Nanocomposites for X-Ray Photodynamic Therapy
Abstract
1. Introduction
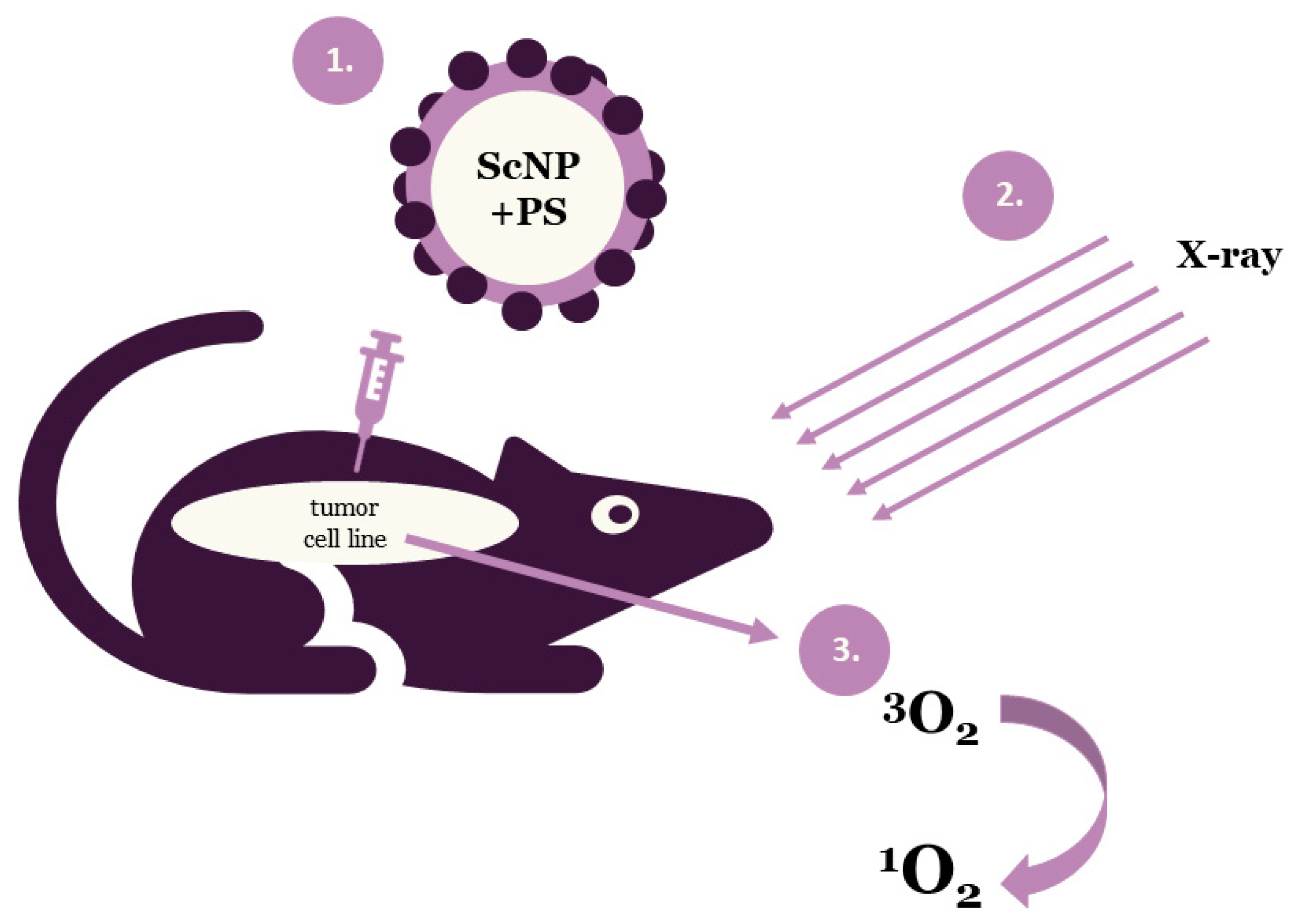
2. Nanoparticles Used in X-Ray Photodynamic Therapy
2.1. Types of Photosensitizers
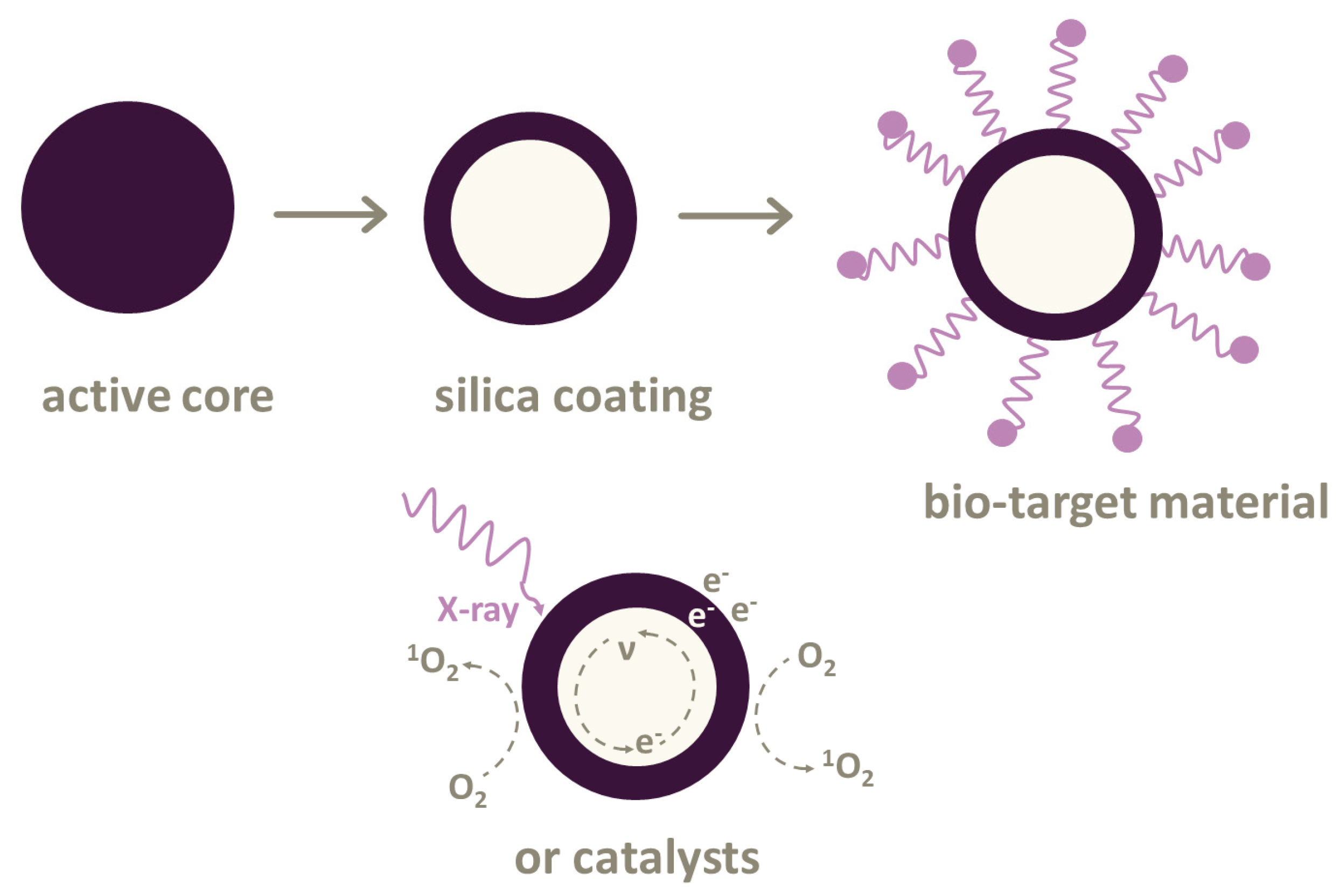
2.2. Types of Scintillating Nanoparticles
2.3. Combination of Photosensitizers and Nanoparticles
2.4. Metal–Organic Frameworks as Photosensitizers
3. Reactive Oxygen Species
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDT | Photodynamic therapy |
| XPDT | X-ray photodynamic therapy |
| PS | Photosensitizer |
| ScNP | Scintillating nanoparticles |
| FRET | Förster resonance energy transfer |
| ROS | Reactive oxygen speeches |
| RB | Rose Bengal |
| PSGC | Pollen-structured gold cluster |
| AIE-Au | Aggregation-induced emission heterogeneous Au clustoluminogens |
| MTN | Mesoporous titanium dioxide nanoparticle |
| RGD | Arginylglycylaspartic acid |
| PLNP | Persistent luminescence nanoparticle |
| MOF | Metal–organic framework |
| SBUs | Secondary building units |
| PEG | Polyethylene glycol |
References
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef]
- Ren, X.H.; Li, G.Y.; Du, H.; Ma, J.P.; Geng, Y.; Dong, Y.B. Thermally activated delayed fluorescent (TADF) coordination polymer with the generation of singlet oxygen. Acta Crystallogr. C Struct. Chem. 2019, 75, 758–767. [Google Scholar] [CrossRef]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic targeting of nanotheranostics enhances cerenkov radiation-induced photodynamic therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef]
- Castro, K.; Moura, N.M.M.; Figueira, F.; Ferreira, R.I.; Simoes, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Silvestre, A.J.D.; Freire, C.S.R.; Tome, J.P.C.; et al. New materials based on cationic porphyrins conjugated to chitosan or titanium dioxide: Synthesis, characterization and antimicrobial efficacy. Int. J. Mol. Sci. 2019, 20, 2522. [Google Scholar] [CrossRef]
- Xu, J.; Gao, J.; Wei, Q. Combination of photodynamic therapy with radiotherapy for cancer treatment. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating nanoparticles as energy mediators for enhanced photodynamic therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef]
- Wilson, B.C. Photodynamic therapy for cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, M.; Chuang, Y.C.; Lo, L.W. Evolution of nanoparticle-mediated photodynamic therapy: From superficial to deep-seated cancers. Molecules 2019, 24, 520. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; Andre, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C.; et al. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Dˇedic, R. New trends in photodynamic therapy research. WDS Proc. Contrib. Pap. 2012, 3, 46–51. [Google Scholar]
- Wang, A.Z.; Tepper, J.E. Nanotechnology in radiation oncology. J. Clin. Oncol. 2014, 32, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Kruger, C.A.; Kadanyo, S.; Mishra, A. Nanoparticles for advanced photodynamic therapy of cancer. Photomed. Laser Surg. 2017, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C.; et al. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-based photodynamic therapy strategies for deep tumor treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Guo, T. X-Ray Nanochemistry: Concepts and Development; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Figueiredo, P.; Bauleth-Ramos, T.; Hirvonen, J.; Sarmento, B.; Santos, H.A. The emerging role of multifunctional theranostic materials in cancer nanomedicine. In Handbook of Nanomaterials for Cancer Theranostics; Elsevier Science Publishing Company: Oxford, UK, 2018; pp. 1–31. [Google Scholar] [CrossRef]
- Conde, J. Front matter. In Handbook of Nanomaterials for Cancer Theranostics; Elsevier Science Publishing Company: Oxford, UK, 2018; pp. i–ii. [Google Scholar] [CrossRef]
- Pogue, B.W.; Wilson, B.C. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23, 1–17. [Google Scholar] [CrossRef]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomedicine 2011, 7, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Elmenoufy, A.H.; Tang, Y.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.D.; Chuang, Y.J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tang, W.; Lau, J.; Shen, Z.; Xie, J.; Shi, J.; Chen, X. Breaking the depth dependence by nanotechnology-enhanced x-ray-excited deep cancer theranostics. Adv. Mater. 2019, 31, e1806381. [Google Scholar] [CrossRef] [PubMed]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef] [PubMed]
- Kaščáková, S.; Giuliani, A.; Lacerda, S.; Pallier, A.; Mercère, P.; Tóth, É.; Réfrégiers, M. X-ray-induced radiophotodynamic therapy (RPDT) using lanthanide micelles: Beyond depth limitations. Nano Res. 2015, 8, 2373–2379. [Google Scholar] [CrossRef]
- Chen, H.P.; Tung, F.I.; Chen, M.H.; Liu, T.Y. A magnetic vehicle realized tumor cell-targeted radiotherapy using low-dose radiation. J. Control Release 2016, 226, 182–192. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, J.; Elmenoufy, A.; Yang, X. A highly efficient fret system capable for deep photodynamic therapy established on x-ray excited mesoporous LaF3:Tb scintillating nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 12261–12269. [Google Scholar] [CrossRef]
- Lei, S.; Pei, L.; Jing, W.; Lun, M. The effectiveness and safety of X-PDT for cutaneous squamous cell carcinoma and melanoma. Nanomedicine 2019, 14, 2027–2043. [Google Scholar]
- Shrestha, S.; Wu, J.; Sah, B.; Vanasse, A.; Cooper, L.N.; Ma, L.; Li, G.; Zheng, H.; Chen, W.; Antosh, M.P.; et al. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 16823–16828. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Sun, Y.-J.; Chung, P.-H.; Chen, W.-Y.; Swieszkowski, W.; Tian, W.; Lin, F.-H. Development of Ce-doped TiO2 activated by X-ray irradiation for alternative cancer treatment. Ceram. Int. 2017, 43, 12675–12683. [Google Scholar] [CrossRef]
- Yang, C.C.; Tsai, M.H.; Li, K.Y.; Hou, C.H.; Lin, F.H. Carbon-doped TiO2 activated by x-ray irradiation for the generation of reactive oxygen species to enhance photodynamic therapy in tumor treatment. Int. J. Mol. Sci. 2019, 20, 2072. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Martinčík, J.; Nikl, M.; Ruml, T.; Lang, K. Octahedral molybdenum clusters as radiosensitizers for X-ray induced photodynamic therapy. J. Mater. Chem. B 2018, 6, 4301–4307. [Google Scholar] [CrossRef] [PubMed]
- Tew, L.S.; Cai, M.T.; Lo, L.W.; Khung, Y.L.; Chen, N.T. Pollen-structured gold nanoclusters for x-ray induced photodynamic therapy. Materials 2018, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Bakhmetyev, V.V.; Minakova, T.S.; Mjakin, S.V.; Lebedev, L.A.; Vlasenko, A.B.; Nikandrova, A.A.; Ekimova, I.A.; Eremina, N.S.; Sychov, M.M.; Ringuede, A.; et al. Synthesis and surface characterization of nanosized Y2O3:Eu and YAG:Eu luminescent phosphors which are useful in photodynamic therapy of cancer. Eur. J. Nanomed. 2016, 8. [Google Scholar] [CrossRef]
- Scaffidi, J.P.; Gregas, M.K.; Lauly, B.; Zhang, Y. Activity of psoralen-functionalized nanoscintillators against cancer cells upon x-ray excitation. ACS Nano 2011, 5, 4679–4687. [Google Scholar] [CrossRef]
- Achilefu, S.; Sudheendra, L.; Das, G.K.; Li, C.; Cherry, S.R.; Kennedy, I.M.; Raghavachari, R. Lanthanide-doped nanoparticles for hybrid x-ray/optical imaging. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V, San Francisco, CA, USA, 4–6 February 2013. [Google Scholar]
- Chen, H.; Sun, X.; Wang, G.D.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J.; Shen, B. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater. Horiz. 2017, 4, 1092–1101. [Google Scholar] [CrossRef]
- Sun, W.; Luo, L.; Feng, Y.; Cai, Y.; Zhuang, Y.; Xie, R.J.; Chen, X.; Chen, H. Aggregation-induced emission gold clustoluminogens for enhanced low-dose x-ray-induced photodynamic therapy. Angew. Chem. Int. Ed. Engl. 2019. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, K.; Tan, Z.; Liu, Y.; Zhao, Z.; Zhu, G.; Ma, K.; Cui, C.; Wang, L.; Kang, T.; et al. Overcoming drug resistance with functional mesoporous titanium dioxide nanoparticles combining targeting, drug delivery and photodynamic therapy. J. Mater. Chem. B 2018, 6, 7750–7759. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, K.; Bu, W.; Ni, D.; Liu, Y.; Feng, J.; Shi, J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. Engl. 2015, 54, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shi, T.; Luo, L.; Chen, X.; Lv, P.; Lv, Y.; Zhuang, Y.; Zhu, J.; Liu, G.; Chen, X.; et al. Monodisperse and uniform mesoporous silicate nanosensitizers achieve low-dose x-ray-induced deep-penetrating photodynamic therapy. Adv. Mater. 2019, 31, e1808024. [Google Scholar] [CrossRef] [PubMed]
- Parak, W.J.; Osinski, M.; Liang, X.-J.; Kudinov, K.; Bekah, D.; Cooper, D.; Shastry, S.; Hill, C.; Bradforth, S.; Nadeau, J.; et al. Lanthanum fluoride nanoparticles for radiosensitization of tumors. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XI, San Francisco, CA, USA, 13–18 February 2016. [Google Scholar]
- Chen, M.H.; Jenh, Y.J.; Wu, S.K.; Chen, Y.S.; Hanagata, N.; Lin, F.H. Non-invasive photodynamic therapy in brain cancer by use of Tb3+-doped LaF3 nanoparticles in combination with photosensitizer through x-ray irradiation: A proof-of-concept study. Nanoscale Res. Lett. 2017, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.; Procházková, L.; Pelikánová, I.T.; Vlk, M.; Palkovský, M.; Jarý, V.; Nikl, M.; Múčka, V.; Mihóková, E.; Čuba, V.; et al. Preliminary study on singlet oxygen production using CeF3:Tb3+@SiO2-PpIX. Radiat. Meas. 2016, 90, 325–328. [Google Scholar] [CrossRef]
- Ahmad, F.; Wang, X.; Jiang, Z.; Yu, X.; Liu, X.; Mao, R.; Chen, X.; Li, W. Codoping enhanced radioluminescence of nanoscintillators for x-ray-activated synergistic cancer therapy and prognosis using metabolomics. ACS Nano 2019, 13, 10419–10433. [Google Scholar] [CrossRef]
- Hubenko, K.; Yefimova, S.; Tkacheva, T.; Maksimchuk, P.; Borovoy, I.; Klochkov, V.; Kavok, N.; Opolonin, O.; Malyukin, Y. Reactive oxygen species generation in aqueous solutions containing GdVO4:Eu3+ nanoparticles and their complexes with methylene blue. Nanoscale Res. Lett. 2018, 13, 100. [Google Scholar] [CrossRef]
- Song, L.; Li, P.-P.; Yang, W.; Lin, X.-H.; Liang, H.; Chen, X.-F.; Liu, G.; Li, J.; Yang, H.-H. Low-dose X-ray activation of W(VI)-doped persistent luminescence nanoparticles for deep-tissue photodynamic therapy. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Ma, L.; Zou, X.; Bui, B.; Chen, W.; Song, K.H.; Solberg, T. X-ray excited ZnS:Cu,Co afterglow nanoparticles for photodynamic activation. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-induced singlet oxygen activation with nanoscintillator-coupled porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Kang, X.; Ma, P.; Dai, Y.; Hou, Z.; Cheng, Z.; Li, C.; Lin, J. Hollow structured upconversion luminescent NaYF4:Yb3+, Er3+ nanospheres for cell imaging and targeted anti-cancer drug delivery. Biomaterials 2013, 34, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Liao, Y.-T.; Kang, T.-C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016, 18, 5957–5961. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martinez, M.; An, J.; Sole-Font, I.; Rosi, N.L.; Maspoch, D. Metal-biomolecule frameworks (MBioFs). Chem. Commun. 2011, 47, 7287–7302. [Google Scholar] [CrossRef]
- Hsu, S.H.; Li, C.T.; Chien, H.T.; Salunkhe, R.R.; Suzuki, N.; Yamauchi, Y.; Ho, K.C.; Wu, K.C. Platinum-free counter electrode comprised of metal-organic-framework (MOF)-derived cobalt sulfide nanoparticles for efficient dye-sensitized solar cells (DSSCs). Sci. Rep. 2014, 4, 6983. [Google Scholar] [CrossRef]
- Song, M.-R.; Li, D.-Y.; Nian, F.-Y.; Xue, J.-P.; Chen, J.-J. Zeolitic imidazolate metal organic framework-8 as an efficient pH-controlled delivery vehicle for zinc phthalocyanine in photodynamic therapy. J. Mater. Sci. 2017, 53, 2351–2361. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Veroneau, S.S.; Feng, X.; Nash, G.T.; Luo, T.; Xu, Z.; Lin, W. Titanium-based nanoscale metal-organic framework for type i photodynamic therapy. J. Am. Chem. Soc. 2019, 141, 4204–4208. [Google Scholar] [CrossRef]
- Maggiorella, L.; Barouch, G.; Devaux, C.; Pottier, A.; Deutsch, E.; Bourhis, J.; Levy, E.B.L. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012, 8, 1167–1181. [Google Scholar] [CrossRef]
- Jayaraman, V.; Bhavesh, G.; Chinnathambi, S.; Ganesan, S.; Aruna, P. Synthesis and characterization of hafnium oxide nanoparticles for bio-safety. Mater. Express 2014, 4, 375–383. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Lan, G.; Tang, H.; Pelizzari, C.; Fu, Y.X.; Spiotto, M.T.; et al. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2018, 2, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lan, G.; Chan, C.; Quigley, B.; Lu, K.; Aung, T.; Guo, N.; La Riviere, P.; Weichselbaum, R.R.; Lin, W.; et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018, 9, 2351. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liotta, L.A.; Petricoin, E.F. Cancer metabolism and mass spectrometry-based proteomics. Cancer Lett. 2015, 356, 176–183. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Belanova, A.A.; Chmykhalo, V.K.; Makarenko, M.S.; Lyangasova, O.V.; Belousova, M.M.; Aleksandrova, A.A.; Zolotukhin, P.V. Effects of JUN and NFE2L2 knockdown on oxidative status and NFE2L2/AP-1 targets expression in HeLa cells in basal conditions and upon sub-lethal hydrogen peroxide treatment. Mol. Biol. Rep. 2019, 46, 27–39. [Google Scholar] [CrossRef]
- Mezencev, R.; Matyunina, L.V.; Jabbari, N.; McDonald, J.F. Snail-induced epithelial-to-mesenchymal transition of MCF-7 breast cancer cells: Systems analysis of molecular changes and their effect on radiation and drug sensitivity. BMC Cancer 2016, 16, 236. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Siddiqui, F.A.; Gupta, V.; Chattopadhyay, S.; Gopinath, P. Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2. Mol. Cancer 2013, 12, 72. [Google Scholar] [CrossRef]
- Schulze, A.; Harris, A.L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012, 491, 364–373. [Google Scholar] [CrossRef]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.; Winkler, D.A. Getting to the source: Selective drug targeting of cancer stem cells. ChemMedChem 2014, 9, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Boskovic, Z.V.; Theriault, J.R.; Wang, A.J.; Stern, A.M.; Wagner, B.K.; Shamji, A.F.; Schreiber, S.L. Discovery of small-molecule enhancers of reactive oxygen species that are nontoxic or cause genotype-selective cell death. ACS Chem. Biol. 2013, 8, 923–929. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.W.; Xiao, X.; Shan, Y.; Xue, B.; Jiang, G.; He, Q.; Chen, J.; Xu, H.G.; Zhao, R.X.; et al. Piperlongumine induces autophagy by targeting p38 signaling. Cell Death Dis. 2013, 4, e824. [Google Scholar] [CrossRef]
- Jog, N.R.; Caricchio, R. The role of necrotic cell death in the pathogenesis of immune mediated nephropathies. Clin. Immunol. 2014, 153, 243–253. [Google Scholar] [CrossRef]
- Siboni, G.; Amit-Patito, I.; Weizman, E.; Waintraub-Porat, M.; Weitman, H.; Ehrenberg, B.; Malik, Z. Specificity of photosensitizer accumulation in undifferentiated versus differentiated colon carcinoma cells. Cancer Lett. 2003, 196, 57–64. [Google Scholar] [CrossRef]
- Yoshiro, N.; Satoshi, N.; Shukichi, S. New method of photosensitizer. Accumulation for photodynamic therapy in an experimental liver tumor. Lasers Surg. Med. 1989, 9, 254–263. [Google Scholar]
- Zelenkov, P.; Baumgartner, R.; Bise, K.; Heide, M.; Meier, R.; Stocker, S.; Sroka, R.; Goldbrunner, R.; Stummer, W. Acute morphological sequelae of photodynamic therapy with 5-aminolevulinic acid in the C6 spheroid model. J. Neurooncol. 2007, 82, 49–60. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.; Oliveira, T.C.; Gomes, V.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L.; et al. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef]
| Photosensitizer | Scintillating Nanoparticle | Absorbed Dose of Ionizing Radiation (Gy) | Biological Experiments | Reference |
|---|---|---|---|---|
| Cu–Cy | - | 5 Gy for 30 min | Squamous cells carcinoma | [34] |
| TiO2:Ce | - | 0.133 Gy for 100 s | A549 | [35] |
| TiO2:C | - | 0.133 Gy for 100 s | A549 | [36] |
| (n-Bu4N)2(Mo6I8(OCOCF3)6) | - | 1 and 2 Gy | HeLa and MRC | [37] |
| RB | CeF3:Tb3+, Gd3+ | 3 Gy | Mgc803, HEK293T and 4T1 | [50] |
| ZnGa2O4:Cr | ZnPcS4 | ~0.18 Gy for 2 min | HeLa | [52] |
| ZnS:Cu, Co | TBrRh123 (tetrabromorhodamine-123) | 2 Gy | PC3 | [53] |
| nMOF Hf-TCPP | Hf as radio-sensitizer; | 6 Gy for 3 min | 4T1, HeLa, and NIH3T3 | [55] |
| nMOF Hf6-DBA (Hf6(μ3-O)4(μ3-OH)4(DBA)6) and Hf12-DBA (Hf12(μ3-O)8(μ3-OH)8(μ2-OH)6(DBA)9) | Hf as radio-sensitizer | 2 Gy | CT26 | [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. https://doi.org/10.3390/ijms21114004
Gadzhimagomedova Z, Zolotukhin P, Kit O, Kirsanova D, Soldatov A. Nanocomposites for X-Ray Photodynamic Therapy. International Journal of Molecular Sciences. 2020; 21(11):4004. https://doi.org/10.3390/ijms21114004
Chicago/Turabian StyleGadzhimagomedova, Zaira, Peter Zolotukhin, Oleg Kit, Daria Kirsanova, and Alexander Soldatov. 2020. "Nanocomposites for X-Ray Photodynamic Therapy" International Journal of Molecular Sciences 21, no. 11: 4004. https://doi.org/10.3390/ijms21114004
APA StyleGadzhimagomedova, Z., Zolotukhin, P., Kit, O., Kirsanova, D., & Soldatov, A. (2020). Nanocomposites for X-Ray Photodynamic Therapy. International Journal of Molecular Sciences, 21(11), 4004. https://doi.org/10.3390/ijms21114004







