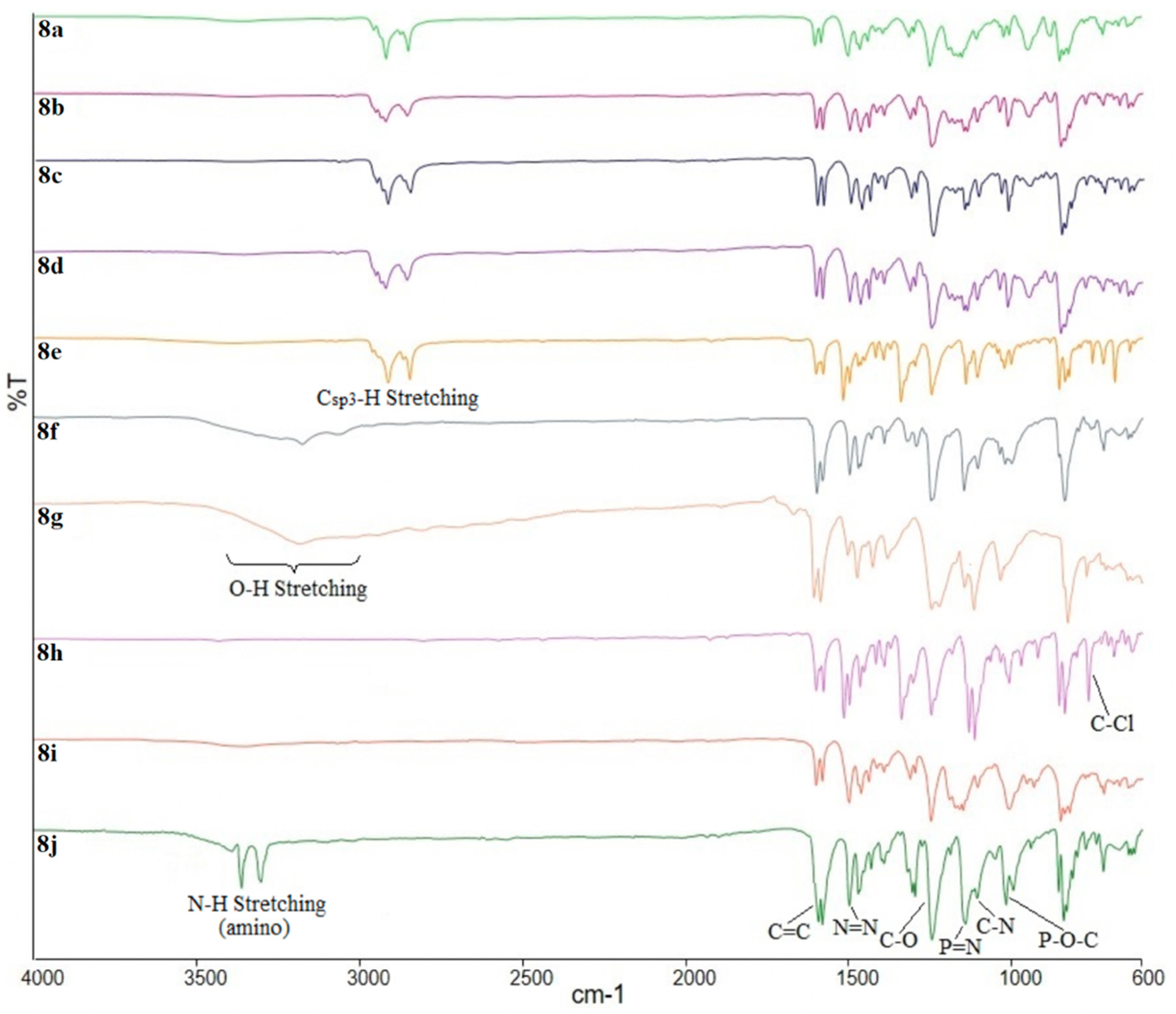
3.3.11. Synthesis of Hexakis{4-((E)-((4-((E)-4-substituted-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8a–i
Hexakis{4-((E)-((4-((E)-4-heptyloxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8a: Intermediate 7 (1.00 g, 1.04 mmol) and intermediate 3a (2.26 g, 7.27 mmol) were mixed in 50 mL ethanol and the solution was poured into a 100 mL round bottom flask. 10 mL of 3% aqueous NaOH was added and the mixture was refluxed for 24 h. The reaction progress was monitored by TLC. Upon completion, the mixture was cooled into an ice bath. The precipitate formed was filtered, washed with cold ethanol, and dried overnight in open air to give a red precipitate. The same method was used to synthesize 8b–i. Yield: 1.85 g (67.77%), mp: 116.9–118.5 °C, red powder. FTIR (cm−1): 2921 and 2860 (Csp3-H stretching), 1603 (aromatic C=C stretching), 1496 (N=N stretching), 1248 (C-O stretching), 1189 (P=N stretching), 1156 (C-N stretching), 997 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.23 (d, J = 10.0 Hz, 2H), 8.15 (d, J = 10.0 Hz, 2H), 7.76 (d, J = 10.0 Hz, 2H), 7.72 (d, J = 5.0 Hz, 2H), 7.02 (d, J = 10.0 Hz, 2H), 6.94 (d, J = 10.0 Hz, 2H), 4.03 (t, J = 7.5 Hz, 2H), 1.70–1.75 (m, 2H), 1.39–1.44 (m, 2H), 1.23–1.33 (m, 6H), 0.85 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.40, 161.26, 160.72, 150.47, 147.16, 146.26, 130.90, 124.55, 124.19, 123.66, 116.35, 115.50, 68.75, 31.52, 29.13, 28.71, 25.82, 22.26, 13.96. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.16 (s, 1P). CHN elemental analysis: Calculated for C150H162N27O12P3: C: 68.55%, H: 6.21%, N: 14.39%; Found: C: 68.18%, H: 6.17%, N: 14.23%.
Hexakis{4-((E)-((4-((E)-4-nonyloxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8b: Yield: 2.10 g (72.41%), mp: 116.1–118.3 °C, dark red powder. FTIR (cm−1): 2921 and 2851 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1496 (N=N stretching), 1248 (C-O stretching), 1181 (P=N stretching), 1151 (C-N stretching), 998 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.23 (d, J = 10.0 Hz, 2H), 8.15 (d, J = 10.0 Hz, 2H), 7.76 (d, J = 10.0 Hz, 2H), 7.72 (d, J = 5.0 Hz, 2H), 7.02 (d, J = 10.0 Hz, 2H), 6.94 (d, J = 10.0 Hz, 2H), 4.03 (t, J = 7.5 Hz, 2H), 1.70–1.75 (m, 2H), 1.39–1.44 (m, 2H), 1.22–1.35 (m, 10H), 0.85 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.52, 161.27, 160.78, 150.32, 147.18, 146.25, 130.87, 124.56, 124.20, 123.61, 116.36, 115.54, 68.76, 31.61, 29.25, 29.14, 29.10, 28.91, 25.87, 22.33, 14.01. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.19 (s, 1P). CHN elemental analysis: Calculated for C162H186N27O12P3: C: 69.58%, H: 6.70%, N: 13.52%; Found: C: 69.44%, H: 6.68%, N: 13.44%.
Hexakis{4-((E)-((4-((E)-4-decyloxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8c: Yield: 2.18 g (72.91%), mp: 113.3–115.1 °C, orange powder. FTIR (cm−1): 2919 and 2851 (Csp3-H stretching), 1603 (aromatic C=C stretching), 1493 (N=N stretching), 1246 (C-O stretching), 1178 (P=N stretching), 1146 (C-N stretching), 997 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.22 (d, J = 10.0 Hz, 2H), 8.14 (d, J = 10.0 Hz, 2H), 7.74 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 10.0 Hz, 2H), 6.99 (d, J = 10.0 Hz, 2H), 6.93 (d, J = 10.0 Hz, 2H), 4.01 (t, J = 5.0 Hz, 2H), 1.68–1.73 (m, 2H), 1.37–1.42 (m, 2H), 1.19–1.32 (m, 12H), 0.82 (t, J = 5.0 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.47, 161.25, 160.73, 150.41, 147.17, 146.26, 130.89, 124.53, 124.18, 123.62, 116.33, 115.48, 68.74, 31.62, 29.28, 29.25, 29.12, 29.08, 28.96, 25.85, 22.32, 13.96. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.18 (s, 1P). CHN elemental analysis: Calculated for C168H198N27O12P3: C: 70.05%, H: 6.93%, N: 13.13%; Found: C: 69.89%, H: 6.90%, N: 13.02%.
Hexakis{4-((E)-((4-((E)-4-dodecyloxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8d: Yield: 2.20 g (69.62%), mp: 109.7–111.4 °C, dark orange powder. FTIR (cm−1): 2916 and 2851 (Csp3-H stretching), 1601 (aromatic C=C stretching), 1493 (N=N stretching), 1248 (C-O stretching), 1192 (P=N stretching), 1151 (C-N stretching), 995 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.21 (d, J = 5.0 Hz, 2H), 8.14 (d, J = 10.0 Hz, 2H), 7.74 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 5.0 Hz, 2H), 7.01 (d, J = 10.0 Hz, 2H), 6.94 (d, J = 5.0 Hz, 2H), 4.05 (t, J = 5.0 Hz, 2H), 1.71–1.76 (m, 2H), 1.41–1.46 (m, 2H), 1.23–1.36 (m, 16H), 0.85 (t, J = 7.5 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.57, 161.33, 160.76, 150.42, 147.40, 146.45, 130.81, 124.46, 124.14, 123.49, 116.40, 115.65, 68.94, 31.60, 29.38, 29.30, 29.29, 29.24, 29.16, 29.05, 28.94, 25.85, 22.26, 13.85. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.15 (s, 1P). CHN elemental analysis: Calculated for C180H222N27O12P3: C: 70.91%, H: 7.34%, N: 12.40%; Found: C: 70.66%, H: 7.33%, N: 12.33%.
Hexakis{4-((E)-((4-((E)-4-tetradecyloxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8e: Yield: 2.55 g (76.35%), mp: 115.9–117.5 °C, orange powder. FTIR (cm−1): 2916 and 2851 (Csp3-H stretching), 1603 (aromatic C=C stretching), 1493 (N=N stretching), 1251 (C-O stretching), 1176 (P=N stretching), 1149 (C-N stretching), 995 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.22 (d, J = 10.0 Hz, 2H), 8.15 (d, J = 10.0 Hz, 2H), 7.75 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 10.0 Hz, 2H), 7.01 (d, J = 5.0 Hz, 2H), 6.93 (d, J = 5.0 Hz, 2H), 4.04 (t, J = 5.0 Hz, 2H), 1.71–1.76 (m, 2H), 1.40–1.46 (m, 2H), 1.22–1.37 (m, 20H), 0.85 (t, J = 5.0 Hz, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.47, 161.30, 160.72, 150.52, 147.33, 146.40, 130.90, 124.48, 124.14, 123.57, 116.34, 115.55, 68.85, 31.62, 29.41, 29.37, 29.36, 29.35, 29.33, 29.27, 29.17, 29.08, 28.97, 25.87, 22.30, 13.87. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.17 (s, 1P). CHN elemental analysis: Calculated for C192H246N27O12P3: C: 71.68%, H: 7.71%, N: 11.76%; Found: C: 71.22%, H: 7.67%, N: 11.68%.
Hexakis{4-((E)-((4-((E)-4-hydroxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8f: Yield: 1.28 g (60.38%), mp: 180.7–182.9 °C, dark orange powder. FTIR (cm−1): 3255 (O-H stretching), 1601 (aromatic C=C stretching), 1496 (N=N stretching), 1252 (C-O stretching), 1178 (P=N stretching), 1150 (C-N stretching), 1017 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.10 (d, J = 5.0 Hz, 2H), 7.94 (d, J = 5.0 Hz, 2H), 7.84 (d, J = 10.0 Hz, 2H), 7.82 (d, J = 10.0 Hz, 2H), 6.96 (d, J = 10.0 Hz, 2H), 6.61 (d, J = 10.0 Hz, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 167.37, 162.03, 156.05, 155.04, 145.82, 136.22, 130.98, 126.81, 125.76, 122.50, 116.51, 112.87. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.19 (s, 1P). CHN elemental analysis: Calculated for C108H78N27O12P3: C: 63.62%, H: 3.86%, N: 18.55%; Found: C: 63.50%, H: 3.90%, N: 18.39%.
Hexakis{4-((E)-((4-((E)-4-carboxy-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8g: Yield: 1.36 g (59.39%), mp: 190.1–192.9 °C, orange powder. FTIR (cm−1): 3251 (O-H stretching), 1684 (C=O stretching), 1603 (aromatic C=C stretching), 1493 (N=N stretching), 1251 (C-O stretching), 1176 (P=N stretching), 1148 (C-N stretching), 1007 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.10 (d, J = 10.0 Hz, 2H), 7.85 (d, J = 10.0 Hz, 2H), 7.83 (d, J = 10.0 Hz, 2H), 6.97 (d, J = 10.0 Hz, 2H), 6.54 (d, J = 10.0 Hz, 2H), 6.51 (d, J = 5.0 Hz, 2H), one exchangeable proton was not observed. 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 167.51, 162.08, 154.97, 149.41, 145.80, 132.72, 131.57, 130.99, 125.77, 122.51, 116.55, 116.10, one carbon environments not observed due to coincident resonances. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.23 (s, 1P). CHN elemental analysis: Calculated for C114H78N27O18P3: C: 62.04%, H: 3.56%, N: 17.14%; Found: C: 61.79%, H: 3.55%, N: 17.02%.
Hexakis{4-((E)-((4-((E)-4-chloro-phenyl)diazenyl)phenyl)diazenyl)phenoxy}triazaphosphazene, 8h: Yield: 1.57 g (70.40%), mp: 177.5–178.1 °C, yellow powder. FTIR (cm−1): 1606 (aromatic C=C stretching), 1491 (N=N stretching), 1255 (C-O stretching), 1180 (P=N stretching), 1149 (C-N stretching), 996 (P-O-C stretching), 790 (C-Cl bending). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.09 (d, J = 10.0 Hz, 2H), 7.91 (d, J = 5.0 Hz, 2H), 7.83 (d, J = 5.0 Hz, 2H), 7.81 (d, J = 10.0 Hz, 2H), 7.46 (d, J = 5.0 Hz, 2H), 6.95 (d, J = 10.0 Hz, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 167.33, 166.97, 162.04, 155.04, 145.82, 138.29, 131.53, 130.96, 129.05, 125.75, 122.49, 116.49. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.20 (s, 1P). CHN elemental analysis: Calculated for C108H72Cl6N27O6P3: C: 60.35%, H: 3.38%, N: 17.59%; Found: C: 60.11%, H: 3.35%, N: 17.40%.
Hexakis{4-((E)-((4-((E)-4-nitro-phenyl)diazenyl)phenyl)diazenyl)phenoxy}triazaphosphazene, 8i: Yield: 1.68 g (73.04%), mp: 193.5–195.3 °C, red powder. FTIR (cm−1): 1603 (aromatic C=C stretching), 1496 (N=N stretching), 1253 (C-O stretching), 1179 (P=N stretching), 1148 (C-N stretching), 1006 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 7.94 (d, J = 5.0 Hz, 2H), 7.65 (d, J = 10.0 Hz, 2H), 7.61 (d, J = 5.0 Hz, 2H), 7.48 (d, J = 10.0 Hz, 2H), 6.92 (d, J = 5.0 Hz, 2H), 6.72 (d, J = 5.0 Hz, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 166.94, 159.70, 151.84, 146.58, 144.31, 138.20, 131.44, 128.94, 124.63, 123.97, 116.29, 114.34. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.18 (s, 1P). CHN elemental analysis: Calculated for C108H72N33O18P3: C: 58.62%, H: 3.28%, N: 20.89%; Found: C: 58.66%, H: 3.25%, N: 20.65%.
3.3.12. Synthesis of Hexakis(4-acetamido-phenoxy)cyclotriphosphazene, 9
4-Acetamidophenol (10.57 g, 0.07 mol), phosphonitrilic chloride trimer (3.48 g, 0.01 mol), and potassium carbonate, K2CO3 (13.82 g, 0.1 mol) were mixed in 150 mL of acetone and the solution was transferred into a 250 mL round bottom flask. The reaction mixture was refluxed for 4 days. The reaction progress was monitored using TLC. Upon completion, the mixture was poured into a 250 mL of cooled water. The precipitate formed was filtered using Buchner funnel, washed with cold water, and then dried overnight in open air to give a white solid powder. Yield: 9.15 g (88.41%), mp: 178.8–180.7 °C, white powder. FTIR (cm−1): 3343 (N-H stretching), 2890 and 2821 (Csp3-H stretching), 1703 (C=O stretching), 1602 (aromatic C=C stretching), 1253 (C-O stretching), 1179 (P=N stretching), 1155 (C-N stretching), 971 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 9.92 (s, 1H), 7.44 (d, J = 10.0 Hz, 2H), 6.80 (d, J = 10.0 Hz, 2H), 2.04 (s, 3H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 168.35, 145.09, 136.32, 120.52, 120.19, 23.80. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 9.18 (s, 1P). CHN elemental analysis: Calculated for C48H48N9O12P3: C: 55.66%, H: 4.67%, N: 12.17%; Found: C: 55.37%, H: 4.63%, N: 12.10%.
3.3.13. Synthesis of Hexakis(4-amino-phenoxy)cyclotriphosphazene, 10
A solution of intermediate 9 (8.00 g, 7.73 mmol) in 100 mL methanol and a solution of sodium hydroxide, NaOH (4.64 g, 0.12 mol) in 20 mL water were mixed in a 250 mL round bottom flask. The mixture was refluxed for 24 h. The reaction progress was monitored using TLC. Upon completion, the mixture was poured into a 250 mL of cooled water. The precipitate formed was filtered, washed with cold water, and dried overnight in open air to give a white precipitate. Yield: 4.80 g (79.34%), mp: 190.1–192.9 °C, white powder. FTIR (cm−1): 3451 and 3217 (N-H stretching), 1606 (aromatic C=C stretching), 1258 (C-O stretching), 1181 (P=N stretching), 1162 (C-N stretching), 970 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 6.52 (d, J = 10.0 Hz, 2H), 6.44 (d, J = 5.0 Hz, 2H), 4.89 (s, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 145.56, 140.83, 120.91, 114.26. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 14.73 (s, 1P). CHN elemental analysis: Calculated for C36H36N9O6P3: C: 55.18%, H: 4.63%, N: 16.09%; Found: C: 54.78%, H: 4.60%, N: 16.10%.
3.3.14. Synthesis of Hexakis{4-((E)-((4-((E)-4-amino-phenyl)diazenyl)phenyl)diazenyl)phenoxy} triazaphosphazene, 8j
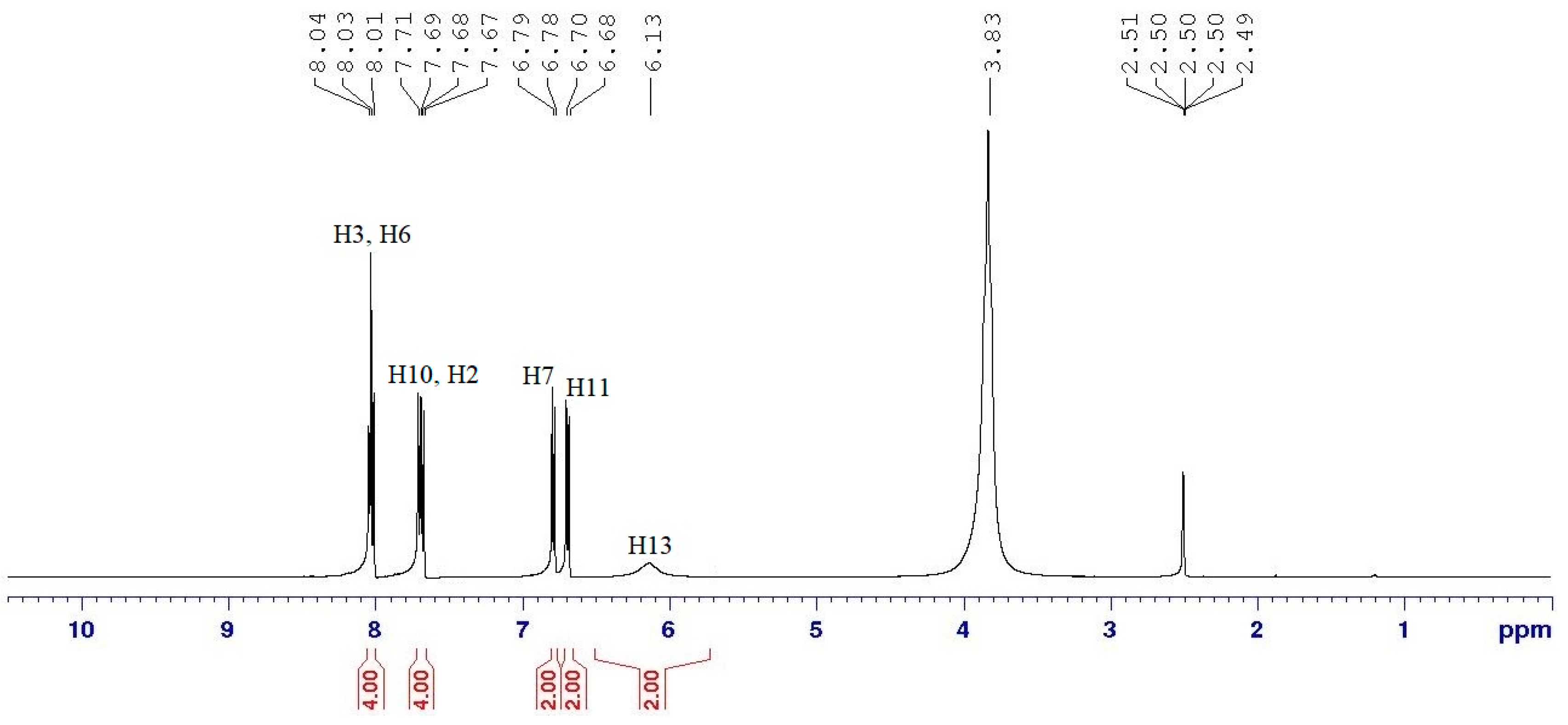
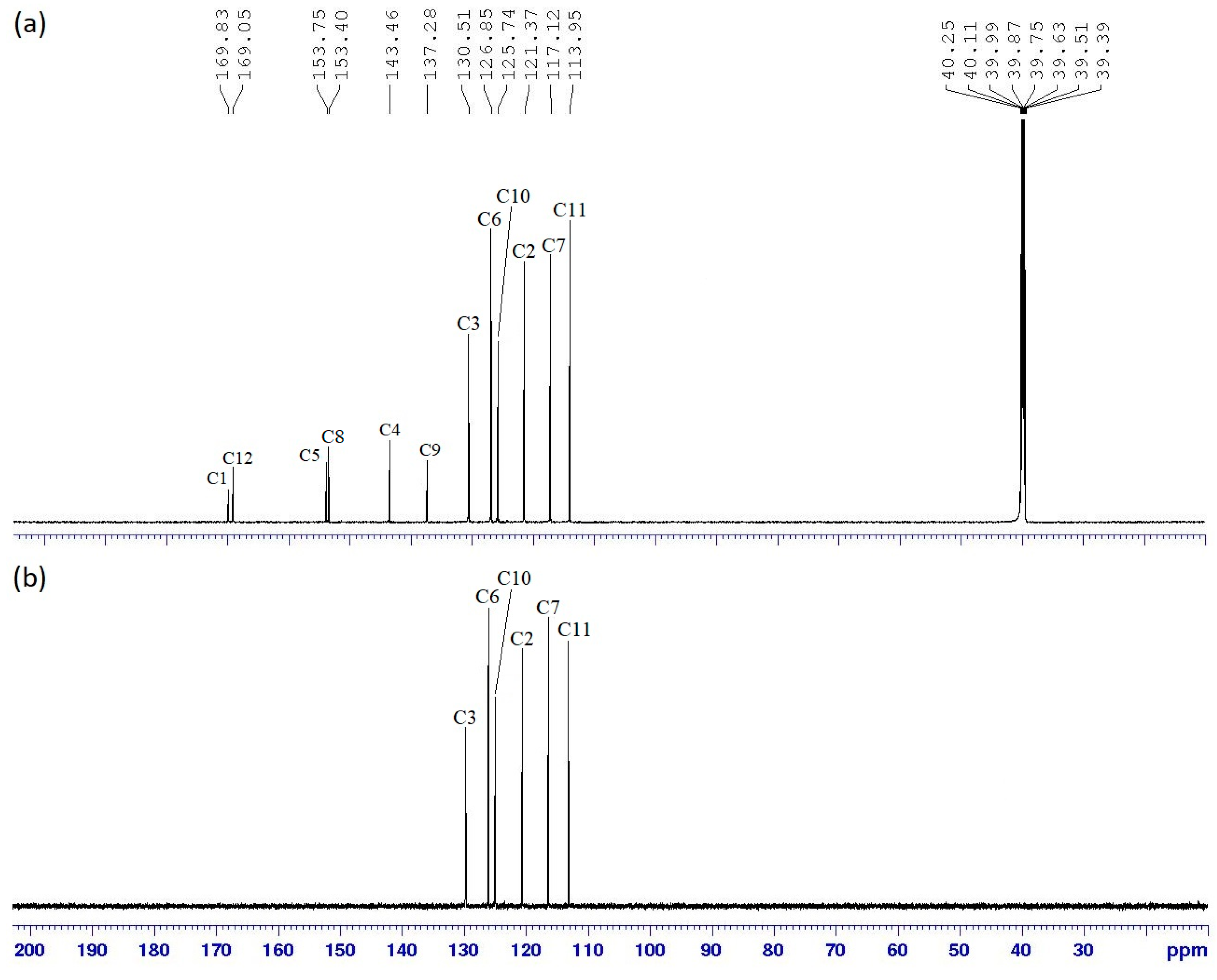
Intermediate 10 (1.00 g, 1.28 mmol) and intermediate 3i (2.16 g, 8.94 mmol) were used in the reaction. Synthetic protocol for 8a was used in this reaction. Yield: 1.31 g (51.98%), mp: 197.7–199.6 °C, orange powder. FTIR (cm−1): 3385 and 3272 (N-H stretching), 1601 (aromatic C=C stretching), 1490 (N=N stretching), 1251 (C-O stretching), 1176 (P=N stretching), 1152 (C-N stretching), 1013 (P-O-C stretching). 1H-NMR (500 MHz, DMSO-d6) δ, ppm: 8.03 (d, J = 5.0 Hz, 2H), 8.02 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 10.0 Hz, 2H), 7.68 (d, J = 5.0 Hz, 2H), 6.79 (d, J = 5.0 Hz, 2H), 6.69 (d, J = 10.0 Hz, 2H), 6.13 (s, 2H). 13C-NMR (125 MHz, DMSO-d6) δ, ppm: 169.83, 169.05, 153.75, 153.40, 143.46, 137.28, 130.51, 126.85, 125.74, 121.37, 117.12, 113.95. 31P-NMR (500 MHz, DMSO-d6) δ, ppm: 11.22 (s, 1P). CHN elemental analysis: Calculated for C108H84N33O6P3: C: 63.81%, H: 4.16%, N: 22.74%; Found: C: 63.44%, H: 4.18%, N: 22.67%.