Apolipoprotein A-I Supports MSCs Survival under Stress Conditions
Abstract
1. Introduction
2. Results
2.1. Analysis of apoA-I Secondary Structure Reveals a High Content of α-Helices
2.2. ApoA-I Did Not Increase the Proliferation and Nuclease Activity of MSCs in a Microenvironment Mimicking the Stem Cell Niche
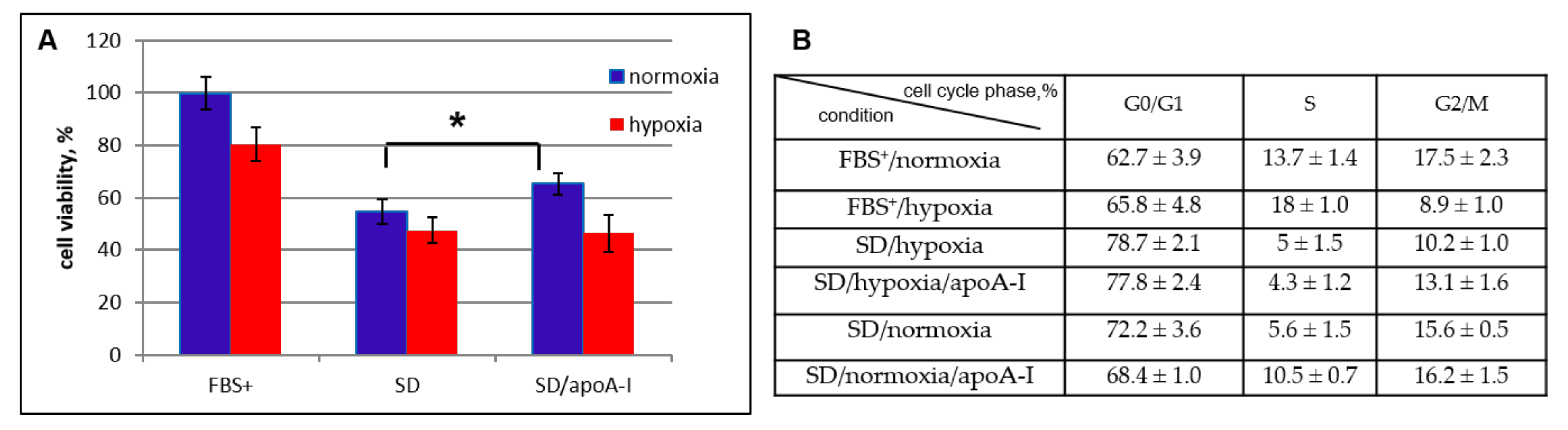
2.2.1. Viability Cell Assay and Cell Cycle Analysis
2.2.2. The Nuclease Activity Assay
2.3. ApoA-I Enhance Proliferation Rate of MSCs under Serum Deprivation Condition
3H-Thymidine Incorporation Assay
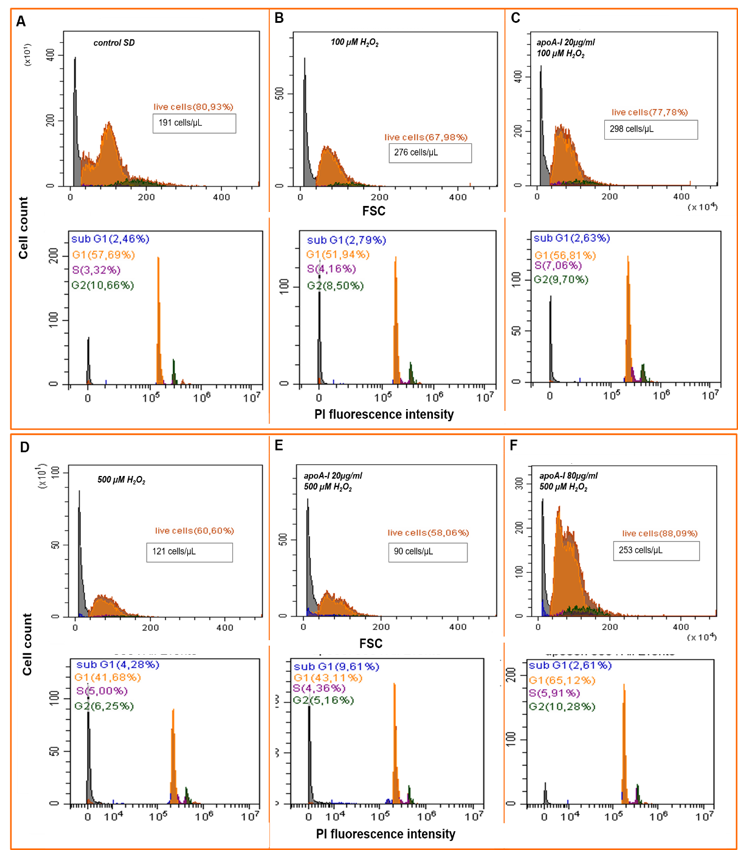
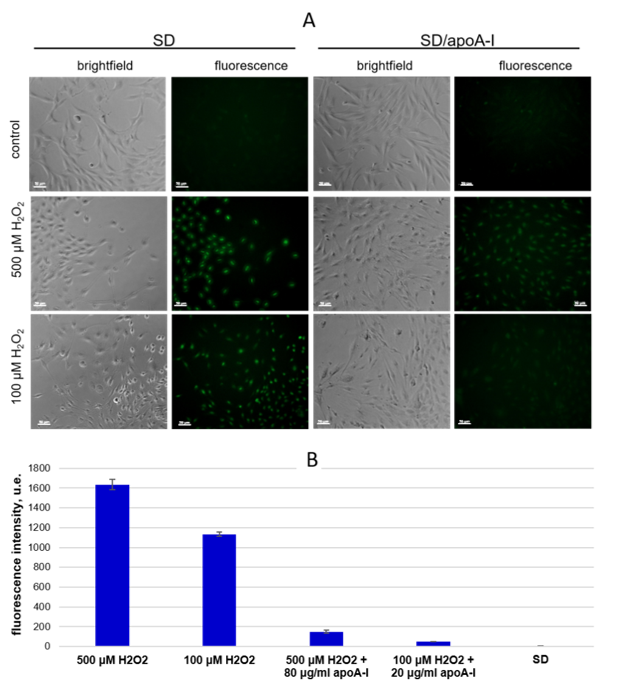
2.4. ApoA-I Enhances MSCs Viability in the Model of Oxidative Stress by Down Regulation of Intracellular Reactive Oxygen Species
2.4.1. Cell Cycler Analysis
2.4.2. Detection of Intracellular ROS Species
2.5. ApoA-I Protects MSCs under Changing Conditions
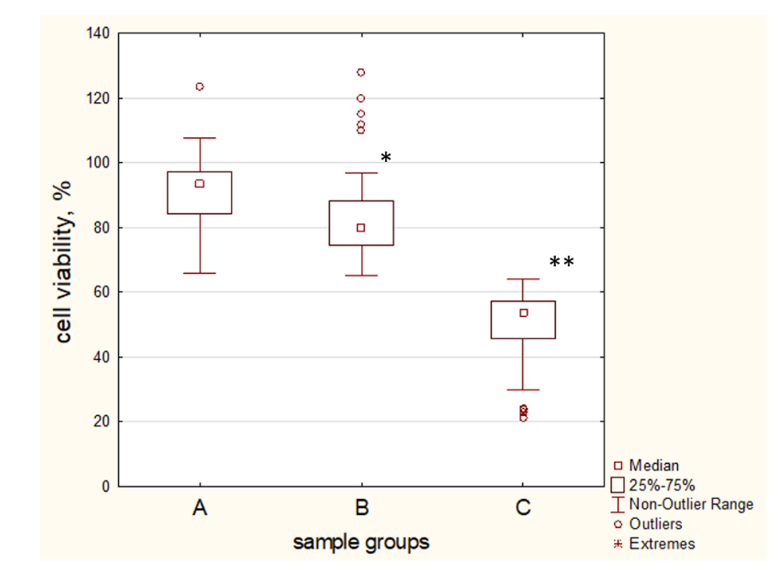
2.6. Blood Plasma Samples of T2D Patient Influence MSCs Proliferation Rate and Survival in a Negative Manner
2.7. Patient T2D Blood Plasma Samples Have a High Variability of Antioxidant Protection against H2O2-Induced Oxidation
2.8. The Influence of apoA-I on Antioxidant Properties of T2D Patient’s Plasmas
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Blood Plasma Samples of T2D Patients
4.3. ApoA-I Isolation and Characterization of Its Secondary Structure
4.4. Cultivation Conditions
4.5. Serum Deprivation
4.6. Hypoxic Conditions
4.7. Oxidative Stress Conditions
4.8. Plasmid Nicking Assay of DNase Activity
4.9. MTT Assay
4.10. 3H-Thymidine Incorporation Assay
4.11. Cell Cycle Analysis
4.12. Detection of Reactive Oxygen Species (ROS) by CellROX Reagent
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal Stem/Stromal Cells |
| ApoA-I | Apolipoprotein A-I |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| ROS | Reactive Oxygen Species |
| SD | Serum Deprivation |
| DNA | Deoxyribonucleic Acid |
| FBS | Fetal Bovine Serum |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| FTIR | Fourier Transform Infrared Spectroscopy |
References
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory effects of MSCs in bone healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Mancardi, G.; Chiesa, S. Is there a role for mesenchymal stem cells in autoimmune diseases? Autoimmunity 2008, 41, 592–595. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Kim, N.R.; Park, K.B.; Do, Y.S.; Roh, K.; Kang, K.S.; Kim, D.I. A Phase I Study of Human Cord Blood-Derived Mesenchymal Stem Cell Therapy in Patients with Peripheral Arterial Occlusive Disease. Int. J. Stem Cells 2013, 6, 37–44. [Google Scholar] [CrossRef][Green Version]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49–54.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Cao, J.X.; Li, D.; Zhang, X.Y.; Liu, J.L.; Li, J.L.; Wang, M.; Liu, Y.; Xu, B.L.; Wang, H.B. Clinical efficacy of autologous stem cell transplantation for the treatment of patients with type 2 diabetes mellitus: A meta-analysis. Cytotherapy 2015, 17, 956–968. [Google Scholar] [CrossRef]
- Bhansali, S.; Dutta, P.; Kumar, V.; Yadav, M.K.; Jain, A.; Mudaliar, S.; Bhansali, S.; Sharma, R.R.; Jha, V.; Marwaha, N.; et al. Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell and Mononuclear Cell Transplantation in Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled Comparative Study. Stem Cells Dev. 2017, 26, 471–481. [Google Scholar] [CrossRef]
- Jiang, R.; Han, Z.; Zhuo, G.; Qu, X.; Li, X.; Wang, X.; Shao, Y.; Yang, S.; Han, Z.C. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: A pilot study. Front. Med. China 2011, 5, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, Z.; Xu, X.; Liao, L.; Chen, J.; Huang, L.; Wu, W.; Luo, F.; Wu, C.; Pugliese, A.; et al. Umbilical Cord Mesenchymal Stromal Cell with Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016, 39, 149–157. [Google Scholar] [CrossRef]
- Karantalis, V.; Difede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; Mcniece, I.; Conte, J.; et al. Autologous Mesenchymal Stem Cells Produce Concordant Improvements in Regional Function, Tissue Perfusion and Fibrotic Burden when Administered to Patients Undergoing Coronary Artery Bypass Grafting—The PROMETHEUS Trial Vasileios. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, A.; Sen, D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res. Ther. 2016, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, M.C.; Malpasso, G.; Musarò, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Mesenchymal Stem Cells 2016, 1416, 149–158. [Google Scholar]
- Kawada, H.; Fujita, J.; Kinjo, K.; Matsuzaki, Y.; Tsuma, M.; Miyatake, H.; Muguruma, Y.; Tsuboi, K.; Itabashi, Y.; Ikeda, Y.; et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004, 104, 3581–3587. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Gualerzi, A.; Banfi, G.; Brini, A.T. Differential proteomic analysis predicts appropriate applications for the secretome of adipose-derived mesenchymal stem/stromal cells and dermal fibroblasts. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Danielyan, L.; Schäfer, R.; Schulz, A.; Ladewig, T.; Lourhmati, A.; Buadze, M.; Schmitt, A.L.; Verleysdonk, S.; Kabisch, D.; Koeppen, K.; et al. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: The critical role of erythropoietin. Cell Death Differ. 2009, 16, 1599–1614. [Google Scholar] [CrossRef]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Sereti, K.I.; Wu, B.M.; Ardehali, R. Translational aspects of cardiac cell therapy. J. Cell. Mol. Med. 2015, 19, 1757–1772. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, J.; Li, S.; Liu, W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Li, F.; Guo, X.; Chen, S.-Y. Function and Therapeutic Potential of Mesenchymal Stem Cells in Atherosclerosis. Front. Cardiovasc. Med. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Scioli, M.G.; Cervelli, V.; Arcuri, G.; Gentile, P.; Doldo, E.; Bielli, A.; Bonanno, E.; Orlandi, A. High Insulin-Induced Down-Regulation of Erk-1/IGF-1R/FGFR-1 Signaling Is Required for Oxidative Stress-Mediated Apoptosis of Adipose-Derived Stem Cells. J. Cell. Physiol. 2014, 229, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular senescence in type 2 diabetes: A therapeutic opportunity. Diabetes 2015, 64, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Cipak Gasparovic, A.; Zarkovic, N.; Zarkovic, K.; Semen, K.; Kaminskyy, D.; Yelisyeyeva, O.; Bottari, S.P. Biomarkers of oxidative and nitro-oxidative stress: Conventional and novel approaches. Br. J. Pharmacol. 2017, 174, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zerrad-Saadi, A.; Therond, P.; Chantepie, S.; Couturier, M.; Rye, K.A.; Chapman, M.J.; Kontush, A. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: Relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2169–2175. [Google Scholar] [CrossRef]
- Morgantini, C.; Natali, A.; Boldrini, B.; Imaizumi, S.; Navab, M.; Fogelman, A.M.; Ferrannini, E.; Reddy, S.T. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 2011, 60, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell. Biochem. 2018, 440, 167–187. [Google Scholar] [CrossRef]
- Wong, N.K.P.; Nicholls, S.J.; Tan, J.T.M.; Bursill, C.A. The role of high-density lipoproteins in diabetes and its vascular complications. Int. J. Mol. Sci. 2018, 19, 1680. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 10–13. [Google Scholar] [CrossRef]
- Bhansali, A.; Upreti, V.; Khandelwal, N.; Marwaha, N.; Gupta, V.; Sachdeva, N.; Sharma, R.R.; Saluja, K.; Dutta, P.; Walia, R.; et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009, 18, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.F.; Gourraud, P.A.; Cantagrel, A.; Davignon, J.L.; Constantin, A. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Jt. Bone Spine 2011, 78, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lindhardsen, J.; Ahlehoff, O.; Gislason, G.H.; Madsen, O.R.; Olesen, J.B.; Torp-Pedersen, C.; Hansen, P.R. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: A Danish nationwide cohort study. Ann. Rheum. Dis. 2011, 70, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gao, X.; Yao, Z.; Xu, Y. Low apoA-I is associated with insulin resistance in patients with impaired glucose tolerance: A cross-sectional study. Lipids Health Dis. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Tang, S.; Tabet, F.; Cochran, B.J.; Cuesta Torres, L.F.; Wu, B.J.; Barter, P.J.; Rye, K.A. Apolipoprotein A-I enhances insulin-dependent and insulin-independent glucose uptake by skeletal muscle. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Florentin, M.; Liberopoulos, E.N.; Wierzbicki, A.S.; Mikhailidis, D.P. Multiple actions of high-density lipoprotein. Curr. Opin. Cardiol. 2008, 23, 370–378. [Google Scholar] [CrossRef]
- Gordon, S.M.; Hofmann, S.; Askew, D.S.; Davidson, W.S. High density lipoprotein: It’s not just about lipid transport anymore. Trends Endocrinol. Metab. 2011, 22, 9–15. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The role and function of HDL in patients with chronic kidney disease and the risk of cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 610. [Google Scholar] [CrossRef]
- Hyka, N.; Dayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K.; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I inhibits the production of interleukin-1β and tumor necrosis factor-α by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef]
- Van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-κB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.J.; Zabalawi, M.; Owen, J.S.; Shah, D.; Grayson, J.M.; Major, A.S.; Bhat, S.; Gibbs, D.P.; Thomas, M.J.; Sorci-Thomas, M.G. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr -/-, ApoA-I-/- mice. J. Biol. Chem. 2010, 285, 36158–36169. [Google Scholar] [CrossRef]
- Castaing-Berthou, A.; Malet, N.; Radojkovic, C.; Cabou, C.; Gayral, S.; Martinez, L.O.; Laffargue, M. PI3Kβ Plays a Key Role in Apolipoprotein A-I-Induced Endothelial Cell Proliferation Through Activation of the Ecto-F 1 -ATPase/P2Y 1 Receptors. Cell. Physiol. Biochem. 2017, 42, 579–593. [Google Scholar] [CrossRef]
- Usynin, I.F.; Dudarev, A.N.; Gorodetskaya, A.Y.; Miroshnichenko, S.M.; Tkachenko, T.A.; Tkachenko, V.I. Apolipoprotein A-I Stimulates Cell Proliferation in Bone Marrow Cell Culture. Bull. Exp. Biol. Med. 2018, 3, 308–311. [Google Scholar]
- Dunbar, R.L.; Movva, R.; Bloedon, L.A.T.; Duffy, D.; Norris, R.B.; Navab, M.; Fogelman, A.M.; Rader, D.J. Oral Apolipoprotein A-I Mimetic D-4F Lowers HDL-Inflammatory Index in High-Risk Patients: A First-in-Human Multiple-Dose, Randomized Controlled Trial. Clin. Transl. Sci. 2017, 10, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; Halpern, S.; Crowe, T.; et al. Effect of Recombinant ApoA-I Milano on Coronary Atherosclerosis in Patients With Acute Coronary Syndromes. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef]
- Drew, B.G.; Carey, A.L.; Natoli, A.K.; Formosa, M.F.; Vizi, D.; Reddy-Luthmoodoo, M.; Weir, J.M.; Barlow, C.K.; Van Hall, G.; Meikle, P.J.; et al. Reconstituted high-density lipoprotein infusion modulates fatty acid metabolism in patients with type 2 diabetes mellitus. J. Lipid Res. 2011, 52, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Toledo, J.D.; Tricerri, M.A.; Garda, H.A. The central type Y amphipathic α-helices of apolipoprotein AI are involved in the mobilization of intracellular cholesterol depots. Arch. Biochem. Biophys. 2008, 473, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Segrest, J.P.; Jones, M.K.; De Loof, H.; Brouillette, C.G.; Venkatachalapathi, Y.V.; Anantharamaiah, G.M. The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. J. Lipid Res. 1992, 33, 141–166. [Google Scholar]
- Usynin, I.F.; Dudarev, A.N.; Miroshnichenko, S.M.; Tkachenko, T.A.; Gorodetskaya, A.Y. Effect of Native and Modified Apolipoprotein A-I on DNA Synthesis in Cultures of Different Cells. Bull. Exp. Biol. Med. 2017, 2, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, J.; Cong, X.; Hu, S.; Chen, X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 2006, 24, 416–425. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Nordberg, J.; Skowronski, E.; Babior, B.M. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. USA 1996, 93, 654–658. [Google Scholar] [CrossRef]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chattopadhyay, A.; Navab, M.; Grijalva, V.; Su, F.; Fogelman, A.M.; Reddy, S.T.; Farias-Eisner, R. Apolipoprotein A-I mimetic peptides inhibit expression and activity of hypoxia-inducible factor-1α in human ovarian cancer cell lines and a mouse ovarian cancer model. J. Pharmacol. Exp. Ther. 2012, 342, 255–262. [Google Scholar] [CrossRef]
- Mohammad, K.; Dakik, P.; Medkour, Y.; Mitrofanova, D.; Titorenko, V.I. Quiescence entry, maintenance, and exit in adult stem cells. Int. J. Mol. Sci. 2019, 20, 2158. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Jonas, K.; Kopeć, G. HDL Cholesterol as a Marker of Disease Severity and Prognosis in Patients with Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2019, 20, 3514. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Torkhovskaya, T.I.; Gorudko, I.V.; Sokolov, A. The Role of Halogenative Stress in Atherogenic Modification of Low-Density Lipoproteins. BIOCHEMISTRY-MOSCOW 2020, 85, 34–55. [Google Scholar] [CrossRef]
- Tümpel, S.; Rudolph, K.L. Quiescence: Good and Bad of Stem Cell Aging. Trends Cell Biol. 2019, 29, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Vono, R.; Jover Garcia, E.; Spinetti, G.; Madeddu, P. Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs. Antioxidants Redox Signal. 2018, 29, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, K.; Naskalski, J.W.; Fedak, D.; Pawlica-Gosiewska, D.; Grudzień, U.; Dumnicka, P.; Małecki, M.T.; Solnica, B. Markers of Antioxidant Defense in Patients with Type 2 Diabetes. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.; Miroshnichenko, S.; Kovalskii, A.; Permyakova, E.; Popov, Z.; Dvořáková, E.; Kiryukhantsev-Korneev, P.; Obrosov, A.; Polčak, J.; Zajíčková, L.; et al. Immobilization of platelet-rich plasma onto COOH plasma-coated PCL nanofibers boost viability and proliferation of human mesenchymal stem cells. Polymers (Basel) 2017, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, S.; Timofeeva, V.; Permykova, E.; Ershov, S.; Kiryukhantsev-Korneev, P.; Dvořaková, E.; Shtansky, D.V.; Zajíčková, L.; Solovieva, A.; Manakhov, A. Plasma-coated polycaprolactone nanofibers with covalently bonded platelet-rich plasma enhance adhesion and growth of human fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Mojica-Henshaw, M.P.; Jacobson, P.; Morris, J.; Kelley, L.; Pierce, J.; Boyer, M.; Reems, J.A. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy 2013, 15, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Major, B.; Vácz, G.; Kuten, O.; Hornyák, I.; Hinsenkamp, A.; Kardos, D.; Bagó, M.; Cseh, D.; Sárközi, A.; et al. The effects of hyperacute serum on the elements of the human subchondral bone marrow niche. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Domingo-Espín, J.; Lindahl, M.; Nilsson-Wolanin, O.; Cushman, S.W.; Stenkula, K.G.; Lagerstedt, J.O. Dual actions of apolipoprotein A-I on glucose-stimulated insulin secretion and insulin-independent peripheral tissue glucose uptake lead to increased heart and skeletal muscle glucose disposal. Diabetes 2016, 65, 1838–1848. [Google Scholar] [CrossRef]
- Poteryaeva, O.N.; Usynin, I.F. Antidiabetic role of high density lipoproteins. Biomeditsinskaya Khimiya 2018, 64, 463–471. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroshnichenko, S.; Usynin, I.; Dudarev, A.; Nimaev, V.; Solovieva, A. Apolipoprotein A-I Supports MSCs Survival under Stress Conditions. Int. J. Mol. Sci. 2020, 21, 4062. https://doi.org/10.3390/ijms21114062
Miroshnichenko S, Usynin I, Dudarev A, Nimaev V, Solovieva A. Apolipoprotein A-I Supports MSCs Survival under Stress Conditions. International Journal of Molecular Sciences. 2020; 21(11):4062. https://doi.org/10.3390/ijms21114062
Chicago/Turabian StyleMiroshnichenko, Svetlana, Ivan Usynin, Alexey Dudarev, Vadim Nimaev, and Anastasiya Solovieva. 2020. "Apolipoprotein A-I Supports MSCs Survival under Stress Conditions" International Journal of Molecular Sciences 21, no. 11: 4062. https://doi.org/10.3390/ijms21114062
APA StyleMiroshnichenko, S., Usynin, I., Dudarev, A., Nimaev, V., & Solovieva, A. (2020). Apolipoprotein A-I Supports MSCs Survival under Stress Conditions. International Journal of Molecular Sciences, 21(11), 4062. https://doi.org/10.3390/ijms21114062





