A New Method for Collecting Large Amounts of Symbiotic Gastrodermal Cells from Octocorals
Abstract
1. Introduction
2. Results and Discussion
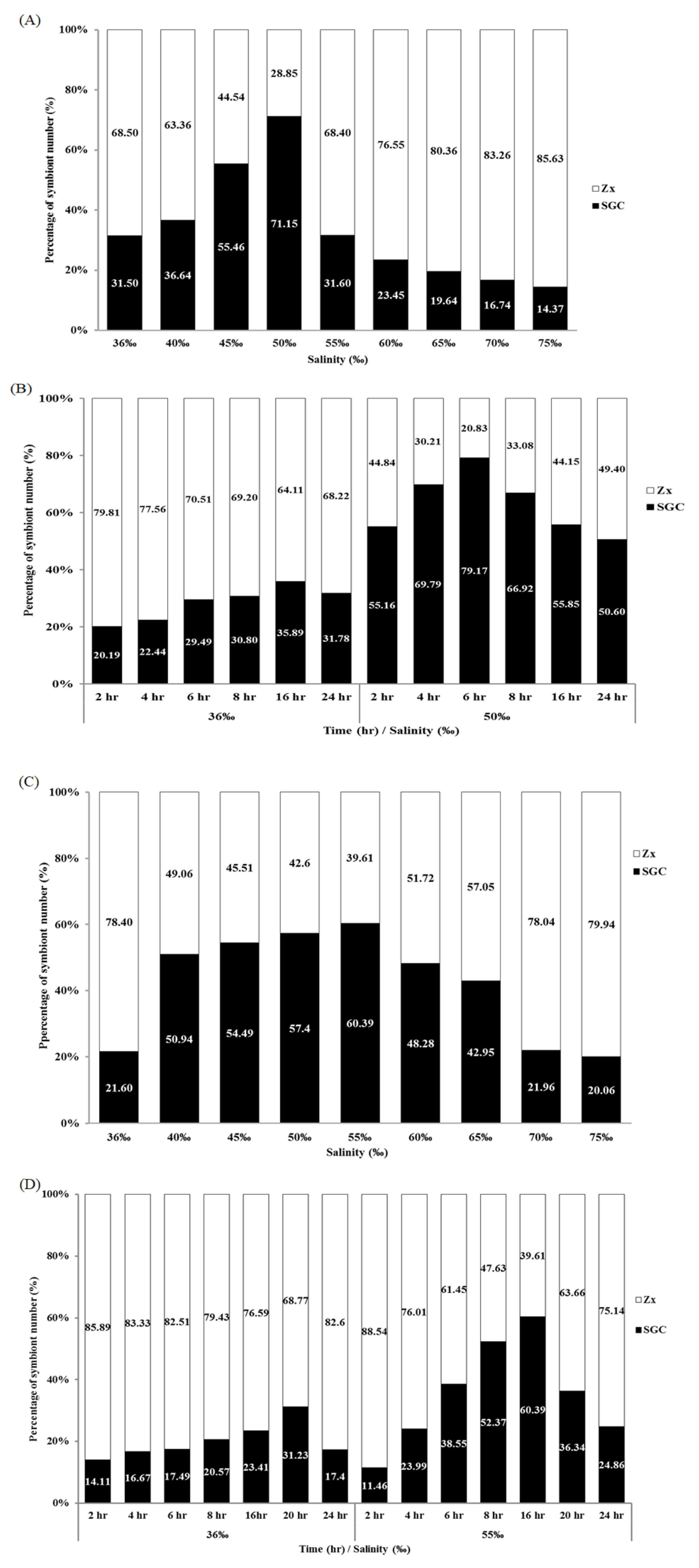
2.1. Incubation of Octocorals in a High-Salinity Solution Increased the Amount of SGCs Released from the Tentacles
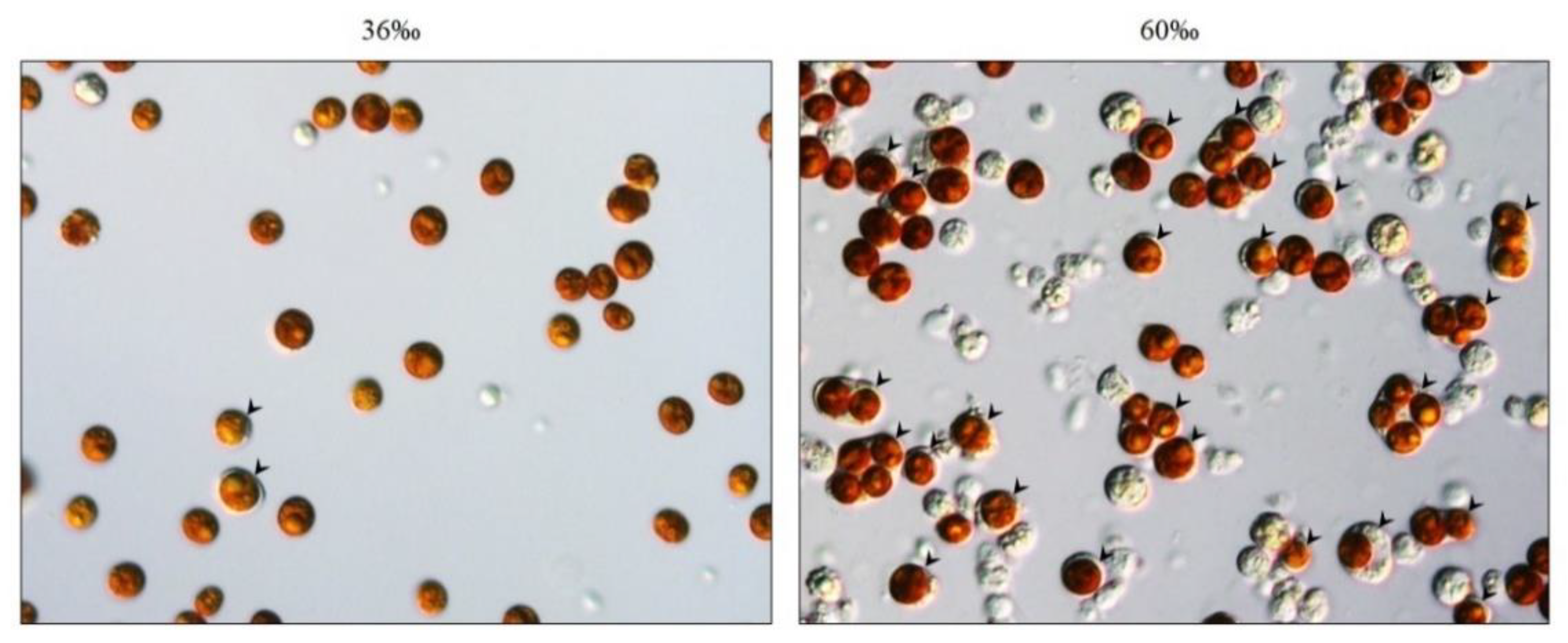
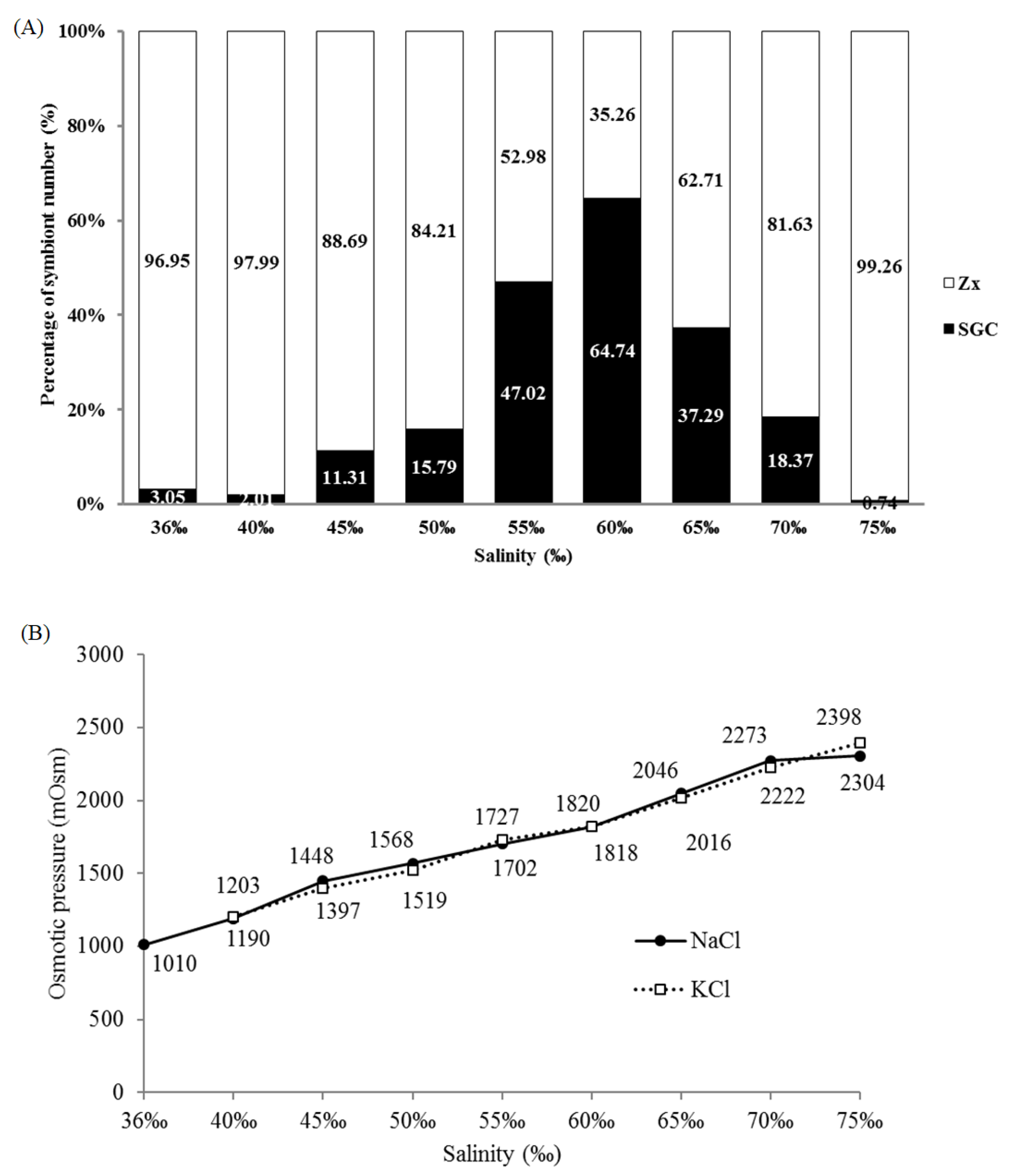
2.2. Salinity and Osmotic Pressure Affect the Ratio of SGCs Released in S. flexibilis
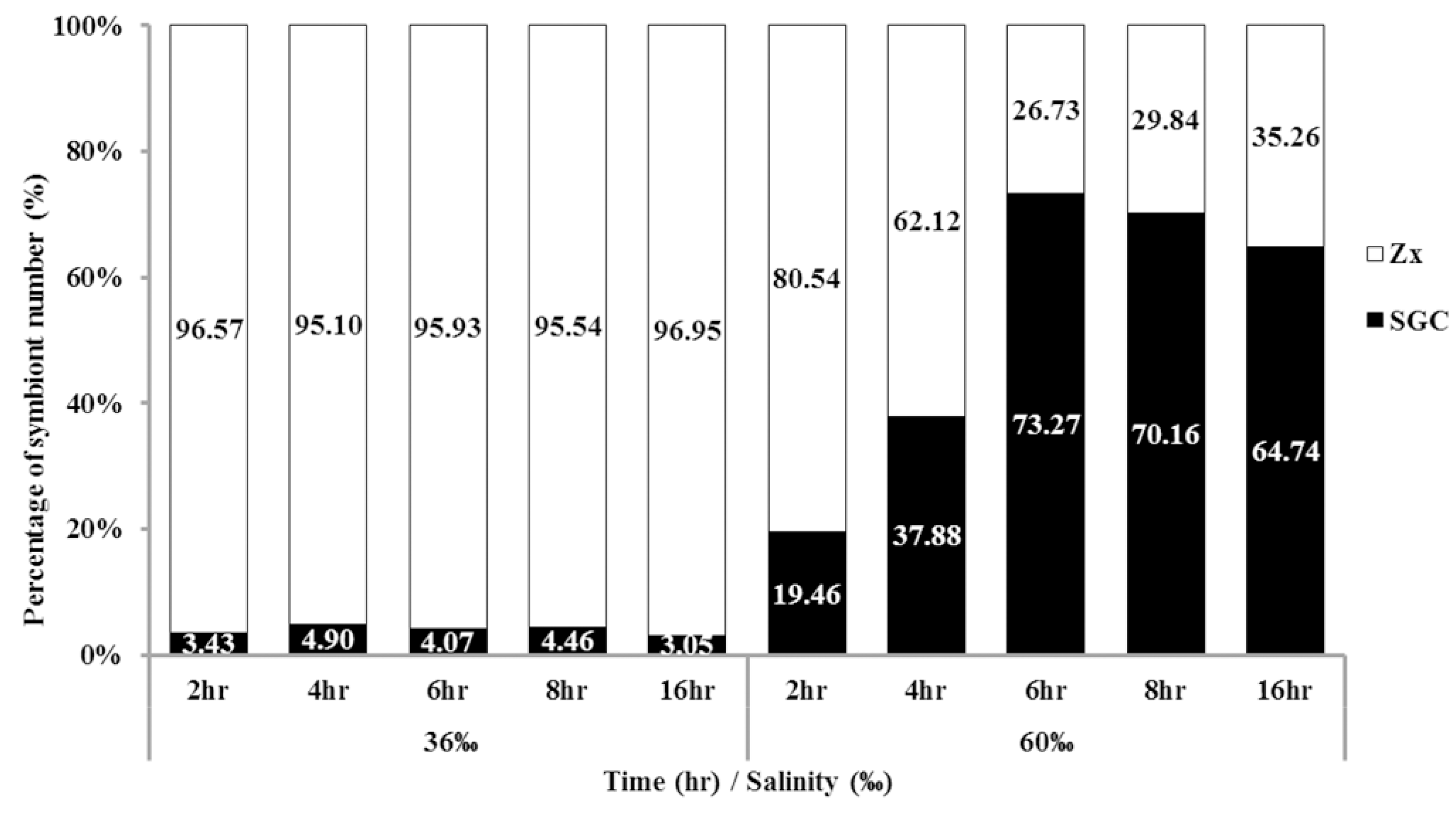
2.3. Time Affects the Ratio of SGCs Released from S. flexibilis
2.4. Salinity Affects Tissue Morphology in S. flexibilis
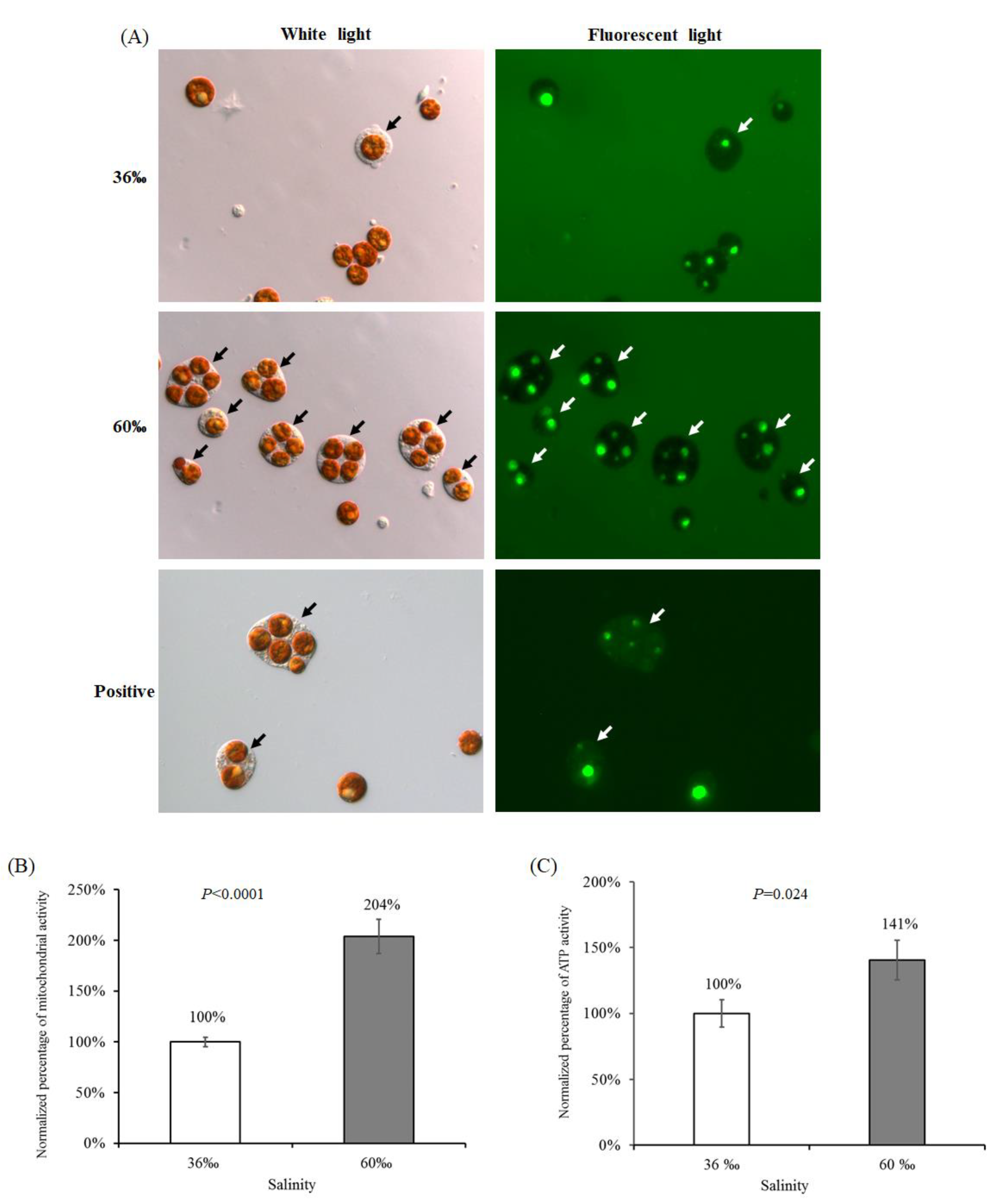
2.5. The Viability of Cells Treated with 60‰ FSW in S. flexibilis
2.6. Salinity and Time Affect the Ratio of SGCs Released from P. thyrsoides and S. compressa
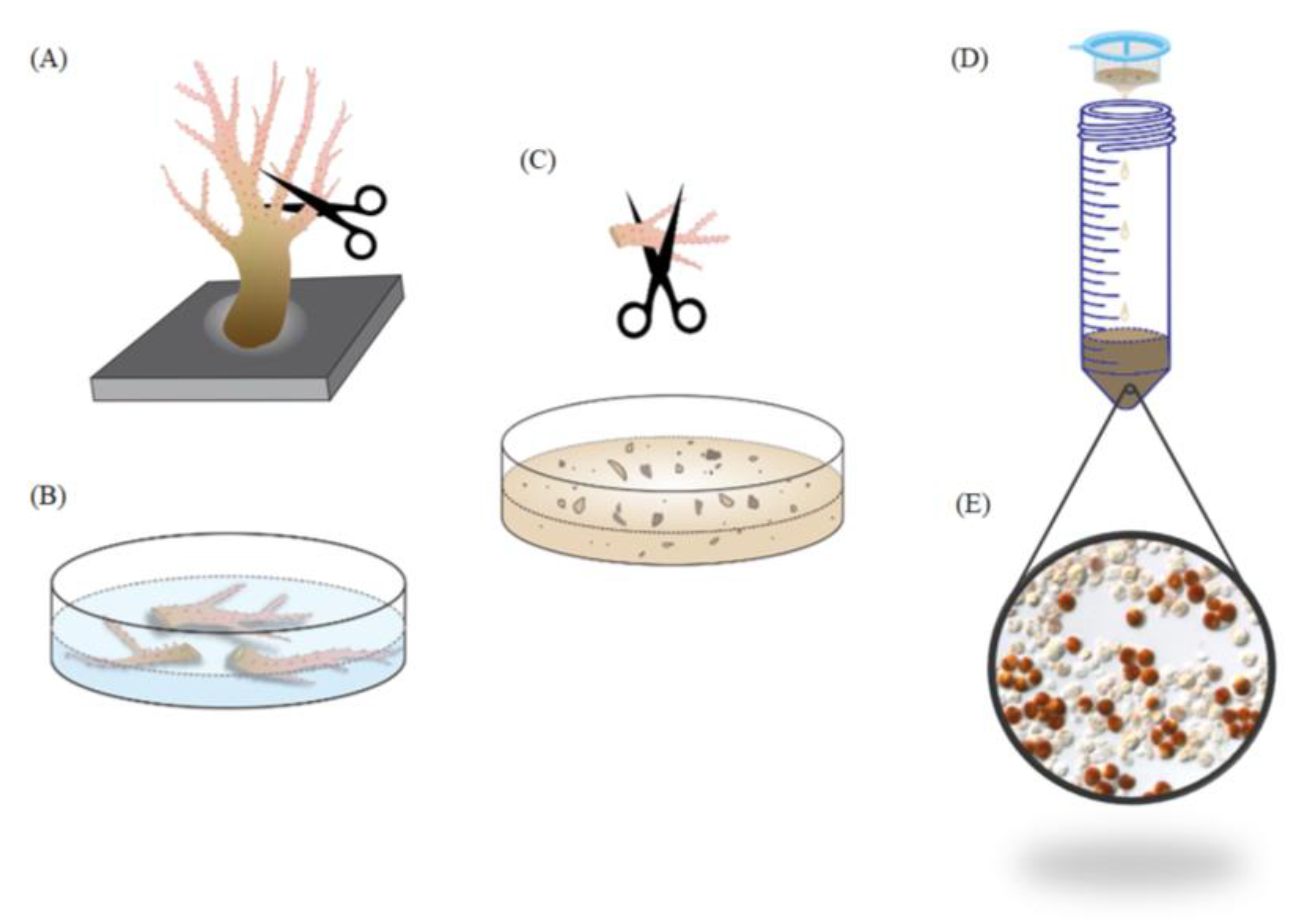
3. Materials and Methods
3.1. Reagents and Culture Media
3.2. Coral Collection and Maintenance
3.3. Quantifying the Ratio of SGCs
3.4. Hematoxylin & Eosin Staining (H&E Staining)
3.5. Viability Assay
3.5.1. Cell Intactness Assay
3.5.2. Mitochondrial Activity Assay
3.5.3. Adenosine Triphosphate (ATP) Activity Assay
3.6. Measurement of Osmolality
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SGCs | Symbiotic Gastrodermal Cells |
| FSW | Filtered Sea Water |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| ATP | Adenosine Triphosphate |
References
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Trench, R.K. Microalgal-invertebrate symbiosis, a review. Endocytobiosis Cell Res. 1993, 9, 135–175. [Google Scholar]
- Peters, E.C. Diseases of Corals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 85–107. ISBN 978-0-813-82411-6. [Google Scholar] [CrossRef]
- Rosental, B.; Kozhekbaeva, Z.; Fernhoff, N.; Tsai, J.M.; Traylor-Knowles, N. Coral cell separation and isolation by fluorescence-activated cell sorting (FACS). BMC Cell Biol. 2017, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Lin, H.P.; Yeh, C.C.; Fang, L.S. Use of a fluorescent membrane probe to identify zooxanthellae in hospite among dissociated endoderm cell culture from coral. Protoplasma 2005, 226, 175–179. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, L.H.; Peng, S.E.; Hsiao, Y.Y.; Chang, C.Y.; Fang, L.S.; Chen, C.S. New cell model for endosymbiosis research: Spontaneous dissociation of endoderm cells based on tissue polarity in coral. Platax 2007, 4, 9–25. [Google Scholar]
- Chen, C.S.; Yeh, S.P.; Wang, L.H.; Li, H.H.; Chen, U.W. Increased susceptibility of algal symbionts to photo-inhibition resulting from the perturbation of coral gastrodermal membrane trafficking. Sci. China Life Sci. 2012, 55, 599–611. [Google Scholar] [CrossRef][Green Version]
- Muscatine, L.; Pool, R.R.; Trench, R.K. Symbiosis of algae and invertebrates: Aspects of the symbiont surface and the host-symbiont interface. Trans. Am. Microsc. Soc. 1975, 94, 450–469. [Google Scholar] [CrossRef]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Peng, S.E.; Chen, W.N.; Chen, H.K.; Lu, C.Y.; Mayfield, A.B.; Fang, L.S.; Chen, C.S. Lipid bodies in coral-dinoflagellate endosymbiosis: Proteomic and ultrastructural studies. Proteomics 2011, 11, 3540–3555. [Google Scholar] [CrossRef]
- Li, H.H.; Huang, Z.Y.; Ye, S.P.; Lu, C.Y.; Cheng, P.C.; Chen, S.H.; Chen, C.S. Membrane labeling of coral gastrodermal cells by biotinylation: The proteomic identification of surface proteins involving cnidaria-dinoflagellate endosymbiosis. PLoS ONE 2014, 9, e85119. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Huang, Z.Y.; Lin, C.Y.; Wang, L.H.; Chou, P.H.; Chen, C.S.; Li, H.H. Generation of clade- and symbiont-specific antibodies to characterize marker molecules during Cnidaria-Symbiodinium endosymbiosis. Sci. Rep. 2017, 7, 5488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Liu, Y.H.; Ju, Y.W.; Hsiao, Y.Y.; Fang, L.S.; Chen, C.S. Cell cycle propagation is driven by light–dark stimulation in a cultured symbiotic dinoflagellate isolated from corals. Coral. Reefs 2008, 27, 823–835. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, L.H.; Chen, W.N.U.; Fang, L.S.; Chen, C.S. Developmentally regulated localization of endosymbiotic dinofagellates in different tissue layers of coral larvae. Coral. Reefs 2008, 27, 365–372. [Google Scholar] [CrossRef]
- Luo, Y.L.; Wang, L.H.; Chen, W.N.U.; Peng, S.E.; Tzen, J.T.C.; Hsiao, Y.Y.; Huang, H.J.; Fang, L.S.; Chen, C.S. Ratiometric imaging of gastrodermal lipid bodies in coral–dinoflagellate endosymbiosis. Coral. Reefs 2009, 28, 289–301. [Google Scholar] [CrossRef]
- Chen, H.K.; Song, S.N.; Wang, L.H.; Mayfield, A.B.; Chen, Y.J.; Chen, W.N.; Chen, C.S. A compartmental comparison of major lipid species in a coral-Symbiodinium endosymbiosis: Evidence that the coral host regulates lipogenesis of its cytosolic lipid bodies. PLoS ONE 2015, 10, e0132519. [Google Scholar] [CrossRef]
- Weis, V.M.; Davy, S.K.; Hoegh-Guldberg, O.; Rodriguez-Lanetty, M.; Pringle, J.R. Cell biology in model systems as the key to understanding corals. Trends Ecol. Evol. 2008, 23, 369–376. [Google Scholar] [CrossRef]
- Lehnert, E.M.; Burriesci, M.S.; Pringle, J.R. Developing the anemone Aiptasia as a tractable model for cnidarian-dinoflagellate symbiosis: The transcriptome of aposymbiotic A. pallida. BMC Genom. 2012, 13, 271. [Google Scholar] [CrossRef]
- Grajales, A.; Rodriguez, E. Morphological revision of the genus Aiptasia and the family Aiptasiidae (Cnidaria, Actiniaria, Metridioidea). Zootaxa 2014, 3826, 55–100. [Google Scholar] [CrossRef]
- Chen, W.N.; Hsiao, Y.J.; Mayfield, A.B.; Young, R.; Hsu, L.L.; Peng, S.E. Transmission of a heterologous clade C Symbiodinium in a model anemone infection system via asexual reproduction. Peer J. 2016, 4, e2358. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, E.A.; Guse, A.; Pringle, J.R. Similar specificities of symbiont uptake by adults and larvae in an anemone model system for coral biology. J. Exp. Biol. 2014, 217, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.S.; Huang, H.D.; Dai, C.F.; Hsiao, Y.C.; Benayahu, Y. Sclerite calcification and reef-building in the fleshy octocoral genus Sinularia (Octocorallia: Alcyonacea). Coral. Reefs 2011, 30, 925–933. [Google Scholar] [CrossRef]
- Shoham, E.; Benayahu, Y. Higher species richness of octocorals in the upper mesophotic zone in Eilat (Gulf of Aqaba) compared to shallower reef zones. Coral. Reefs 2016, 36, 71–81. [Google Scholar] [CrossRef]
- Khalesi, M.K.; Vera-Jimenez, N.I.; Aanen, D.K.; Beeftink, H.H.; Wijffels, R.H. Cell cultures from the symbiotic soft coral Sinularia flexibilis. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 330–338. [Google Scholar] [CrossRef]
- Aceret, T.L.; Coll, J.C.; Uchio, Y.; Sammarco, P.W. Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia). Comp. Biochem. Physiol. Part C Pharm. Toxicol. Endocrinol. 1998, 120, 121–126. [Google Scholar] [CrossRef]
- Neoh, C.A.; Wang, R.Y.; Din, Z.H.; Su, J.H.; Chen, Y.K.; Tsai, F.J.; Weng, S.H.; Wu, Y.J. Induction of apoptosis by sinulariolide from soft coral through mitochondrial-related and p38MAPK pathways on human bladder carcinoma cells. Mar. Drugs 2012, 10, 2893–2911. [Google Scholar] [CrossRef]
- Li, H.H.; Su, J.H.; Chiu, C.C.; Lin, J.J.; Yang, Z.Y.; Hwang, W.I.; Chen, Y.K.; Lo, Y.H.; Wu, Y.J. Proteomic investigation of the sinulariolide-treated melanoma cells A375: Effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Mar. Drugs 2013, 11, 2625–2642. [Google Scholar] [CrossRef]
- Chen, Y.J.; Su, J.H.; Tsao, C.Y.; Hung, C.T.; Chao, H.H.; Lin, J.J.; Liao, M.H.; Yang, Z.Y.; Huang, H.H.; Tsai, F.J.; et al. Sinulariolide induced hepatocellular carcinoma apoptosis through activation of mitochondrial-related apoptotic and PERK/eIF2alpha/ATF4/CHOP pathway. Molecules 2013, 18, 10146–10161. [Google Scholar] [CrossRef]
- Wu, Y.J.; Neoh, C.A.; Tsao, C.Y.; Su, J.H.; Li, H.H. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through mapks and pi3k/akt signaling pathways. Int. J. Mol. Sci. 2015, 16, 16469–16482. [Google Scholar] [CrossRef]
- Lin, J.J.; Su, J.H.; Tsai, C.C.; Chen, Y.J.; Liao, M.H.; Wu, Y.J. 11-epi-Sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling pathways. Mar. Drugs 2014, 12, 4783–4798. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Wang, R.Y.L.; Chen, J.C.; Chiu, C.C.; Liao, M.H.; Wu, Y.J. Cytotoxicity of 11-epi-sinulariolide acetate isolated from cultured soft corals on HA22T cells through the endoplasmic reticulum stress pathway and mitochondrial dysfunction. Int. J. Mol. Sci. 2016, 17, 1787. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, A.J.; Matson, J.A.; Bilayet Hossain, M.; van der Helm, D. Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral, Sinularia flexibilis. Tetrahedron Lett. 1977, 18, 2923–2926. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chen, N.F.; Chen, W.F.; Hung, H.C.; Lee, H.P.; Lin, Y.Y.; Wang, H.M.; Sung, P.J.; Sheu, J.H.; Wen, Z.H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Jean, Y.H.; Lee, H.P.; Chen, W.F.; Sun, Y.M.; Su, J.H.; Lu, Y.; Huang, S.Y.; Hung, H.C.; Sung, P.J.; et al. A soft coral-derived compound, 11-epi-sinulariolide acetate suppresses inflammatory response and bone destruction in adjuvant-induced arthritis. PLoS ONE 2013, 8, e62926. [Google Scholar] [CrossRef]
- Chen, N.F.; Huang, S.Y.; Lu, C.H.; Chen, C.L.; Feng, C.W.; Chen, C.H.; Hung, H.C.; Lin, Y.Y.; Sung, P.J.; Sung, C.S.; et al. Flexibilide obtained from cultured soft coral has anti-neuroinflammatory and analgesic effects through the upregulation of spinal transforming growth factor-beta1 in neuropathic rats. Mar. Drugs 2014, 12, 3792–3817. [Google Scholar] [CrossRef]
- Tsai, T.C.; Chen, H.Y.; Sheu, J.H.; Chiang, M.Y.; Wen, Z.H.; Dai, C.F.; Su, J.H. Structural elucidation and structure-anti-inflammatory activity relationships of cembranoids from cultured soft sorals Sinularia sandensis and Sinularia flexibilis. J. Agric. Food Chem. 2015, 63, 7211–7218. [Google Scholar] [CrossRef]
- Khalesi, M.K.; Beeftink, R.H.; Wijffels, R.H. The soft coral Sinularia flexibilis: Potential for drug development. Adv. Coral Husb. Public Aquar. 2001, 2, 47–60. [Google Scholar]
- Mayfield, A.B.; Gates, R.D. Osmoregulation in anthozoan-dinoflagellate symbiosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 1–10. [Google Scholar] [CrossRef]
- Goiran, C.; Allemand, D.; Galgani, I. Transient Na+ stress in symbiotic dinoflagellates after isolation from coral-host cells and subsequent immersion in seawater. Mar. Biol. 1997, 129, 581–589. [Google Scholar] [CrossRef]
- Suescun-Bolivar, L.P.; Iglesias-Prieto, R.; Thome, P.E. Induction of glycerol synthesis and release in cultured Symbiodinium. PLoS ONE 2012, 7, e47182. [Google Scholar] [CrossRef] [PubMed]
- Ochsenkuhn, M.A.; Rothig, T.; D’Angelo, C.; Wiedenmann, J.; Voolstra, C.R. The role of floridoside in osmoadaptation of coral-associated algal endosymbionts to high-salinity conditions. Sci. Adv. 2017, 3, e1602047. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-Y.; Lin, L.-Y.; Chen, Y.; Liu, E.-R.; Li, H.-H. A New Method for Collecting Large Amounts of Symbiotic Gastrodermal Cells from Octocorals. Int. J. Mol. Sci. 2020, 21, 3911. https://doi.org/10.3390/ijms21113911
Chiu H-Y, Lin L-Y, Chen Y, Liu E-R, Li H-H. A New Method for Collecting Large Amounts of Symbiotic Gastrodermal Cells from Octocorals. International Journal of Molecular Sciences. 2020; 21(11):3911. https://doi.org/10.3390/ijms21113911
Chicago/Turabian StyleChiu, Hsiang-Yi, Li-Yi Lin, Ying Chen, En-Ru Liu, and Hsing-Hui Li. 2020. "A New Method for Collecting Large Amounts of Symbiotic Gastrodermal Cells from Octocorals" International Journal of Molecular Sciences 21, no. 11: 3911. https://doi.org/10.3390/ijms21113911
APA StyleChiu, H.-Y., Lin, L.-Y., Chen, Y., Liu, E.-R., & Li, H.-H. (2020). A New Method for Collecting Large Amounts of Symbiotic Gastrodermal Cells from Octocorals. International Journal of Molecular Sciences, 21(11), 3911. https://doi.org/10.3390/ijms21113911



