From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
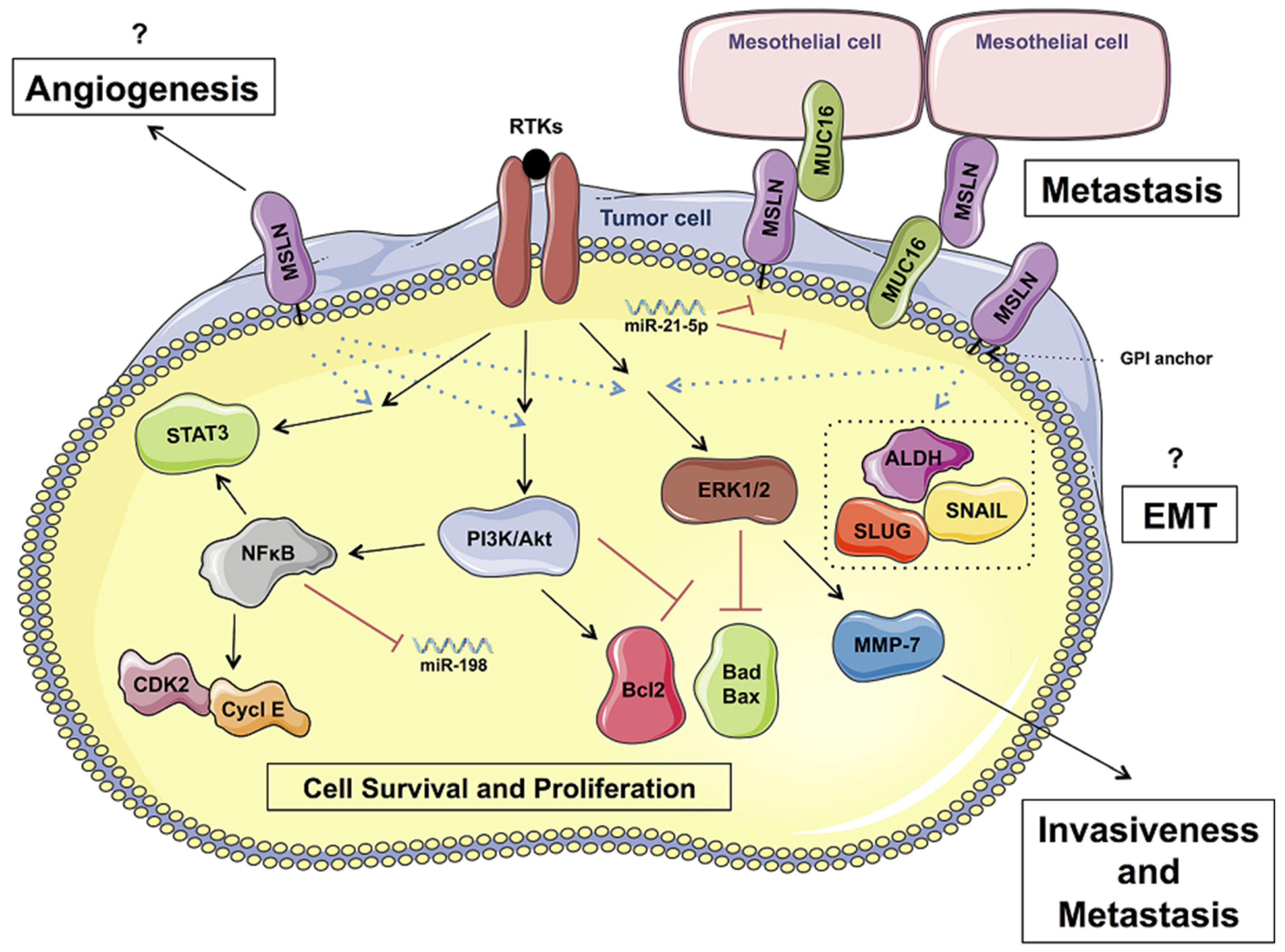
2. Role of MSLN in PDAC Progression
2.1. Structure of MSLN and Physiological Functions
2.2. Expression of MSLN in PDAC
2.3. Cell Proliferation and Anti-Apoptosis
2.4. Promoting Tumor Invasion and Metastasis
2.5. Resistance to Chemotherapy
2.6. Genetic Regulation of MSLN Expression in Tumor Cells
3. Predictive Value of MSLN and Anti-MSLN Diagnosis-Dedicated Agents in PDAC
3.1. Predictive Value of MSLN Expression
3.2. SMRP Assay
3.3. Imaging Probes for the Phenotyping of MSLN-Expressing Tumors
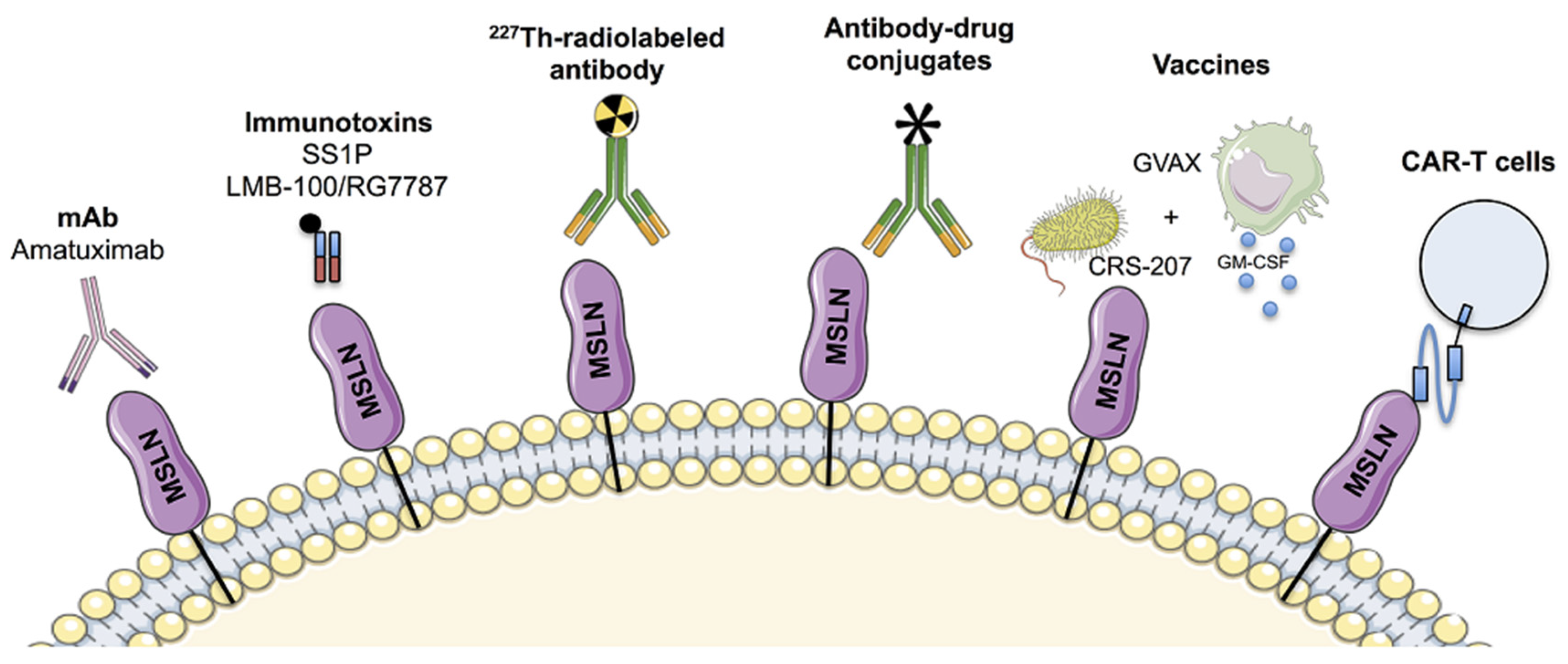
4. Anti-MSLN Targeting Drugs in Development
4.1. Antibodies-Based Approach
4.1.1. Immunotoxins SS1P and LMB-100/RG7787
4.1.2. Monoclonal Antibody: Amatuximab
4.1.3. Antibody-Drug Conjugates
4.1.4. 227Th-Radiolabeled Antibody
4.2. Cancer Vaccines
4.3. CAR-T Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | Antibody drug-conjugates |
| CAR-T | Chimeric antigen receptor T cells |
| Fab | Fragment antigen binding |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GPI | Glycosylphosphatidylinositol |
| GVAX | GM-CSF gene-transfected tumor cell vaccine |
| mAb | Monoclonal antibody |
| MAV | Metabolic Active Volume |
| MPF | Megakaryocyte-potentiating factor |
| MSLN | Mesothelin |
| MUC16 | Mucin 16 |
| OCT-2 | Homeobox transcription factors POU2F2 |
| OS | Overall survival |
| PD-1 | programm death 1 |
| PD-L1 | programm death ligand 1 |
| PD | Progressive disease |
| PDAC | Pancreatic ductal adenocarcinoma |
| PR | Partial response |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RTK | Receptor Tyrosine Kinase |
| SD | Stable disease |
| SMRP | Serum mesothelin-related peptide |
| Tat | Targeted alpha therapy |
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Quante, A.S.; Ming, C.; Rottmann, M.; Engel, J.; Boeck, S.; Heinemann, V.; Westphalen, C.B.; Strauch, K. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016, 5, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Rahn, S.; Zimmermann, V.; Viol, F.; Knaack, H.; Stemmer, K.; Peters, L.; Lenk, L.; Ungefroren, H.; Saur, D.; Schäfer, H.; et al. Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018, 415, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Font-Burgada, J.; Sun, B.; Karin, M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016, 23, 48–62. [Google Scholar] [CrossRef]
- Delitto, D.; Zhang, D.; Han, S.; Black, B.S.; Knowlton, A.E.; Vlada, A.C.; Sarosi, G.A.; Behrns, K.E.; Thomas, R.M.; Lu, X.; et al. Nicotine Reduces Survival via Augmentation of Paracrine HGF-MET Signaling in the Pancreatic Cancer Microenvironment. Clin. Cancer Res. 2016, 22, 1787–1799. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Huang, H.; Jiang, Q.; Zhao, D.; Tian, Y.; Ma, J.; Yuan, W.; Sun, Y.; Che, X.; et al. Effects of alcohol drinking and smoking on pancreatic ductal adenocarcinoma mortality: A retrospective cohort study consisting of 1783 patients. Sci. Rep. 2017, 7, 9572. [Google Scholar] [CrossRef]
- Pihlak, R.; Valle, J.W.; McNamara, M.G. Germline mutations in pancreatic cancer and potential new therapeutic options. Oncotarget 2017, 8, 73240–73257. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 2401–2409. [Google Scholar] [CrossRef]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Shahbazi, R.; Ulubayram, K.; Ozpolat, B. Targeted Therapies for Pancreatic Cancer and Hurdles Ahead. Anticancer Res. 2018, 38, 6591–6606. [Google Scholar] [CrossRef] [PubMed]
- Manji, G.A.; Olive, K.P.; Saenger, Y.M.; Oberstein, P. Current and Emerging Therapies in Metastatic Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Talar-Wojnarowska, R.; Dąbrowski, A.; Degowska, M.; Durlik, M.; Gąsiorowska, A.; Głuszek, S.; Jurkowska, G.; Kaczka, A.; Lampe, P.; et al. Diagnostic and therapeutic recommendations in pancreatic ductal adenocarcinoma. Recommendations of the Working Group of the Polish Pancreatic Club. Prz. Gastroenterol. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Hilmi, M.; Bartholin, L.; Neuzillet, C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J. Gastroenterol. 2018, 24, 2137–2151. [Google Scholar] [CrossRef]
- Patnaik, A.; Kang, S.P.; Rasco, D.; Papadopoulos, K.P.; Elassaiss-Schaap, J.; Beeram, M.; Drengler, R.; Chen, C.; Smith, L.; Espino, G.; et al. Phase I Study of Pembrolizumab (MK-3475; Anti–PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015, 21, 4286–4293. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef]
- Nichetti, F.; Marra, A.; Corti, F.; Guidi, A.; Raimondi, A.; Prinzi, N.; de Braud, F.; Pusceddu, S. The Role of Mesothelin as a Diagnostic and Therapeutic Target in Pancreatic Ductal Adenocarcinoma: A Comprehensive Review. Target Oncol. 2018, 13, 333–351. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Hassan, R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014, 74, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Pastan, I.; Willingham, M.C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer 1992, 50, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef]
- Hassan, R.; Bera, T.; Pastan, I. Mesothelin: A new target for immunotherapy. Clin. Cancer Res. 2004, 10, 3937–3942. [Google Scholar] [CrossRef]
- Hassan, R.; Ho, M. Mesothelin targeted cancer immunotherapy. Eur. J. Cancer 2008, 44, 46–53. [Google Scholar] [CrossRef]
- Sathyanarayana, B.K.; Hahn, Y.; Patankar, M.S.; Pastan, I.; Lee, B. Mesothelin, Stereocilin, and Otoancorin are predicted to have superhelical structures with ARM-type repeats. BMC Struct. Biol. 2009, 9, 1. [Google Scholar] [CrossRef]
- Bera, T.K.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell. Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef]
- Gubbels, J.A.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef]
- Haridas, D.; Ponnusamy, M.P.; Chugh, S.; Lakshmanan, I.; Seshacharyulu, P.; Batra, S.K. MUC16: Molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014, 28, 4183–4199. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Wang, P.; Liang, S.; Iwaisako, K.; Liu, X.; Xu, J.; Zhang, M.; Sun, M.; Cong, M.; Karin, D.; et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Invest. 2017, 127, 1254–1270. [Google Scholar] [CrossRef]
- Muniyan, S.; Haridas, D.; Chugh, S.; Rachagani, S.; Lakshmanan, I.; Gupta, S.; Seshacharyulu, P.; Smith, L.M.; Ponnusamy, M.P.; Batra, S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer 2016, 7, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Marcos-Silva, L.; Ricardo, S.; Ponte, F.; Costa, A.; Lopes, J.M.; David, L. Peritoneal dissemination of ovarian cancer: Role of MUC16-mesothelin interaction and implications for treatment. Expert Rev. Anticancer Ther. 2018, 18, 177–186. [Google Scholar] [CrossRef]
- Chang, K.; Pai, L.H.; Batra, J.K.; Pastan, I.; Willingham, M.C. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992, 52, 181–186. [Google Scholar]
- Tchou, J.; Wang, L.-C.; Selven, B.; Zhang, H.; Conejo-Garcia, J.; Borghaei, H.; Kalos, M.; Vondeheide, R.H.; Albelda, S.M.; June, C.H.; et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res. Treat. 2012, 133, 799–804. [Google Scholar] [CrossRef]
- Tozbikian, G.; Brogi, E.; Kadota, K.; Catalano, J.; Akram, M.; Patil, S.; Ho, A.Y.; Reis-Filho, J.S.; Weigelt, B.; Norton, L.; et al. Mesothelin Expression in Triple Negative Breast Carcinomas Correlates Significantly with Basal-Like Phenotype, Distant Metastases and Decreased Survival. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Should anti-mesothelin therapies be explored in lung cancer? Expert Rev. Anticancer Ther. 2016, 16, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Wang, J.; Zhang, T.; Guo, Y.; Chang, H.; Wang, S.; Zhu, B. Overexpression of Mesothelin in Pancreatic Ductal Adenocarcinoma (PDAC). Int. J. Med. Sci. 2020, 17, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Moon, E.A.; Howell, V.M.; Mehta, S.; Pavlakis, N.; Chan, D.; Ahadi, M.S.; Gill, A.J.; Samra, J.; Mittal, A. Tissue biomarker panel as a surrogate marker for squamous subtype of pancreatic cancer. Eur. J Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Nahm, C.B.; Turchini, J.; Jamieson, N.; Moon, E.; Sioson, L.; Itchins, M.; Arena, J.; Colvin, E.; Howell, V.M.; Pavlakis, N.; et al. Biomarker panel predicts survival after resection in pancreatic ductal adenocarcinoma: A multi-institutional cohort study. Eur. J. Surg. Oncol. 2019, 45, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Sopha, S.C.; Gopal, P.; Merchant, N.B.; Revetta, F.L.; Gold, D.V.; Washington, K.; Shi, C. Diagnostic and therapeutic implications of a novel immunohistochemical panel detecting duodenal mucosal invasion by pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2013, 6, 2476–2486. [Google Scholar]
- Montemagno, C.; Cassim, S.; Trichanh, D.; Savary, C.; Pouyssegur, J.; Pagès, G.; Fagret, D.; Broisat, A.; Ghezzi, C. 99mTc-A1 as a Novel Imaging Agent Targeting Mesothelin-Expressing Pancreatic Ductal Adenocarcinoma. Cancers 2019, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bharadwaj, U.; Zhang, R.; Zhang, S.; Mu, H.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol. Cancer Ther. 2008, 7, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Li, M.; Chen, C.; Yao, Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol. Cancer Res. 2008, 6, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis 2011, 32, 1013–1024. [Google Scholar] [CrossRef]
- Wang, K.; Bodempudi, V.; Liu, Z.; Borrego-Diaz, E.; Yamoutpoor, F.; Meyer, A.; Woo, R.A.; Pan, W.; Dudek, A.Z.; Olyaee, M.S.; et al. Inhibition of Mesothelin as a Novel Strategy for Targeting Cancer Cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin confers pancreatic cancer cell resistance to TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and IL-6/Mcl-1 overexpression. Mol. Cancer 2011, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jia, W.; Tang, Y.; Zhao, H.; Jiang, Y.; Sun, S. Mesothelin regulates growth and apoptosis in pancreatic cancer cells through p53-dependent and -independent signal pathway. J. Exp. Clin. Cancer Res. 2012, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Uehara, N.; Matsuoka, Y.; Tsubura, A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol. Cancer Res. 2008, 6, 186–193. [Google Scholar] [CrossRef]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef]
- Shimizu, A.; Hirono, S.; Tani, M.; Kawai, M.; Okada, K.-I.; Miyazawa, M.; Kitahata, Y.; Nakamura, Y.; Noda, T.; Yokoyama, S.; et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012, 103, 739–746. [Google Scholar] [CrossRef]
- Chen, S.-H.; Hung, W.-C.; Wang, P.; Paul, C.; Konstantopoulos, K. Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation. Sci. Rep. 2013, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, L.; Riedel, H.; Wang, K.; Yang, Y.; Dinu, C.Z.; Rojanasakul, Y. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol. Cancer 2017, 16. [Google Scholar] [CrossRef]
- Avula, L.R.; Rudloff, M.; El-Behaedi, S.; Arons, D.; Albalawy, R.; Chen, X.; Zhang, X.; Alewine, C. Mesothelin enhances tumor vascularity in newly forming pancreatic peritoneal metastases. Mol. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Melaiu, O.; Stebbing, J.; Lombardo, Y.; Bracci, E.; Uehara, N.; Bonotti, A.; Cristaudo, A.; Foddis, R.; Mutti, L.; Barale, R.; et al. MSLN Gene Silencing Has an Anti-Malignant Effect on Cell Lines Overexpressing Mesothelin Deriving from Malignant Pleural Mesothelioma. PLoS ONE 2014, 9, e85935. [Google Scholar] [CrossRef]
- Cheng, W.-F.; Huang, C.-Y.; Chang, M.-C.; Hu, Y.-H.; Chiang, Y.-C.; Chen, Y.-L.; Hsieh, C.-Y.; Chen, C.-A. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br. J. Cancer 2009, 100, 1144–1153. [Google Scholar] [CrossRef]
- Ren, Y.R.; Patel, K.; Paun, B.C.; Kern, S.E. Structural Analysis of the Cancer-specific Promoter in Mesothelin and in Other Genes Overexpressed in Cancers. J. Biol. Chem. 2011, 286, 11960–11969. [Google Scholar] [CrossRef]
- De Santi, C.; Vencken, S.; Blake, J.; Haase, B.; Benes, V.; Gemignani, F.; Landi, S.; Greene, C.M. Identification of MiR-21-5p as a Functional Regulator of Mesothelin Expression Using MicroRNA Capture Affinity Coupled with Next Generation Sequencing. PLoS ONE 2017, 12, e0170999. [Google Scholar] [CrossRef] [PubMed]
- Marin-Muller, C.; Li, D.; Bharadwaj, U.; Li, M.; Chen, C.; Hodges, S.E.; Fisher, W.E.; Mo, Q.; Hung, M.-C.; Yao, Q. A Tumorigenic Factor Interactome Connected Through Tumor Suppressor MicroRNA-198 in Human Pancreatic Cancer. Clin. Cancer Res. 2013, 19, 5901–5913. [Google Scholar] [CrossRef]
- Wu, X.; Li, D.; Liu, L.; Liu, B.; Liang, H.; Yang, B. Serum soluble mesothelin-related peptide (SMRP): A potential diagnostic and monitoring marker for epithelial ovarian cancer. Arch. Gynecol. Obs. 2014, 289, 1309–1314. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Creaney, J.; Lake, R.; Nowak, A.; Musk, A.W.; de Klerk, N.; Winzell, P.; Hellstrom, K.E.; Hellstrom, I. Soluble mesothelin-related protein—A blood test for mesothelioma. Lung Cancer 2005, 49 (Suppl. 1), S109–S111. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.L.; Geschwindt, R.D.; Glover, C.L.; Tran, L.; Hellstrom, I.; Hellstrom, K.-E.; Miller, M.C.; Verch, T.; Allard, W.J.; Pass, H.I.; et al. MESOMARK: A potential test for malignant pleural mesothelioma. Clin. Chem. 2007, 53, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.; Zhang, J.; Hollevoet, K.; Steinberg, S.M.; Pastan, I.; Onda, M.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Hassan, R. Serum mesothelin and megakaryocyte potentiating factor in pancreatic and biliary cancers. Clin. Chem. Lab. Med. 2012, 50, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Wu, C.; Brechbiel, M.W.; Margulies, I.; Kreitman, R.J.; Pastan, I. 111Indium-labeled monoclonal antibody K1: Biodistribution study in nude mice bearing a human carcinoma xenograft expressing mesothelin. Int. J. Cancer 1999, 80, 559–563. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sasaki, T.; Takenaka, F.; Yakushiji, H.; Fujii, Y.; Kishi, Y.; Kita, S.; Shen, L.; Kumon, H.; Matsuura, E. A Novel PET Imaging Using 64Cu-Labeled Monoclonal Antibody against Mesothelin Commonly Expressed on Cancer Cells. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef]
- Ter Weele, E.J.; Terwisscha van Scheltinga, A.G.T.; Kosterink, J.G.W.; Pot, L.; Vedelaar, S.R.; Lamberts, L.E.; Williams, S.P.; Lub-de Hooge, M.N.; de Vries, E.G.E. Imaging the distribution of an antibody-drug conjugate constituent targeting mesothelin with 89Zr and IRDye 800CW in mice bearing human pancreatic tumor xenografts. Oncotarget 2015, 6, 42081–42090. [Google Scholar] [CrossRef][Green Version]
- Van Scheltinga, A.G.T.T.; Ogasawara, A.; Pacheco, G.; Vanderbilt, A.N.; Tinianow, J.N.; Gupta, N.; Li, D.; Firestein, R.; Marik, J.; Scales, S.J.; et al. Preclinical Efficacy of an Antibody–Drug Conjugate Targeting Mesothelin Correlates with Quantitative 89Zr-ImmunoPET. Mol. Cancer Ther. 2017, 16, 134–142. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Menke-van der Houven van Oordt, C.W.; ter Weele, E.J.; Bensch, F.; Smeenk, M.M.; Voortman, J.; Hoekstra, O.S.; Williams, S.P.; Fine, B.M.; Maslyar, D.; et al. ImmunoPET with Anti-Mesothelin Antibody in Patients with Pancreatic and Ovarian Cancer before Anti-Mesothelin Antibody-Drug Conjugate Treatment. Clin. Cancer Res. 2016, 22, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, L.; Thomas, A.; Adler, S.; Mena, E.; Kurdziel, K.; Maltzman, J.; Wallin, B.; Hoffman, K.; Pastan, I.; Paik, C.H.; et al. Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using single photon emission computed tomography-computed tomography (SPECT-CT) imaging. Oncotarget 2015, 6, 4496–4504. [Google Scholar] [CrossRef] [PubMed]
- Yakushiji, H.; Kobayashi, K.; Takenaka, F.; Kishi, Y.; Shinohara, M.; Akehi, M.; Sasaki, T.; Ohno, E.; Matsuura, E. Novel single-chain variant of antibody against mesothelin established by phage library. Cancer Sci. 2019, 110, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Hassan, R.; Zhang, J.; Wang, Q.-C.; Onda, M.; Bera, T.; Pastan, I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin. Cancer Res. 2005, 11, 3814–3820. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; Fitzgerald, D.J.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 2006, 6, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Williams-Gould, J.; Steinberg, S.M.; Liewehr, D.J.; Yokokawa, J.; Tsang, K.Y.; Surawski, R.J.; Scott, T.; Camphausen, K. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin. Cancer Res. 2006, 12, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Mossoba, M.E.; Onda, M.; Taylor, J.; Massey, P.R.; Treadwell, S.; Sharon, E.; Hassan, R.; Pastan, I.; Fowler, D.H. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin. Cancer Res. 2011, 17, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Bullock, S.; Premkumar, A.; Kreitman, R.J.; Kindler, H.; Willingham, M.C.; Pastan, I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007, 13, 5144–5149. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Sharon, E.; Thomas, A.; Zhang, J.; Ling, A.; Miettinen, M.; Kreitman, R.J.; Steinberg, S.M.; Hollevoet, K.; Pastan, I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014, 120, 3311–3319. [Google Scholar] [CrossRef]
- Hassan, R.; Miller, A.C.; Sharon, E.; Thomas, A.; Reynolds, J.C.; Ling, A.; Kreitman, R.J.; Miettinen, M.M.; Steinberg, S.M.; Fowler, D.H.; et al. Major Cancer Regressions in Mesothelioma After Treatment with an Anti-Mesothelin Immunotoxin and Immune Suppression. Sci. Transl. Med. 2013, 5, 208ra147. [Google Scholar] [CrossRef]
- Hollevoet, K.; Mason-Osann, E.; Liu, X.; Imhof-Jung, S.; Niederfellner, G.; Pastan, I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol. Cancer Ther. 2014, 13, 2040–2049. [Google Scholar] [CrossRef]
- Zhang, J.; Khanna, S.; Jiang, Q.; Alewine, C.; Miettinen, M.; Pastan, I.; Hassan, R. Efficacy of Anti-mesothelin Immunotoxin RG7787 plus Nab-Paclitaxel against Mesothelioma Patient-Derived Xenografts and Mesothelin as a Biomarker of Tumor Response. Clin. Cancer Res. 2017, 23, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Kolyvas, E.; Rudloff, M.; Poruchynsky, M.; Landsman, R.; Hollevoet, K.; Venzon, D.; Alewine, C. Mesothelin-targeted immunotoxin RG7787 has synergistic anti-tumor activity when combined with taxanes. Oncotarget 2017, 8, 9189–9199. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Zhou, Q.; Hassan, R.; Pastan, I. Panbinostat decreases cFLIP and enhances killing of cancer cells by immunotoxin LMB-100 by stimulating the extrinsic apoptotic pathway. Oncotarget 2017, 8, 87307–87316. [Google Scholar] [CrossRef]
- Hassan, R.; Ebel, W.; Routhier, E.L.; Patel, R.; Kline, J.B.; Zhang, J.; Chao, Q.; Jacob, S.; Turchin, H.; Gibbs, L.; et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007, 7, 20. [Google Scholar]
- Mizukami, T.; Kamachi, H.; Fujii, Y.; Matsuzawa, F.; Einama, T.; Kawamata, F.; Kobayashi, N.; Hatanaka, Y.; Taketomi, A. The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model. Oncotarget 2018, 9, 33844–33852. [Google Scholar] [CrossRef][Green Version]
- Hassan, R.; Cohen, S.J.; Phillips, M.; Pastan, I.; Sharon, E.; Kelly, R.J.; Schweizer, C.; Weil, S.; Laheru, D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin. Cancer Res. 2010, 16, 6132–6138. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Kindler, H.L.; Jahan, T.; Bazhenova, L.; Reck, M.; Thomas, A.; Pastan, I.; Parno, J.; O’Shannessy, D.J.; Fatato, P.; et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin. Cancer Res. 2014, 20, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Hussein, Z.; Hassan, R.; Wustner, J.; Maltzman, J.D.; Wallin, B.A. Population pharmacokinetics and exposure-response relationship of amatuximab, an anti-mesothelin monoclonal antibody, in patients with malignant pleural mesothelioma and its application in dose selection. Cancer Chemother. Pharmacol. 2016, 77, 733–743. [Google Scholar] [CrossRef]
- Hassan, R.; Blumenschein, G.R.; Moore, K.N.; Santin, A.D.; Kindler, H.L.; Nemunaitis, J.J.; Seward, S.M.; Thomas, A.; Kim, S.K.; Rajagopalan, P.; et al. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 2020, JCO1902085. [Google Scholar] [CrossRef]
- Scales, S.J.; Gupta, N.; Pacheco, G.; Firestein, R.; French, D.M.; Koeppen, H.; Rangell, L.; Barry-Hamilton, V.; Luis, E.; Chuh, J.; et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol. Cancer Ther. 2014, 13, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Weekes, C.D.; Lamberts, L.E.; Borad, M.J.; Voortman, J.; McWilliams, R.R.; Diamond, J.R.; de Vries, E.G.E.; Verheul, H.M.; Lieu, C.H.; Kim, G.P.; et al. Phase I Study of DMOT4039A, an Antibody-Drug Conjugate Targeting Mesothelin, in Patients with Unresectable Pancreatic or Platinum-Resistant Ovarian Cancer. Mol. Cancer Ther. 2016, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Y.; Subramanyam, B.; Sarapa, N.; Golfier, S.; Dinter, H. Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors. Clin. Cancer Drugs 2016, 3, 76–86. [Google Scholar] [CrossRef]
- Clarke, J.; Chu, S.-C.; Siu, L.L.; Machiels, J.-P.; Markman, B.; Heinhuis, K.; Millward, M.; Lolkema, M.; Patel, S.P.; de Souza, P.; et al. Abstract B057: BMS-986148, an anti-mesothelin antibody-drug conjugate (ADC), alone or in combination with nivolumab demonstrates clinical activity in patients with select advanced solid tumors. Mol. Cancer Ther. 2019, 18, B057. [Google Scholar] [CrossRef]
- Targeted Alpha Therapy Working Group; Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Lindén, O.; Noordzij, W.; Park, J.; Saad, F. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Wickstroem, K.; Hagemann, U.B.; Cruciani, V.; Wengner, A.M.; Kristian, A.; Ellingsen, C.; Siemeister, G.; Bjerke, R.M.; Karlsson, J.; Ryan, O.B.; et al. Synergistic Effect of a Mesothelin-Targeted 227Th Conjugate in Combination with DNA Damage Response Inhibitors in Ovarian Cancer Xenograft Models. J. Nucl. Med. 2019, 60, 1293–1300. [Google Scholar] [CrossRef]
- Laheru, D.; Lutz, E.; Burke, J.; Biedrzycki, B.; Solt, S.; Onners, B.; Tartakovsky, I.; Nemunaitis, J.; Le, D.; Sugar, E.; et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin. Cancer Res. 2008, 14, 1455–1463. [Google Scholar] [CrossRef]
- Lutz, E.; Yeo, C.J.; Lillemoe, K.D.; Biedrzycki, B.; Kobrin, B.; Herman, J.; Sugar, E.; Piantadosi, S.; Cameron, J.L.; Solt, S.; et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011, 253, 328–335. [Google Scholar] [CrossRef]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A Live-attenuated Listeria Vaccine (ANZ-100) and a Live-attenuated Listeria Vaccine Expressing Mesothelin (CRS-207) for Advanced Cancers: Phase 1 Studies of Safety and Immune Induction. Clin. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.; Morse, M.A.; Zeh, H.J.; Weekes, C.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chen, Y.-L.; Chiang, Y.-C.; Chen, T.-C.; Tang, Y.-C.; Chen, C.-A.; Sun, W.-Z.; Cheng, W.-F. Mesothelin-specific cell-based vaccine generates antigen-specific immunity and potent antitumor effects by combining with IL-12 immunomodulator. Gene Ther. 2016, 23, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, M.; Wada, S.; Hirono, S.; Yoshida, S.; Yada, E.; Sasada, T.; Shirabe, K.; Kuwano, H.; Yamaue, H. Identification of a novel HLA-A24-restricted cytotoxic T lymphocyte epitope peptide derived from mesothelin in pancreatic cancer. Oncotarget 2018, 9, 31448–31458. [Google Scholar] [CrossRef] [PubMed]
- Nomi, T.; Sho, M.; Akahori, T.; Hamada, K.; Kubo, A.; Kanehiro, H.; Nakamura, S.; Enomoto, K.; Yagita, H.; Azuma, M.; et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 2007, 13, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.; Rucki, A.A.; Wu, A.A.; Olino, K.; Xiao, Q.; Chai, Y.; Wamwea, A.; Bigelow, E.; Lutz, E.; Liu, L.; et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J. Immunother. 2015, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Crocenzi, T.; Durham, J.N.; Sugar, E.A.; Wu, A.A.; Onners, B.; Nauroth, J.M.; Anders, R.A.; Fertig, E.J.; Laheru, D.A.; et al. Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-mesothelin (CRS-207) With or Without Nivolumab in Patients with Pancreatic Cancer. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.J.; Rafiq, S.; Brentjens, R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016, 13, 370–383. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, R.; Li, P. Incorporation of functional elements enhances the antitumor capacity of CAR T cells. Exp. Hematol. Oncol. 2017, 6. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Stromnes, I.M.; Schmitt, T.M.; Hulbert, A.; Brockenbrough, J.S.; Nguyen, H.; Cuevas, C.; Dotson, A.M.; Tan, X.; Hotes, J.L.; Greenberg, P.D.; et al. T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell 2015, 28, 638–652. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Lv, S.; Liu, X.; Cui, L.; Jiang, D.; Zhang, Q.; Li, L.; Qin, W.; Jin, H.; et al. Engineered CAR T cells targeting mesothelin by piggyBac transposon system for the treatment of pancreatic cancer. Cell. Immunol. 2018, 329, 31–40. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, S.; Zhao, J.; Deng, C.; Teng, R.; Zhao, Y.; Chen, J.; Dong, J.; Yin, M.; Bai, Y.; et al. Engineered T lymphocytes eliminate lung metastases in models of pancreatic cancer. Oncotarget 2018, 9, 13694–13705. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Cherkassky, L.; Villena-Vargas, J.; Colovos, C.; Servais, E.; Plotkin, J.; Jones, D.R.; Sadelain, M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014, 6, 261ra151. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, R.; Wu, D.; Zheng, D.; Wu, Z.; Shi, J.; Wei, X.; Wu, Q.; Long, Y.; Lin, S.; et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J. Hematol. Oncol. 2019, 12, 18. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Morello, A.; Sadelain, M.; Adusumilli, P.S. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 2016, 6, 133–146. [Google Scholar] [CrossRef] [PubMed]
| Agent | Title | Status | Phase | NCT/References |
|---|---|---|---|---|
| SS1P | Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. | Completed (Results available) | I | [78] |
| LMB-100/RG7787 | Mesothelin-targeted immunotoxin LMB-100 alone or in combination with Nab-Paclitaxel in people with previously treated metastatic and/or locally advanced PDAC and mesothelin-expressing solid tumors. | Active | I/II | NCT02810418 |
| Mesothelin-targeted immunotoxin LMB-100 in combination with tofacitinib in persons with previously treated metastatic PDAC and other mesothelin-expressing solid tumors. | Recruiting | I | NCT04034238 | |
| Amatuximab | Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. | Completed (Results available) | I | [87] |
| Anetumab ravtansine | First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. | Completed (Results available) | Ib | [90] |
| Phase II anetumab ravtansine in pre-treated mesothelin-expressing pancreatic cancer | Completed | II | NCT03023722 | |
| DMOT4039A | Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. | Completed (Results available) | I | [92] |
| BMS-986148 | A study of BMS-986148 in patients with selected advanced solid tumors. | Completed | I/II | NCT02341625 |
| BAY2287411 | First in human study of BAY2287411 injection, a Thorium-227 labeled antibody-chelator conjugate, in patients with tumors known to express mesothelin. | Recruiting | I | NCT03507452 |
| CRS-207 | A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. | Completed (Results available) | I | [99] |
| Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). | Completed (Results available) | IIb | [100] | |
| Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-mesothelin (CRS-207) With or Without Nivolumab in Patients with Pancreatic Cancer. | Completed (Results available) | II | [105] | |
| CAR-T cells | Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. | Completed (Results available) | I | [114] |
| CAR T Cell immunotherapy for pancreatic cancer. | Active | I | NCT03323944 | |
| A study of mesothelin redirected autologous T cells for advanced pancreatic carcinoma. | Unknown | I | NCT02706782 | |
| Evaluate the safety and efficacy of CAR-T in the treatment of pancreatic cancer. | Unknown | I | NCT03267173 | |
| PD-1 antibody expressing mesoCAR-T cells for mesothelin positive advanced solid tumor. | Recruiting | I/II | NCT03615313 | |
| CTLA-4 and PD-1 antibodies expressing mesothelin-CAR-T cells for mesothelin positive advanced solid tumor. | Unknown | I/II | NCT03182803 | |
| Study of PD-1 gene-knocked out mesothelin-directed CAR-T cells with the conditioning of PC in mesothelin positive multiple solid tumors. | Recruiting | I | NCT03747965 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montemagno, C.; Cassim, S.; Pouyssegur, J.; Broisat, A.; Pagès, G. From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 4067. https://doi.org/10.3390/ijms21114067
Montemagno C, Cassim S, Pouyssegur J, Broisat A, Pagès G. From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma. International Journal of Molecular Sciences. 2020; 21(11):4067. https://doi.org/10.3390/ijms21114067
Chicago/Turabian StyleMontemagno, Christopher, Shamir Cassim, Jacques Pouyssegur, Alexis Broisat, and Gilles Pagès. 2020. "From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma" International Journal of Molecular Sciences 21, no. 11: 4067. https://doi.org/10.3390/ijms21114067
APA StyleMontemagno, C., Cassim, S., Pouyssegur, J., Broisat, A., & Pagès, G. (2020). From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma. International Journal of Molecular Sciences, 21(11), 4067. https://doi.org/10.3390/ijms21114067







