Pharmacogenetics in Model-Based Optimization of Bevacizumab Therapy for Metastatic Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Data
2.2. PK Model
2.3. Binding QSS Model
2.4. PK/PD Model
3. Discussion
4. Materials and Methods
4.1. Cohort
4.2. Samples
4.3. Genotyping
4.4. Measurement of Bevacizumab Levels
4.5. Measurement of Free VEGF-A
4.6. Model Development and Co-Variate Assessment
4.7. PK Model
4.8. Binding QSS Model
4.9. PK/PD Model
4.10. Model Evaluation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell Mol. Med. 2005, 9, 777–794. [Google Scholar] [CrossRef]
- Pavlidis, E.T.; Pavlidis, T.E. Role of bevacizumab in colorectal cancer growth and its adverse effects: A review. World J. Gastroenterol. 2013, 19, 5051–5060. [Google Scholar] [CrossRef]
- Piao, Y.; Henry, V.; Tiao, N.; Park, S.Y.; Martinez-Ledesma, J.; Dong, J.W.; Balasubramaniyan, V.; de Groot, J.F. Targeting intercellular adhesion molecule-1 prolongs survival in mice bearing bevacizumab-resistant glioblastoma. Oncotarget 2017, 8, 96970–96983. [Google Scholar] [CrossRef]
- Zuckerman, L.A.; Pullen, L.; Miller, J. Functional Consequences of Costimulation by ICAM-1 on IL-2 Gene Expression and T Cell Activation. The Journal of Immunology 1998, 160, 3259–3268. [Google Scholar]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Cohen, M.H.; Gootenberg, J.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 2007, 12, 356–361. [Google Scholar] [CrossRef]
- Han-Chung, W.; Chia-Ting, H.; Chang, D.-K. Anti-Angiogenic Therapeutic Drugs for Treatment of Human Cancer. J. Cancer Mol. 2008, 4, 37–45. [Google Scholar]
- Hwang, C.; Heath, E.I. Angiogenesis inhibitors in the treatment of prostate cancer. J. Hematol. Oncol. 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.; Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B. 3rd Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Claret, L.; Gupta, M.; Han, K.; Joshi, A.; Sarapa, N.; He, J.; Powell, B.; Bruno, R. Evaluation of tumor-size response metrics to predict overall survival in Western and Chinese patients with first-line metastatic colorectal cancer. J. Clin. Oncol. 2013, 31, 2110–2114. [Google Scholar] [CrossRef]
- Ouerdani, A.; Goutagny, S.; Kalamarides, M.; Troconiz, I.F.; Ribba, B. Mechanism-based modeling of the clinical effects of bevacizumab and everolimus on vestibular schwannomas of patients with neurofibromatosis type 2. Cancer Chemother. Pharmacol. 2016, 77, 1263–1273. [Google Scholar] [CrossRef]
- Rocchetti, M.; Germani, M.; Del Bene, F.; Poggesi, I.; Magni, P.; Pesenti, E.; De Nicolao, G. Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth after administration of an anti-angiogenic agent, bevacizumab, as single-agent and combination therapy in tumor xenografts. Cancer Chemother. Pharmacol. 2013, 71, 1147–1157. [Google Scholar] [CrossRef]
- Sharan, S.; Woo, S. Systems pharmacology approaches for optimization of antiangiogenic therapies: Challenges and opportunities. Front. Pharmacol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Titz, B.; Kozak, K.R.; Jeraj, R. Computational modelling of anti-angiogenic therapies based on multiparametric molecular imaging data. Phys. Med. Biol. 2012, 57, 6079–6101. [Google Scholar] [CrossRef]
- Gibiansky, L.; Gibiansky, E. Target-mediated drug disposition model: Approximations, identifiability of model parameters and applications to the population pharmacokinetic-pharmacodynamic modeling of biologics. Expert. Opin. Drug. Metab. Toxicol. 2009, 5, 803–812. [Google Scholar] [CrossRef]
- Levy, G. Pharmacologic target-mediated drug disposition. Clin. Pharmacol. Ther. 1994, 56, 248–252. [Google Scholar] [CrossRef]
- Mager, D.E.; Jusko, W.J. General Pharmacokinetic Model for Drugs Exhibiting Target-Mediated Drug Disposition. J. Pharmacokinet. Pharmacodyn. 2001, 28, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.C.; Schindler, E.; Friberg, L.E. Population pharmacokinetic-pharmacodynamic modelling in oncology: A tool for predicting clinical response. Br. J. Clin. Pharmacol. 2015, 79, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Mould, D.R.; Sweeney, K.R. The pharmacokinetics and pharmacodynamics of monoclonal antibodies--mechanistic modeling applied to drug development. Curr. Opin. Drug Discov. Devel. 2007, 10, 84–96. [Google Scholar] [PubMed]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development. CPT. Pharmacomet. Syst. Pharmacol. 2012, 1, e6. [Google Scholar] [CrossRef]
- Caulet, M.; Lecomte, T.; Bouche, O.; Rollin, J.; Gouilleux-Gruart, V.; Azzopardi, N.; Leger, J.; Borg, C.; Douillard, J.Y.; Manfredi, S.; et al. Bevacizumab Pharmacokinetics Influence Overall and Progression-Free Survival in Metastatic Colorectal Cancer Patients. Clin. Pharmacokinet. 2016, 55, 1381–1394. [Google Scholar] [CrossRef]
- Han, K.; Peyret, T.; Marchand, M.; Quartino, A.; Gosselin, N.H.; Girish, S.; Allison, D.E.; Jin, J. Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother. Pharmacol. 2016, 78, 341–351. [Google Scholar] [CrossRef]
- Li, J.; Gupta, M.; Jin, D.; Xin, Y.; Visich, J.; Allison, D.E. Characterization of the long-term pharmacokinetics of bevacizumab following last dose in patients with resected stage II and III carcinoma of the colon. Cancer Chemother. Pharmacol. 2013, 71, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Bruno, R.; Eppler, S.; Novotny, W.; Lum, B.; Gaudreault, J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 2008, 62, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Panoilia, E.; Schindler, E.; Samantas, E.; Aravantinos, G.; Kalofonos, H.P.; Christodoulou, C.; Patrinos, G.P.; Friberg, L.E.; Sivolapenko, G. A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients. Cancer Chemother. Pharmacol. 2015, 75, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Barbolosi, D.; Ciccolini, J.; Lacarelle, B.; Barlesi, F.; Andre, N. Computational oncology--mathematical modelling of drug regimens for precision medicine. Nat. Rev. Clin. Oncol. 2016, 13, 242–254. [Google Scholar] [CrossRef]
- Gibiansky, L.; Gibiansky, E.; Kakkar, T.; Ma, P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J. Pharmacokinet. Pharmacodyn. 2008, 35, 573–591. [Google Scholar] [CrossRef]
- Koutras, A.K.; Antonacopoulou, A.G.; Eleftheraki, A.G.; Dimitrakopoulos, F.I.; Koumarianou, A.; Varthalitis, I.; Fostira, F.; Sgouros, J.; Briasoulis, E.; Bournakis, E.; et al. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharm. J. 2012, 12, 468–475. [Google Scholar] [CrossRef]
- Papachristos, A.; Kemos, P.; Katsila, T.; Panoilia, E.; Patrinos, G.P.; Kalofonos, H.; Sivolapenko, G.B. VEGF-A and ICAM-1 Gene Polymorphisms as Predictors of Clinical Outcome to First-Line Bevacizumab-Based Treatment in Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2019. [Google Scholar] [CrossRef]
- Schneider, B.P.; Wang, M.; Radovich, M.; Sledge, G.W.; Badve, S.; Thor, A.; Flockhart, D.A.; Hancock, B.; Davidson, N.; Gralow, J.; et al. Association of Vascular Endothelial Growth Factor and Vascular Endothelial Growth Factor Receptor-2 Genetic Polymorphisms With Outcome in a Trial of Paclitaxel Compared With Paclitaxel Plus Bevacizumab in Advanced Breast Cancer: ECOG 2100. J. Clin. Oncol. 2008, 26, 4672–4678. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Lafky, J.M.; Uhm, J.H.; Giannini, C.; Kumar, S.K.; Kimlinger, T.K.; Northfelt, D.W.; Flynn, P.J.; Jaeckle, K.A.; et al. Phase II Study of Bevacizumab in Combination with Sorafenib in Recurrent Glioblastoma (N0776): A North Central Cancer Treatment Group Trial. Clin. Cancer Res. 2013, 19, 4816. [Google Scholar] [CrossRef]
- Dowlati, A.; Gray, R.; Sandler, A.B.; Schiller, J.H.; Johnson, D.H. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non–small cell lung cancer treated with chemotherapy with or without bevacizumab—an eastern cooperative oncology group study. Clin. Cancer Res. 2008, 14, 1407–1412. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okamoto, W.; Makiyama, A.; Shitara, K.; Denda, T.; Ogura, T.; Nakano, Y.; Nishina, T.; Komoda, M.; Hara, H.; et al. Plasma ICAM-1 (pICAM-1) and plasma IL-8 (pIL-8) level as biomarker of metastatic colorectal cancer patients (mCRC) treated with mFOLFOX6/XELOX plus bevacizumab (BV) (WJOG7612GTR). J. Clin. Oncol. 2018, 36, 670. [Google Scholar] [CrossRef]
- Zhang, L.; Beal, S.L.; Sheinerz, L.B. Simultaneous vs. sequential analysis for population PK/PD data II: Robustness of methods. J. Pharm. Pharm. [CrossRef] [PubMed]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.; van Schaik, R.H.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte--an update of guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Ceze, N.; Lecomte, T.; Degenne, D.; Duveau, A.C.; Watier, H.; Dorval, E.; Paintaud, G. An enzyme-linked immunosorbent assay to study bevacizumab pharmacokinetics. Ther. Drug Monit. 2010, 32, 647–652. [Google Scholar] [CrossRef]
- Quantikine ELISA. Human VEGF Immunoassay. Available online: https://resources.rndsystems.com/pdfs/datasheets/dve00.pdf (accessed on 10 July 2018).
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/avastin#overview-section (accessed on 10 July 2018).
- EMA Summary of Product Characteristics. Avastin®. Available online: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf (accessed on 10 July 2018).
- Mousa, L.; Salem, M.E.; Mikhail, S. Biomarkers of Angiogenesis in Colorectal Cancer. Biomark. Cancer 2015, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
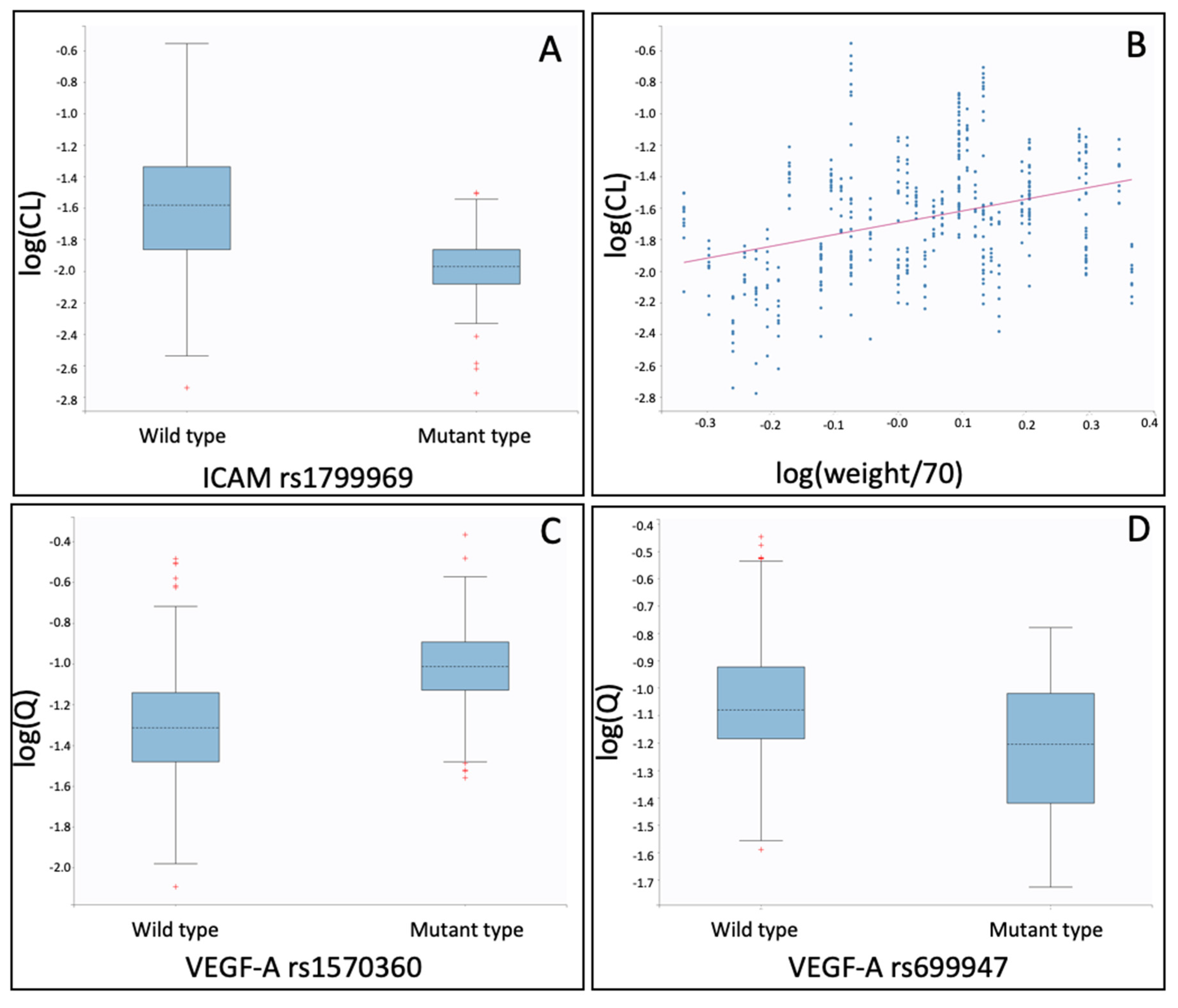
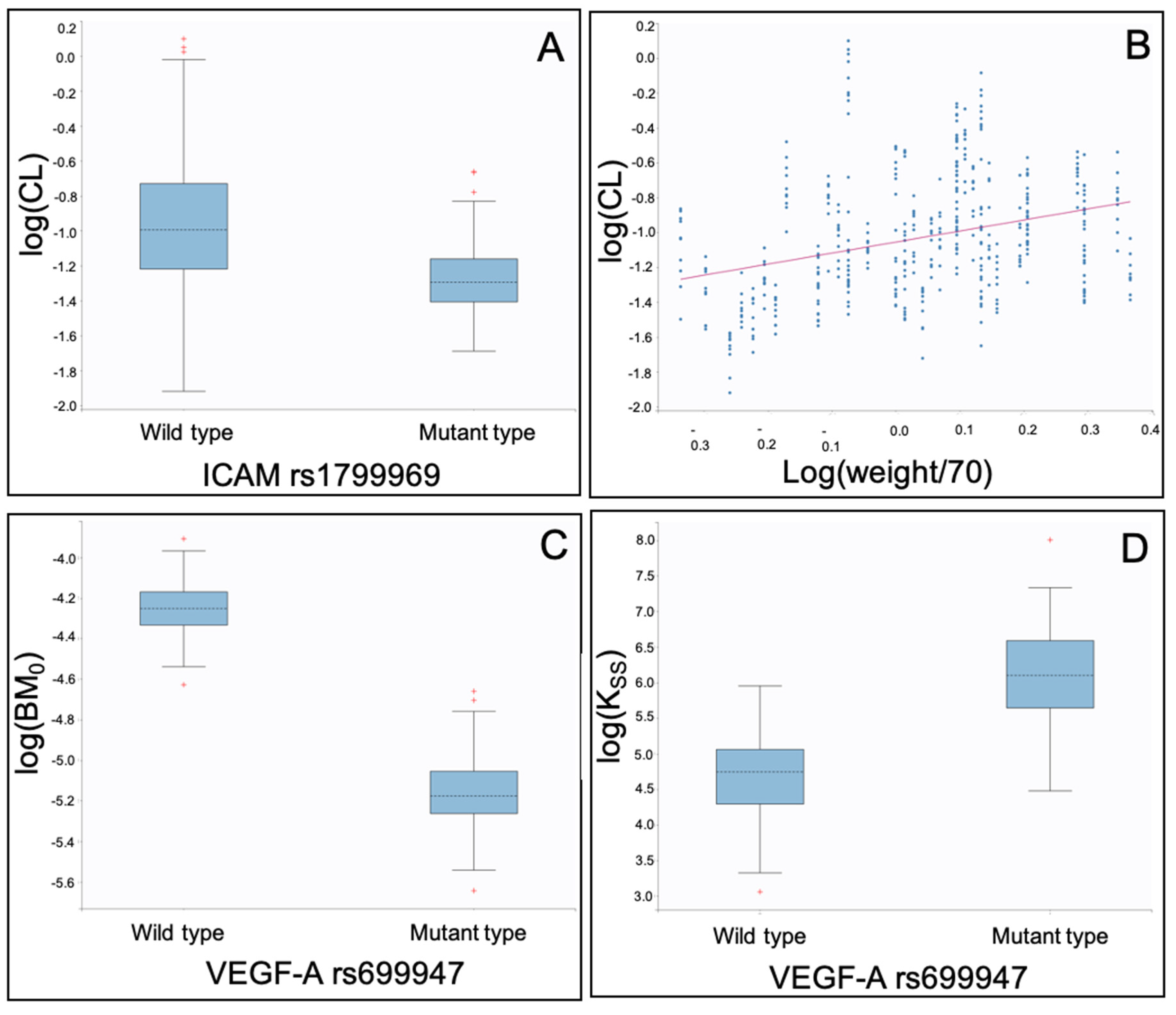

| Fixed Effects (Unit) | Parameter Value | Standard Error | RSE (%) | p Value |
|---|---|---|---|---|
| CLpop(L/day) | 0.200 | 0.0157 | 7.8 | |
| ICAM-1 rs1799969 mutant on CL | −0.423 | 0.0298 | 7.0 | <2.2e–16 |
| log(weight/70) on CL | 1.04 | 0.0701 | 6.8 | <2.2e–16 |
| V1pop (L) | 3.09 | 0.196 | 6.4 | |
| Qpop (L/day) | 0.35 | 0.0142 | 4.1 | |
| VEGF-A rs1570360 mutant on Q | 0.378 | 0.0167 | 4.4 | <2.2e–16 |
| VEGF-A rs699947 mutant on Q | −0.429 | 0.0192 | 4.5 | <2.2e–16 |
| V2pop (L) | 2.39 | 0.804 | 33.6 | |
| Standard Deviation of the Random Effects | Parameter Value | Standard Error | RSE (%) | |
| ωCL | 0.319 | 0.0648 | 20.3 | |
| ωV1 | 0.174 | 0.0487 | 28.0 | |
| ωQ | 0.160 | 0.039 | 24.4 | |
| ωV2 | 0.676 | 0.253 | 37.4 | |
| Proportional Error Model | Parameter Value | Standard Error | RSE (%) | |
| σprop | 0.246 | 0.0198 | 8.1 | |
| Correlation | Coefficient | Standard Error | RSE (%) | |
| p(Q,CL) | −0.999 | 0.22 | 22.0 |
| Fixed Effects (Units) | Parameter Value | Standard Error | RSE (%) | p Value |
|---|---|---|---|---|
| V1pop (L) | 5.83 | 0.335 | 5.7 | |
| Koutpop (day −1) | 0.116 | 0.0285 | 24.6 | |
| KSSpop (nM) | 135 | 46.5 | 34.4 | |
| VEGF-A rs699947 mutant on KSS | 1.22 | 0.394 | 32.3 | 0.00198 |
| BM0pop (nM) (or ng/L) | 0.0137 (616.5) | 0.00256 | 18.8 | |
| VEGF-A rs699947 mutant on BM0 | −0.851 | 0.242 | 28.5 | 0.000445 |
| CL(L/day) | 0.344 | 0.0205 | 5.9 | |
| ICAM-1 rs1799969 mutant on CL | −0.33 | 0.139 | 42.2 | 0.0177 |
| log(weight/70) on CL | 1.01 | 0.314 | 31.1 | 0.00129 |
| Qpop (L/days) | 0.136 | 0.00795 | 5.8 | |
| V2pop (L) | 3.17 | 1.23 | 38.7 | |
| Standard Deviation of the Random Effects | Parameter Value | Standard Error | RSE(%) | |
| ωV1 | 0.169 | 0.0535 | 31.6 | |
| ωΒΜ0 | 0.24 | 0.0547 | 22.8 | |
| ωCL | 0.309 | 0.046 | 14.9 | |
| ωQ | 0.201 | 0.0721 | 35.9 | |
| ωV2 | 0.555 | 0.223 | 40.2 | |
| Proportional Error Model | Parameter Value | Standard Error | RSE (%) | |
| σBEVA | 0.253 | 0.0186 | 7.3 | |
| σVEGF | 0.290 | 0.0279 | 9.6 | |
| Correlation | Coefficient | Standard Error | RSE (%) | |
| p(Q,CL) | -0.999 | 0.367 | 36.8 |
| Fixed Effects (Units) | Parameter Value | Standard Error | RSE (%) | p Value |
|---|---|---|---|---|
| CLpop (L/days) | 0.388 | 0.0288 | 7.4 | |
| ICAM-1 rs1799969 mutant on CL | −0.423 | 0.153 | 36.1 | 0.00566 |
| log(weight/70) on CL | 0.78 | 0.243 | 31.2 | 0.0228 |
| V1pop (L) | 5.48 | 0.28 | 5.1 | |
| Qpop (L/days) | 0.315 | 0.0362 | 11.5 | |
| VEGF-A rs699947 mutant on Q | −0.414 | 0.13 | 31.4 | <2.2e–16 |
| V2(L) | 8.81 | 2.3 | 26.1 | |
| E0pop (ng/L) | 684 | 105 | 15.4 | |
| Imaxpop | 0.951 | 0.0251 | 2.6 | |
| IC50pop (mg/L) | 29.1 | 7.49 | 25.7 | |
| Standard Deviation of the Random Effects | Parameter Value | Standard Error | RSE (%) | |
| ωCL | 0.338 | 0.0586 | 17.4 | |
| ωV1 | 0.176 | 0.0646 | 36.7 | |
| ωQ | 0.601 | 0.173 | 28.8 | |
| ωV2 | 0.579 | 0.189 | 32.6 | |
| ωE0 | 0.167 | 0.0591 | 35.4 | |
| Proportional Error Model | Parameter Value | Standard Error | RSE (%) | |
| σBEVA | 0.238 | 0.02 | 8.4 | |
| σVEGF | 0.264 | 0.0297 | 11.3 | |
| Correlation | Coefficient | Standard Error | RSE (%) | |
| p(Q,CL) | −0.979 | 0.0695 | 7.1 |
| Model | Parameter | Co-Variate |
|---|---|---|
| PK model | CL | ICAM-1 rs1799969 |
| Weight | ||
| Q | VEGF-A rs1570360 | |
| VEGF-A rs699947 | ||
| Binding QSS model | Kss | VEGF-A rs699947 |
| BM0 | VEGF-A rs699947 | |
| CL | ICAM-1 rs1799969 | |
| Weight | ||
| PK/PD model | CL | ICAM-1 rs1799969 |
| Weight | ||
| Q | VEGF-A rs699947 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papachristos, A.; Karatza, E.; Kalofonos, H.; Sivolapenko, G. Pharmacogenetics in Model-Based Optimization of Bevacizumab Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3753. https://doi.org/10.3390/ijms21113753
Papachristos A, Karatza E, Kalofonos H, Sivolapenko G. Pharmacogenetics in Model-Based Optimization of Bevacizumab Therapy for Metastatic Colorectal Cancer. International Journal of Molecular Sciences. 2020; 21(11):3753. https://doi.org/10.3390/ijms21113753
Chicago/Turabian StylePapachristos, Apostolos, Eleni Karatza, Haralabos Kalofonos, and Gregory Sivolapenko. 2020. "Pharmacogenetics in Model-Based Optimization of Bevacizumab Therapy for Metastatic Colorectal Cancer" International Journal of Molecular Sciences 21, no. 11: 3753. https://doi.org/10.3390/ijms21113753
APA StylePapachristos, A., Karatza, E., Kalofonos, H., & Sivolapenko, G. (2020). Pharmacogenetics in Model-Based Optimization of Bevacizumab Therapy for Metastatic Colorectal Cancer. International Journal of Molecular Sciences, 21(11), 3753. https://doi.org/10.3390/ijms21113753





