Delayed NK Cell Reconstitution and Reduced NK Activity Increased the Risks of CMV Disease in Allogeneic-Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Results
2.1. Patient Population
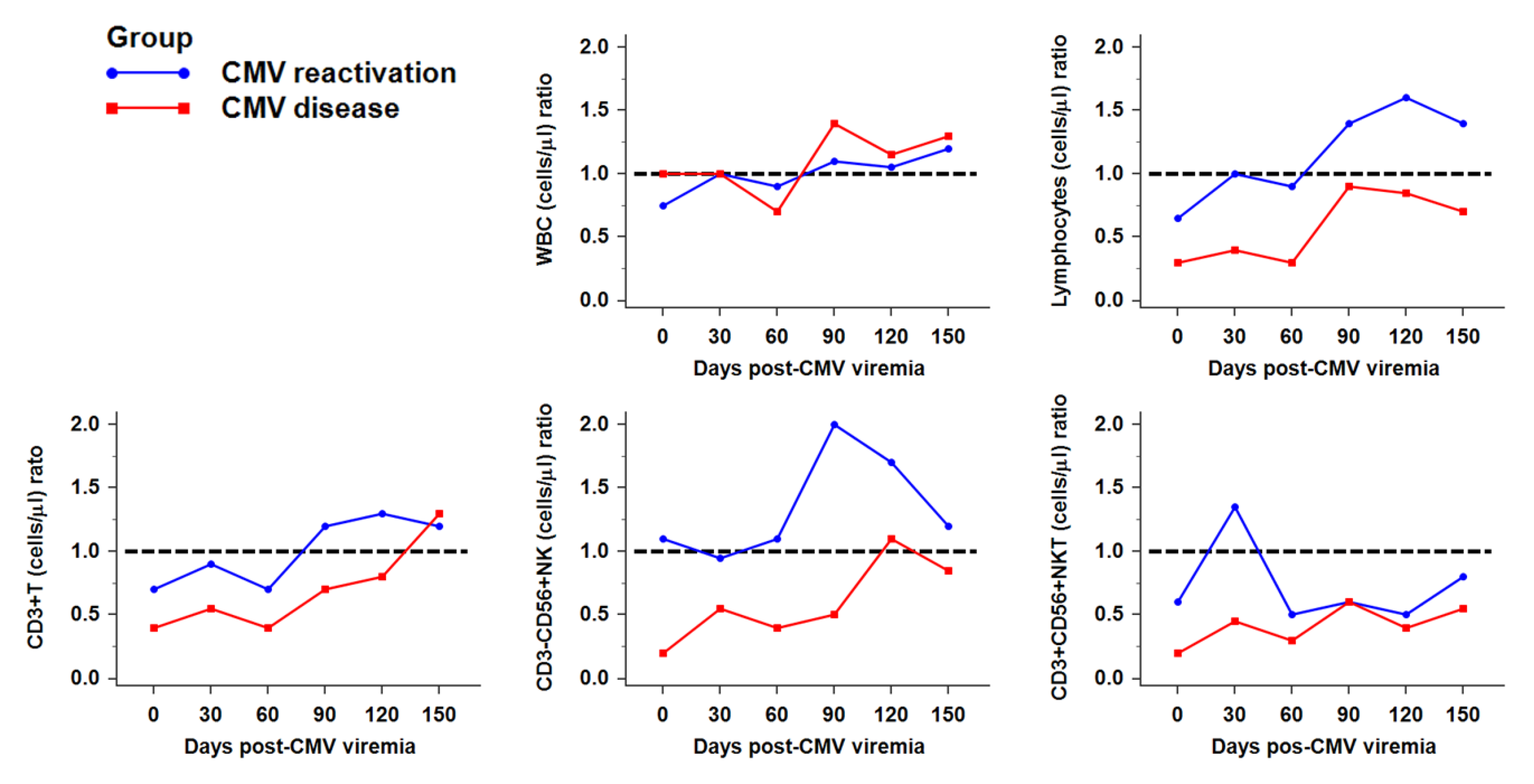
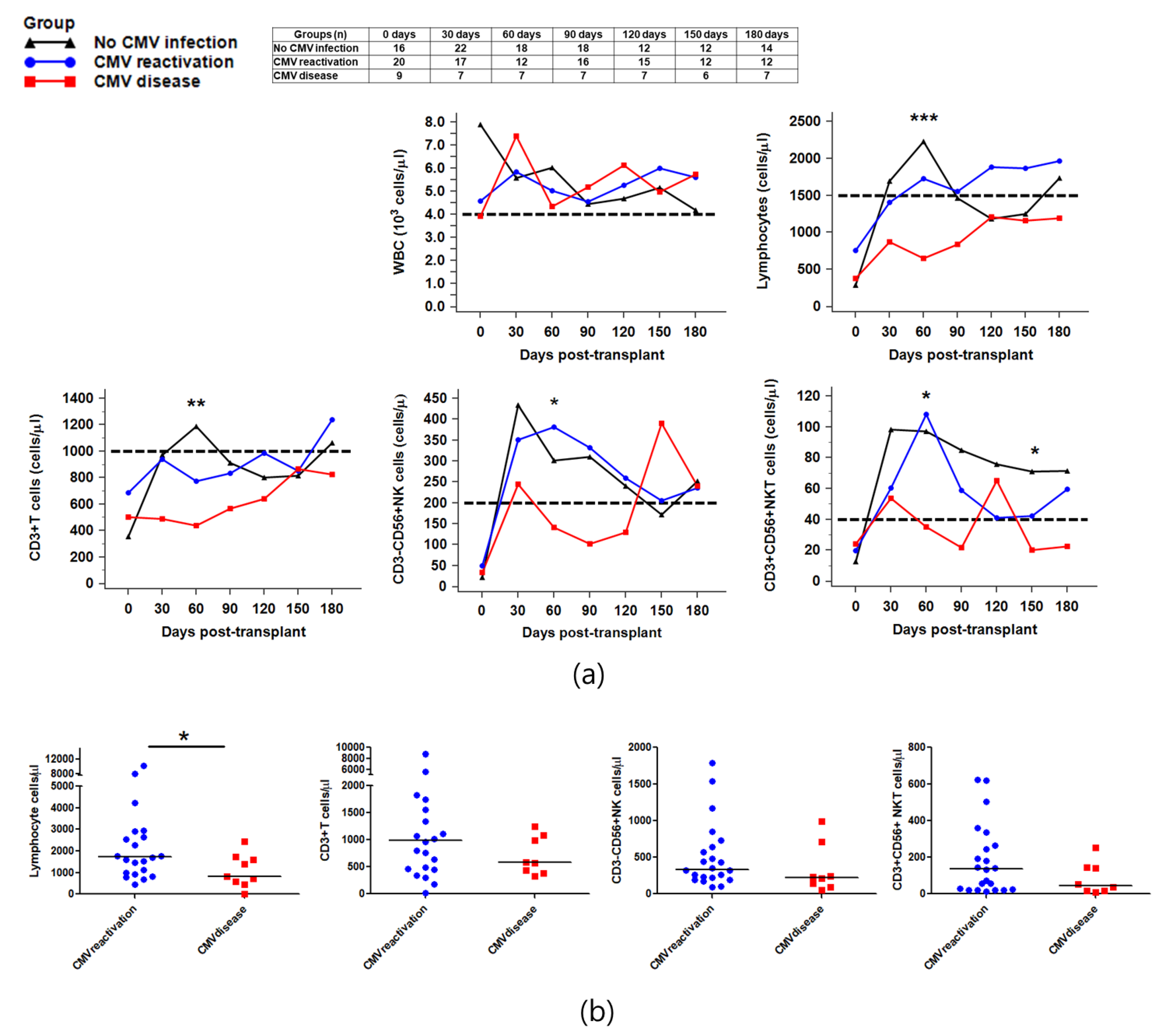
2.2. Reconstitution of Immune Cells against CMV Infection after HSCT
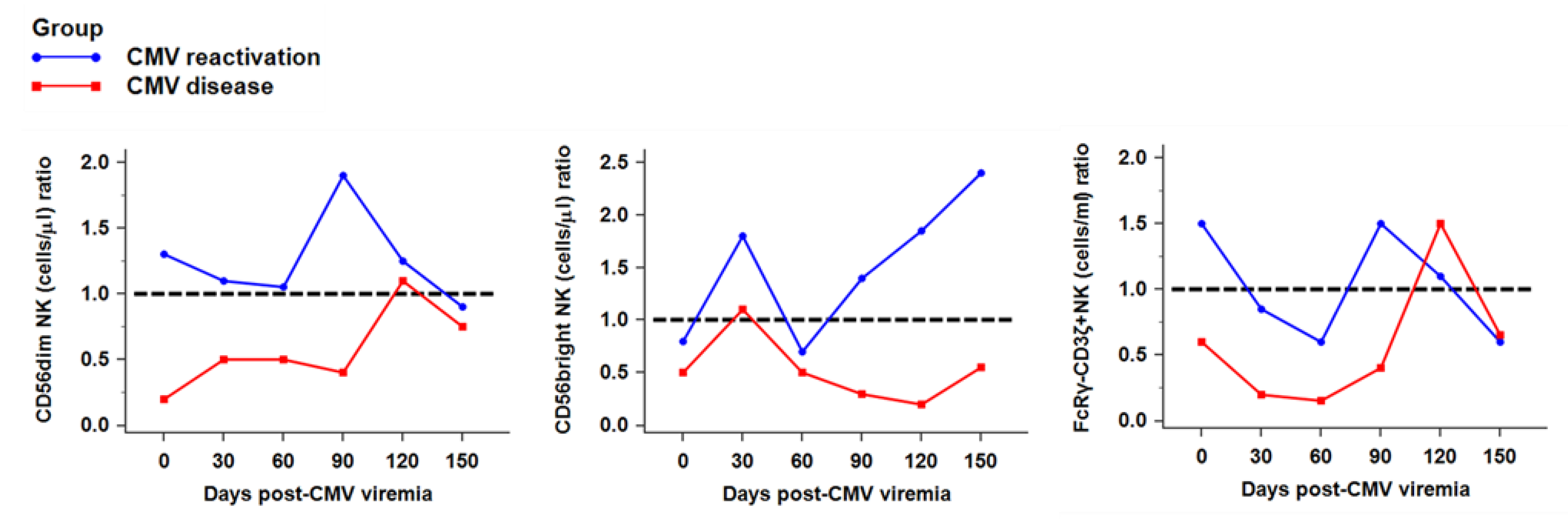
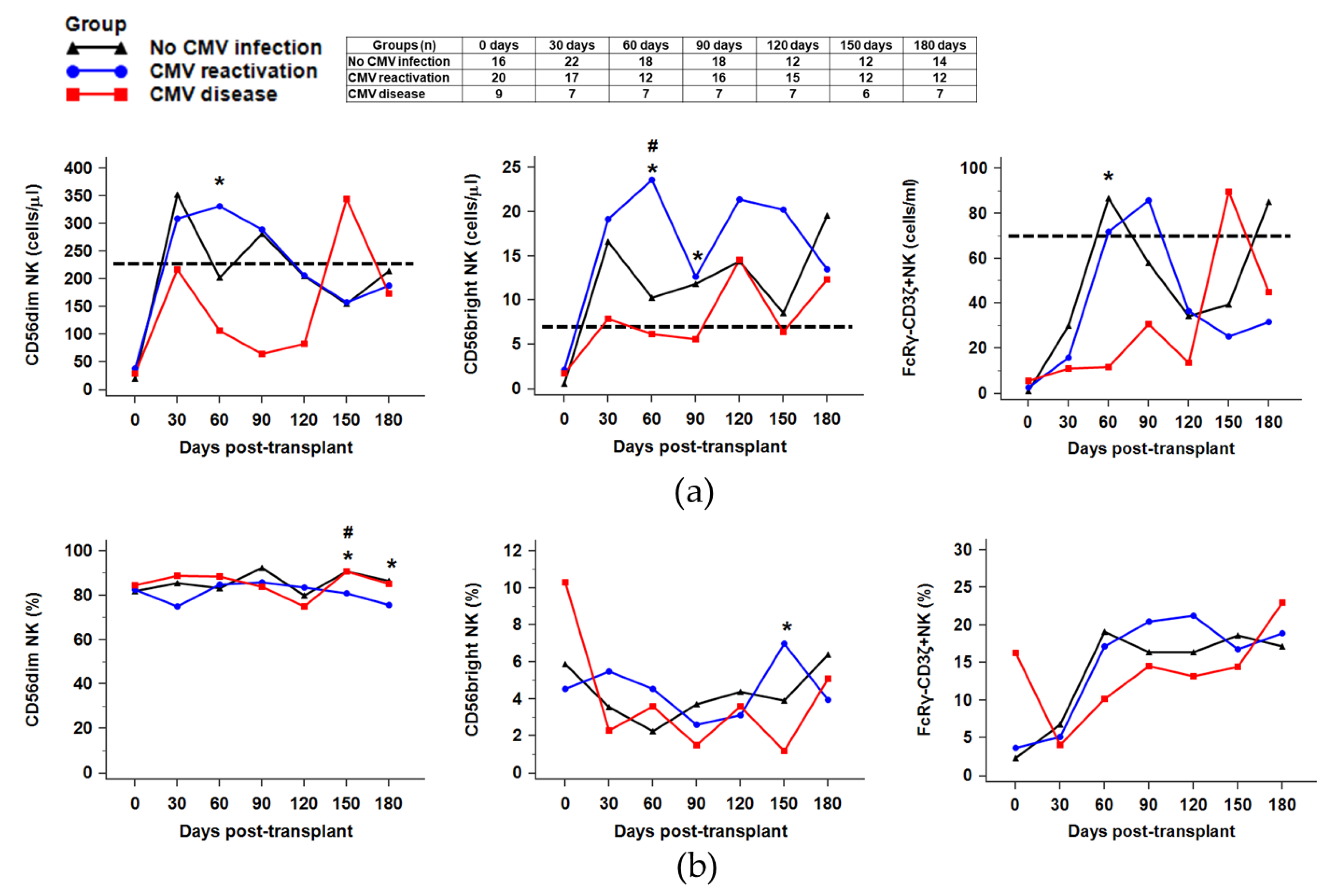
2.3. Recovery of NK Subsets against CMV Infection
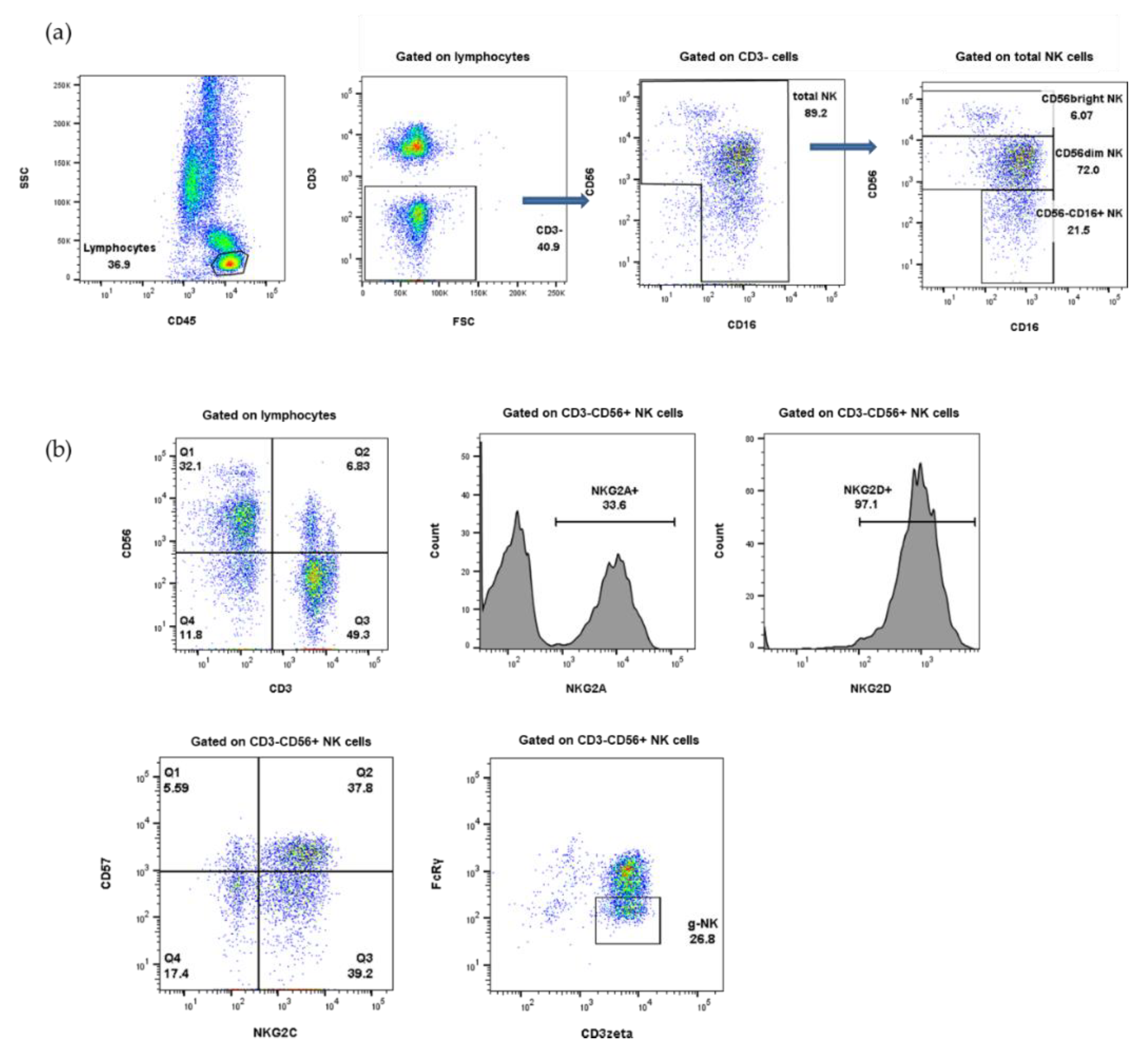
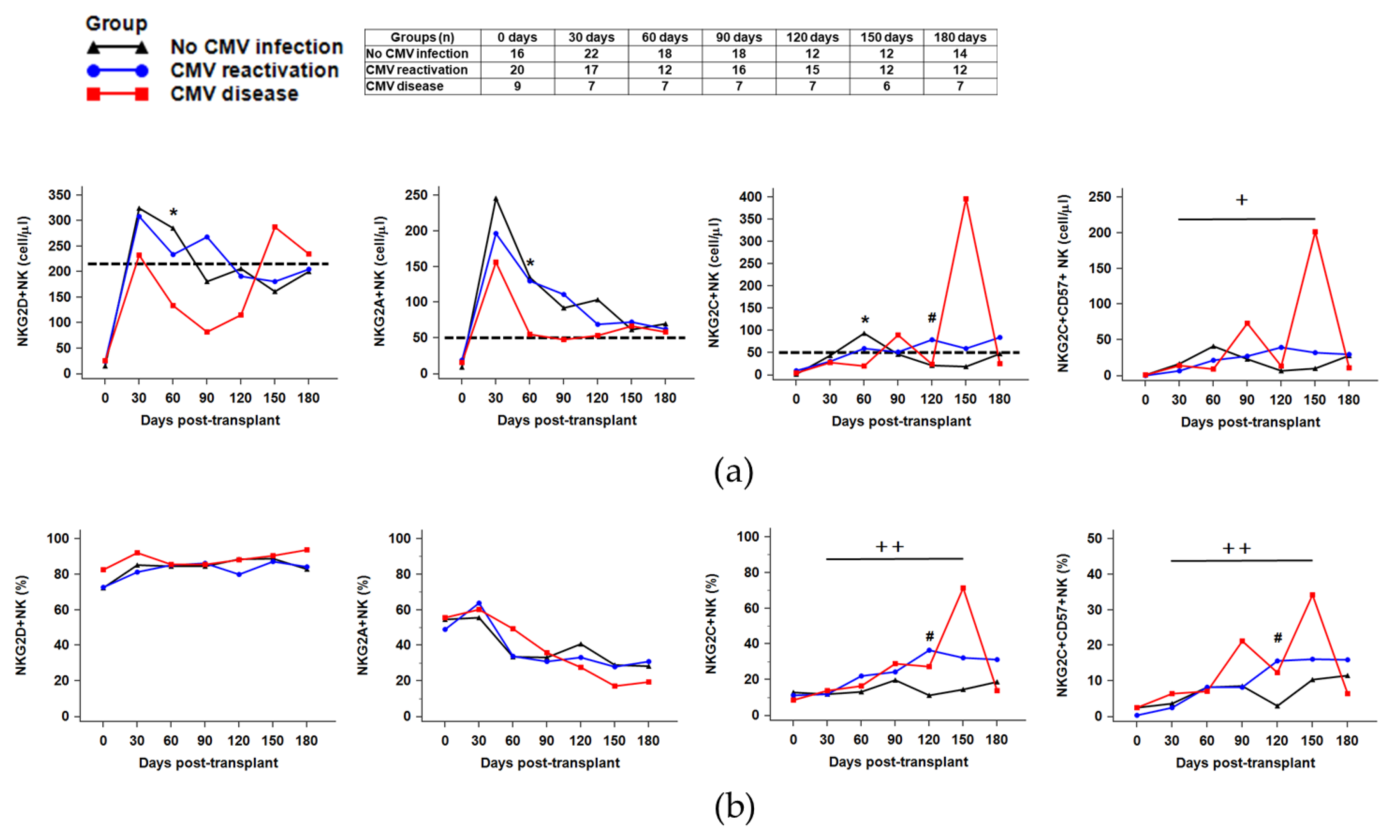
2.4. Distribution of NK Receptors (NKG2D, NKG2A, NKG2C)-Positive Population in Response to CMV Reactivation or CMV Disease after HSCT
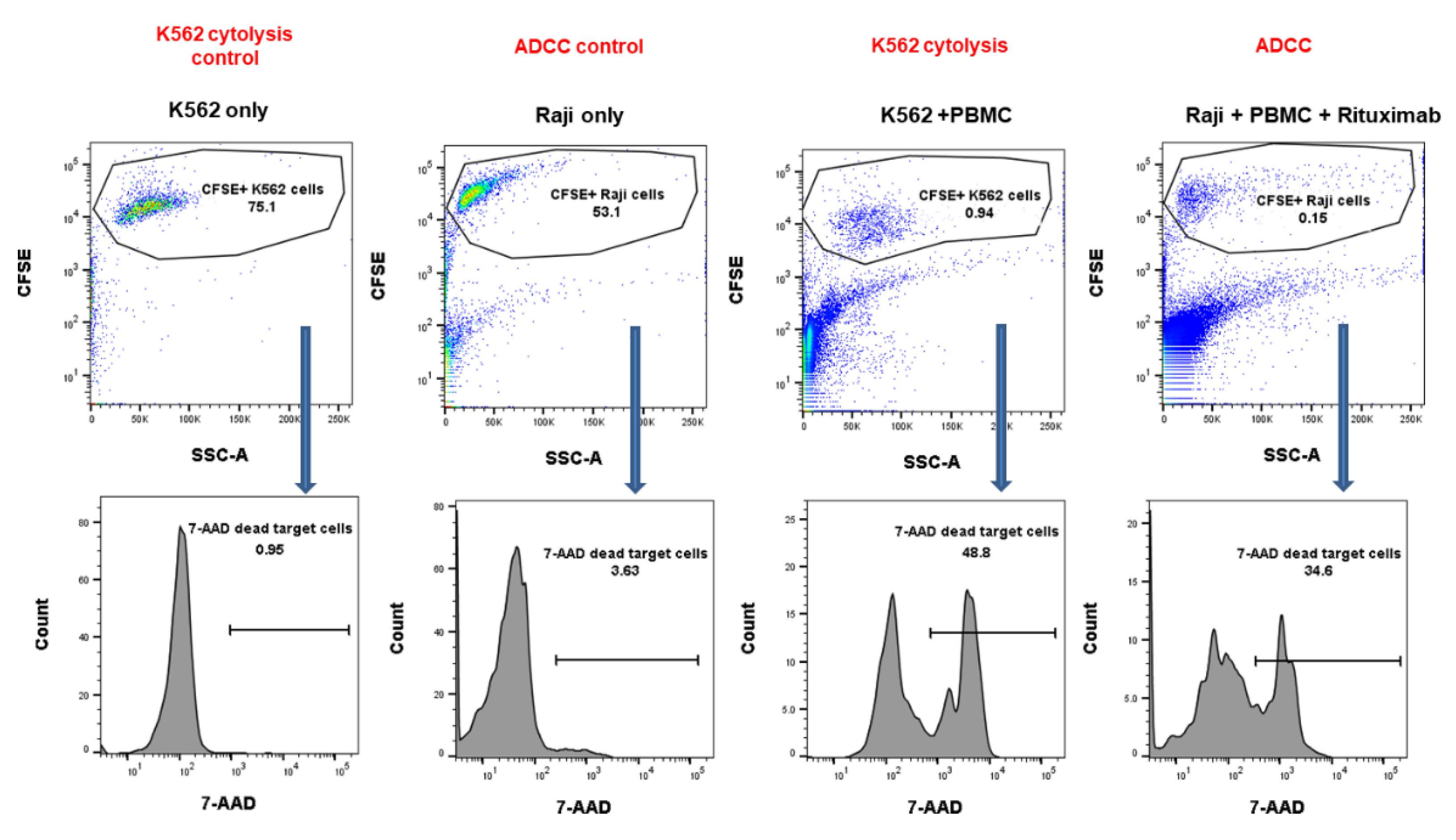
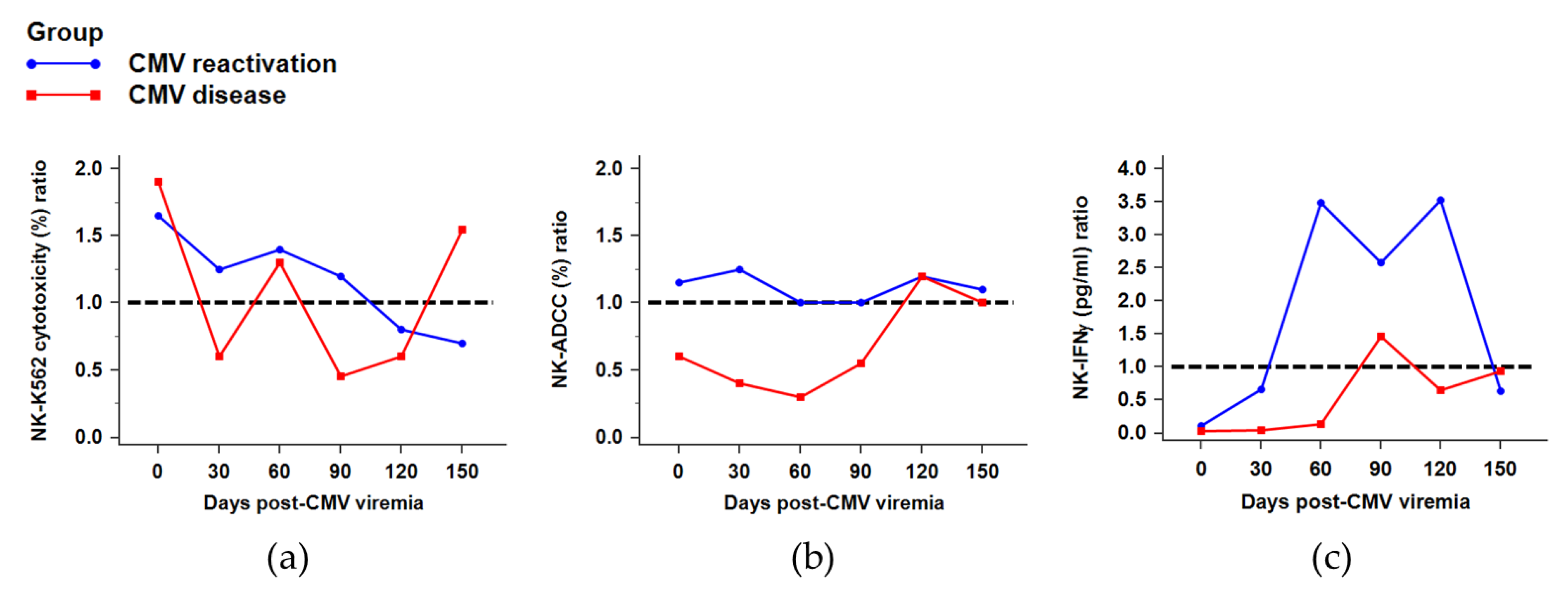
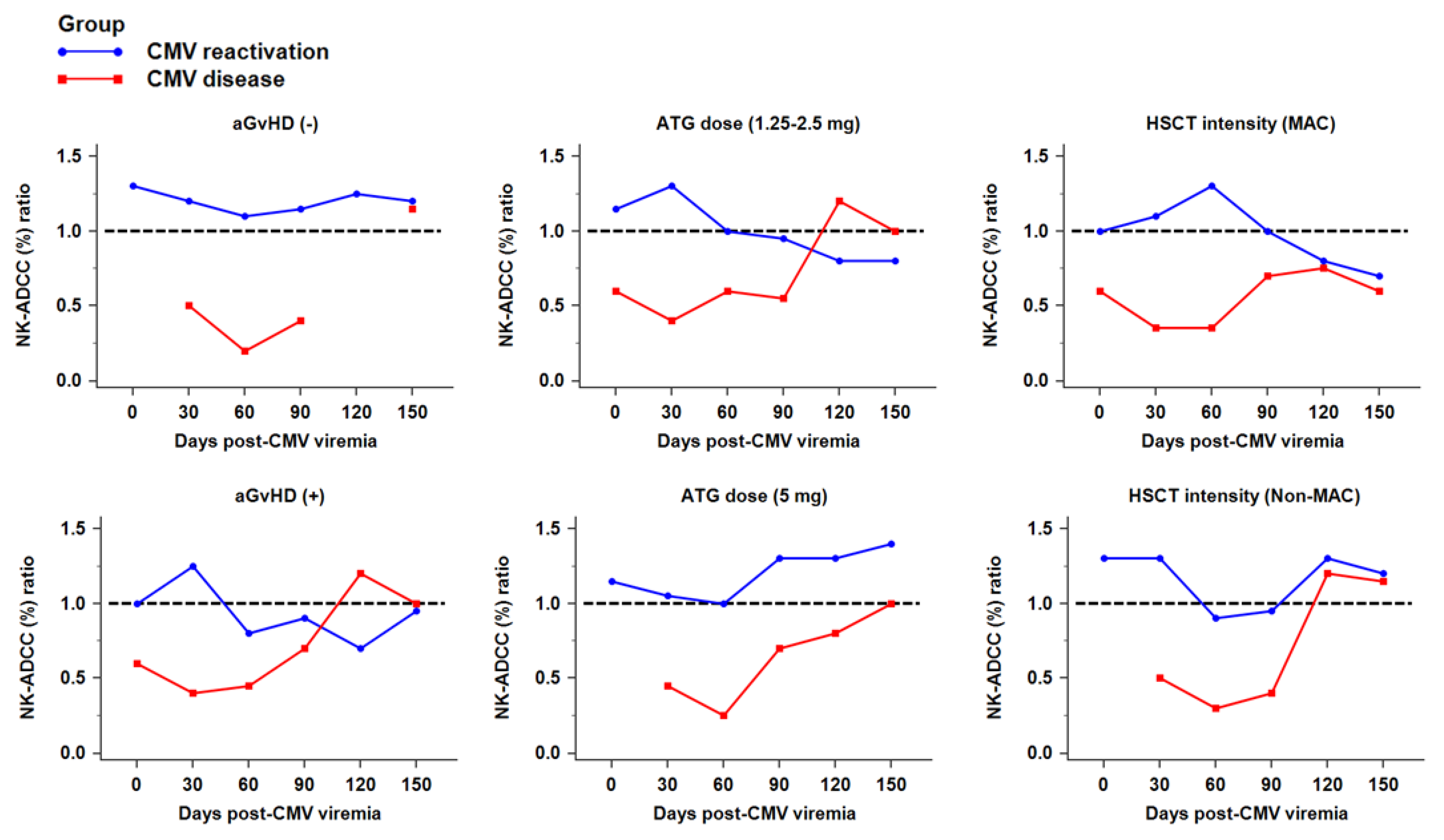
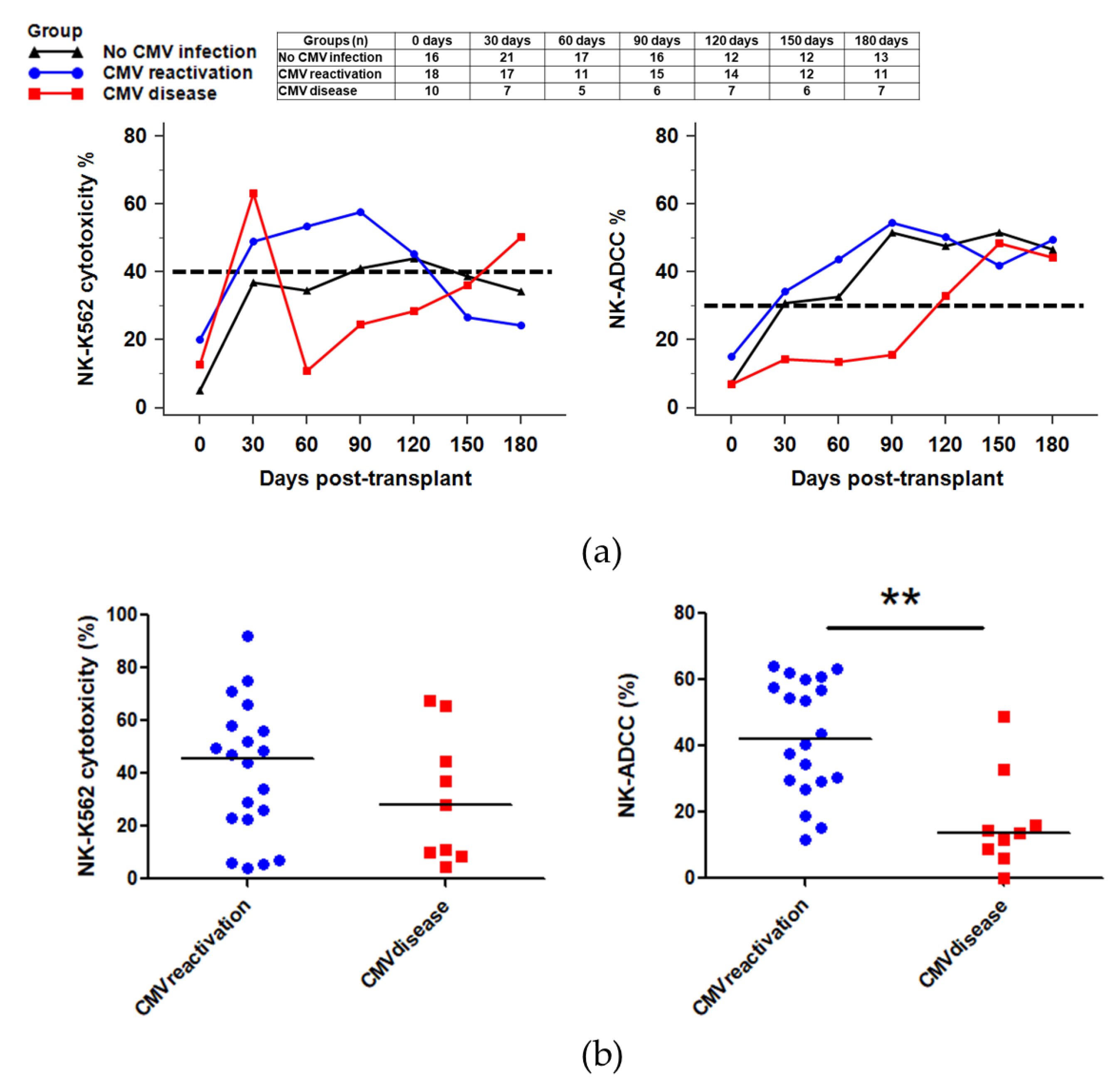
2.5. NK Cytotoxicity against CMV Infection after HSCT
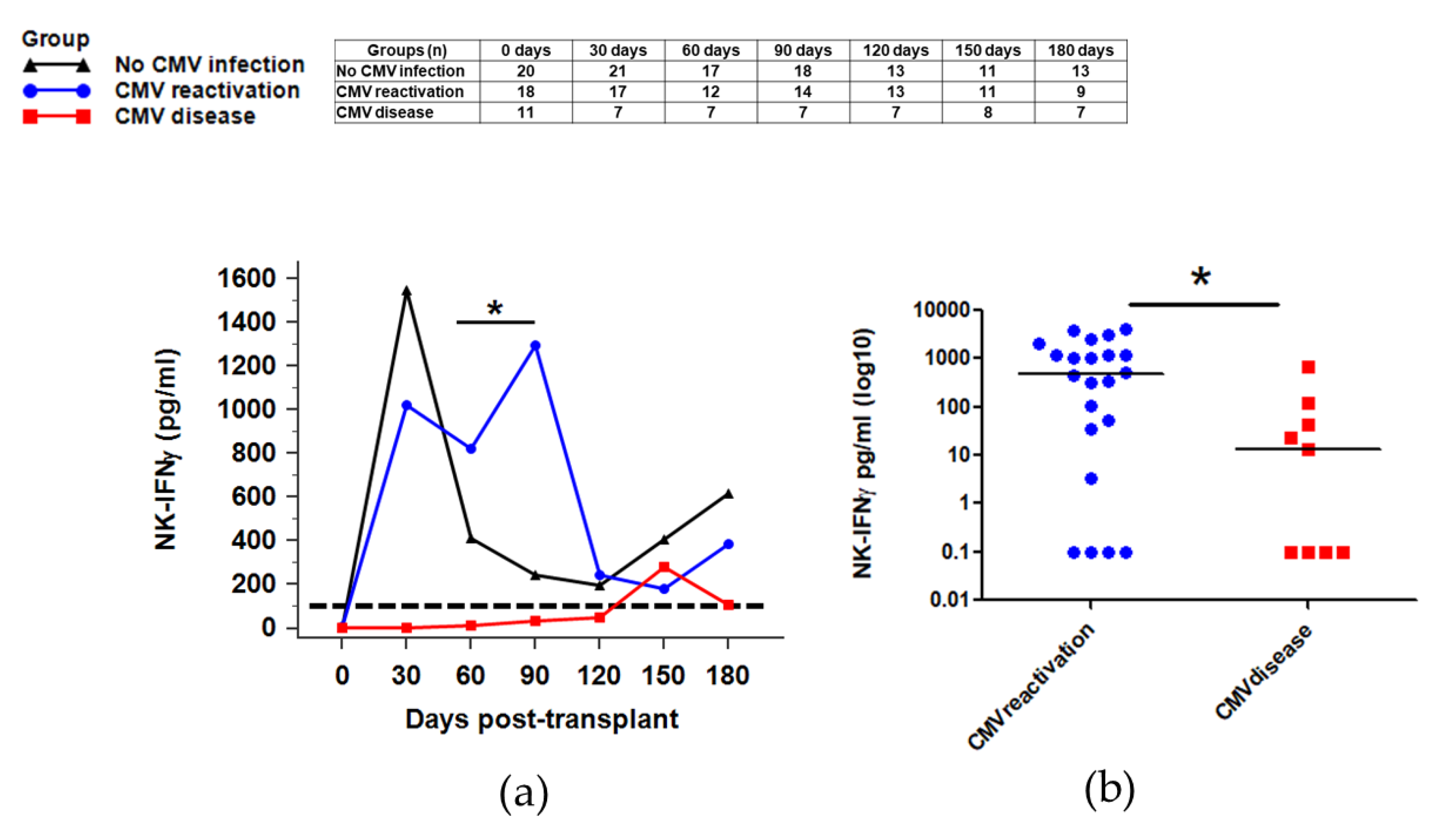
2.6. NK Function for IFNγ Secretion against CMV Infection after HSCT
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunophenotyping
4.3. NK Target Cell Lines (K562 and Raji)
4.4. NK Cytotoxicity
4.5. NK-IFNγ Secretion Assay
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CMV | Cytomegalovirus |
| HSCT | Hematopoietic stem cell transplantation |
| AML | Acute myeloid leukemia |
| NK cell | Natural killer cell |
| ADCC | Antibody-dependent cellular cytotoxicity |
| IFNγ | Interferon gamma |
Appendix A

References
- Saber, W.; Opie, S.; Rizzo, J.D.; Zhang, M.J.; Horowitz, M.M.; Schriber, J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood 2012, 119, 3908–3916. [Google Scholar] [CrossRef]
- Cho, B.S.; Yoon, J.H.; Shin, S.H.; Yahng, S.A.; Lee, S.E.; Eom, K.S.; Kim, Y.J.; Lee, S.; Min, C.K.; Cho, S.G.; et al. Comparison of allogeneic stem cell transplantation from familial-mismatched/haploidentical donors and from unrelated donors in adults with high-risk acute myelogenous leukemia. Biol. Blood Marrow Transplant. 2012, 18, 1552–1563. [Google Scholar] [CrossRef][Green Version]
- Ruggeri, A.; Ciceri, F.; Gluckman, E.; Labopin, M.; Rocha, V. Alternative donors hematopoietic stem cells transplantation for adults with acute myeloid leukemia: Umbilical cord blood or haploidentical donors? Best Pract. Res. Clin. Haematol. 2010, 23, 207–216. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [PubMed]
- Cho, S.Y.; Lee, D.G.; Kim, H.J. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2666. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, K.H.; Ryu, J.H.; Choi, A.R.; Yu, J.H.; Lim, J.; Han, K.; Kim, S.I.; Yang, C.W.; Chung, B.H.; et al. Cytomegalovirus (CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in seropositive kidney transplant recipients. PLoS ONE 2017, 12, e0189488. [Google Scholar] [CrossRef] [PubMed]
- Seggewiss, R.; Einsele, H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: An update. Blood 2010, 115, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Rong, G.; Xu, T.; Zhao, H.; Chen, D.; Wu, L.; Huang, P.; Wang, F. Peripheral blood lymphocyte responses to cytomegalovirus seropositivity after allogeneic-hematopoietic stem cell transplantation. OncoTargets Ther. 2018, 11, 5143–5150. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, M.; Nichols, W.G.; Papanicolaou, G.; Rubin, R.; Wingard, J.R.; Zaia, J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol. Blood Marrow Transplant. 2003, 9, 543–558. [Google Scholar] [CrossRef]
- Maertens, J.; Lyon, S. Current and future options for cytomegalovirus reactivation in hematopoietic cell transplantation patients. Future Microbiol. 2017, 12, 839–842. [Google Scholar] [CrossRef]
- Nam, M.; Shin, S.; Park, K.U.; Kim, I.; Yoon, S.S.; Kwon, T.K.; Song, E.Y. Association of FOXP3 Single Nucleotide Polymorphisms with Clinical Outcomes After Allogenic Hematopoietic Stem Cell Transplantation. Ann. Lab. Med. 2018, 38, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect. Dis. Clin. N. Am. 2010, 24, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Falco, M.; Muccio, L.; Bertaina, A.; Locatelli, F.; Moretta, A. Impact of HCMV Infection on NK Cell Development and Function after HSCT. Front. Immunol. 2013, 4, 458. [Google Scholar] [CrossRef] [PubMed]
- Muccio, L.; Falco, M.; Bertaina, A.; Locatelli, F.; Frassoni, F.; Sivori, S.; Moretta, L.; Moretta, A.; Della Chiesa, M. Late Development of FcepsilonRgamma(neg) Adaptive Natural Killer Cells Upon Human Cytomegalovirus Reactivation in Umbilical Cord Blood Transplantation Recipients. Front. Immunol. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Adams, N.M.; Geary, C.D.; Santosa, E.K.; Lumaquin, D.; Le Luduec, J.B.; Sottile, R.; van der Ploeg, K.; Hsu, J.; Whitlock, B.M.; Jackson, B.T.; et al. Cytomegalovirus Infection Drives Avidity Selection of Natural Killer Cells. Immunity 2019, 50, 1381–1390.e5. [Google Scholar] [CrossRef]
- Jin, F.; Lin, H.; Gao, S.; Wang, H.; Yan, H.; Guo, J.; Hu, Z.; Jin, C.; Wang, Y.; Wang, Z.; et al. Characterization of IFNgamma-producing natural killer cells induced by cytomegalovirus reactivation after haploidentical hematopoietic stem cell transplantation. Oncotarget 2017, 8, 51–63. [Google Scholar] [CrossRef]
- Muccio, L.; Bertaina, A.; Falco, M.; Pende, D.; Meazza, R.; Lopez-Botet, M.; Moretta, L.; Locatelli, F.; Moretta, A.; Della Chiesa, M. Analysis of memory-like natural killer cells in human cytomegalovirus-infected children undergoing alphabeta+T and B cell-depleted hematopoietic stem cell transplantation for hematological malignancies. Haematologica 2016, 101, 371–381. [Google Scholar] [CrossRef]
- Davis, Z.B.; Cooley, S.A.; Cichocki, F.; Felices, M.; Wangen, R.; Luo, X.; DeFor, T.E.; Bryceson, Y.T.; Diamond, D.J.; Brunstein, C.; et al. Adaptive Natural Killer Cell and Killer Cell Immunoglobulin-Like Receptor-Expressing T Cell Responses are Induced by Cytomegalovirus and Are Associated with Protection against Cytomegalovirus Reactivation after Allogeneic Donor Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1653–1662. [Google Scholar]
- Almehmadi, M.; Flanagan, B.F.; Khan, N.; Alomar, S.; Christmas, S.E. Increased numbers and functional activity of CD56(+) T cells in healthy cytomegalovirus positive subjects. Immunology 2014, 142, 258–268. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Verges, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012, 119, 2665–2674. [Google Scholar] [CrossRef]
- Ullah, M.A.; Hill, G.R.; Tey, S.K. Functional Reconstitution of Natural Killer Cells in Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Felices, M.; Cichocki, F.; Cooley, S.; Verneris, M.R.; Miller, J.S. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT). Immunol. Rev. 2014, 258, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Buhlmann, L.; Buser, A.S.; Cantoni, N.; Gerull, S.; Tichelli, A.; Gratwohl, A.; Stern, M. Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011, 46, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Cooley, S.; Verneris, M.R.; Curtsinger, J.; Luo, X.; Waller, E.K.; Weisdorf, D.J.; Miller, J.S. NK cell education after allogeneic transplantation: Dissociation between recovery of cytokine-producing and cytotoxic functions. Blood 2011, 118, 2784–2792. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Falco, M.; Podesta, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus? Blood 2012, 119, 399–410. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Hendricks, D.W.; Balfour, H.H., Jr.; Dunmire, S.K.; Schmeling, D.O.; Hogquist, K.A.; Lanier, L.L. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J. Immunol. 2014, 192, 4492–4496. [Google Scholar] [CrossRef]
- Cichocki, F.; Cooley, S.; Davis, Z.; DeFor, T.E.; Schlums, H.; Zhang, B.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J.; et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef]
- Hwang, I.; Zhang, T.; Scott, J.M.; Kim, A.R.; Lee, T.; Kakarla, T.; Kim, A.; Sunwoo, J.B.; Kim, S. Identification of human NK cells that are deficient for signaling adaptor FcRgamma and specialized for antibody-dependent immune functions. Int. Immunol. 2012, 24, 793–802. [Google Scholar] [CrossRef]
- Rolle, A. Deciphering the biology of NKG2C+ Natural Killer cells. Oncotarget 2015, 6, 19930–19931. [Google Scholar] [CrossRef]
- Lopez-Verges, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.M.; Norris, P.J.; et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Falco, M.; Bertaina, A.; Muccio, L.; Alicata, C.; Frassoni, F.; Locatelli, F.; Moretta, L.; Moretta, A. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C-/- umbilical cord blood. J. Immunol. 2014, 192, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Sivori, S.; Carlomagno, S.; Moretta, L.; Moretta, A. Activating KIRs and NKG2C in Viral Infections: Toward NK Cell Memory? Front. Immunol. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Cichicki, F.; Schlums, H.; Theorell, J.; Tesi, B.; Miller, J.S.; Ljunggren, H.G.; Bryceson, Y.T. Diversification and Functional Specialization of Human NK Cell Subsets. Curr. Top. Microbiol. Immunol. 2016, 395, 63–94. [Google Scholar]
- Lam, V.C.; Lanier, L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017, 44, 43–51. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, S.S.; Lim, J.Y.; Min, G.J.; Park, S.; Jeon, Y.W.; Yahng, S.A.; Shin, S.H.; Lee, S.E.; Yoon, J.H.; et al. Predictive Role of Circulating Immune Cell Subtypes Early after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Acute Leukemia. Int. J. Stem Cells 2019, 12, 73. [Google Scholar] [CrossRef]
- Zhang, T.; Scott, J.M.; Hwang, I.; Kim, S. Cutting edge: Antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J. Immunol. 2013, 190, 1402–1406. [Google Scholar] [CrossRef]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef]
- Trotta, R.; Chen, L.; Ciarlariello, D.; Josyula, S.; Mao, C.; Costinean, S.; Yu, L.; Butchar, J.P.; Tridandapani, S.; Croce, C.M.; et al. miR-155 regulates IFN-gamma production in natural killer cells. Blood 2012, 119, 3478–3485. [Google Scholar] [CrossRef]
- Luetke-Eversloh, M.; Cicek, B.B.; Siracusa, F.; Thom, J.T.; Hamann, A.; Frischbutter, S.; Baumgrass, R.; Chang, H.D.; Thiel, A.; Dong, J.; et al. NK cells gain higher IFN-gamma competence during terminal differentiation. Eur. J. Immunol. 2014, 44, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Nederby, L.; Jakobsen, A.; Hokland, M.; Hansen, T.F. Quantification of NK cell activity using whole blood: Methodological aspects of a new test. J. Immunol. Methods 2018, 458, 21–25. [Google Scholar] [CrossRef]
- Luetke-Eversloh, M.; Hammer, Q.; Durek, P.; Nordstrom, K.; Gasparoni, G.; Pink, M.; Hamann, A.; Walter, J.; Chang, H.D.; Dong, J.; et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014, 10, e1004441. [Google Scholar] [CrossRef]
- Ljungman, P.; Griffiths, P.; Paya, C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin. Infect. Dis. 2002, 34, 1094–1097. [Google Scholar] [CrossRef]
- Park, K.H.; Park, H.; Kim, M.; Kim, Y.; Han, K.; Oh, E.J. Evaluation of NK cell function by flowcytometric measurement and impedance based assay using real-time cell electronic sensing system. Biomed. Res. Int. 2013, 2013, 210726. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget 2017, 8, 70431–70440. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.S.; Lee, J.M.; Park, K.H.; Choi, A.R.; Yoon, J.H.; Ryu, H.; Oh, E.J. Natural Killer Cell Function Tests by Flowcytometry-Based Cytotoxicity and IFN-gamma Production for the Diagnosis of Adult Hemophagocytic Lymphohistiocytosis. Int. J. Mol. Sci. 2019, 20, 5413. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.T.; Chun, S.; Kim, S.H.; Ali, A.K.; Lee, S.H.; Kim, S.; Kim, S.H.; Cho, D. Natural killer cell subsets and receptor expression in peripheral blood mononuclear cells of a healthy Korean population: Reference range, influence of age and sex, and correlation between NK cell receptors and cytotoxicity. Hum. Immunol. 2017, 78, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Kim, D.W.; Phan, M.T.; Kim, J.S.; Yang, J.H.; Choi, J.I.; Lee, J.J.; Shin, M.G.; Ryang, D.W.; Kim, S.K.; et al. Comparison of FcRgamma-Deficient and CD57+ Natural Killer Cells Between Cord Blood and Adult Blood in the Cytomegalovirus-Endemic Korean Population. Ann. Lab. Med. 2015, 35, 423–428. [Google Scholar] [CrossRef]
| Characteristics | All Participants (n = 58) | No CMV Infection (n = 24) | CMV Reactivation (n = 23) | CMV Disease (n = 11) |
|---|---|---|---|---|
| Median age (range) | 48 (18–69) | 45 (18–61) | 48 (32–69) | 55 (22–65) |
| Sex; male, n (%) | 34 (58.6) | 15 (62.5) | 14 (60.9) | 5 (45.5) |
| Donor type, n (%) | ||||
| MUD | 33 (56.9) | 17 (70.8) | 12 (52.2) | 4 (36.4) |
| FMD | 25 (43.1) | 7 (29.2) | 11 (47.8) | 7 (63.6) |
| HSCT intensity, n (%) | ||||
| MAC | 29 (50.0) | 17 (70.8) * | 8 (34.8) | 4 (36.4) |
| Non-MAC | 29 (50.0) | 7 (29.2) | 15 (65.2) | 7 (63.6) |
| GvHD, n (%) | ||||
| Acute GvHD | 35 (60.3) | 13 (54.2) | 13 (56.5) | 9 (81.8) |
| Chronic GvHD | 20 (34.5) | 8 (33.3) | 7 (30.4) | 5 (45.5) |
| GvHD prophylaxis, n (%) | ||||
| Tacrolimus | 57 (98.3) | 23 (95.8) | 23 (100.0) | 11 (100.0) |
| Cyclosporine | 1 (1.7) | 1 (4.2) | 0 (0.0) | 0 (0.0) |
| ATG dose, n (%) | ||||
| 1.25–2.5 mg | 37 (63.8) | 17 (70.8) | 14 (60.9) | 6 (54.5) |
| 5 mg | 21 (36.2) | 7 (29.2) | 9 (39.1) | 5 (45.5) |
| CMV recipient/donor serostatus, n (%) | ||||
| R+/D+ | 55 (94.8) | 22 (91.7) | 22 (95.7) | 11 (100.0) |
| R+/D− | 2 (3.4) | 1 (4.2) | 1 (4.3) | 0 (0.0) |
| R−/D− | 1 (1.7) | 1 (4.2) | 0 (0.0) | 0 (0.0) |
| Status, n (%) during the first 6 Mo | ||||
| Relapse | 3 (5.3) | 2 (8.3) | 1 (4.5) | 0 (0.0) |
| Time to CMV viremia detection, median day (range) | 34 (15–94) | 0 (0–0) | 35 (15–94) | 28 (19–90) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Ryu, J.H.; Bae, H.; Yun, S.; Jang, J.H.; Han, K.; Cho, B.S.; Kim, H.-J.; Lee, H.; Oh, E.-J. Delayed NK Cell Reconstitution and Reduced NK Activity Increased the Risks of CMV Disease in Allogeneic-Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2020, 21, 3663. https://doi.org/10.3390/ijms21103663
Park KH, Ryu JH, Bae H, Yun S, Jang JH, Han K, Cho BS, Kim H-J, Lee H, Oh E-J. Delayed NK Cell Reconstitution and Reduced NK Activity Increased the Risks of CMV Disease in Allogeneic-Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences. 2020; 21(10):3663. https://doi.org/10.3390/ijms21103663
Chicago/Turabian StylePark, Ki Hyun, Ji Hyeong Ryu, Hyunjoo Bae, Sojeong Yun, Joo Hee Jang, Kyungja Han, Byung Sik Cho, Hee-Je Kim, Hyeyoung Lee, and Eun-Jee Oh. 2020. "Delayed NK Cell Reconstitution and Reduced NK Activity Increased the Risks of CMV Disease in Allogeneic-Hematopoietic Stem Cell Transplantation" International Journal of Molecular Sciences 21, no. 10: 3663. https://doi.org/10.3390/ijms21103663
APA StylePark, K. H., Ryu, J. H., Bae, H., Yun, S., Jang, J. H., Han, K., Cho, B. S., Kim, H.-J., Lee, H., & Oh, E.-J. (2020). Delayed NK Cell Reconstitution and Reduced NK Activity Increased the Risks of CMV Disease in Allogeneic-Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences, 21(10), 3663. https://doi.org/10.3390/ijms21103663






