The Cell Membrane of Sulfolobus spp.—Homeoviscous Adaption and Biotechnological Applications
Abstract
1. Introduction
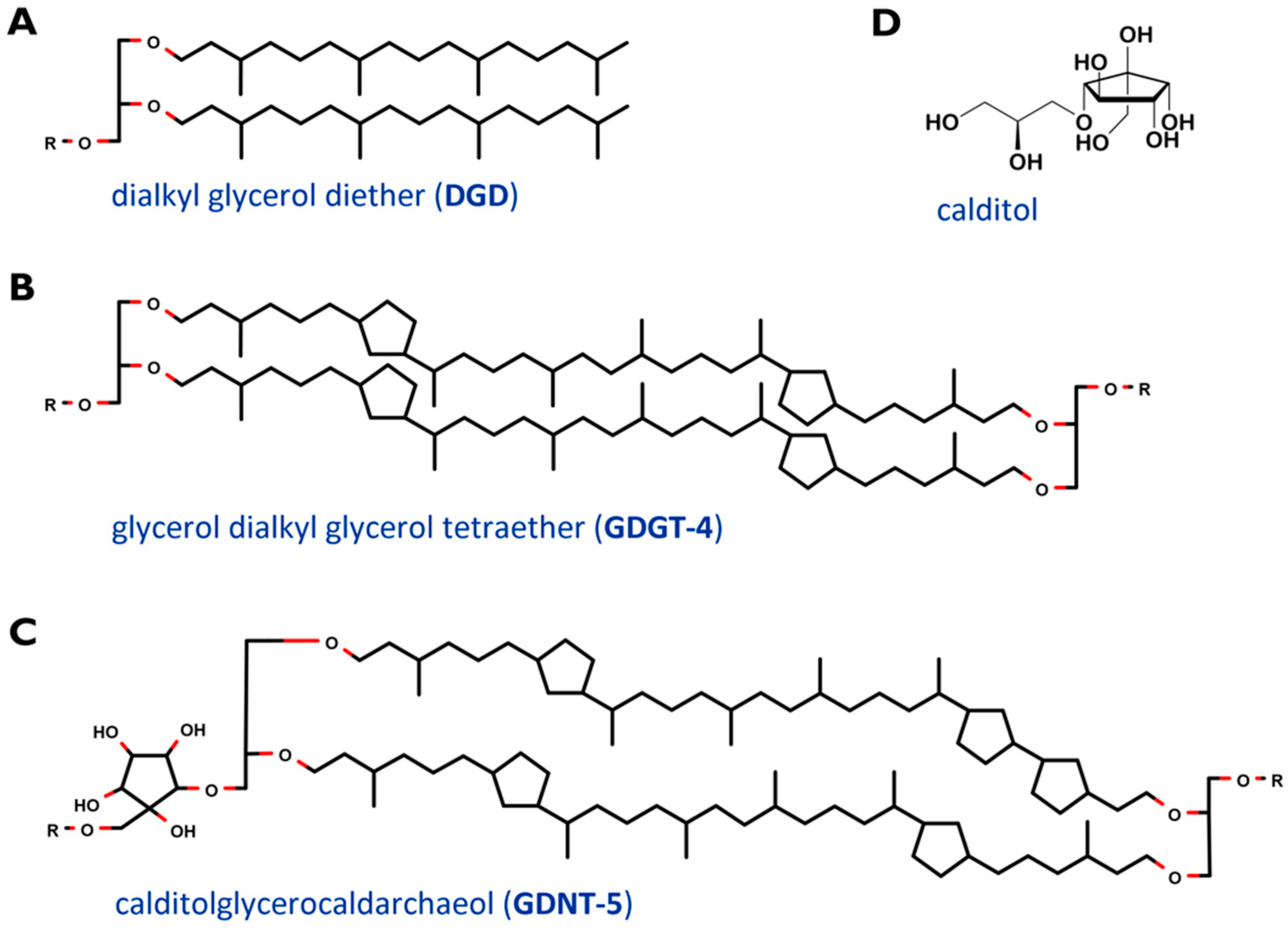
2. Cell Membrane and Lipids of Sulfolobus Spp.
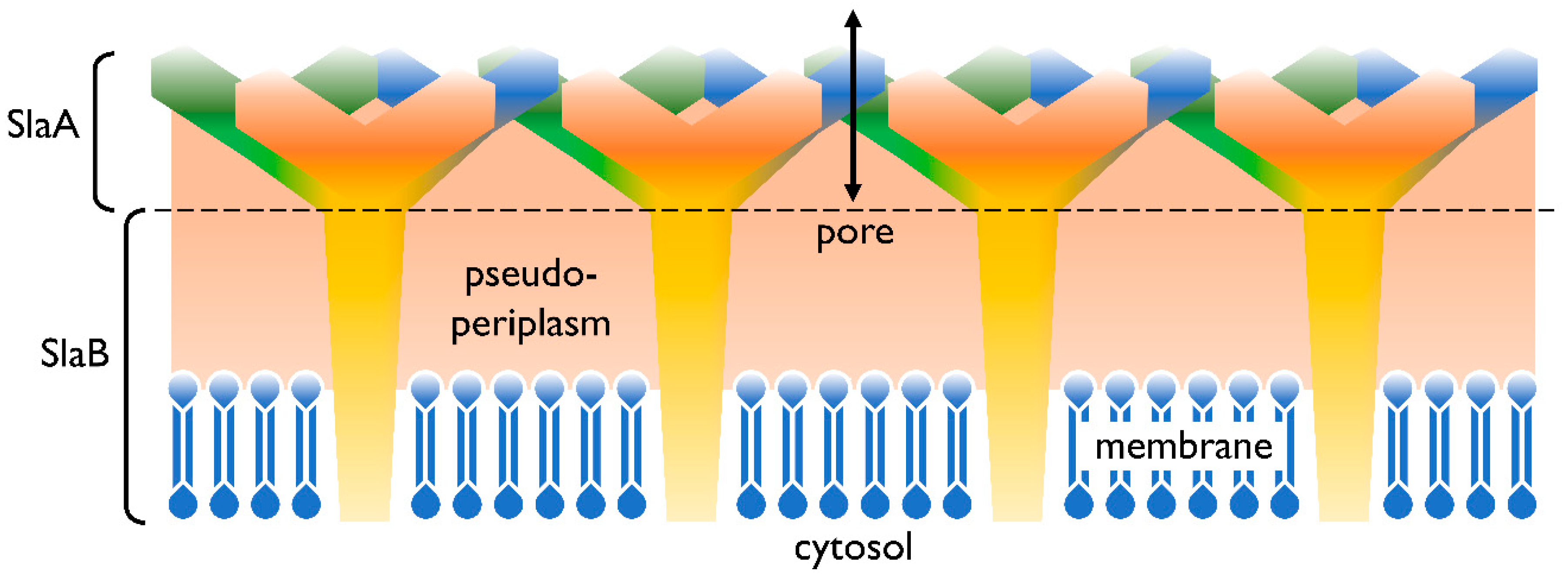
2.1. Cell Membrane Structure
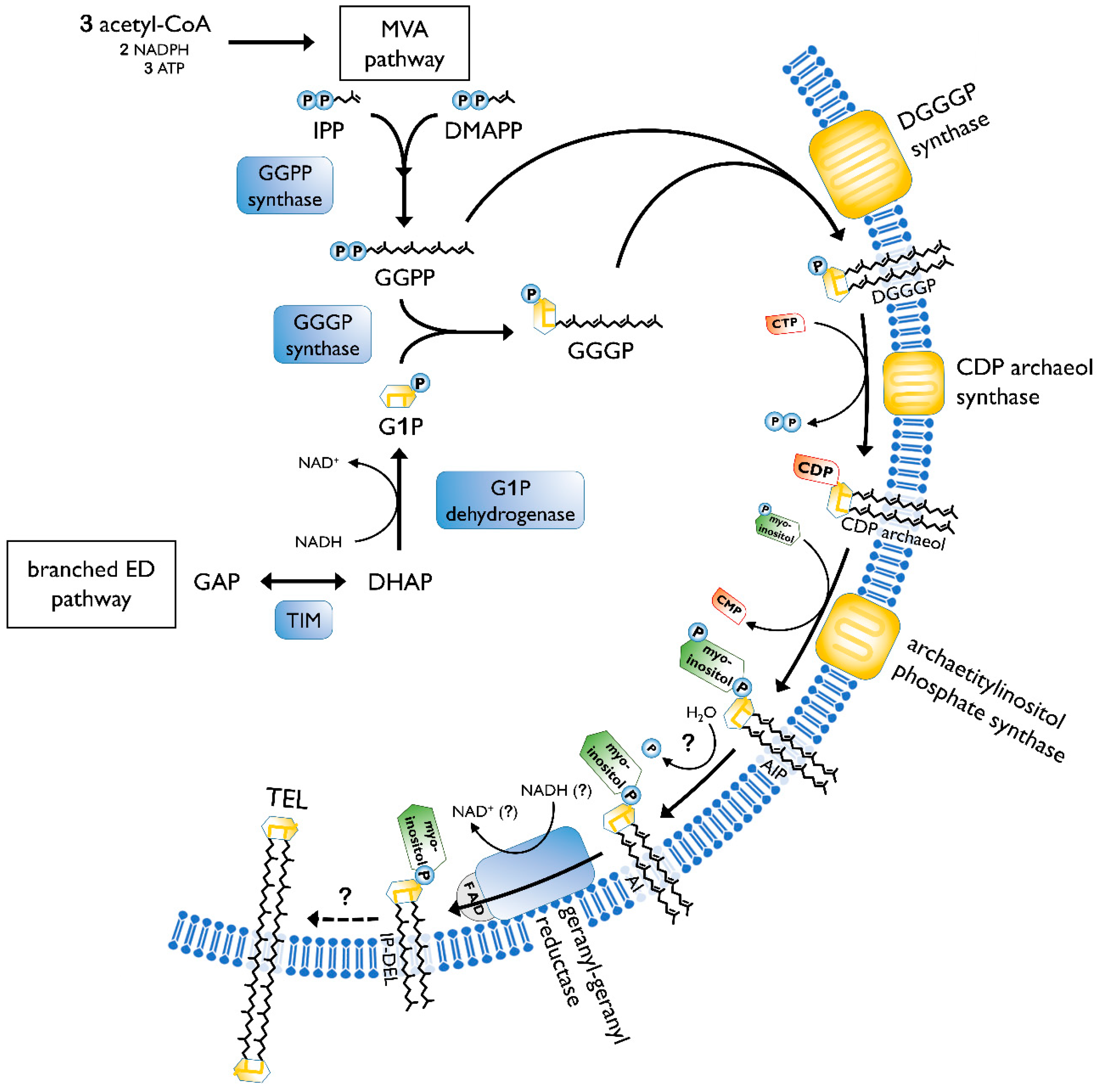
2.2. Biosynthesis of Archaeal Membrane Lipids
2.3. Effect of Cyclopentane Rings, DEL:TEL Ratio and Length of Carbon Chain on the Archaeal Membrane
2.4. Membrane Regulators
3. Homeoviscous Adaption
3.1. Temperature
3.2. pH
3.3. Growth Rate
4. Extraction and Lipid Analysis
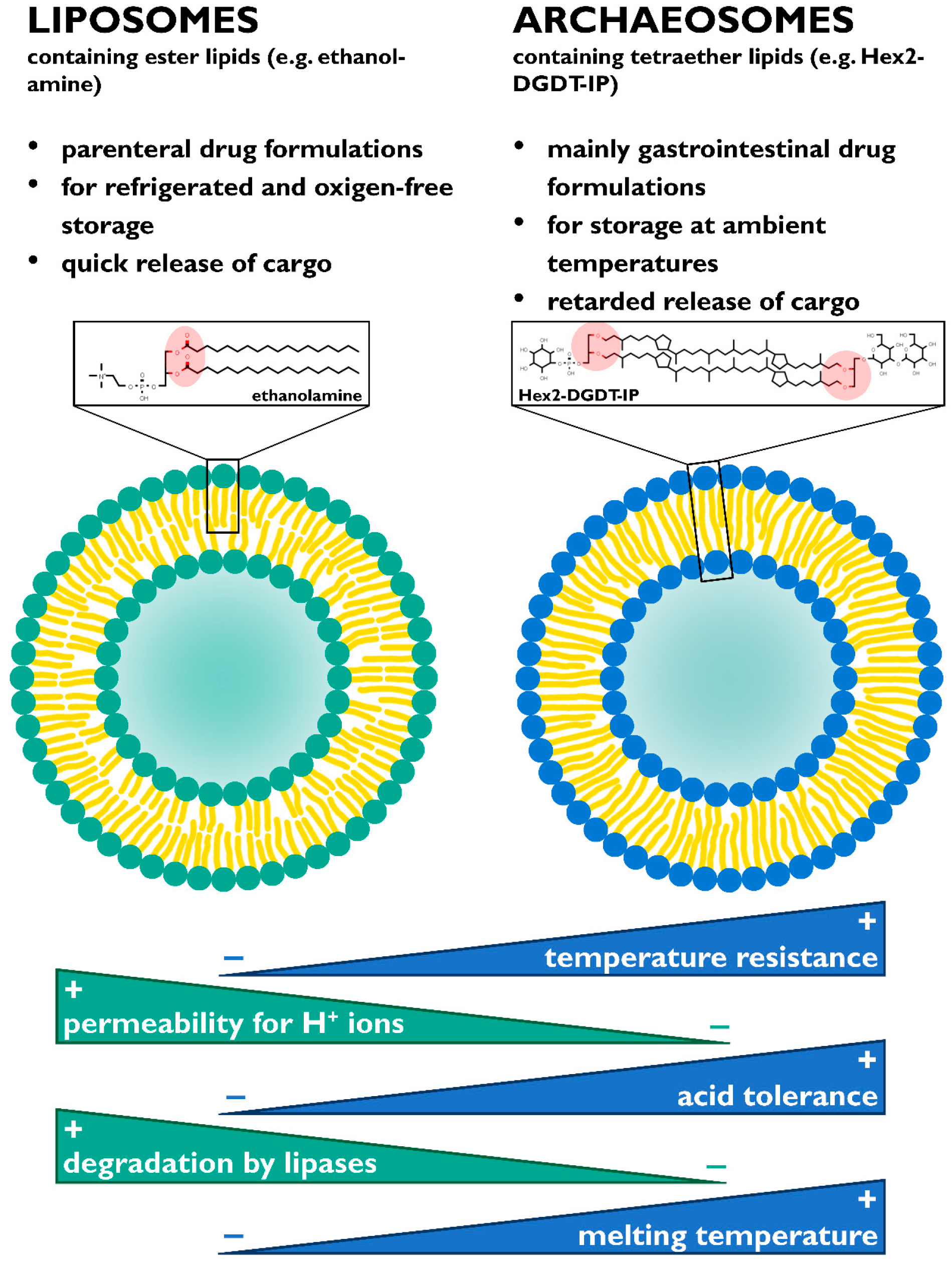
5. Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EDTA | ethylenediamine tetraacetic acid |
| SDS | sodium dodecyl sulfate |
| DMSO | dimethyl sulfoxide |
| NADH | nicotinamide adenine dinucleotide hydrogen |
| NADPH | nicotinamide adenine dinucleotide phosphate hydrogen |
| MVA | mevalonate pathway |
| IPP | isopentyl pyrophosphate |
| DMAPP | dimethylallyl pyrophosphate |
| GPP | geranyl diphosphate |
| GGGP | geranylgeranylglyceryl diphosphate |
| DHAP | dihydroxyacetone phosphate |
| DGGGP | 2,3-O-geranylgeranylglyceryl diphosphate |
| CTP | cytidine triphosphate |
| CDP | cytidine diphosphate |
| Grs | GDGT ring synthases |
| SAM | S-adenosylmethionine |
| DEL | diether lipid |
| TEL | tetraether lipid |
| RI | ring index, cyclization number |
| SARC | super-acid-resistant Crenarchaeota |
| TLC | thin layer chromatography |
| cds | calditol synthase gene |
| DPH | 1,6-diphenylhexatriene |
| AFM | atomic force microscopy |
| Hsp | heat shock proteins |
| MTBE | methyl-tert-butyl-ether |
| MS | mass spectrometry |
| ESI | electrospray ionization |
| EI | electron impact |
| MALDI | matrix assisted laser desorption/ionization |
| LC | liquid chromatography |
| Nomenclature lipids | |
| DGD | archaeol, dialkyl glycerol diether |
| GDGT | caldarchaeol, glycerol dialkyl glycerol tetraether |
| GDNT | calditolglycerocaldarchaeol, glycerol dialkyl nonitoltetraether |
| GDGT-[0–8] | GDGT with 0 - 8 cyclopentane rings in its structure |
| PG | diphytanylglycerol analogue of phosphatidylglycerol |
| PGP | diphytanyl-glycerol analogue of phosphatidylglycerol-phosphate |
| Headgroups | |
| IP | inositolphosphate |
| Me | methyl group |
| Hex | hexose |
| Hex2 | dihexose |
| Sulfono-Hex3 | sulfonated trihexose |
References
- Chattopadhyay, A. Membrane Organization and Dynamics; Springer: Berlin, Germany, 2017; ISBN 978-3-319-66601-3. [Google Scholar]
- Sinensky, M. Homeoviscous adaptation—A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Rilfors, L.; Lindblom, G. Regulation of lipid composition in biological membranes—biophysical studies of lipids and lipid synthesizing enzymes. Colloids Surf. B: Biointerfaces 2002, 26, 112–124. [Google Scholar] [CrossRef]
- Lindblom, G.; Hauksson, J.; Rilfors, L.; Bergenståhl, B.; Wieslander, A.; Eriksson, P.-O. Membrane lipid regulation in Acholeplasma laidlawii grown with saturated fatty acids: Biosynthesis of a triacylglucolipid forming reversed micelles. J. Biol. Chem. 1993, 268, 16198–16207. [Google Scholar] [PubMed]
- Rietveld, A.G.; Killian, J.A.; Dowhan, W.; de Kruijff, B. Polymorphic regulation of membrane phospholipid composition in Escherichia coli. J. Biol. Chem. 1993, 268, 12427–12433. [Google Scholar]
- De Kruijff, B. Polymorphic regulation of membrane lipid composition. Nature 1987, 329, 587–588. [Google Scholar] [CrossRef][Green Version]
- Parsons, J.B.; Rock, C.O. Bacterial Lipids: Metabolism and Membrane Homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef]
- Jensen, S.M.; Brandl, M.; Treusch, A.H.; Ejsing, C.S. Structural characterization of ether lipids from the archaeon Sulfolobus islandicus by high-resolution shotgun lipidomics. J. Mass Spectrom. 2015, 50, 476–487. [Google Scholar] [CrossRef]
- Rampelotto, P.H. Extremophiles and Extreme Environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Archiv. Mikrobiol. 1972, 84, 54–68. [Google Scholar] [CrossRef]
- Moll, R.; Schäfer, G. Chemiosmotic H+ cycling across the plasma membrane of the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. FEBS Lett. 1988, 232, 359–363. [Google Scholar] [CrossRef]
- Patel, G.B.; Sprott, G.D. Archaeobacterial Ether Lipid Liposomes (Archaeosomes) as Novel Vaccine and Drug Delivery Systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef] [PubMed]
- Benvegnu, T.; Lemiègre, L.; Cammas-Marion, S. New generation of liposomes called archaeosomes based on natural or synthetic archaeal lipids as innovative formulations for drug delivery. Recent Pat. Drug Deliv. Formul. 2009, 3, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, J.; Shen, L.; Albers, S.-V.; Siebers, B.; Spadiut, O. Sulfolobus—A Potential Key Organism in Future Biotechnology. Front Microbiol. 2017, 8, 2474. [Google Scholar] [CrossRef] [PubMed]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef]
- Nicolaus, B.; Gambacorta, A.; Basso, A.L.; Riccio, R.; De Rosa, M.; Grant, W.D. Trehalose in Archaebacteria. Syst. Appl. Microbiol. 1988, 10, 215–217. [Google Scholar] [CrossRef]
- Charlesworth, J.; Burns, B. Untapped Resources: Biotechnological Potential of Peptides and Secondary Metabolites in Archaea. Archaea 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Peng, N.; Han, W.; Li, Y.; Liang, Y.; She, Q. Genetic technologies for extremely thermophilic microorganisms of Sulfolobus, the only genetically tractable genus of crenarchaea. Sci. Chin. Life Sci. 2017, 60, 370–385. [Google Scholar] [CrossRef]
- Grogan, D.W. Phenotypic characterization of the archaebacterial genus Sulfolobus: Comparison of five wild-type strains. J. Bacteriol. 1989, 171, 6710–6719. [Google Scholar] [CrossRef]
- Van de Vossenberg, J.L.C.M.; Driessen, A.J.M.; Konings, W.N. The essence of being extremophilic: The role of the unique archaeal membrane lipids. Extremophiles 1998, 2, 163–170. [Google Scholar] [CrossRef]
- Chen, L.; Brügger, K.; Skovgaard, M.; Redder, P.; She, Q.; Torarinsson, E.; Greve, B.; Awayez, M.; Zibat, A.; Klenk, H.-P.; et al. The Genome of Sulfolobus acidocaldarius, a Model Organism of the Crenarchaeota. J. Bacteriol. 2005, 187, 4992–4999. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.M.; Shand, R.F. Halocins and sulfolobicins: The emerging story of archaeal protein and peptide antibiotics. J. Ind. Microbiol. Biotechnol. 2002, 28, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Brügger, K.; Liu, C.; Shah, S.A.; Zheng, H.; Zhu, Y.; Wang, S.; Lillestøl, R.K.; Chen, L.; Frank, J.; et al. Genome Analyses of Icelandic Strains of Sulfolobus islandicus, Model Organisms for Genetic and Virus-Host Interaction Studies. J. Bacteriol. 2011, 193, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.-V.; Meyer, B. The archaeal cell envelope. Nat. Rev. Microbiol. 2011, 9, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.-V.; Jarrell, K.F. The archaellum: How Archaea swim. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Glansdorff, N. Extremophiles—Volume I; EOLSS Publications: Abu Dhabi, UAE, 2009; ISBN 978-1-905839-93-3. [Google Scholar]
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67. [Google Scholar] [CrossRef]
- Weiss, R. Subunit Cell Wall of Sulfolobus acidocaldarius. J. Bacteriol. 1974, 118, 275–284. [Google Scholar] [CrossRef]
- Veith, A.; Klingl, A.; Zolghadr, B.; Lauber, K.; Mentele, R.; Lottspeich, F.; Rachel, R.; Albers, S.-V.; Kletzin, A. Acidianus, Sulfolobus and Metallosphaera surface layers: Structure, composition and gene expression. Mol. Microbiol. 2009, 73, 58–72. [Google Scholar] [CrossRef]
- Gambelli, L.; Meyer, B.H.; McLaren, M.; Sanders, K.; Quax, T.E.F.; Gold, V.A.M.; Albers, S.-V.; Daum, B. Architecture and modular assembly of Sulfolobus S-layers revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 2019, 116, 25278–25286. [Google Scholar] [CrossRef]
- Kate, M. Chapter 9 Membrane lipids of archaea. In New Comprehensive Biochemistry; Kates, M., Kushner, D.J., Matheson, A.T., Eds.; Elsevier: New York, NY, USA, 1993; Volume 26, pp. 261–295. [Google Scholar]
- Klingl, A. S-layer and cytoplasmic membrane—exceptions from the typical archaeal cell wall with a focus on double membranes. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Sugai, A.; Sakuma, R.; Fukuda, I.; Kurosawa, N.; Itoh, Y.H.; Kon, K.; Ando, S.; Itoh, T. The structure of the core polyol of the ether lipids from Sulfolobus acidocaldarius. Lipids 1995, 30, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Blériot, Y.; Untersteller, E.; Fritz, B.; Sinaÿ, P. Total Synthesis of Calditol: Structural Clarification of this Typical Component of Archaea Order Sulfolobales. Chem.—Eur. J. 2002, 8, 240–246. [Google Scholar] [CrossRef]
- De Rosa, M.; De Rosa, S.; Gambacorta, A.; Bu’Lockt, J.D. Structure of calditol, a new branched-chain nonitol, and of the derived tetraether lipids in thermoacidophile archaebacteria of the Caldariella group. Phytochemistry 1980, 19, 249–254. [Google Scholar] [CrossRef]
- Gibson, J.A.E.; Miller, M.R.; Davies, N.W.; Neill, G.P.; Nichols, D.S.; Volkman, J.K. Unsaturated diether lipids in the psychrotrophic archaeon Halorubrum lacusprofundi. Syst. Appl. Microbiol. 2005, 28, 19–26. [Google Scholar] [CrossRef]
- Koga, Y.; Nishihara, M.; Morii, H.; Akagawa-Matsushita, M. Ether polar lipids of methanogenic bacteria: Structures, comparative aspects, and biosyntheses. Microbiol. Rev. 1993, 57, 164–182. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.-L.; Wei, J.H.; Summons, R.E.; Welander, P.V. Calditol-linked membrane lipids are required for acid tolerance in Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA 2018, 115, 12932–12937. [Google Scholar] [CrossRef]
- Morii, H.; Koga, Y. Tetraether type polar lipids increase after logarithmic growth phase of Methanobacterium thermoautotrophicum in compensation for the decrease of diether lipids. FEMS Microbiol. Lett. 1993, 109, 283–287. [Google Scholar] [CrossRef]
- Tornabene, T.G.; Langworthy, T.A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science 1979, 203, 51–53. [Google Scholar] [CrossRef]
- Jensen, S.M.; Neesgaard, V.L.; Skjoldbjerg, S.L.N.; Brandl, M.; Ejsing, C.S.; Treusch, A.H. The Effects of Temperature and Growth Phase on the Lipidomes of Sulfolobus islandicus and Sulfolobus tokodaii. Life 2015, 5, 1539–1566. [Google Scholar] [CrossRef]
- Quehenberger, J.; Pittenauer, E.; Allmaier, G.; Spadiut, O. The influence of the specific growth rate on the lipid composition of Sulfolobus acidocaldarius. Extremophiles 2020. [Google Scholar] [CrossRef]
- Nicolaus, B.; Lanzotti, V.; Trincone, A.; De Rosa, M.; Grant, W.D.; Gambacorta, A. Glycine betaine and polar lipid composition in halophilic archaebacteria in response to growth in different salt concentrations. FEMS Microbiol. Lett. 1989, 59, 157–160. [Google Scholar] [CrossRef][Green Version]
- Angelini, R.; Corral, P.; Lopalco, P.; Ventosa, A.; Corcelli, A. Novel ether lipid cardiolipins in archaeal membranes of extreme haloalkaliphiles. Biochim. Biophys. Acta 2012, 1818, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Esposito, E.; Gambacorta, A.; Nicolaus, B.; Bu’Lock, J.D. Effects of Temperature on ether lipid composition of Caldariella acidophila. Phytochemistry 1980, 19, 827–831. [Google Scholar] [CrossRef]
- Bode, M.L.; Buddoo, S.R.; Minnaar, S.H.; du Plessis, C.A. Extraction, isolation and NMR data of the tetraether lipid calditoglycerocaldarchaeol (GDNT) from Sulfolobus metallicus harvested from a bioleaching reactor. Chem. Phys. Lipids 2008, 154, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.D.; Gambacorta, A.; Nicolaus, B.; Chappe, B.; Albrecht, P. Isoprenoid ethers; backbone of complex lipids of the archaebacterium Sulfolobus solfataricus. Biochim. Biophys. Acta (BBA) Metab. 1983, 753, 249–256. [Google Scholar] [CrossRef]
- Gliozzi, A.; Paoli, G.; De Rosa, M.; Gambacorta, A. Effect of isoprenoid cyclization on the transition temperature of lipids in thermophilic archaebacteria. Biochim. Biophys. Acta (BBA)—Biomembr. 1983, 735, 234–242. [Google Scholar] [CrossRef]
- Jain, S.; Caforio, A.; Driessen, A.J.M. Biosynthesis of archaeal membrane ether lipids. Front. Microbiol. 2014, 5, 641. [Google Scholar] [CrossRef]
- Boucher, Y.; Kamekura, M.; Doolittle, W.F. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol. Microbiol. 2004, 52, 515–527. [Google Scholar] [CrossRef]
- Koga, Y.; Morii, H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol. Mol. Biol. Rev. 2007, 71, 97–120. [Google Scholar] [CrossRef]
- Koga, Y. Biosynthesis and evolution of archaeal membranes and ether phospholipids. In Biogenesis of Fatty Acids, Lipids and Membranes; Geiger, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 539–552. ISBN 978-3-319-50430-8. [Google Scholar]
- Morii, H.; Kiyonari, S.; Ishino, Y.; Koga, Y. A novel biosynthetic pathway of archaetidyl-myo-inositol via archaetidyl-myo-inositol phosphate from CDP-archaeol and D-glucose 6-phosphate in methanoarchaeon Methanothermobacter thermautotrophicus cells. J. Biol. Chem. 2009, 284, 30766–30774. [Google Scholar] [CrossRef]
- Villanueva, L.; Damsté, J.S.S.; Schouten, S. A re-evaluation of the archaeal membrane lipid biosynthetic pathway. Nat. Rev. Microbiol. 2014, 12, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, X.-L.; Farley, K.R.; Wei, J.H.; Metcalf, W.W.; Summons, R.E.; Welander, P.V. GDGT cyclization proteins identify the dominant archaeal sources of tetraether lipids in the ocean. Proc. Nat. Acad. Sci. USA 2019, 116. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Delago, A.; Nußbaum, P.; Meyer, B.; Albers, S.-V.; Eichler, J. Gene deletions leading to a reduction in the number of cyclopentane rings in Sulfolobus acidocaldarius tetraether lipids. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, A.; Caracciolo, G.; Trabasso, D.; Izzo, I.; Spinella, A.; Sodano, G. Biosynthesis of calditol, the cyclopentanoid containing moiety of the membrane lipids of the archaeon Sulfolobus solfataricus. Tetrahedron Lett. 2002, 43, 451–453. [Google Scholar] [CrossRef]
- Yamauchi, N.; Ueoka, H.; Kamada, N.; Murae, T. Resemblance of Carbocycle Formation from Carbohydrates between Archaea and Eucarya/Eubacteria. Biosynthesis of Calditol, the Characteristic Lipid-Content Molecule in Sulfolobus acidocaldarius. Bull. Chem. Soc. Jpn. 2004, 77, 771–778. [Google Scholar] [CrossRef]
- Macalady, J.L.; Vestling, M.M.; Baumler, D.; Boekelheide, N.; Kaspar, C.W.; Banfield, J.F. Tetraether-linked membrane monolayers in Ferroplasma spp: A key to survival in acid. Extremophiles 2004, 8, 411–419. [Google Scholar] [CrossRef]
- Schouten, S.; Hopmans, E.C.; Sinninghe Damsté, J.S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org. Geochem. 2013, 54, 19–61. [Google Scholar] [CrossRef]
- Zhai, Y.; Lee-Gau Chong, P.; Taylor, L.J.-A.; Erlkamp, M.; Grobelny, S.; Czeslik, C.; Watkins, E.; Winter, R. Physical Properties of Archaeal Tetraether Lipid Membranes As Revealed by Differential Scanning and Pressure Perturbation Calorimetry, Molecular Acoustics, and Neutron Reflectometry: Effects of Pressure and Cell Growth Temperature. Langmuir 2012, 28, 5211–5217. [Google Scholar] [CrossRef]
- Heremans, K. Chapter 1—Pressure—Temperature effects on protein conformational states. In Chemistry at Extreme Conditions; Manaa, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–27. ISBN 978-0-444-51766-1. [Google Scholar]
- Chong, P.L.-G.; Sulc, M.; Winter, R. Compressibilities and Volume Fluctuations of Archaeal Tetraether Liposomes. Biophys. J. 2010, 99, 3319–3326. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Chong, P.L.-G. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Chong, P.L.-G.; Ayesa, U.; Prakash Daswani, V.; Hur, E.C. On physical properties of tetraether lipid membranes: Effects of cyclopentane rings. Archaea 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.G.; Patel, G.B.; Sprott, G.D.; Beveridge, T.J. Stability of pressure-extruded liposomes made from archaeobacterial ether lipids. Appl. Microbiol. Biotechnol. 1994, 42, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.G.; Patel, G.B.; Sprott, G.D. Heat sterilization of archaeal liposomes. Can. J. Microbiol. 1996, 42, 183–186. [Google Scholar] [CrossRef]
- Patel, G.B.; Agnew, B.J.; Deschatelets, L.; Fleming, L.P.; Sprott, G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000, 194, 39–49. [Google Scholar] [CrossRef]
- Oger, P.M.; Cario, A. Adaptation of the membrane in Archaea. Biophys. Chem. 2013, 183, 42–56. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Saunders, L.P.; Sen, S.; Wilkinson, B.J.; Gatto, C. Insights into the Mechanism of Homeoviscous Adaptation to Low Temperature in Branched-Chain Fatty Acid-Containing Bacteria through Modeling FabH Kinetics from the Foodborne Pathogen Listeria monocytogenes. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Akasaka, K.; Matsuki, H. High Pressure Bioscience: Basic Concepts, Applications and Frontiers; Springer: Berlin, Germany, 2015; ISBN 978-94-017-9918-8. [Google Scholar]
- Nichols, D.S.; Miller, M.R.; Davies, N.W.; Goodchild, A.; Raftery, M.; Cavicchioli, R. Cold Adaptation in the Antarctic Archaeon Methanococcoides burtonii Involves Membrane Lipid Unsaturation. J. Bacteriol. 2004, 186, 8508–8515. [Google Scholar] [CrossRef]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef]
- Salvador-Castell, M.; Tourte, M.; Oger, P.M. In Search for the Membrane Regulators of Archaea. Int. J. Mol. Sci. 2019, 20, 4434. [Google Scholar] [CrossRef]
- Pearson, A.; Pi, Y.; Zhao, W.; Li, W.-J.; Li, Y.; Inskeep, W.; Perevalova, A.; Romanek, C.; Li, S.; Zhang, C. Factors Controlling the Distribution of Archaeal Tetraethers in Terrestrial Hot Springs. Appl. Environ. Microbiol. 2008, 74, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Weber, Y.; Chiu, B.K.; Elling, F.J.; Cobban, A.B.; Pearson, A.; Leavitt, W.D. Energy flux controls tetraether lipid cyclization in Sulfolobus acidocaldarius. Environ. Microbiol. 2020, 22, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bischof, L.F.; Haurat, M.F.; Hoffmann, L.; Albersmeier, A.; Wolf, J.; Neu, A.; Pham, T.K.; Albaum, S.A.; Jakobi, T.; Schouten, S.; et al. Early Response of Sulfolobus acidocaldarius to Nutrient Limitation. Front. Microbiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y. Thermal Adaptation of the Archaeal and Bacterial Lipid Membranes. Archaea 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Hafenbradl, D.; Keller, M.; Stetter, K.O. Lipid analysis of Methanopyrus kandleri. FEMS Microbiol. Lett. 1996, 136, 199–202. [Google Scholar] [CrossRef]
- Cario, A.; Grossi, V.; Schaeffer, P.; Oger, P.M. Membrane homeoviscous adaptation in the piezo-hyperthermophilic archaeon Thermococcus barophilus. Front. Microbiol. 2015, 6, 1152. [Google Scholar] [CrossRef]
- Matsuno, Y.; Sugai, A.; Higashibata, H.; Fukuda, W.; Ueda, K.; Uda, I.; Sato, I.; Itoh, T.; Imanaka, T.; Fujiwara, S. Effect of Growth Temperature and Growth Phase on the Lipid Composition of the Archaeal Membrane from Thermococcus kodakaraensis. Biosci. Biotechnol. Biochem. 2009, 73, 104–108. [Google Scholar] [CrossRef]
- Wuchter, C.; Schouten, S.; Coolen, M.J.L.; Damasté, J.S.S. Temperature-dependent variation in the distribution of tetraether membrane lipids of marine Crenarchaeota: Implications for TEX86 paleothermometry. Paleoceanography 2004, 19. [Google Scholar] [CrossRef]
- Boyd, E.S.; Pearson, A.; Pi, Y.; Li, W.-J.; Zhang, Y.G.; He, L.; Zhang, C.L.; Geesey, G.G. Temperature and pH controls on glycerol dibiphytanyl glycerol tetraether lipid composition in the hyperthermophilic crenarchaeon Acidilobus sulfurireducens. Extremophiles 2011, 15, 59–65. [Google Scholar] [CrossRef]
- Pineda De Castro, L.F.; Dopson, M.; Friedman, R. Biological Membranes in Extreme Conditions: Simulations of Anionic Archaeal Tetraether Lipid Membranes. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, B.; Cai, G.; Fu, L.; Wu, Y.; Wang, X.; Ren, B.; Ma, H. A proton shelter inspired by the sugar coating of acidophilic archaea. Sci. Rep. 2012, 2, 892. [Google Scholar] [CrossRef] [PubMed]
- Auernik, K.S.; Cooper, C.R.; Kelly, R.M. Life in hot acid: Pathway analyses in extremely thermoacidophilic archaea. Curr. Opin. Biotechnol. 2008, 19, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef]
- Shimada, H.; Nemoto, N.; Shida, Y.; Oshima, T.; Yamagishi, A. Effects of pH and Temperature on the Composition of Polar Lipids in Thermoplasma acidophilum HO-62. J. Bacteriol. 2008, 190, 5404–5411. [Google Scholar] [CrossRef]
- Chong, P.L.-G. Archaebacterial bipolar tetraether lipids: Physico-chemical and membrane properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar] [CrossRef]
- McCarthy, S.; Johnson, T.; Pavlik, B.L.; Payne, S.; Schackwitz, W.; Martin, J.; Lipzen, A.; Keffeler, E.; Blum, P. Expanding the Limits of Thermoacidophily in the Archaeon Sulfolobus solfataricus by Adaptive Evolution. Appl. Environ. Microbiol. 2016, 82. [Google Scholar] [CrossRef]
- Buetti-Dinh, A.; Dethlefsen, O.; Friedman, R.; Dopson, M. Transcriptomic analysis reveals how a lack of potassium ions increases Sulfolobus acidocaldarius sensitivity to pH changes. Microbiology 2016, 162, 1422–1434. [Google Scholar] [CrossRef]
- Roy, M.; Gupta, S. The oligomeric plasticity of Hsp20 of Sulfolobus acidocaldarius protects environment-induced protein aggregation and membrane destabilization. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 2549–2565. [Google Scholar] [CrossRef]
- Yang, L.L.; Haug, A. Structure of membrane lipids and physico-biochemical properties of the plasma membrane from Thermoplasma acidophilum, adapted to growth at 37 °C. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1979, 573, 308–320. [Google Scholar] [CrossRef]
- Uda, I.; Sugai, A.; Itoh, Y.H.; Itoh, T. Variation in molecular species of polar lipids from Thermoplasma acidophilum depends on growth temperature. Lipids 2001, 36, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Eggers, L.F.; Schwudke, D. Liquid extraction: Folch. In Encyclopedia of Lipidomics; Wenk, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–6. ISBN 978-94-007-7864-1. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, A.; Eggers, L.F.; Schwudke, D. Liquid extraction: Bligh and dyer. In Encyclopedia of Lipidomics; Wenk, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–4. ISBN 978-94-007-7864-1. [Google Scholar]
- Jensen, S.K. Improved Bligh and Dyer extraction procedure. Lipid Technol. 2008, 20, 280–281. [Google Scholar] [CrossRef]
- Liu, X.; Moon, S.H.; Mancuso, D.J.; Jenkins, C.M.; Guan, S.; Sims, H.F.; Gross, R.W. Oxidized Fatty Acid Analysis by Charge Switch Derivatization, Selected Reaction Monitoring and Accurate Mass Quantification. Anal. Biochem. 2013, 442. [Google Scholar] [CrossRef]
- Huguet, C.; Martens-Habbena, W.; Urakawa, H.; Stahl, D.A.; Ingalls, A.E. Comparison of extraction methods for quantitative analysis of core and intact polar glycerol dialkyl glycerol tetraethers (GDGTs) in environmental samples. Limnol. Oceanogr. Methods 2010, 8, 127–145. [Google Scholar] [CrossRef]
- Ståhlman, M. Lipid extraction: Quantitative recovery. In Encyclopedia of Lipidomics; Wenk, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–2. [Google Scholar]
- Chen, I.S.; Shen, C.S.J.; Sheppard, A.J. Comparison of methylene chloride and chloroform for the extraction of fats from food products. J. Am. Oil Chem. Soc. 1981, 58, 599–601. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Kulakovskaya, E.; Kulakovskaya, T. Chapter 2—Methods for studying yeast extracellular glycolipids. In Extracellular Glycolipids of Yeasts; Kulakovskaya, E., Kulakovskaya, T., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 15–28. ISBN 978-0-12-420069-2. [Google Scholar]
- Fuchs, B.; Süß, R.; Teuber, K.; Eibisch, M.; Schiller, J. Lipid analysis by thin-layer chromatography—A review of the current state. J. Chromatogr. A 2011, 1218, 2754–2774. [Google Scholar] [CrossRef]
- Roberts, L.D.; McCombie, G.; Titman, C.M.; Griffin, J.L. A matter of fat: An introduction to lipidomic profiling methods. J. Chromatogr. B 2008, 871, 174–181. [Google Scholar] [CrossRef]
- Knittelfelder, O.L.; Weberhofer, B.P.; Eichmann, T.O.; Kohlwein, S.D.; Rechberger, G.N. A versatile ultra-high performance LC-MS method for lipid profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 951, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Byrdwell, W.C. Chapter 4—APCI-MS in lipid analysis. In Advances in Lipid Methodology; Adlof, R.O., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 171–253. ISBN 978-0-9531949-6-4. [Google Scholar]
- Khoury, S.; Canlet, C.; Lacroix, M.Z.; Berdeaux, O.; Jouhet, J.; Bertrand-Michel, J. Quantification of Lipids: Model, Reality, and Compromise. Biomolecules 2018, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.P.; Thompson, D.H. Efficient Synthesis of 40- and 48-Membered Tetraether Macrocyclic Bisphosphocholines. Org. Lett. 1999, 1, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Huguet, C.; Hopmans, E.C.; Febo-Ayala, W.; Thompson, D.H.; Sinninghe Damsté, J.S.; Schouten, S. An improved method to determine the absolute abundance of glycerol dibiphytanyl glycerol tetraether lipids. Org. Geochem. 2006, 37, 1036–1041. [Google Scholar] [CrossRef]
- Gambacorta, A.; Gliozzi, A.; De Rosa, M. Archael lipids and their biotechnological applications. World J. Microbiol. Biotechnol. 1995, 11, 115–131. [Google Scholar] [CrossRef]
- Brown, D.A.; Venegas, B.; Cooke, P.H.; English, V.; Chong, P.L.-G. Bipolar tetraether archaeosomes exhibit unusual stability against autoclaving as studied by dynamic light scattering and electron microscopy. Chem. Phys. Lipids 2009, 159, 95–103. [Google Scholar] [CrossRef]
- Benvegnu, T.; Lemiègre, L.; Cammas-Marion, S. Archaeal Lipids: Innovative Materials for Biotechnological Applications. Eur. J. Org. Chem. 2008, 2008, 4725–4744. [Google Scholar] [CrossRef]
- Lo, S.-L.; Chang, E.L. Purification and characterization of a liposomal-forming tetraether lipid fraction. Biochem. Biophys. Res. Commun. 1990, 167, 238–243. [Google Scholar] [CrossRef]
- Komatsu, H.; Chong, P.L.-G. Low Permeability of Liposomal Membranes Composed of Bipolar Tetraether Lipids from Thermoacidophilic Archaebacterium Sulfolobus acidocaldarius. Biochemistry 1998, 37, 107–115. [Google Scholar] [CrossRef]
- Kaur, G.; Garg, T.; Rath, G.; Goyal, A.K. Archaeosomes: An excellent carrier for drug and cell delivery. Drug Deliv. 2016, 23, 2497–2512. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.M.; Christensen, C.J.; Petersen, J.M.; Treusch, A.H.; Brandl, M. Liposomes containing lipids from Sulfolobus islandicus withstand intestinal bile salts: An approach for oral drug delivery? Int. J. Pharm. 2015, 493, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, J.; Sun, W.; Xu, Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem. Biophys. Res. Commun. 2010, 394, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Parmentier, J.; Thewes, B.; Gropp, F.; Fricker, G. Oral peptide delivery by tetraether lipid liposomes. Int. J. Pharm. 2011, 415, 150–157. [Google Scholar] [CrossRef]
- Uhl, P.; Helm, F.; Hofhaus, G.; Brings, S.; Kaufman, C.; Leotta, K.; Urban, S.; Haberkorn, U.; Mier, W.; Fricker, G. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur. J. Pharm. Biopharm. 2016, 103, 159–166. [Google Scholar] [CrossRef]
- Jung, I.-W.; Han, H.-K. Effective mucoadhesive liposomal delivery system for risedronate: Preparation and in vitro/in vivo characterization. Int. J. Nanomed. 2014, 9, 2299–2306. [Google Scholar] [CrossRef]
- Parmentier, J.; Hofhaus, G.; Thomas, S.; Cuesta, L.C.; Gropp, F.; Schröder, R.; Hartmann, K.; Fricker, G. Improved Oral Bioavailability of Human Growth Hormone by a Combination of Liposomes Containing Bio-Enhancers and Tetraether Lipids and Omeprazole. J. Pharm. Sci. 2014, 103, 3985–3993. [Google Scholar] [CrossRef]
- Moghimipour, E.; Kargar, M.; Ramezani, Z.; Handali, S. The Potent In Vitro Skin Permeation of Archaeosome Made from Lipids Extracted of Sulfolobus acidocaldarius. Archaea 2013, 2013, e782012. [Google Scholar] [CrossRef]
- Mahmoud, G.; Jedelská, J.; Strehlow, B.; Bakowsky, U. Bipolar tetraether lipids derived from thermoacidophilic archaeon Sulfolobus acidocaldarius for membrane stabilization of chlorin e6 based liposomes for photodynamic therapy. Eur. J. Pharm. Biopharm. 2015, 95. [Google Scholar] [CrossRef]
- Engelhardt, K.H.; Pinnapireddy, S.R.; Baghdan, E.; Jedelská, J.; Bakowsky, U. Transfection Studies with Colloidal Systems Containing Highly Purified Bipolar Tetraether Lipids from Sulfolobus acidocaldarius. Available online: https://www.hindawi.com/journals/archaea/2017/8047149/ (accessed on 3 February 2020).
| Membrane Lipids | Sulfolobales | Natronococcus | Methanobacterium | Reference |
|---|---|---|---|---|
| C20-20 DGD | Σ15%–30% | Σ57%–77% | Σ12.8%–50% | [40,41,42,43,44] |
| DGD | ~5% | n.d. | + | [38,42,43] |
| IP-DGD | 10%-30% | n.d. | + | [38,42,43] |
| PG | - | 8–26% | - | [44] |
| PGP | - | 49–51% | - | [44] |
| PGP-Me | - | + | - | [45] |
| C20-25 DGD | - | Σ22%–44% | - | [44] |
| PG | - | 8%–9% | - | [44] |
| PGP | - | 14%–35% | - | [44] |
| C40-40 TEL (GDNT/GDGT) * | Σ70–85% | - | Σ50%–83% (GDGT only) | [40,41,42,43] |
| TEL | 7–9% | - | n.d. | [42,43] |
| Hex2-TEL | 57.8% | - | n.d. | [42,43] |
| IP-TEL | 3–36% | - | n.d. | [42,43] |
| Sulfono-Hex3-TEL-IP | 1–11% | - | n.d. | [42,43] |
| Hex-TEL-IP | + | - | n.d. | [9] |
| Hex2-TEL-IP | 4%–82% | - | n.d. | [42,43] |
| C40-40 GDNT | Σ 68–80% | - | - | [34,46,47,48,49] |
| Hex-GDNT | + | - | - | [46,47] |
| IP-GDNT | + | - | - | [46,47] |
| Cultivation Conditions | Modifications | ||
|---|---|---|---|
| Ring Index (RI) | DEL:TEL Ratio | Headgroups | |
| Temperature | positive [42,46] | impacted, but no distinct trend determined [42] | impacted, but no distinct trend determined |
| pH | not investigated | not investigated | calditol important for acid resistance [39] |
| Growth Rate | Negative [43,78,79] | negative [43] impacted, but no distinct trend determined [42] | impacted, but no distinct trend determined [42,43] |
| Field of Application | Specific Use | Properties Influenced by TELs | Reference |
|---|---|---|---|
| Oral Drug Delivery | Delivery of insulin; antibiotic, cancer, hepatitis B and D, as well as osteoporosis treatment | Protection against drug degradation in the gastrointestinal tract | [124,125,126,127,128,129] |
| Dermal Delivery | Delivery of a methylene blue as drug model through rat skin | Improved skin permeation | [130] |
| Intravenous Drug Delivery | Photodynamic therapy for anti-cancer treatment | Increased membrane rigidity for controlled release | [131] |
| Gene Delivery | Transfection of mammalian cells with TEL containing lipid nanoparticles | Increased stability and transfection efficiency | [132] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastädter, K.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. The Cell Membrane of Sulfolobus spp.—Homeoviscous Adaption and Biotechnological Applications. Int. J. Mol. Sci. 2020, 21, 3935. https://doi.org/10.3390/ijms21113935
Rastädter K, Wurm DJ, Spadiut O, Quehenberger J. The Cell Membrane of Sulfolobus spp.—Homeoviscous Adaption and Biotechnological Applications. International Journal of Molecular Sciences. 2020; 21(11):3935. https://doi.org/10.3390/ijms21113935
Chicago/Turabian StyleRastädter, Kerstin, David J. Wurm, Oliver Spadiut, and Julian Quehenberger. 2020. "The Cell Membrane of Sulfolobus spp.—Homeoviscous Adaption and Biotechnological Applications" International Journal of Molecular Sciences 21, no. 11: 3935. https://doi.org/10.3390/ijms21113935
APA StyleRastädter, K., Wurm, D. J., Spadiut, O., & Quehenberger, J. (2020). The Cell Membrane of Sulfolobus spp.—Homeoviscous Adaption and Biotechnological Applications. International Journal of Molecular Sciences, 21(11), 3935. https://doi.org/10.3390/ijms21113935





