Clinical Trials of Stem Cell Treatment for Spinal Cord Injury
Abstract
1. Introduction
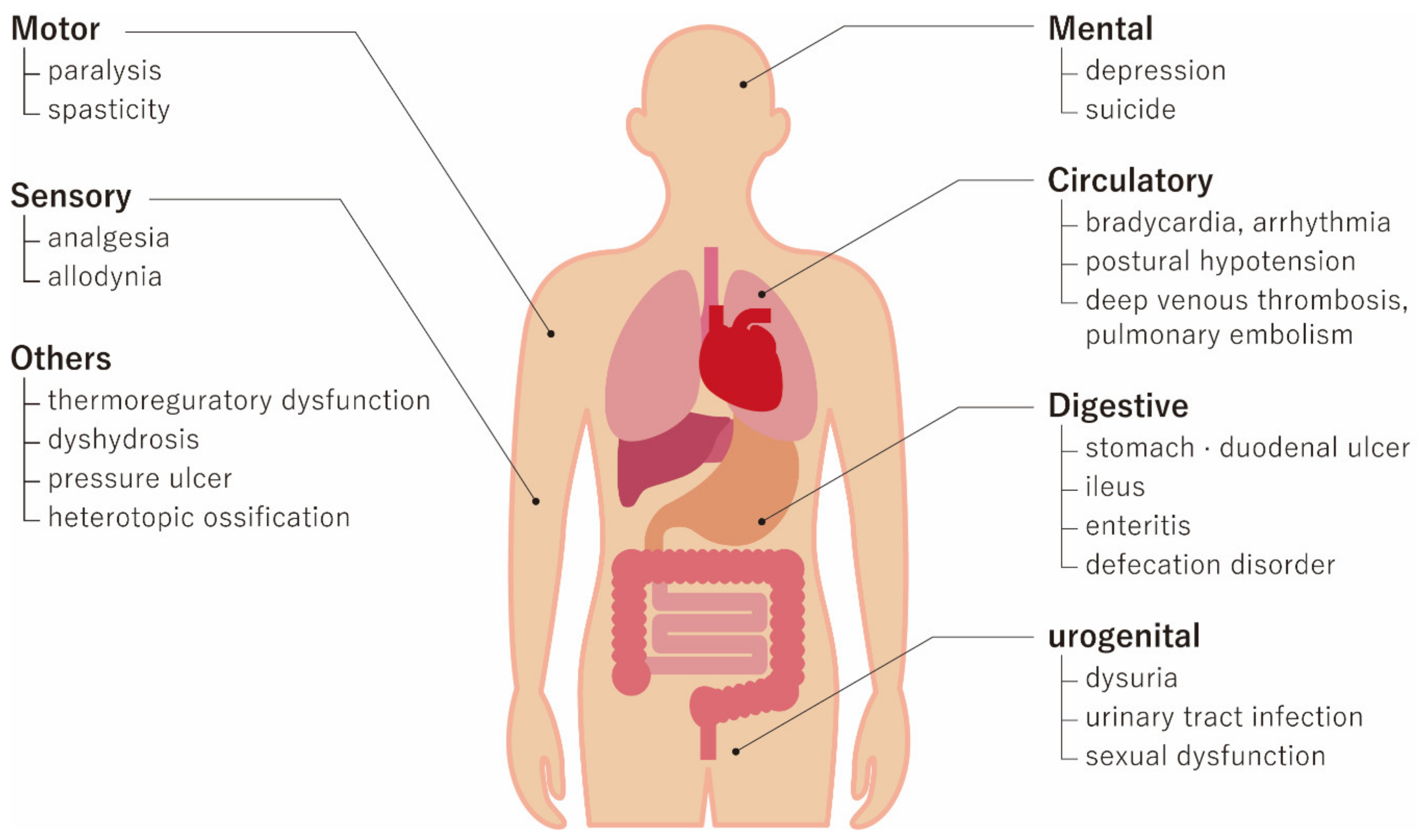
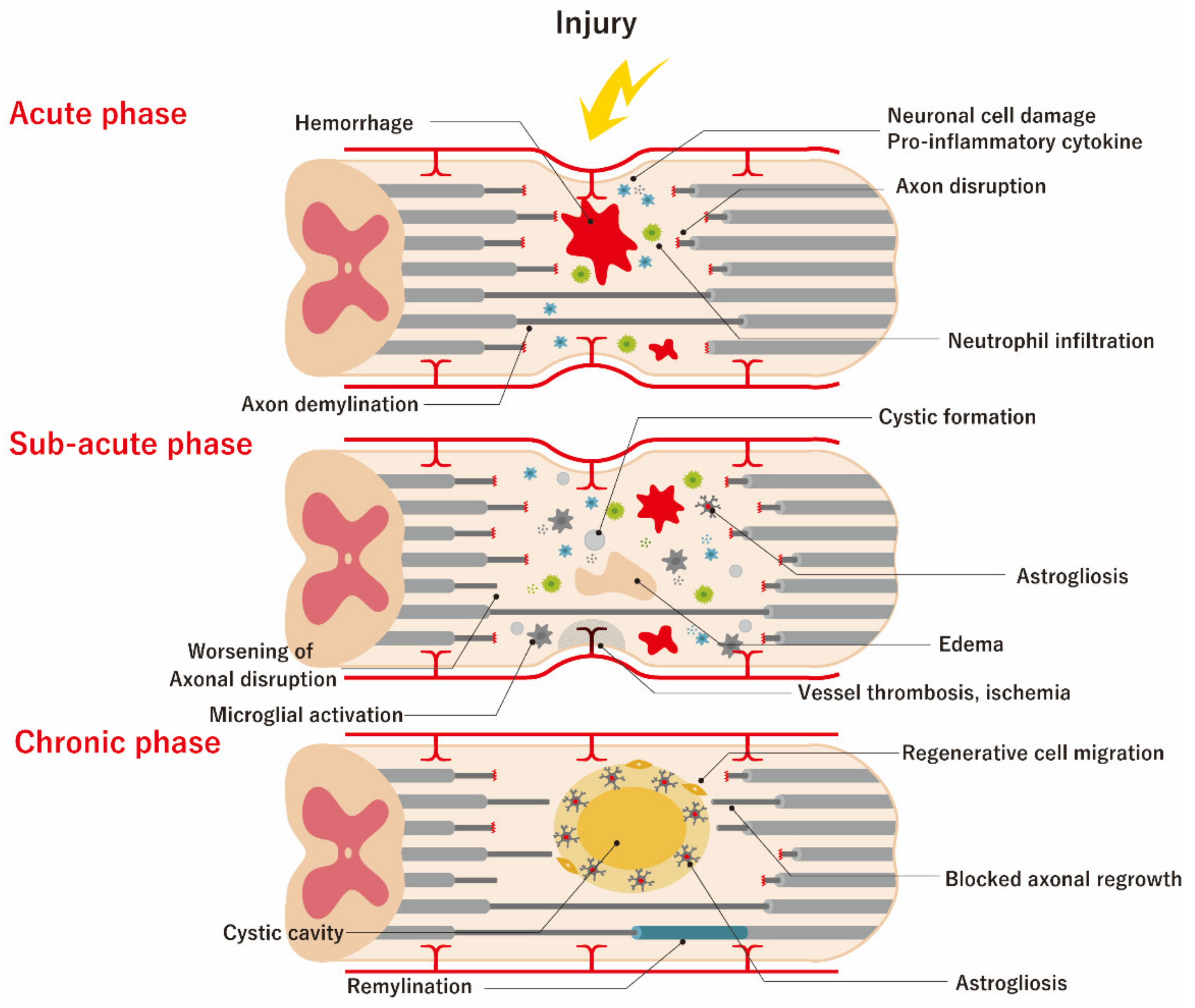
2. Pathophysiology of SCI and Therapeutic Targets
3. Mechanisms of Action of Stem Cell Transplantation
4. Key Segment of Clinical Trials
4.1. Overview of Clinical Trial Results
4.1.1. Acute Phase of SCI
4.1.2. Sub-Acute Phase of SCI
4.1.3. Chronic Phase of SCI
4.2. Source Stem Cell Types
4.2.1. MSCs and MNCs
4.2.2. Hematopoietic Stem Cells
4.2.3. OECs
4.2.4. Schwann Cells
4.2.5. NSCs
4.2.6. ESCs
4.2.7. iPSCs
4.3. Cell Dose and Route
4.4. Patient Characteristics and Outcome Measures
4.5. Results, Pitfalls, and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef]
- Thompson, C.; Mutch, J.; Parent, S.; Mac-Thiong, J.M. The changing demographics of traumatic spinal cord injury: An 11-year study of 831 patients. J. Spinal Cord Med. 2015, 38, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Hagen, E.M. Acute complications of spinal cord injuries. World J. Orthop 2015, 6, 17–23. [Google Scholar] [CrossRef] [PubMed]
- DeVivo, M.J. Causes and costs of spinal cord injury in the United States. Spinal Cord 1997, 35, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Wilson, J.R.; O’Higgins, M. Introduction: Spinal cord injury at the cutting edge of clinical translation: A focus issue collaboration between NACTN and AOSpine North America. J. Neurosurg. Spine 2012, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.C.; Noonan, V.; Cadotte, D.W.; Fehlings, M.G. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: An evidence-based examination of pre-clinical and clinical studies. J. Neurotrauma 2011, 28, 1371–1399. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192. [Google Scholar] [CrossRef]
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J.C.; Marshall, L.F.; et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 1992, 76, 23–31. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Holford, T.R.; Leo-Summers, L.; Aldrich, E.F.; Fazl, M.; Fehlings, M.G.; Herr, D.L.; Hitchon, P.W.; Marshall, L.F.; et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J. Neurosurg. 1988, 89, 699–706. [Google Scholar] [CrossRef]
- Pointillart, V.; Petitjean, M.E.; Wiart, L.; Vital, J.M.; Lassie, P.; Thicoipe, M.; Dabadie, P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000, 38, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transpl. 2018, 27, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E.; Homola, A.; Mazanec, R.; Lachmann, H.; Konradova, S.L.; Kobylka, P.; Padr, R.; Neuwirth, J.; Komrska, V.; Vavra, V.; et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transpl. 2006, 15, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Karamouzian, S.; Nematollahi-Mahani, S.N.; Nakhaee, N.; Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012, 114, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef]
- Hur, J.W.; Cho, T.H.; Park, D.H.; Lee, J.B.; Park, J.Y.; Chung, Y.G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2016, 39, 655–664. [Google Scholar] [CrossRef]
- Yoon, S.H.; Shim, Y.S.; Park, Y.H.; Chung, J.K.; Nam, J.H.; Kim, M.O.; Park, H.C.; Park, S.R.; Min, B.H.; Kim, E.Y.; et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells 2007, 25, 2066–2073. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, K.N.; Yoo, J.; Kim, I.S.; Yun, S.; Lee, H.; Jung, K.; Hwang, K.; Kim, M.; Lee, I.S.; et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast 2015, 2015, 630932. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bartlett Bunge, M.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef]
- Levi, A.D.; Okonkwo, D.O.; Park, P.; Jenkins, A.L., 3rd; Kurpad, S.N.; Parr, A.M.; Ganju, A.; Aarabi, B.; Kim, D.; Casha, S.; et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery 2018, 82, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, Y.; Rao, S.; Ghosh, D.; Balaraju, S.; Radhika, C.R.; Satish Kumar, K.V. Autologous mesenchymal stem cells in chronic spinal cord injury. Br. J. Neurosurg. 2011, 25, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Geffner, L.F.; Santacruz, P.; Izurieta, M.; Flor, L.; Maldonado, B.; Auad, A.H.; Montenegro, X.; Gonzalez, R.; Silva, F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transpl. 2008, 17, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Moviglia, G.A.; Fernandez Vina, R.; Brizuela, J.A.; Saslavsky, J.; Vrsalovic, F.; Varela, G.; Bastos, F.; Farina, P.; Etchegaray, G.; Barbieri, M.; et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy 2006, 8, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, E.R.; Stupak, V.V.; Muradov, G.M.; Sizikov, M.Y.; Shevela, E.Y.; Leplina, O.Y.; Tikhonova, M.A.; Kulagin, A.D.; Lisukov, I.A.; Ostanin, A.A.; et al. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull. Exp. Biol Med. 2007, 143, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Cristante, A.F.; Barros-Filho, T.E.; Tatsui, N.; Mendrone, A.; Caldas, J.G.; Camargo, A.; Alexandre, A.; Teixeira, W.G.; Oliveira, R.P.; Marcon, R.M. Stem cells in the treatment of chronic spinal cord injury: Evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord 2009, 47, 733–738. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Kishk, N.A.; Gabr, H.; Hamdy, S.; Afifi, L.; Abokresha, N.; Mahmoud, H.; Wafaie, A.; Bilal, D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil. Neural. Repair 2010, 24, 702–708. [Google Scholar] [CrossRef]
- Frolov, A.A.; Bryukhovetskiy, A.S. Effects of hematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transpl. 2012, 21, S49–S55. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transpl. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Fernandez, C.; Tapiador, N.; Sevilla, M.; Morejon, C.; Montilla, J.; et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy 2017, 19, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vazquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Pratas-Vital, J.; Escada, P.; Hasse-Ferreira, A.; Capucho, C.; Peduzzi, J.D. Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. J. Spinal Cord Med. 2006, 29, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A.; Feron, F.; Cochrane, J.; Bassingthwaighte, L.; Bayliss, C.; Davies, W.; Fronek, P.; Gray, C.; Kerr, G.; Licina, P.; et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 2008, 131, 2376–2386. [Google Scholar] [CrossRef]
- Saberi, H.; Moshayedi, P.; Aghayan, H.R.; Arjmand, B.; Hosseini, S.K.; Emami-Razavi, S.H.; Rahimi-Movaghar, V.; Raza, M.; Firouzi, M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008, 443, 46–50. [Google Scholar] [CrossRef]
- Deda, H.; Inci, M.C.; Kurekci, A.E.; Kayihan, K.; Ozgun, E.; Ustunsoy, G.E.; Kocabay, S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy 2008, 10, 565–574. [Google Scholar] [CrossRef]
- Lima, C.; Escada, P.; Pratas-Vital, J.; Branco, C.; Arcangeli, C.A.; Lazzeri, G.; Maia, C.A.; Capucho, C.; Hasse-Ferreira, A.; Peduzzi, J.D. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil. Neural. Repair 2010, 24, 10–22. [Google Scholar] [CrossRef]
- Dai, G.; Liu, X.; Zhang, Z.; Yang, Z.; Dai, Y.; Xu, R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013, 1533, 73–79. [Google Scholar] [CrossRef]
- Mendonca, M.V.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. 2014, 5, 126. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Fernandez, C.; Rodriguez-Boto, G.; Marin, E.; Tapiador, N.; Sevilla, M.; Carballido, J.; et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy 2018, 20, 796–805. [Google Scholar] [CrossRef]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950 e946. [Google Scholar] [CrossRef]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma. 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Al-Zoubi, A.; Jafar, E.; Jamous, M.; Al-Twal, F.; Al-Bakheet, S.; Zalloum, M.; Khalifeh, F.; Radi, S.A.; El-Khateeb, M.; Al-Zoubi, Z. Transplantation of purified autologous leukapheresis-derived CD34+ and CD133+ stem cells for patients with chronic spinal cord injuries: Long-term evaluation of safety and efficacy. Cell Transpl. 2014, 23, S25–S34. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.Y.; Sung, I.Y.; Choi, G.H.; Jeon, M.H.; Kim, K.K.; Jeon, S.R. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery 2012, 70, 1238–1247. [Google Scholar] [CrossRef]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Montilla, J.; Bustamante, S.; Carballido, J.; Marin, E.; Martinez, F.; et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016, 18, 1025–1036. [Google Scholar] [CrossRef]
- Kumar, A.A.; Kumar, S.R.; Narayanan, R.; Arul, K.; Baskaran, M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp. Clin. Transpl. 2009, 7, 241–248. [Google Scholar]
- Choo, A.M.; Liu, J.; Lam, C.K.; Dvorak, M.; Tetzlaff, W.; Oxland, T.R. Contusion, dislocation, and distraction: Primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J. Neurosurg. Spine 2007, 6, 255–266. [Google Scholar] [CrossRef]
- LaPlaca, M.C.; Simon, C.M.; Prado, G.R.; Cullen, D.K. CNS injury biomechanics and experimental models. Prog Brain Res. 2007, 161, 13–26. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Martin, A.R.; Fehlings, M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Wilson, J.R.; Forgione, N.; Fehlings, M.G. Emerging therapies for acute traumatic spinal cord injury. CMAJ 2013, 185, 485–492. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef]
- Teng, Y.D. Functional Multipotency of Stem Cells and Recovery Neurobiology of Injured Spinal Cords. Cell Transpl. 2019, 28, 451–459. [Google Scholar] [CrossRef]
- Liu, M.; Wu, W.; Li, H.; Li, S.; Huang, L.T.; Yang, Y.Q.; Sun, Q.; Wang, C.X.; Yu, Z.; Hang, C.H. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J. Spinal Cord Med. 2015, 38, 745–753. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Tao, Y.; Zhang, S.; Wang, J.; Feng, X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 2014, 266, 91–101. [Google Scholar] [CrossRef]
- Morita, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Oka, S.; Oshigiri, T.; Takebayashi, T.; Yamashita, T.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience 2016, 335, 221–231. [Google Scholar] [CrossRef]
- Kwon, B.K.; Tetzlaff, W.; Grauer, J.N.; Beiner, J.; Vaccaro, A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004, 4, 451–464. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Capocelli, A.L., Jr.; Anzil, A.P.; Kotzen, R.M.; Milhorat, R.H. Pathological basis of spinal cord cavitation in syringomyelia: Analysis of 105 autopsy cases. J. Neurosurg. 1995, 82, 802–812. [Google Scholar] [CrossRef]
- Nori, S.; Ahuja, C.S.; Fehlings, M.G. Translational Advances in the Management of Acute Spinal Cord Injury: What is New? What is Hot? Neurosurgery 2017, 64, 119–128. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Badhiwala, J.H.; Ahuja, C.S.; Fehlings, M.G. Time is spine: A review of translational advances in spinal cord injury. J. Neurosurg. Spine 2018, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kuroda, S.; Shichinohe, H.; Ikeda, J.; Seki, T.; Hida, K.; Tada, M.; Sawada, K.; Iwasaki, Y. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology 2003, 23, 169–180. [Google Scholar] [CrossRef]
- Novikova, L.N.; Brohlin, M.; Kingham, P.J.; Novikov, L.N.; Wiberg, M. Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy 2011, 13, 873–887. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Zhao, S.; Jin, Y.; Zhou, F.; Yuan, P.; Cao, L.; Wang, J.; Qiu, Y.; Sun, C.; et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019, 10, 597. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef]
- Shu, Y. Neuronal signaling in central nervous system. Sheng Li Xue Bao 2011, 63, 1–8. [Google Scholar]
- Neirinckx, V.; Cantinieaux, D.; Coste, C.; Rogister, B.; Franzen, R.; Wislet-Gendebien, S. Concise review: Spinal cord injuries: How could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells 2014, 32, 829–843. [Google Scholar] [CrossRef]
- Ceci, M.; Mariano, V.; Romano, N. Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment. Rev. Neurosci. 2018, 30, 45–66. [Google Scholar] [CrossRef]
- Sharma, A.; Gokulchandran, N.; Chopra, G.; Kulkarni, P.; Lohia, M.; Badhe, P.; Jacob, V.C. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transpl. 2012, 21, Suppl 1. S79–S90. [Google Scholar] [CrossRef]
- Feron, F.; Perry, C.; Cochrane, J.; Licina, P.; Nowitzke, A.; Urquhart, S.; Geraghty, T.; Mackay-Sim, A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 2005, 128, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Jeon, S.R. Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review. Korean J. Neurotrauma 2016, 12, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transpl. 2017, 26, 585–603. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Arsenijevic, N.; Stojkovic, M. Stem Cells Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular, T. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bellagamba, B.C.; Grudzinski, P.B.; Ely, P.B.; Nader, P.J.H.; Nardi, N.B.; da Silva Meirelles, L. Induction of Expression of CD271 and CD34 in Mesenchymal Stromal Cells Cultured as Spheroids. Stem Cells Int. 2018, 2018, 7357213. [Google Scholar] [CrossRef]
- Stagg, J.; Pommey, S.; Eliopoulos, N.; Galipeau, J. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood 2006, 107, 2570–2577. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; Francois, M.; Boivin, M.N.; Stagg, J.; Galipeau, J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J. Immunol. 2007, 179, 1549–1558. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Chiba, Y.; Kuroda, S.; Maruichi, K.; Osanai, T.; Hokari, M.; Yano, S.; Shichinohe, H.; Hida, K.; Iwasaki, Y. Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model. Neurosurgery 2009, 64, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.T.; Neuhuber, B.; Coleman, C.; Kushner, R.; Swanger, S.A.; Kopen, G.C.; Wagner, J.; Shumsky, J.S.; Fischer, I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil. Neural Repair 2006, 20, 278–296. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.H.; Song, S.U.; Yi, T.G.; Yoon, S.H.; Park, S.R.; Choi, B.H. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J. Tissue Eng. Regen. Med. 2018, 12, e1034–e1045. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Nakai, Y.; Seo, T.B.; Homma, T.; Yamada, Y.; Ohta, M.; Suzuki, Y.; Nakatani, T.; Fukushima, M.; Hayashibe, M.; et al. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS ONE 2013, 8, e73494. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Zurita, M.; Bonilla, C.; Aguayo, C.; Vela, A.; Rico, M.A.; Vaquero, J. Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral hemorrhage. Cytotherapy 2011, 13, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, M.; Alviano, F.; Pirondi, S.; Lanzoni, G.; Fernandez, M.; Lizzo, G.; Giardino, L.; Giuliani, A.; Costa, R.; Marchionni, C.; et al. Human mesenchymal stem cells produce bioactive neurotrophic factors: Source, individual variability and differentiation issues. Int. J. Immunopathol. Pharm. 2014, 27, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Zhang, R.P.; Li, J.D. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir. (Wien.) 2014, 156, 1409–1418. [Google Scholar] [CrossRef]
- Watanabe, S.; Uchida, K.; Nakajima, H.; Matsuo, H.; Sugita, D.; Yoshida, A.; Honjoh, K.; Johnson, W.E.; Baba, H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells 2015, 33, 1902–1914. [Google Scholar] [CrossRef]
- Wu, S.; Suzuki, Y.; Ejiri, Y.; Noda, T.; Bai, H.; Kitada, M.; Kataoka, K.; Ohta, M.; Chou, H.; Ide, C. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 2003, 72, 343–351. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, T.T.; Peng, Y.R.; Hu, S.Q.; Xu, T. The treatment of spinal cord injury in rats using bone marrow-derived neural-like cells induced by cerebrospinal fluid. Neurosci. Lett. 2018, 666, 85–91. [Google Scholar] [CrossRef]
- Zhao, T.; Yan, W.; Xu, K.; Qi, Y.; Dai, X.; Shi, Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy 2013, 15, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.B.; Dominguez, C.; Pernold, K.; Reger, R.; Wiesenfeld-Hallin, Z.; Olson, L.; Prockop, D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 2009, 27, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Neuhuber, B.; Timothy Himes, B.; Shumsky, J.S.; Gallo, G.; Fischer, I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005, 1035, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.I.; Min, J.; Choi, K.H.; Kim, S.W.; Kim, K.S.; Jeon, S.R. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir. (Wien.) 2011, 153, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef]
- Boyd, J.G.; Doucette, R.; Kawaja, M.D. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. Faseb J. 2005, 19, 694–703. [Google Scholar] [CrossRef]
- Li, L.; Adnan, H.; Xu, B.; Wang, J.; Wang, C.; Li, F.; Tang, K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur. Spine J. 2015, 24, 919–930. [Google Scholar] [CrossRef]
- Bunge, M.B.; Wood, P.M. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb. Clin. Neurol. 2012, 109, 523–540. [Google Scholar] [CrossRef]
- Duncan, I.D.; Aguayo, A.J.; Bunge, R.P.; Wood, P.M. Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J. Neurol. Sci. 1981, 49, 241–252. [Google Scholar] [CrossRef]
- Saberi, H.; Firouzi, M.; Habibi, Z.; Moshayedi, P.; Aghayan, H.R.; Arjmand, B.; Hosseini, K.; Razavi, H.E.; Yekaninejad, M.S. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J. Neurosurg. Spine 2011, 15, 515–525. [Google Scholar] [CrossRef]
- Zhu, T.; Tang, Q.; Gao, H.; Shen, Y.; Chen, L.; Zhu, J. Current status of cell-mediated regenerative therapies for human spinal cord injury. Neurosci. Bull. 2014, 30, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Zahir, T.; Wang, X.; Yue, C.; Keating, A.; Tator, C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 2008, 155, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.; Frame, J.; Siegenthaler, M.; Nistor, G.; Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 2010, 28, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef]
- Li, J.Y.; Christophersen, N.S.; Hall, V.; Soulet, D.; Brundin, P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008, 31, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373. [Google Scholar] [CrossRef]
- Tsuji, O.; Miura, K.; Okada, Y.; Fujiyoshi, K.; Mukaino, M.; Nagoshi, N.; Kitamura, K.; Kumagai, G.; Nishino, M.; Tomisato, S.; et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 12704–12709. [Google Scholar] [CrossRef]
- Suzuki, H.; Ahuja, C.S.; Salewski, R.P.; Li, L.; Satkunendrarajah, K.; Nagoshi, N.; Shibata, S.; Fehlings, M.G. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS ONE 2017, 12, e0182339. [Google Scholar] [CrossRef]
- Bakshi, A.; Hunter, C.; Swanger, S.; Lepore, A.; Fischer, I. Minimally invasive delivery of stem cells for spinal cord injury: Advantages of the lumbar puncture technique. J. Neurosurg. Spine 2004, 1, 330–337. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Oya, S.; Santos, M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: Systemic or local administration? Neurosci. Lett. 2006, 398, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Samdani, A.F.; Betz, R.R.; Fischer, I.; Neuhuber, B. Grafting of human bone marrow stromal cells into spinal cord injury: A comparison of delivery methods. Spine (Phila Pa 1976) 2009, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.A.; Kim, J.M.; Kim, H.I.; Yi, S.; Ha, Y.; Yoon, D.H.; Kim, K.N. Comparison of functional and histological outcomes after intralesional, intracisternal, and intravenous transplantation of human bone marrow-derived mesenchymal stromal cells in a rat model of spinal cord injury. Acta Neurochir. (Wien.) 2013, 155, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Amemori, T.; Ruzicka, J.; Romanyuk, N.; Jhanwar-Uniyal, M.; Sykova, E.; Jendelova, P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
| Reference number | Journal | PMID | Author | Country | Cell Type | Cell Type | Dose | Route | Patient Type | Patient Number | Patient Characteristics (ASIA: A-D, or Others) | Major Functional Outcome | Major Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] | Cell Transplant | 29871514 | Xiao | China | Allogenic | UMSC&Scaffold | 4 × 107 | On-spine | Acute | 2 | A | 100% ASIA improvement (C:2) | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Arterial | Sub-acute | 5 | A:4, B:1 | 40% ASIA improvement | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Venous | Sub-acute | 9 | A:7, B:2 | No ASIA improvement, Some SEP/MEP improvement | |
| [14] | Cytotherapy | 19903102 | Pal | India | Autologous | BMSC | 6 × 107/kg | Thecal | Sub-acute | 15 | A11, C4 | No improvement | |
| [15] | Clin Neurol Neurosurg. | 22464434 | Karamouzian | Iran | Autologous | BMSC | 7–12 × 106 | Thecal | Sub-acute | 11 | A | 46% ASIA improvement (C:5), control 15% | |
| [16] | Cytotherapy | 26971680 | Satti | Pakistan | Autologous | BMSC | 1.2 × 106 | Thecal | Sub-acute | 3 | A | Safe | |
| [17] | J Spinal Cord Med | 26208177 | Hur | South Korea | Autologous | AMCS | 9 × 107 | Thecal | Sub-acute | 3 | A2, B1 | ASIA sensory change (improved 2, decline 1) | |
| [18] | Stem Cells | 17464087 | Yoon | South Korea | Autologous | BMMNC | 2 × 108 | Spinal | Sub-acute | 35 | A | 19% ASIA improvement (8% control) | 33% of patients with new pain |
| [19] | Neural Plasticity | 26568892 | Shin | South Korea | Allogenic | NSC | 1 × 108 | Spinal | Sub-acute | 19 | A:17, B:2 | 26% ASIA improvement (A to B:1, A to C:2, B to D:2) | |
| [20] | J Neurotrauma | 28225648 | Anderson | USA | Autologous | Schwann cell | 5, 10, and 15 × 106 | Spinal | Sub-acute | 6 | A | Improvement in FIM and others | |
| [21] | Neurosurgery | 30180779 | Levi | USA | Allogenic | NSC | 4 × 107 | Spinal | Sub-acute | 1 | B | Improvement in motor assessment | |
| [22] | Br J Neurosurg | 21749185 | Bhanot | India | Autologous | BMSC | Spine: 6 to 24 × 107; thecal: 6 to 12 × 107 | Spinal and thecal | Sub-acute | 2 | A | No improvement | |
| [23] | Cell Transplant | 19364066 | Geffner | Ecuador | Autologous | CD34+ | 4 × 108 | Spinal, thecal, and venous | Sub-acute | 3 | A | 67% ASIA improvement (C:2) | |
| [24] | Cytotherapy | 16793729 | Moviglia | Argentina | Autologous | BMSC | 5 to 10 × 109 | Venous | Chronic | 2 | N/A | Motor/SEP recovery | None |
| [25] | Bull Exp Biol Med | 18214319 | Chernykh | Russia | Autologous | BMMNC | 3.6 × 107 | Venous and spinal | Chronic | 18 | N/A | Original Scale improved compare with control | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Arterial | Chronic | 1 | C | 0% ASIA improvement, Some SEP/MEP improvement | |
| [26] | Spinal Cord | 19333245 | Cristante | Brazil | Autologous | CD34+ | 1.5 × 108 | Arterial | Chronic | 29 | Complete | SEP recovery (67%) | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Venous | Chronic | 5 | A:4, B:1 | 0% ASIA improvement, Some MEP improvement | |
| [27] | Stem Cells Dev | 21303266 | Ra | South Korea | Autologous | AMSC | 4 × 108 | Venous | Chronic | 8 | A & B | 12.5% ASIA improvement (A to C:1) | No SAE |
| [14] | Cytotherapy | 19903102 | Pal | India | Autologous | BMSC | 6 × 107 | Thecal | Chronic | 10 | A9, C1 | 0% ASIA improvement, Some motor/sensory improvement | |
| [28] | Neurorehabil Neural Repair | 20660620 | Kishk | Egypt | Autologous | BMSC | 3–6 × 107 | Thecal | Chronic | 43 | A:40, C:3 | 30% ASIA improvement (control 16%) | Neuropathic pain 24/43 |
| [29] | Cell Transplant | 22507680 | Frolov | Russia | Autologous | CD34+ | 24–51 × 106 | Thecal | Chronic | 20 | N/A | SEP/MEP improvement (15–20%) | |
| [30] | Cell Transplant | 23452836 | El-Kheir | Egypt | Autologous | BMSC | 1.2 × 108 | Thecal | Chronic | 50 | A:15, B:35 | 34% ASIA improvement (control 0%) | |
| [16] | Cytotherapy | 26971680 | Satti | Pakistan | Autologous | BMSC | 1.2 × 106 | Thecal | Chronic | 6 | A | Safe | |
| [17] | J Spinal Cord Med | 26208177 | Hur | South Korea | Autologous | AMCS | 9 × 107 | Thecal | Chronic | 11 | A10, D1 | ASIA sensory change (improved 8) | |
| [31] | Cytotherapy | 28089079 | Vaquero | Spain | Autologous | BMSC | 120 × 106 | Thecal | Chronic | 10 | B:4, C:5, D:1 | Improvement in ASIA score | |
| [32] | Cytotherapy | 29853256 | Vaquero | Spain | Autologous | BMSC | 3 × 108 | Thecal | Chronic | 11 | A3: B:4, C:3, D:1 | 27% ASIA improvement | |
| [33] | J Spinal Cord Med | 16859223 | Lima | Spain | Autologous | Olfactory Mucosa | N/A | Spinal | Chronic | 7 | A | 29% ASIA improvement (A to C:2) | Worsening of sensory:1 |
| [34] | Brain | 18689435 | Mackay-Sim | Spain | Autologous | Olfactory Mucosa | 1.2–2.8 × 107 | Spinal | Chronic | 3 | A | No functional improvement | |
| [35] | Neurosci Lett | 18662744 | Saberi | Iran | Autologous | Schwann cell | 3–4.5 × 106 | Spinal | Chronic | 4 | A:2, C:2 | 25% ASIA improvement (1:C to D) | |
| [36] | Cytotherapy | 18615345 | Deda | Turkey | Autologous | CD34+ | 1 × 107 | Spinal | Chronic | 9 | A | 100% ASIA improvement (B:2, C:7) | |
| [37] | Neurorehabil Neural Repair | 19794133 | Lima | Spain | Autologous | Olfactory Mucosa | N/A | Spinal | Chronic | 20 | A:15, B:5 | 55% ASIA improved (A to B:2, A to C:6, B to C:3) | |
| [38] | Brain Res | 23948102 | Dai | China | Autologous | BMSC | 2 × 107 | Spinal | Chronic | 20 | A | 45% ASIA improvement (A to B:9) | |
| [39] | Stem Cell Res Ther | 25406723 | Mendonca | Brazil | Autologous | BMSC | 4–52 × 106 | Spinal | Chronic | 12 | A | 58% ASIA improvement (B:6, C:1) | |
| [40] | J Transl Med | 25209445 | Cheng | China | Allogenic | UMSC | 4 × 107 | Spinal | Chronic | 10 | A | Improvement in ASIA score (cell: 70%, rehabilitation: 36%, control: 0%) | |
| [41] | Cytotherapy | 29784434 | Vaquero | Spain | Autologous | BMSC | 3 × 108 | Spinal | Chronic | 6 | A:3, B:2, D:1 | Improvement in ASIA score | |
| [42] | Cell Stem Cell | 29859175 | Curtis | USA | Allogenic | NSC | 1.2 × 106 | Spinal | Chronic | 4 | A | EMG improvement | |
| [43] | Neurosurgery | 30180779 | Levi | USA | Allogenic | NSC | 2 × 108 and 4 × 108 | Spinal | Chronic | 24 | A, B | Improvement in motor assessment | |
| [44] | Cell Transplant | 25372344 | Al-Zoubi | USA | Autologous | CD34+ | 7.6 × 107 | Spinal and thecal | Chronic | 19 | A | 47% ASIA improvement (B:7, C:2) | |
| [22] | Br J Neurosurg | 21749185 | Bhanot | India | Autologous | BMSC | Spine: 6 to 24 ×107; thecal: 6 to 12 × 107 | Spinal and thecal | Chronic | 11 | A | 9% ASIA improvement (A to B:1) | |
| [45] | Neurosurgery | 22127044 | Park | South Korea | Autologous | BMSC | Spine: 8 × 106; thecal: 4 × 107 | Spinal and thecal | Chronic | 10 | A:4, B:6 | SEP/MEP improvement (30%) | |
| [46] | Neurosurgery | 26891377 | Oh | South Korea | Autologous | BMSC | Spine: 1.6 × 107; thecal: 3.2 × 107 | Spinal and thecal | Chronic | 20 | B | Original Scale improvement (13%) | |
| [47] | Cytotherapy | 27311799 | Vaquero | Spain | Autologous | BMSC | Spine: 5 to 150 × 106; thecal: 30 × 106 | Spinal and thecal | Chronic | 12 | A | 33% ASIA improvement (B:3, C:1) | |
| [23] | Cell Transplant | 19364066 | Geffner | Ecuador | Autologous | CD34+ | 4 × 108 | Spinal, thecal, and venous | Chronic | 5 | A2, B1, C2 | 75% ASIA improvement | |
| [48] | Exp Clin Transplant | 20353375 | Kumar | India | Autologous | BMMNC | 3–5 × 108 | Thecal | N/A | 264 | A:233, B:7, C:22, D: | 30% ASIA improvement | |
| [70] | Cell Transplant | 22507683 | Sharma | India | Autologous | BMMNC | 1 × 106/kg | Thecal | N/A | 4 | N/A | 25% ASIA improvement |
| Reference number | Authors (Year) | Rat SCI Model | Timing | Donor Cell | Delivery Routes | Dose | Evaluation | Results |
|---|---|---|---|---|---|---|---|---|
| [110] | Bakshi et al. (2003) | rat cervical SCI | 24 h | rat BMSC | intraventricular intravenous intrathecal | 200 × 104 200 × 104 200 ×104 | Histology | intraventricular, intrathecal > intravenous |
| [111] | Vaquero et al. (2006) | rat thoracic SCI | 3 mo | rat BMSC | direct intrathecal | 300 × 104 300 × 104 | motor function | direct > intrathecal |
| [112] | Paul et al. (2009) | rat cervical SCI | 24 h | human BMSC | direct intravenous intrathecal | 15 × 104 100 × 104 100 × 104 | Histology | intrathecal > intravenous |
| [113] | Shin et al. (2009) | rat thoracic SCI | 1 wk | human BMSC | intralesional intracisternal intravenous | 30 × 104 100 × 104 100 × 104 | histology and motor function | Function: intracisternal > intralesional > intravenous Histology: intralesional > intracisternal > intravenous |
| [114] | Amemori et al. (2015) | rat thoracic SCI | 1 wk | humani PSC-NPC | direct intrathecal | 50 × 104 50 × 104 | histology and motor function | direct > intrathecal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, K.; Kawabori, M.; Seki, T.; Houkin, K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. Int. J. Mol. Sci. 2020, 21, 3994. https://doi.org/10.3390/ijms21113994
Yamazaki K, Kawabori M, Seki T, Houkin K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. International Journal of Molecular Sciences. 2020; 21(11):3994. https://doi.org/10.3390/ijms21113994
Chicago/Turabian StyleYamazaki, Kazuyoshi, Masahito Kawabori, Toshitaka Seki, and Kiyohiro Houkin. 2020. "Clinical Trials of Stem Cell Treatment for Spinal Cord Injury" International Journal of Molecular Sciences 21, no. 11: 3994. https://doi.org/10.3390/ijms21113994
APA StyleYamazaki, K., Kawabori, M., Seki, T., & Houkin, K. (2020). Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. International Journal of Molecular Sciences, 21(11), 3994. https://doi.org/10.3390/ijms21113994






