Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater
Abstract
1. Introduction
2. Results and Discussion
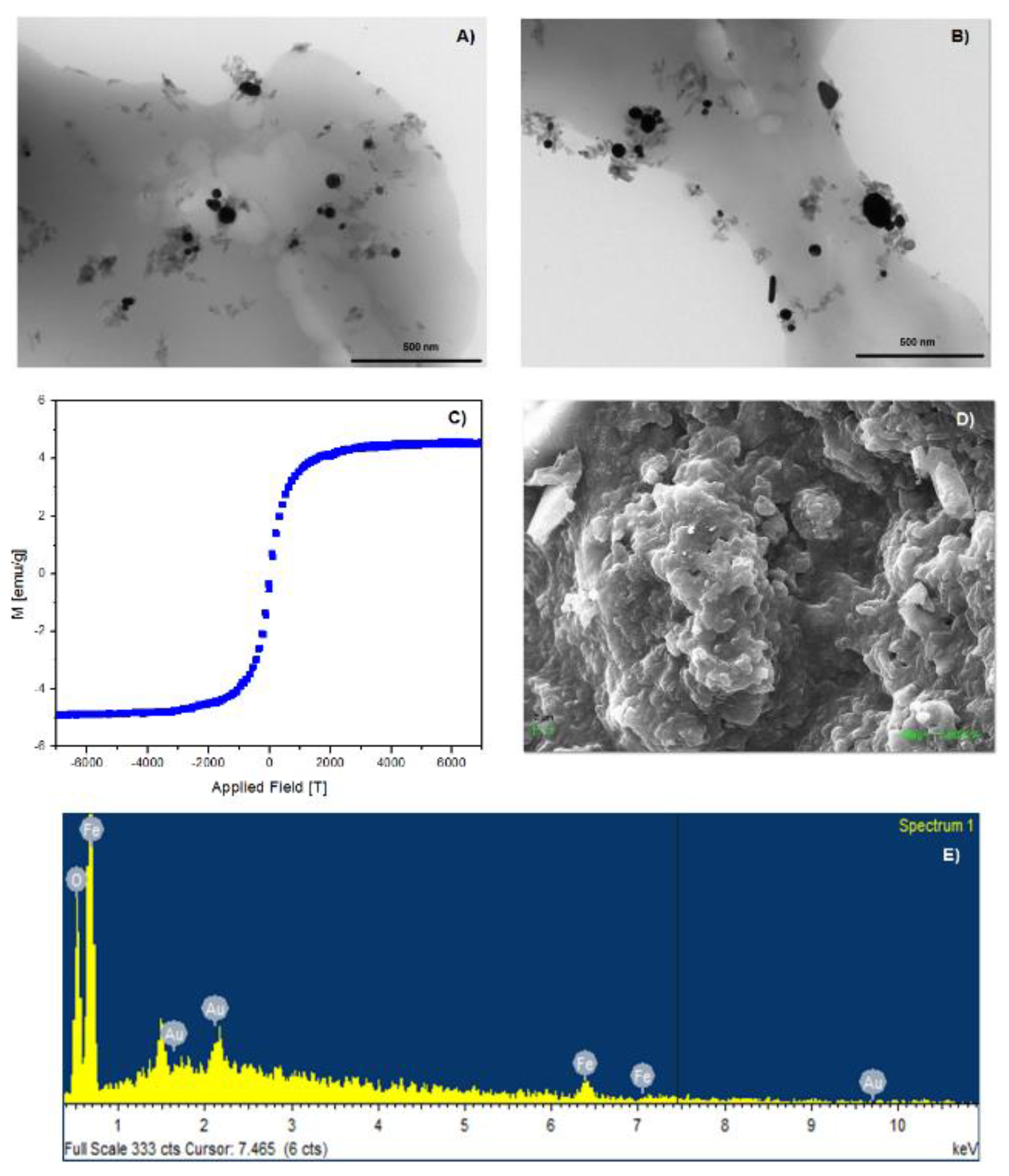
2.1. Characterization of NSs Decorated with Fe3O4 Nanoparticles
2.2. H-NMR Spectra of NS-DTF Inclusion Compound
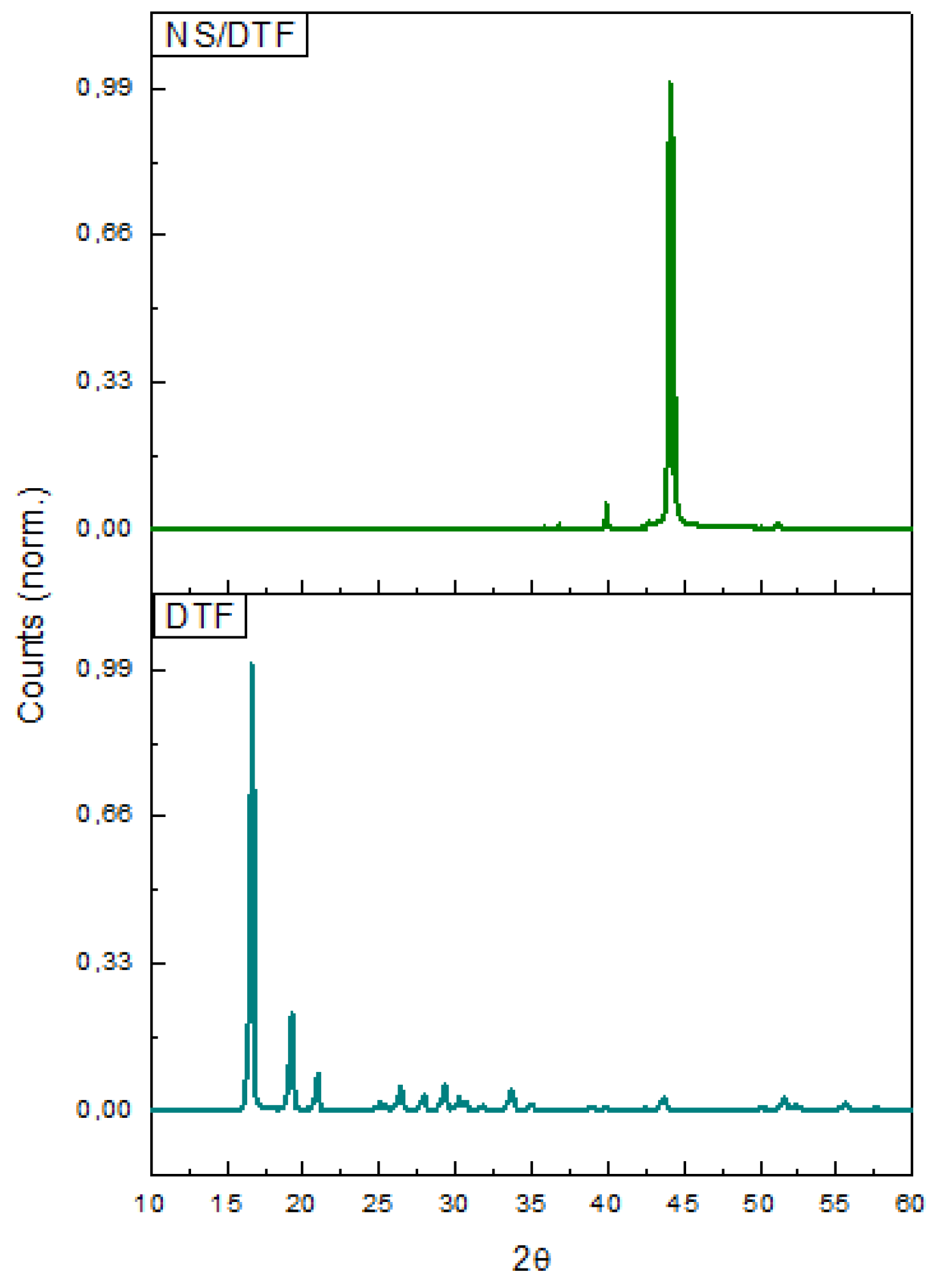
2.3. XRPD of the NS–DTF Inclusion Compound
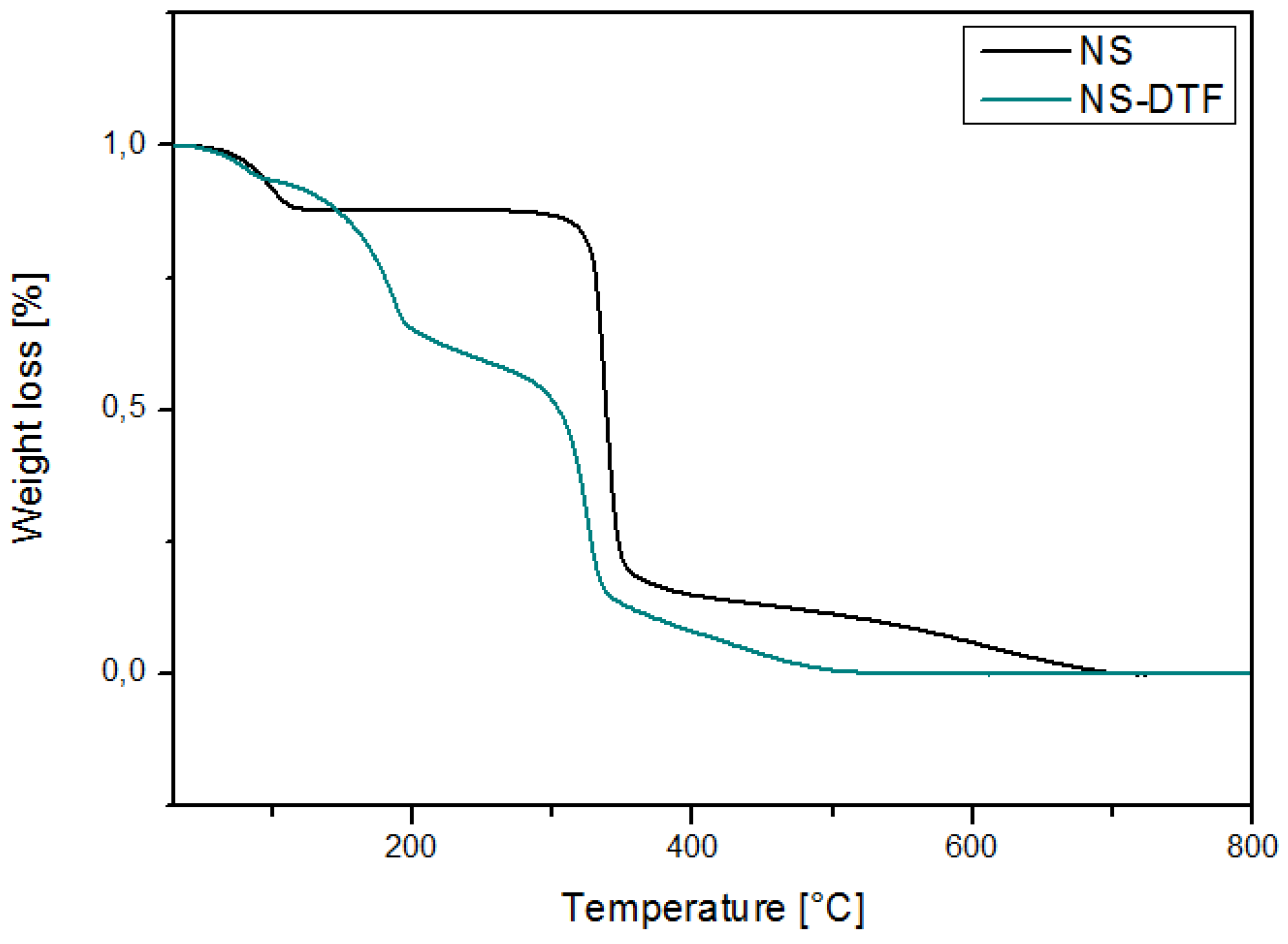
2.4. TGA of the NS-DFT Inclusion Compound
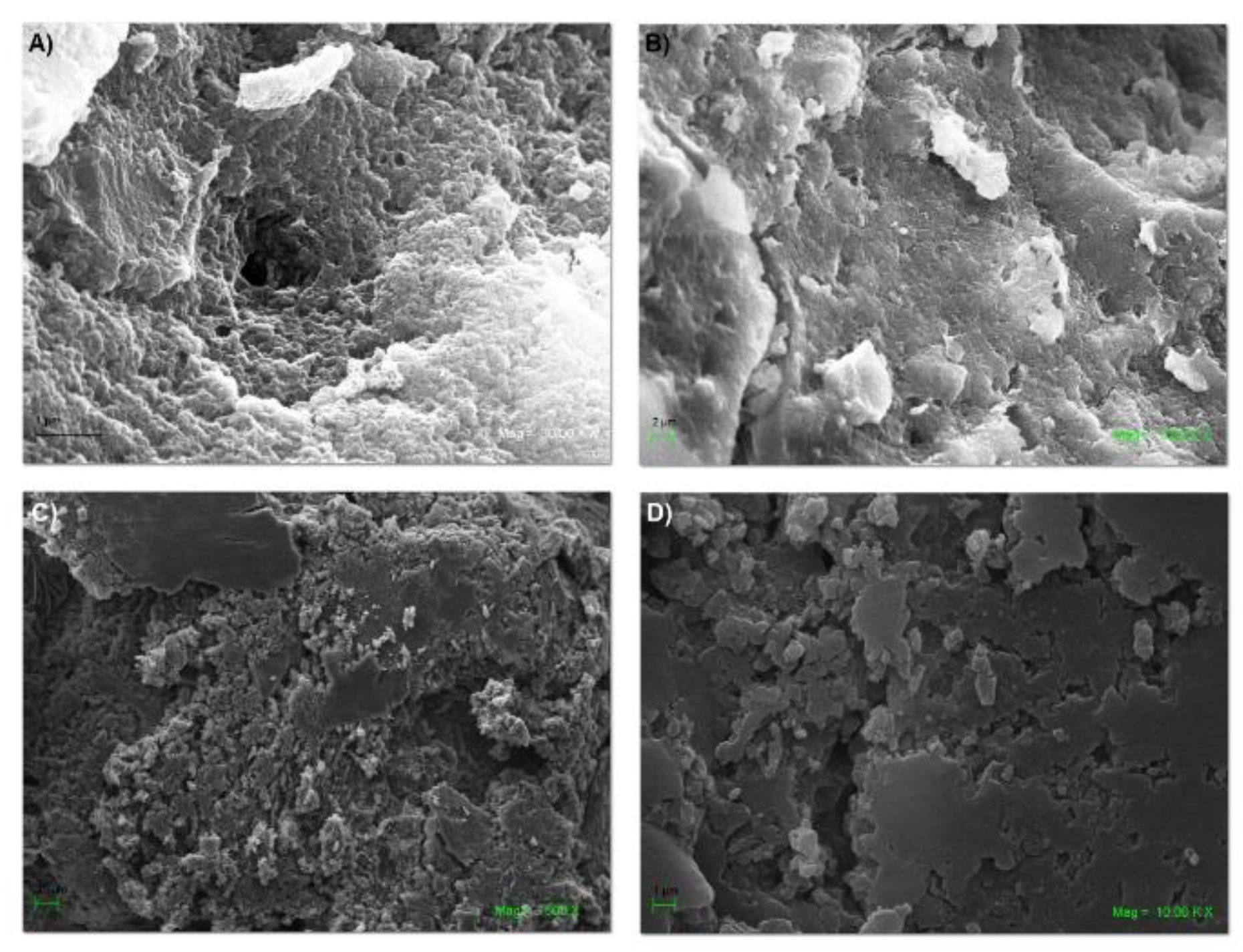
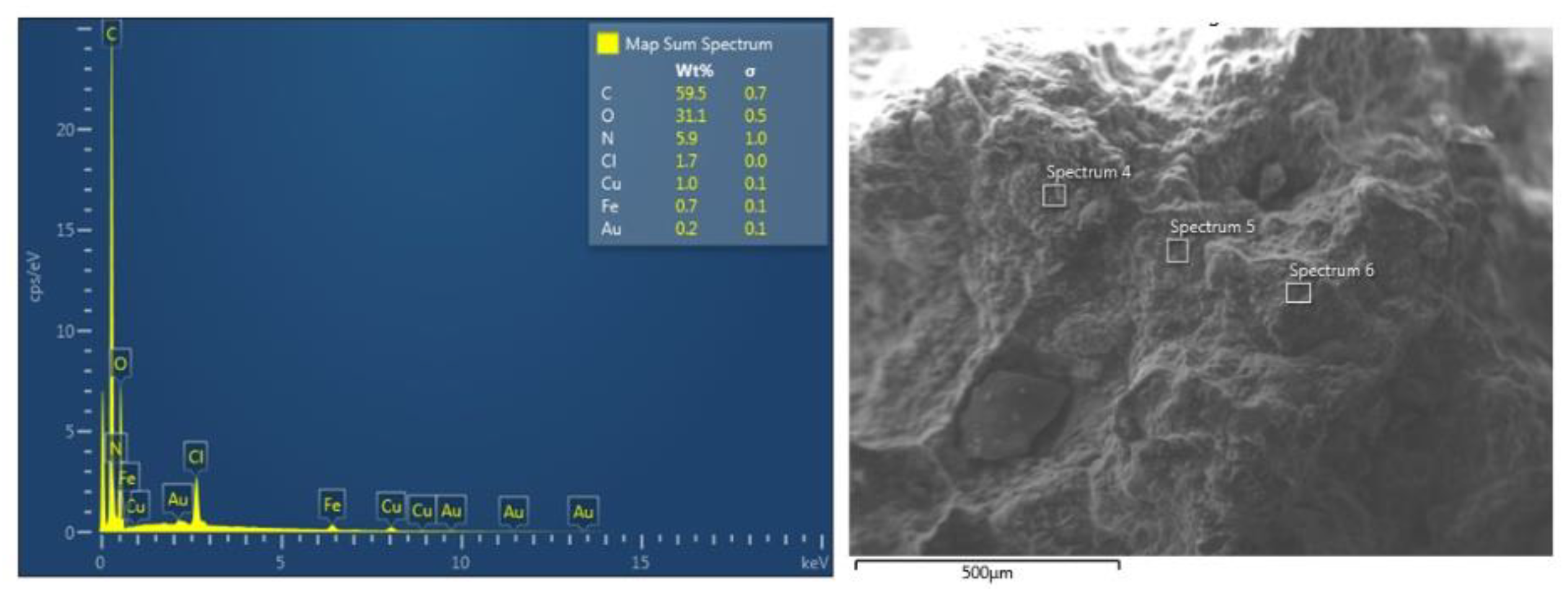
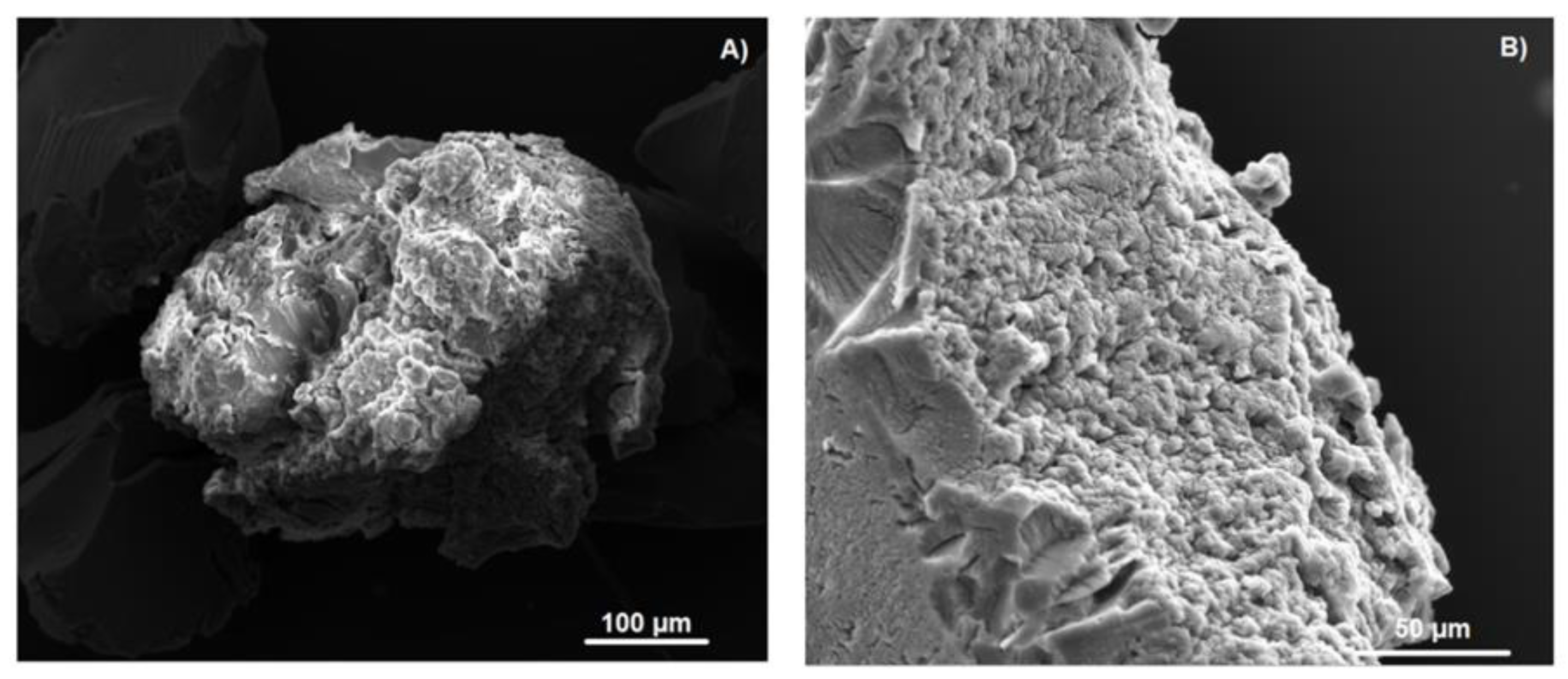
2.5. SEM and EDS of the NS–DTF Complex
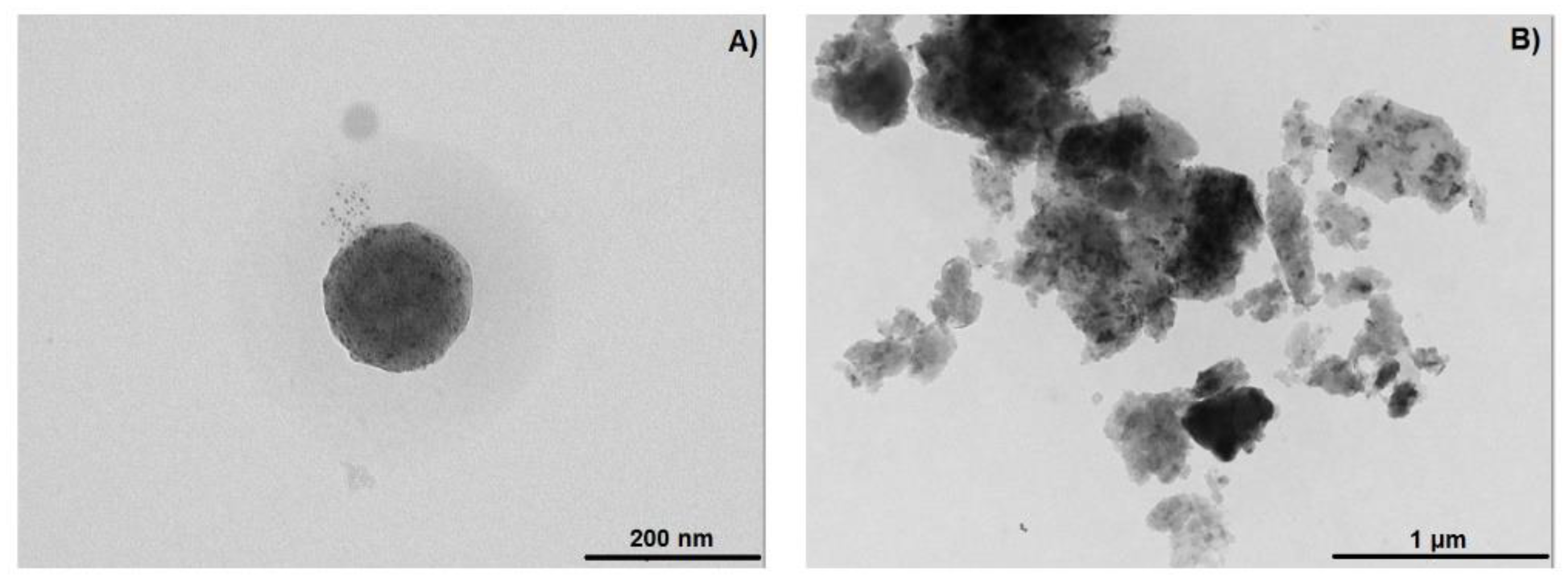
2.6. TEM of the NS–DTF Complex
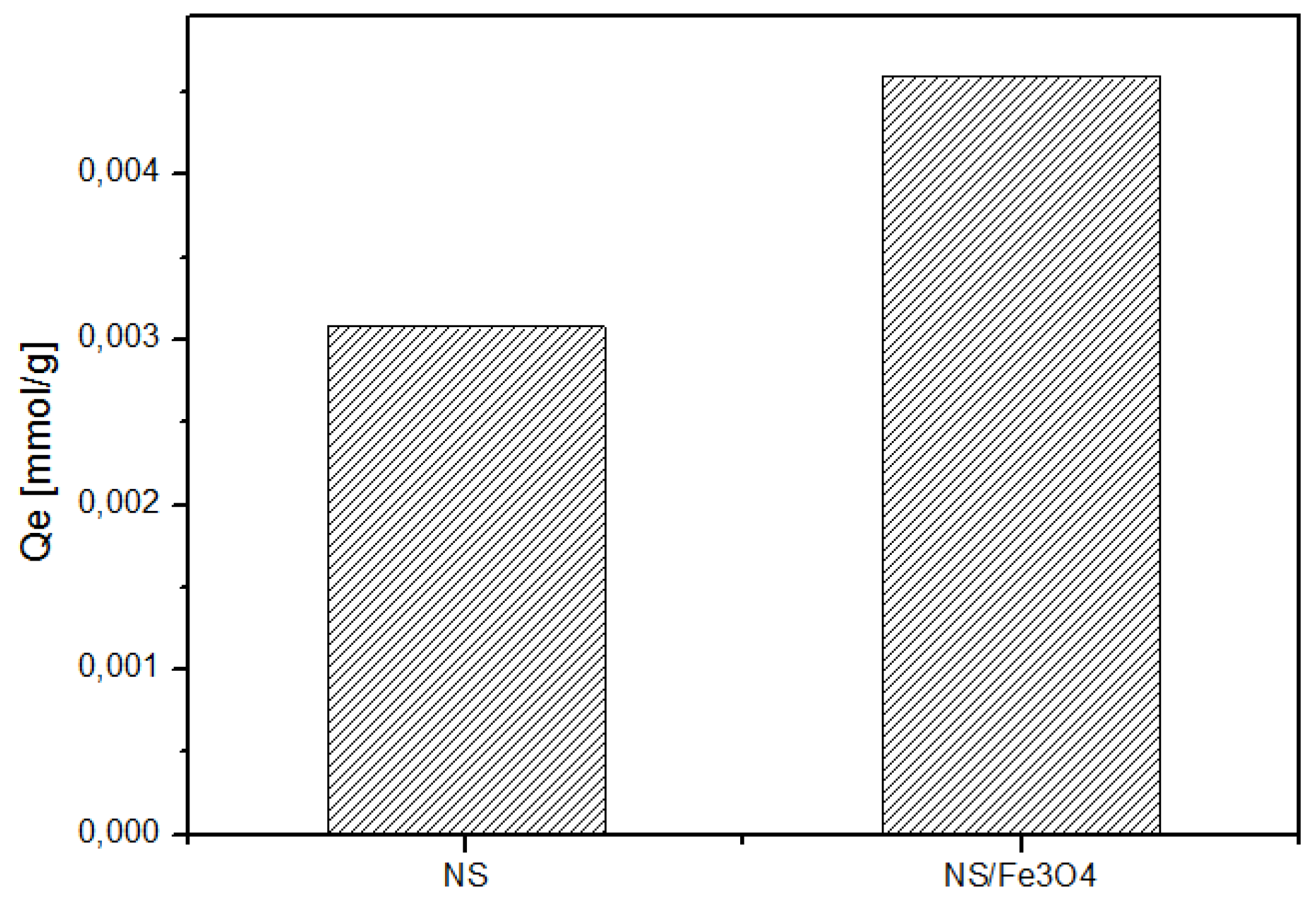
2.7. Sorption Efficiency and Loading Capacity
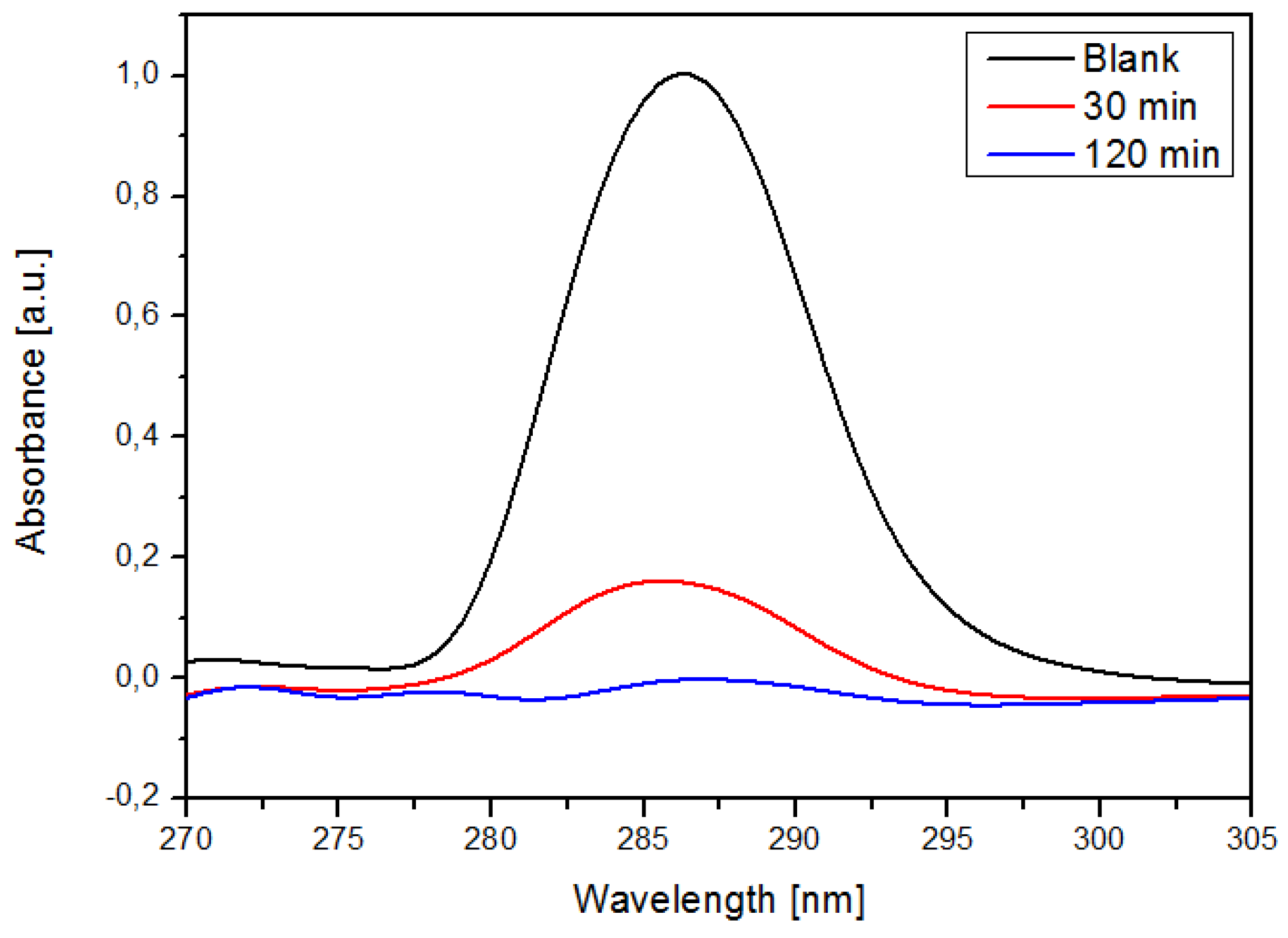
2.7.1. Molar Attenuation
2.7.2. Sorption Efficiency
2.8. Reutilization of Magnetic NSs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe3O4 Nanoparticles
3.3. Synthesis of NSs
3.4. NS/DTF Inclusion Compound
3.5. Decoration of NSs with Fe3O4 Nanoparticles
3.6. Characterization of NSs
3.7. Characterization of Fe3O4 Nanoparticles
3.8. Characterization of NS/DTF Inclusion Compound
3.9. Characterization of NSs Decorated with Fe3O4 Nanoparticles
3.10. Sorption Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl Sulfoxide |
| NSs | Nanosponges |
| β-CD | Beta Cyclodextrin |
| DTF | Dinotefuran |
| 1H-NMR | Proton nuclear magnetic resonance |
| TGA | Thermogravimetric analysis |
| XRPD | X-ray powder diffraction |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| VSM | Vibrating sample magnetometer |
| EDS | Energy Dispersive Spectroscopy |
| DPC | Diphenylcarbonate |
References
- Chambers, R.G.; Chatzimichael, K.; Tzouvelekas, V. Sub-lethal concentrations of neonicotinoid insecticides at the field level affect negatively honey yield: Evidence from a 6-year survey of Greek apiaries. PLoS ONE 2019, 14, e0215363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Nieh, J.C. The neonicotinoid imidacloprid impairs honey bee aversive learning of simulated predation. J. Exp. Boil. 2015, 218, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Muth, F.; Leonard, A.S. A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Sci. Rep. 2019, 9, 4764. [Google Scholar] [CrossRef] [PubMed]
- Matias, D.M.S.; Leventon, J.; Rau, A.-L.; Borgemeister, C.; Von Wehrden, H. A review of ecosystem service benefits from wild bees across social contexts. Ambio 2016, 46, 456–467. [Google Scholar] [CrossRef]
- Tonietto, R.K.; Larkin, D.J. Habitat restoration benefits wild bees: A meta-analysis. J. Appl. Ecol. 2017, 55, 582–590. [Google Scholar] [CrossRef]
- Donkersley, P. Trees for bees. Agric. Ecosyst. Environ. 2019, 79–83. [Google Scholar] [CrossRef]
- Li, D.; Ma, M. Nanosponges: From Inclusion Chemistry to Water Purifying Technology; Chemtech: Toronto, ON, Canada, 1999; pp. 31–37. [Google Scholar]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Investigation of the sorption properties of β-cyclodextrin-based polyurethanes with phenolic dyes and naphthenates. J. Colloid Interface Sci. 2011, 356, 217–226. [Google Scholar] [CrossRef]
- Martel, B.; Le Thuaut, P.; Bertini, S.; Bacquet, M.; Torri, G.; Morcellet, M. Grafting of cyclodextrins onto polypropylene nonwoven fabrics for the manufacture of reactive filters. III. Study of the sorption properties. J. Appl. Polym. Sci. 2002, 85, 1771–1778. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.I.; Jara, P.S. Removal of aromatic chlorinated pesticides from aqueous solution using β-cyclodextrin polymers decorated with Fe3O4 nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef]
- Trotta, F.; Tumiatti, W. Cross-Linked Polymers Based on Cyclodextrins for Removing Polluting Agents. U.S. Patent Application No. 10/510,792, 12 January 2006. [Google Scholar]
- Li, D.; Ma, M. Nanosponges for water purification. Clean Technol. Environ. Policy 2000, 2, 112–116. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Mamba, B.; Krause, R.W.; Malefetse, T.J. Removal of organic contaminants from water using nanosponge cyclodextrin polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Novel materials for environmental remediation of tailing pond waters containing naphthenic acids. Process. Saf. Environ. Prot. 2008, 86, 237–243. [Google Scholar] [CrossRef]
- Xiao, L.; Ling, Y.; Alsbaiee, A.; Li, C.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin polymer network sequesters perfluorooctanoic acid at environmentally relevant concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.R.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef]
- Salgın, S.; Salgın, U.; Vatansever, Ö. Synthesis and characterization of β-Cyclodextrin nanosponge and its application for the removal of p-Nitrophenol from Water. CSAWAC 2017, 45, 1500837. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Wu, C.P.; Tsai, H.H.; Peng, K.C.; Lin, P.Y.; Su, S.Y.; Chen, L.D.; Tsai, F.J. Effect of hydroxypropyl cyclodextrin complexation on the aqueous solubility, structure, thermal stability, antioxidant activity and tyrosinae inhibition of paeonol. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 405–411. [Google Scholar] [CrossRef]
- Peila, R.; Scordino, P.; Shanko, D.; Caldera, F.; Trotta, F.; Ferri, A. Synthesis and characterization of β-cyclodextrin nanosponges for N,N-diethyl-meta-toluamide complexation and their application on polyester fabrics. React. Funct. Polym. 2017, 119, 87–94. [Google Scholar] [CrossRef]
- Szarpak-Jankowska, A.; Burgess, C.; De Cola, L.; Huskens, J. Cyclodextrin-Modified zeolites: Host-Guest surface chemistry for the construction of multifunctional nanocontainers. Chem. Eur. J. 2013, 19, 14925–14930. [Google Scholar] [CrossRef]
- Zugasti, M.E.; Zornoza, A.; Goñi, M.D.M.; Isasi, J.R.; Vélaz, I.; Martin, C.; Sanchez, M.; Martínez-Ohárriz, M.C. Influence of soluble and insoluble cyclodextrin polymers on drug release from hydroxypropyl methylcellulose tablets. Drug Dev. Ind. Pharm. 2009, 35, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. A simple and sensitive electrochemical sensor for new neonicotinoid insecticides in gran samples based on β-cyclodextrin-graphene modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 229, 190–199. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. β-cyclodextrin Functionalized Graphene Nanosheets with high superamolecular recognition capability: Synthesis and Host-Guest Inclusion for Enchanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Zhai, X.; Yang, X.; Zhao, H.; Wang, J.; Dong, A.; Wang, Z. Application of β-cyclodextrin-reduced graphene oxide nanosheets for enchanced electrochemical sensing of the nitenpryram residue in real samples. New J. Chem. 2017, 41, 2169–2177. [Google Scholar] [CrossRef]
- Liua, G.; Li, L.; Xua, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal–organic framework preparation using magnetic graphene oxide–β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold Nanoparticles Interacting with β-Cyclodextrin–Phenylethylamine Inclusion Complex: A Ternary System for Photothermal Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 15177–15188. [Google Scholar] [CrossRef]
- Bernad-Bernad, M.-J.; Gracia-Mora, J.; Díaz, D.; Castillo-Blum, S.E. Thermodynamic study of cyclodextrins complexation with benzimidazolic antihelminitcs in different reaction media. Curr. Drug Discov. Technol. 2008, 5, 146–153. [Google Scholar] [CrossRef]
- Omer, S.M.; Ibrahim, F.; Ismail, A. Formulation and Evaluation of cyclodextrin based nanosponges of griseo-fulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharma. J. 2020, 3, 349–361. [Google Scholar] [CrossRef]
- Olteanu, A.A.; Arama, C.-C.; Bleotu, C.; Lupuleasa, D.; Monciu, C.M. Investigation of cyclodextrin based nanosponges complexes with angiotensin I converting enzyme inhibitors (Enalapril, captopril, cilazapril). Farmacia 2015, 63, 492–503. [Google Scholar]
- Samabasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Gorria, P.; Fernández-García, M.P.; Sevilla, M.; Blanco, J.A.; Fuertes, A.B. Nickel nanoparticles deposited into an activated porous caron: Synthesis, microstructure and magnetic properties. Phys. Status Solidi Rapid. 2009, 3, 4–6. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Lemus, J.; Palomares, F.; Morales, M.; Mazario, E. Superparamagnetic nanosorbent for water purification: Assessment of the adsorptive removal of lead and methyl orange from aqueous solutions. Sci. Total. Environ. 2020, 711, 134644. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, X.C.; Yang, X.; Zhao, H.T.; Dong, A.J.; Zhang, H.; Wang, J.; Liu, G.Y. Rapid and sensitive determination of dinotefuran residue based on electrochemical enchancement of β-Cyclodextrin-Graphene composite. Electroanalysis 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, S. Synthesis, propierties and potential applications of multicomponent magnetic nanoparticles. Adv. Funct. Mater. 2008, 18, 391–400. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Sun, S.; Hou, Y.; Gao, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Trotta, F.; Cavalli, R.; Tumiatti, V.; Zerbinati, O.; Roggero, C.; Vallero, R. Ultrasound Assisted Synthesis of Cyclodextrin Based Nanosponges. U.S. Patent WO 2006/002814 A1, 22 January 2006. [Google Scholar]
- Silva, N.; Moris, S.; Díaz, M.; Yutronic, N.; Lang, E.; Chornik, B.; Kogan, M.J.; Jara, P.S. Evidence of the disassembly of α-Cyclodextrin-octylamine inclusion compounds conjugated to gold nanoparticles via Thermal and Photothermal Effects. Molecules 2016, 21, 1444. [Google Scholar] [CrossRef]
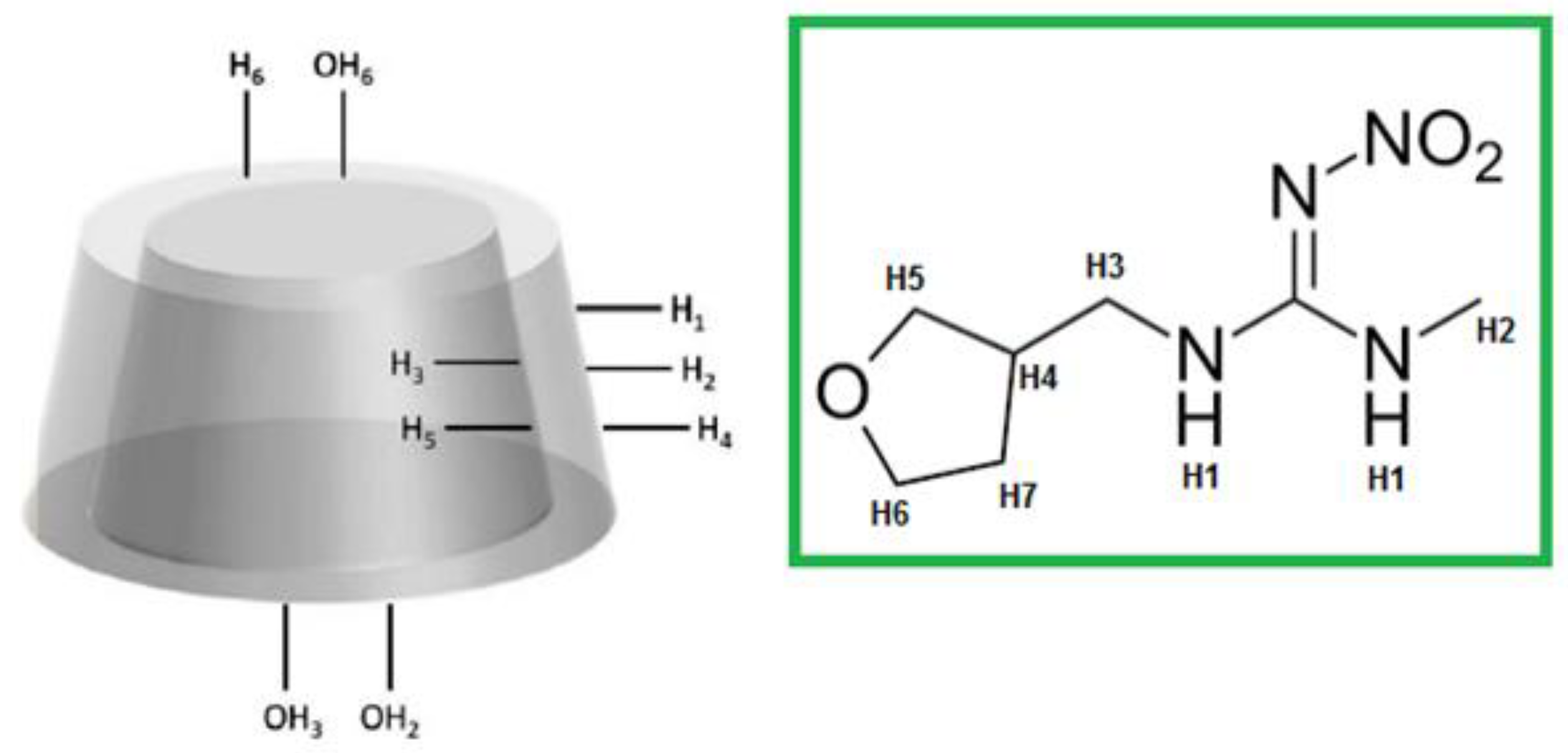



| H DTF | δ DTF (ppm) | δ NS–DTF (ppm) | Δδ (ppm) |
|---|---|---|---|
| H1 | 2.035 | 2.026 | −0.009 |
| H2 | 2.811 | 2.809 | −0.003 |
| H3 | 1.440 | 1.437 | −0.003 |
| H4 | 1.927 | 1.922 | −0.005 |
| H5 | 3.827 | 3.825 | −0.002 |
| H6 | 3.572 | 3.569 | −0.003 |
| H7 | 1.985 | 1.982 | −0.003 |
| H NS | δ NS (ppm) | δ NS–DTF (ppm) | Δδ (ppm) |
| OH 2 | 5.704 | 5.717 | 0.013 |
| OH 3 | 5.670 | 5.671 | 0.001 |
| OH 6 | 4.440 | 4.447 | 0.007 |
| H 1 | 4.827 | 4.830 | 0.003 |
| H 3 | 3.627 | 3.635 | 0.008 |
| H 5 | 3.572 | 3.575 | 0.003 |
| H 6 | 3.572 | 3.575 | 0.003 |
| Sample | First Decomposition | Second Decomposition | Third Decomposition |
|---|---|---|---|
| NS | 113.4 °C | 344.8 °C | - |
| NS-DTF | 115.1 °C | 185.4 °C | 341.7 °C |
| Sample | Contact Time [min] | Ce [mM] | Qe [mmol/g] | Uptake |
|---|---|---|---|---|
| DTF | 30 | 2.83 × 10−3 | 3.59 × 10−3 | 71.7% |
| DTF | 120 | 9.65 × 10−4 | 4.53 × 10−3 | 90.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, S.; Yutronic, N.; Jara, P. Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. Int. J. Mol. Sci. 2020, 21, 4079. https://doi.org/10.3390/ijms21114079
Salazar S, Yutronic N, Jara P. Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. International Journal of Molecular Sciences. 2020; 21(11):4079. https://doi.org/10.3390/ijms21114079
Chicago/Turabian StyleSalazar, Sebastián, Nicolás Yutronic, and Paul Jara. 2020. "Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater" International Journal of Molecular Sciences 21, no. 11: 4079. https://doi.org/10.3390/ijms21114079
APA StyleSalazar, S., Yutronic, N., & Jara, P. (2020). Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. International Journal of Molecular Sciences, 21(11), 4079. https://doi.org/10.3390/ijms21114079





