Abstract
We investigated whether belimumab treatment impacts on levels of autoantibodies and cytokines of interest in systemic lupus erythematosus (SLE). Longitudinally collected serum samples from 78 belimumab-treated Swedish SLE patients were analysed. Serum cytokine levels were determined using Luminex xMAP technology, and nuclear antigen autoantibody specificities using addressable laser bead immunoassay. In patients with detectable levels at baseline, interferon (IFN)-α2 levels were lower at month 6 (median; interquartile range (IQR): 8.9; 1.5–54.9 pg/mL) versus baseline (28.4; 20.9–100.3 pg/mL; p = 0.043). Interleukin (IL)-6 (baseline: 7.1; 2.9–16.1 pg/mL) decreased from month 6 (0.5; 0.5–6.3 pg/mL; p = 0.018) and throughout a 24 month follow-up. IL-10 (baseline: 12.6; 2.8–29.7 pg/mL) showed more rapid decreases from month 3 (1.8; 0.6–9.1 pg/mL; p = 0.003). Levels of anti-dsDNA (p < 0.001), anti-Smith antigen (Sm) (p = 0.002), anti-U1 small nuclear ribonucleoprotein (U1RNP) (p < 0.001), anti-Sm-U1RNP complex (p = 0.028), and anti-ribosomal P (p = 0.012) antibodies decreased from month 3 and remained decreased. Anti-Sm positivity at baseline was associated with higher probability and/or shorter time to achieve sustained SLE responder index-4 response (hazard ratio (HR): 2.52; 95% CI: 1.20–5.29; p = 0.015), independently of other factors. Decline of IL-6 levels through month 3 was greater in responders. In summary, belimumab treatment lowered IFN-α2, IL-6, and IL-10 levels, as well as levels of multiple autoantibodies, however after different time spans. Notably, anti-Sm positivity and early decline in IL-6 levels were associated with favorable treatment outcome.
1. Introduction
Belimumab, a monoclonal antibody against the soluble counterpart of B cell activating factor (BAFF), also known as B lymphocyte stimulator (BLyS), is used for the treatment of active systemic lupus erythematosus (SLE) despite standard of care therapy, that is, antimalarial agents and non-selective immunosuppressants including glucocorticoids [1]. The effects of belimumab on serum levels of anti-double stranded (ds)DNA antibodies and BAFF, as well as the homologous to BAFF plasma cell survival factor a proliferation-inducing ligand (APRIL), have been demonstrated in previous studies [2,3,4]. Furthermore, predictors of response and non-response to belimumab treatment have been implicated; high disease activity and anti-dsDNA positivity at baseline have been associated with increased probability of good response, whereas established organ damage, especially in the cardiovascular and neuropsychiatric domains, has been shown to reduce belimumab efficacy [4,5,6,7,8].
In a recent study, we compared free circulating serum levels of multiple autoantibody specificities with their corresponding levels in circulating immune complexes (IC), and demonstrated that high anti-dsDNA antibody levels in IC, but not in serum, prior to belimumab treatment initiation were associated with clinical improvement [9]. Importantly, B cells also have functions other than antibody production [10], such as antigen presentation and proinflammatory cytokine excretion [11,12], and cell types other than B cells also express receptors for BAFF [13], including certain subsets of T cells [14]. Thus, belimumab may be expected to also exert indirect effects on the equilibrium of immune responses in SLE, warranting thorough survey of its impact on SLE-associated immune pathways.
In the present study, we investigated whether belimumab treatment impacts levels of cytokines outside the BAFF/APRIL pathway but implicated in SLE pathogenesis and lupus drug development, as well as nuclear antigen autoantibody specificities commonly used for diagnosis and surveillance. We also investigated the performance of detectable cytokine levels and autoantibody positivity at baseline as predictors of response to belimumab treatment.
2. Results
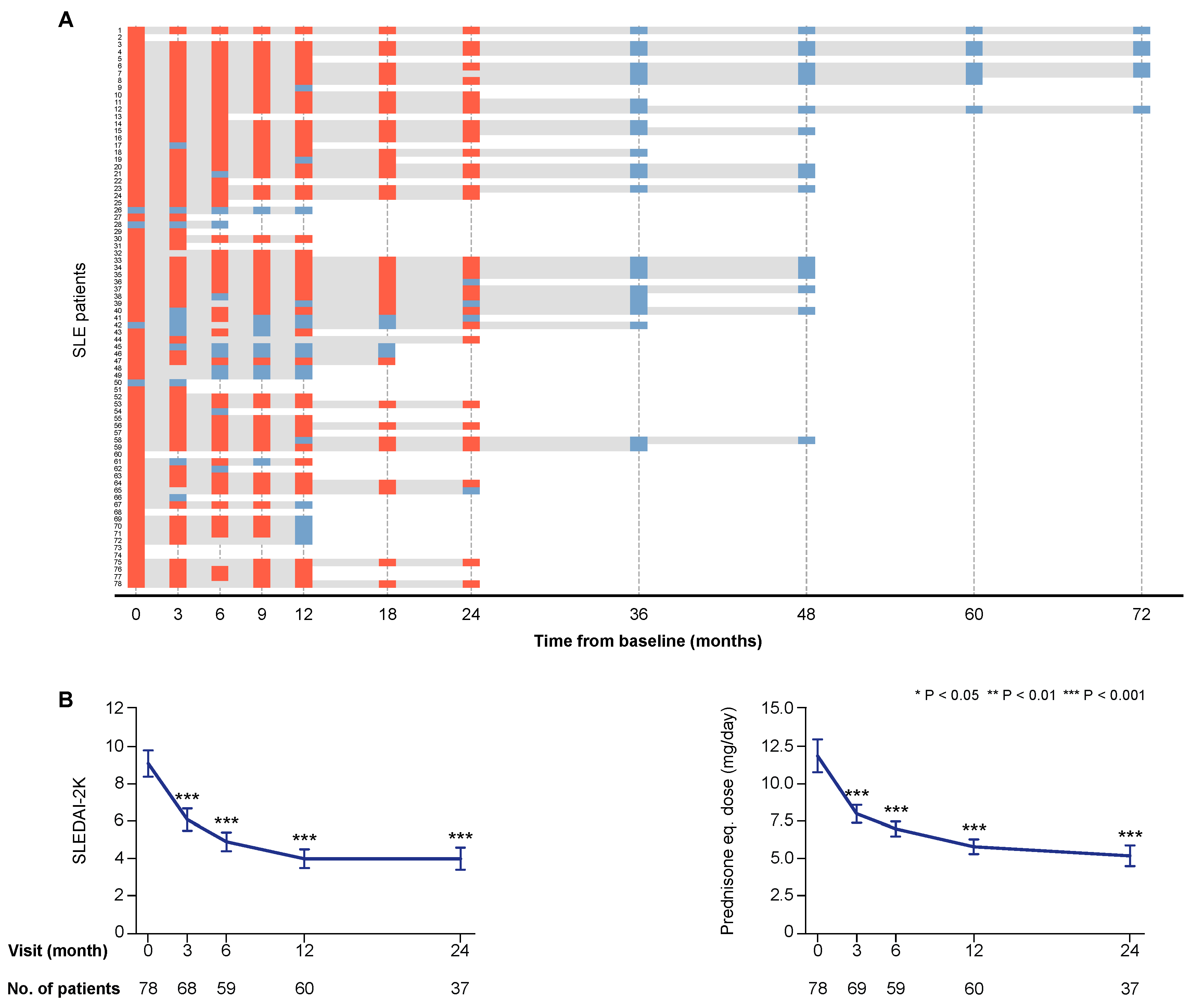
Seventy-three patients (93.6%) were women in line with the general estimation of the female-to-male ratio of SLE patients in Sweden [15], and the median age at the time of enrolment was 41.3 years (interquartile range (IQR): 31.6–51.4 years). The clinical course of the first 55 patients enrolled in the study has been reported previously [4]. The total follow-up time for each study participant, as well as SLE Disease Activity Index 2000 (SLEDAI-2K) scores and prednisone equivalent doses during treatment with belimumab are presented in Figure 1.
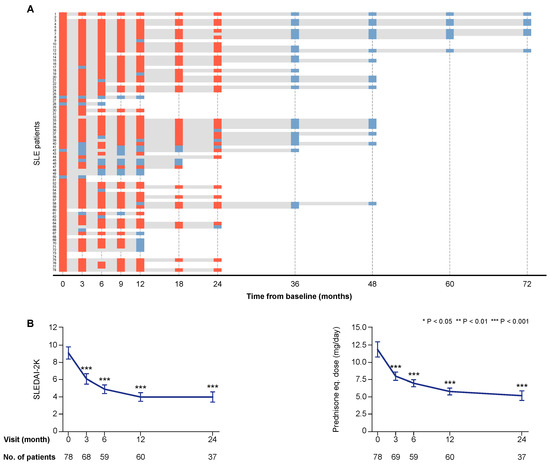
Figure 1.
Follow-up and disease activity over time. Panel (A) illustrates the individual follow-up time for each one of the patients. The bars in grey represent time on treatment. The orange rectangles correspond to follow-up visits when a clinical assessment was performed and serum samples were available for analysis. The light blue rectangles correspond to follow-up visits from which clinical data only were obtained. The charts in panel (B) illustrate systemic lupus erythematosus (SLE) disease activity according to SLE Disease Activity Index 2000 (SLEDAI-2K) scores, and prednisone equivalent dose over time on treatment with belimumab. The blue lines connect mean values of the distributions at given time points, and the whiskers represent standard errors of the mean. The number of patients contributing to the respective measurement is indicated. Asterisks indicate statistically significant changes compared with baseline, on the basis of the non-parametric paired Wilcoxon signed rank test.
We first analysed and graphically illustrated data for all patients regarding serum levels of the examined cytokines (Figure 2), autoantibodies, and circulating IC (Figure 3) over time on belimumab therapy. We next analysed serum levels of the examined cytokines, autoantibodies, and circulating IC during belimumab treatment in patients with detectable (for cytokines) or positive (for autoantibodies and IC) levels at baseline (Figure 4). Serum levels of autoantibody specificities in a proportion of patients and follow-up time from the same cohort have been included in previous investigations [4,9,16]. With regard to patients with undetectable cytokine levels, or negative autoantibody or circulating IC levels at baseline, we only observed a few seroconversions to detectable or positive levels, respectively, during the 24 month follow-up. These are reported in detail in Table S1.
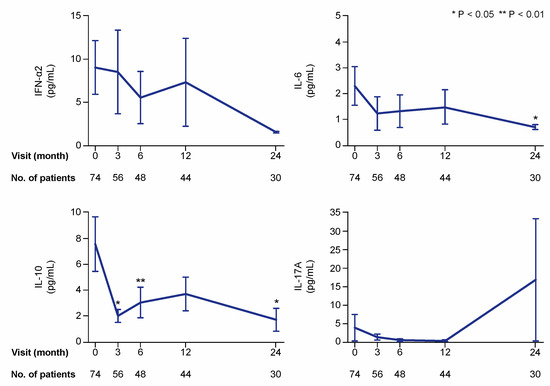
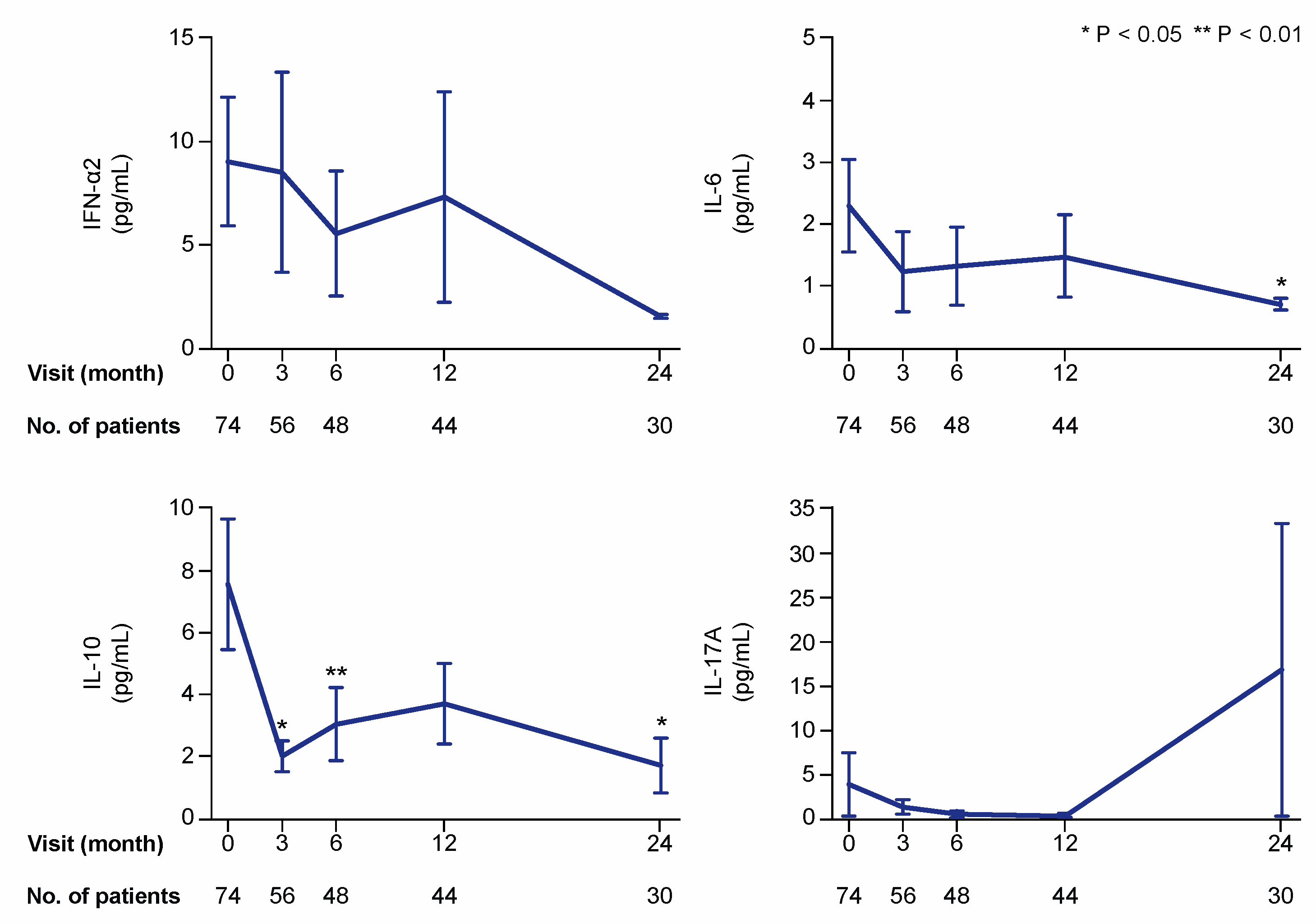
Figure 2.
Selected cytokines during treatment with belimumab. The charts illustrate serum levels of selected cytokines—interferon (IFN)-α2, interleukin (IL)-6, IL-10, and IL-17A—at specific time points during treatment with belimumab. The blue lines connect mean values of the distributions at the different times points, and the whiskers represent standard errors of the mean. The numbers of patients contributing to the measurements are indicated. Asterisks indicate statistically significant changes compared with baseline, on the basis of the non-parametric paired Wilcoxon signed rank test.
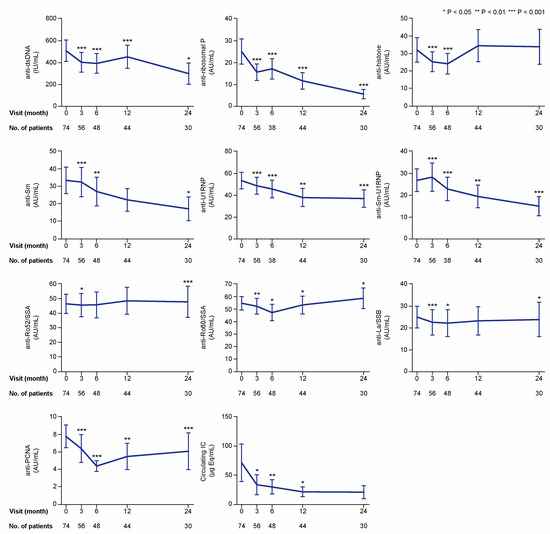
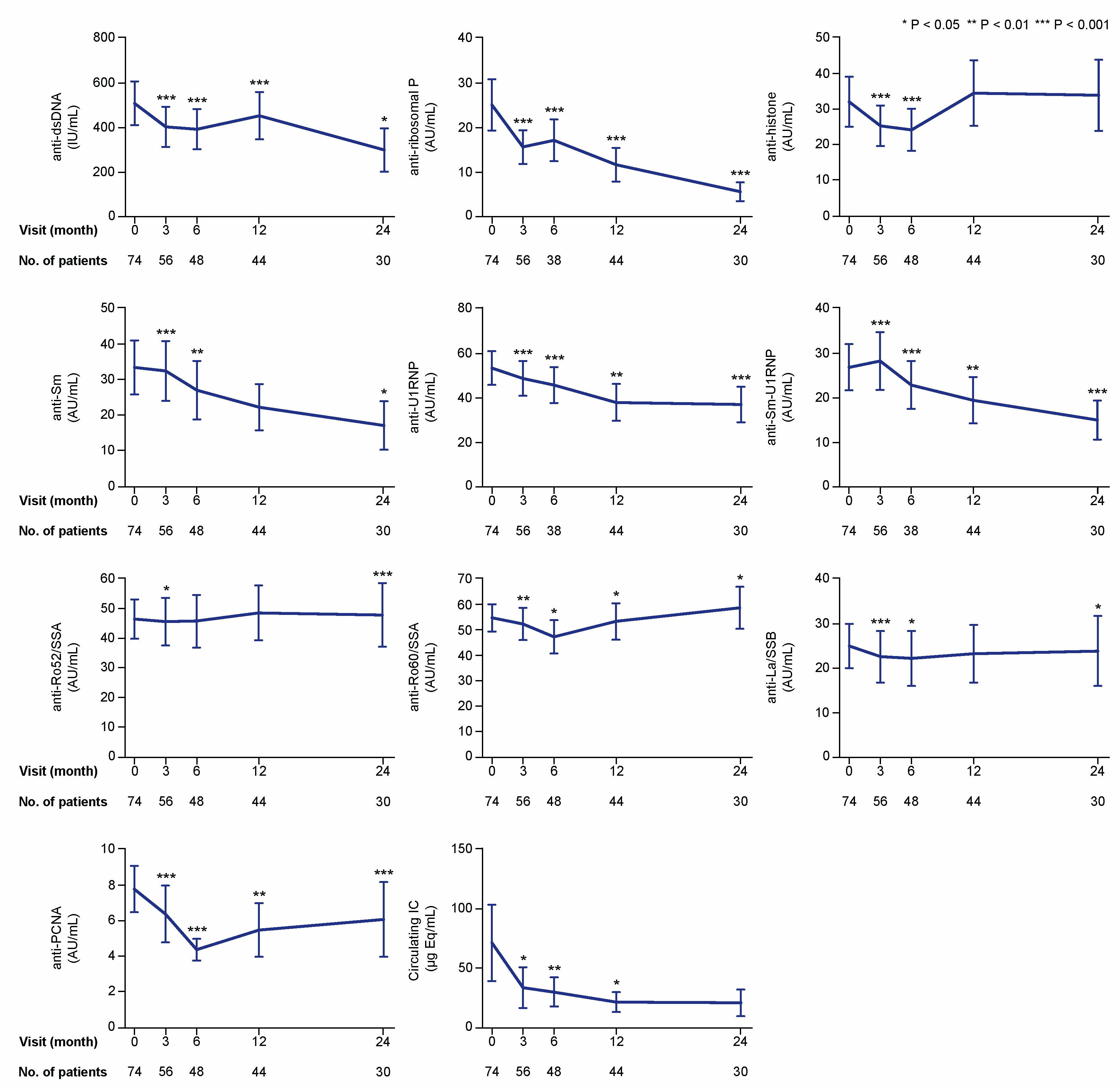
Figure 3.
Autoantibody specificities and IC during treatment with belimumab. The charts illustrate serum levels of multiple nuclear antigen autoantibody specificities at specific time points during treatment with belimumab. The blue lines connect mean values of the distributions at the different times points, and the whiskers represent standard errors of the mean. The numbers of patients contributing to the measurements are indicated. Asterisks indicate statistically significant changes compared with baseline, based on the non-parametric paired Wilcoxon signed rank test. IC: immune complex; IU: international units; AU: arbitrary units.
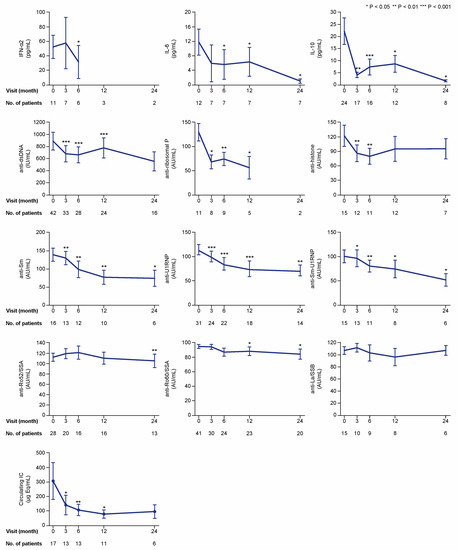
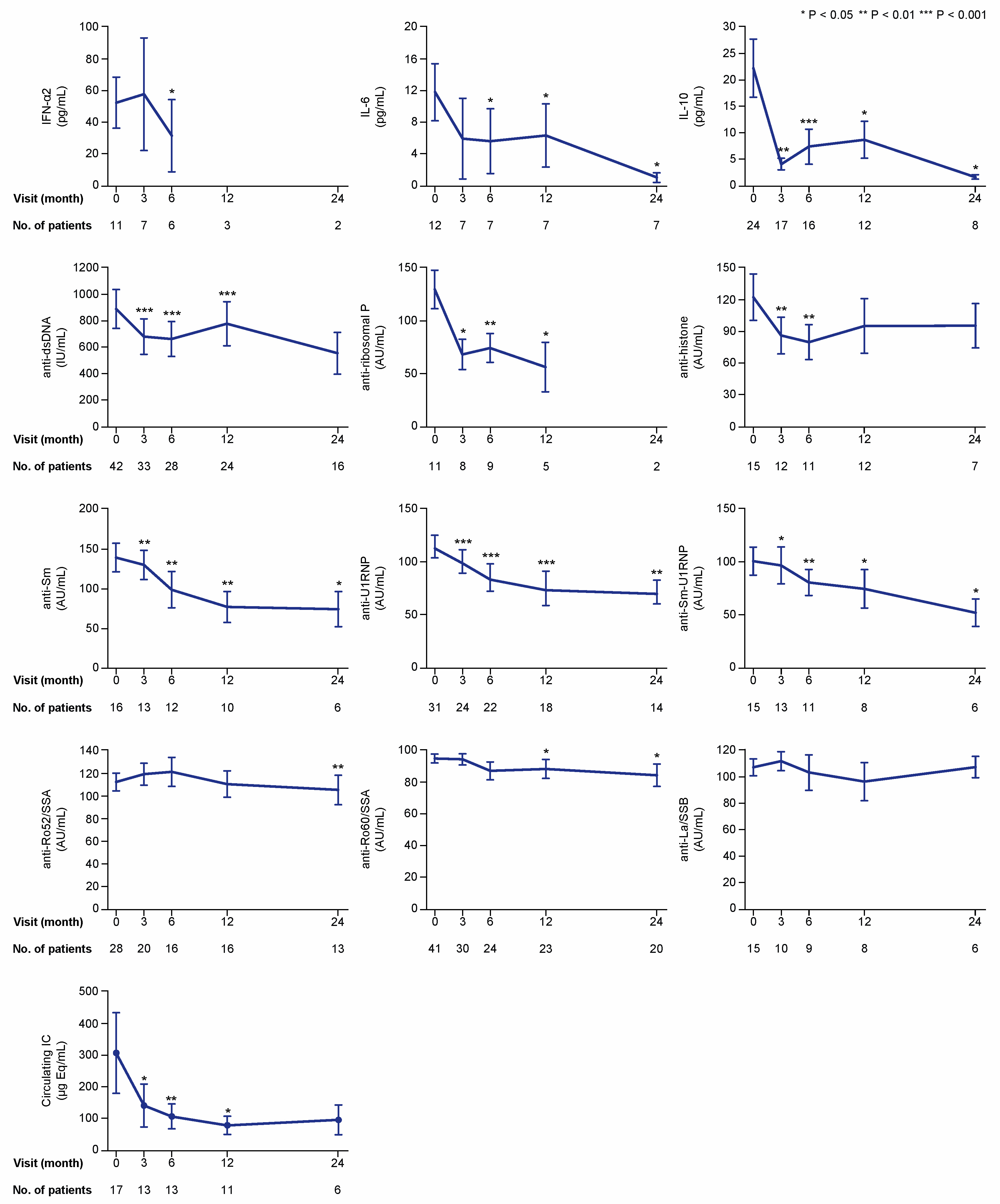
Figure 4.
Cytokine, autoantibody, and immune complex (IC) levels if detectable or positive at baseline. The charts illustrate serum levels of selected cytokines and nuclear antigen autoantibody specificities over time on belimumab treatment in patients with detectable levels for cytokines and levels above the threshold for positivity for autoantibodies and immune complexes. The blue lines connect mean values of the distributions at the different times points, and the whiskers represent standard errors of the mean. The numbers of patients contributing to the measurements are indicated; data samples with fewer than five patients were dispensed from statistical analysis and graphical illustration. Asterisks indicate statistically significant changes compared with baseline, on the basis of the non-parametric paired Wilcoxon signed rank test. IC: immune complex; IFN: interferon; IL: interleukin; IU: international units; AU: arbitrary units.
2.1. Cytokine Levels during Belimumab Therapy
In the first analysis including all patients (also patients with cytokine levels below the lower detection limit of the assay), serum levels of interleukin (IL)-10 showed the most prominent changes over time, with statistically significant decreases as soon as at the 3 month follow-up (mean: 2.0; median 0.6; IQR: 0.6–0.6 pg/mL) compared with baseline (mean: 7.6; median 0.6; IQR: 0.6–2.8 pg/mL; p = 0.016). Serum levels of IL-6 (baseline mean: 2.3; median 0.5; IQR: 0.5–0.5 pg/mL) showed a slower decline, which reached statistical significance at month 24 (mean: 0.7; median 0.5; IQR: 0.5–0.5 pg/mL; p = 0.043). Changes in levels of interferon (IFN)-α2 and IL-17A did not reach statistical significance in this analysis (Figure 2).
At baseline, the number of patients with detectable levels of IFN-α2, IL-10, and IL-6 was 11, 24, and 12, respectively (Figure 4). Because only one patient had detectable levels of IL-17A, this cytokine was excluded from further analysis. In the analysis of patients with detectable baseline levels, serum levels of IFN-α2 were lower at month 6 (median: 8.9; IQR: 1.5–54.9 pg/mL) compared with baseline (median: 28.4; IQR: 20.9–100.3 pg/mL; p = 0.043), but not at month 3 (p = 0.345). Levels of IL-6 showed decreases from baseline (median: 7.1; IQR: 2.9–16.1 pg/mL) to month 6 (median: 0.5; IQR: 0.5–6.3 pg/mL; p = 0.018) and throughout a 24 month follow-up. Levels of IL-10 (baseline median: 12.6; IQR: 2.8–29.7 pg/mL) showed more rapid decreases at month 3 (median: 1.8; IQR: 0.6–9.1 pg/mL; p = 0.003) and remained significantly lower than baseline levels over a 24 month follow-up (Figure 4).
2.2. Autoantibody and IC Levels during Belimumab Therapy
In the first analysis including all patients, serum levels of anti-dsDNA showed profound decreases from baseline values (median: 82.8; IQR: 11.7–499.5 international units (IU)/mL), reaching statistical significance at month 3 (median: 63.9; IQR: 10.1–588.3 IU/mL; p < 0.001), which was maintained throughout a 24 month follow-up (Figure 3). Serum levels of anti-Smith antigen (Sm) levels also decreased over time compared with baseline levels (median: 2.7; IQR: 0.6–19.7 arbitrary units (AU)/mL); these decreases were statistically significant at the 3 month visit (median: 1.8; IQR: 0.5–18.1 AU/mL; p < 0.001) and remained significantly decreased throughout a 24 month follow-up, with the exception of the 12 month visit (p = 0.145). Levels of anti-U1 small nuclear ribonucleoprotein (U1RNP) were significantly decreased compared with baseline levels (median: 17.8; IQR: 3.0–86.1 AU/mL) at month 3 and throughout the follow-up period until the 24 month visit (median: 14.7; IQR: 1.4–59.4 AU/mL; p < 0.001). Similarly, levels of antibodies against the Sm-U1RNP complex were decreased compared with baseline at all studied follow-up time points (Figure 3). Serum levels of circulating IC showed decreases compared with baseline levels (median: 1.2; IQR: 0.1–10.1 μg Eq/mL) at month 3 (median: 0.7; IQR: 0.1–9.8 μg Eq/mL; p = 0.031), and remained decreased at month 6 (p = 0.009) and 12 (p = 0.049), but not at month 24 (p = 0.272).
Numbers of patients with serum autoantibody levels above the thresholds for positivity at baseline were sufficient for further analysis for most of the antibody specificities, that is, anti-dsDNA (n = 42), anti-histone (n = 15), anti-Sm (n = 16), anti-Sm-U1RNP (n = 15), anti-U1RNP (n = 31), anti-ribosomal P (n = 11), anti-Ro52/SSA (n = 28), anti-Ro60/SSA (n = 41), and anti-La/SSB (n = 15). However, only two patients had positive levels of antibodies against proliferating cell nuclear antigen (anti-PCNA), and this specificity was therefore not included in the subsequent analyses. In patients with positive baseline levels, levels of anti-dsDNA (p < 0.001), anti-Sm (p = 0.002), anti-Sm-U1RNP (p = 0.028), anti-U1RNP (p < 0.001), and anti-ribosomal P (p = 0.012) antibodies were found to be reduced at month 3 and remained significantly lower than baseline levels over the 24 month study period (Figure 4). Anti-histone antibody levels showed decreases at month 3 (p = 0.008) and 6 (p = 0.003) from treatment initiation, but were not significantly changed compared with baseline levels at later time points. In patients with baseline circulating IC levels equal to or above the threshold for positivity (10.8 μg Eq/mL) (n = 17), serum IC levels showed decreases compared with baseline levels (median: 76.5; IQR: 30.1–278.3 μg Eq/mL) at month 3 (median: 28.1; IQR: 18.6–180.3 μg Eq/mL; p = 0.028), and remained decreased at month 6 (p = 0.009) and 12 (p = 0.021), but no significant change was observed at month 24 (p = 0.345; Figure 4).
2.3. Autoantibody to total IgG ratios
To investigate whether the decreases in autoantibody levels were specific for the respective autoantibody or reflected a general decrease in IgG levels, we assessed autoantibody to total IgG ratios over time on treatment. Because data on total IgG were available in a limited proportion of the study population (n = 55), and only for the early follow-up period in the majority of these patients, we only investigated changes from baseline to month 6. The median of total IgG levels decreased by 5.1% (p = 0.001). Among autoantibody specificities, anti-dsDNA to total IgG ratios declined from a median of 4.5 (IQR: 0.7–24.5) IU/mg to a median of 3.6 (IQR: 0.7–31.1) IU/mg (p = 0.002), anti-ribosomal P to total IgG ratios declined from a median of 0.14 (IQR: 0.08–0.60) AU/mg to a median of 0.10 (IQR: 0.08–0.38) AU/mg (p < 0.001), and anti-PCNA to total IgG ratios declined from a median of 0.20 (IQR: 0.13–0.33) AU/mg to a median of 0.16 (IQR: 0.10–0.26) AU/mg (p = 0.004), whereas the rest of the autoantibody to total IgG ratios did not differ significantly between baseline and month 6 (p = ns for all).
2.4. Baseline Cytokine, Autoantibody, and IC Profiles as Predictors of Treatment Response
We evaluated the patients’ baseline cytokine (levels above the detection limit of the assay), autoantibody (levels above the threshold for positivity), and IC (positive levels) status with regard to achievement of different outcomes; IL-17A and anti-PCNA were excluded from this analysis due to low numbers of eligible patients.
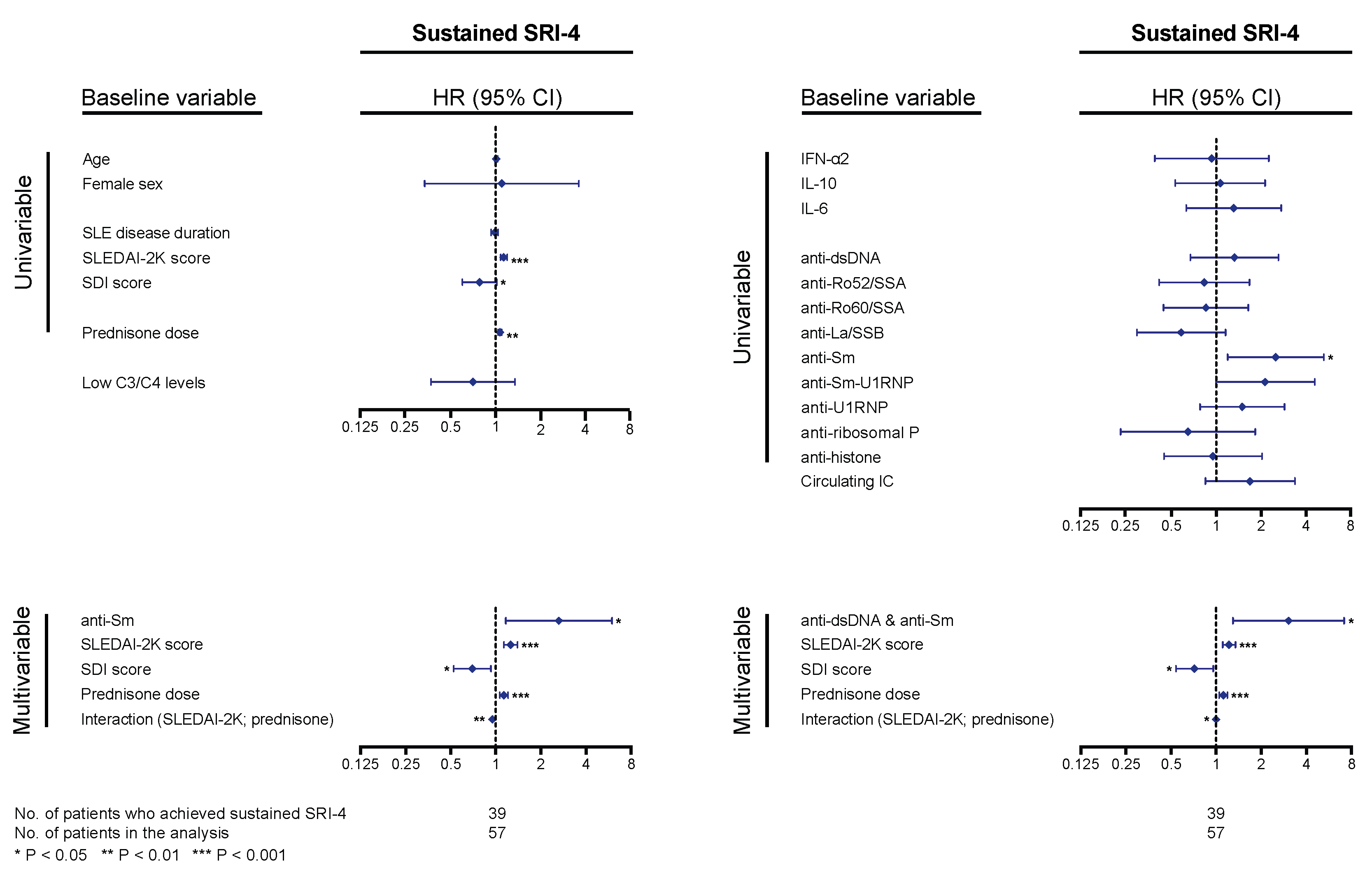
For analysis of the systemic lupus erythematosus responder index 4 (SRI-4) response, at least two follow-up visits, data availability allowing determination of the SRI-4 components, and a baseline SLEDAI-2K score of 4 or higher were required. Of a total of 57 patients qualifying for analysis, 39 patients (68.4%) achieved sustained SRI-4 during follow-up, after a median time of 3.4 (IQR: 2.8–7.9) months; 18 patients (31.6%) did not achieve the outcome after having been followed for a median time of 13.4 (IQR: 10.4–39.6) months. In Cox regression analysis, higher baseline SLEDAI-2K scores and prednisone equivalent doses were associated with higher probability and/or shorter time to sustained SRI-4 response (hazard ratio, HR: 1.10; 95% confidence interval, CI: 1.05–1.16; p < 0.001 and HR: 1.04; 95% CI: 1.01–1.07; p = 0.002, respectively), and higher baseline Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI) scores were associated with lower probability and/or longer time to sustained SRI-4 response (HR: 0.76; 95% CI: 0.58–0.99; p = 0.044). Age, sex, SLE disease duration, and hypocomplementaemia showed no significant association with achievement of SRI-4 response (Figure 5). Notably, anti-Sm antibody positivity was associated with higher probability and/or shorter time to achieve sustained SRI-4 response (HR: 2.52; 95% CI: 1.20–5.29; p = 0.015); this association remained significant after adjustment for baseline SLEDAI-2K scores, prednisone equivalent doses, and baseline SDI scores, including the significant interaction between SLEDAI-2K scores and prednisone doses (Figure 5). Substituting anti-Sm positivity with positivity for both anti-dsDNA and anti-Sm (n = 12) slightly improved the model, yielding a HR of 3.06 (95% CI: 1.30–7.21; p = 0.011; Figure 5). No other cytokines or autoantibody specificities were found to impact on attainment of sustained SRI-4 response in our cohort. No association was found between SRI-4 response attainment and positive levels of circulating IC at baseline.
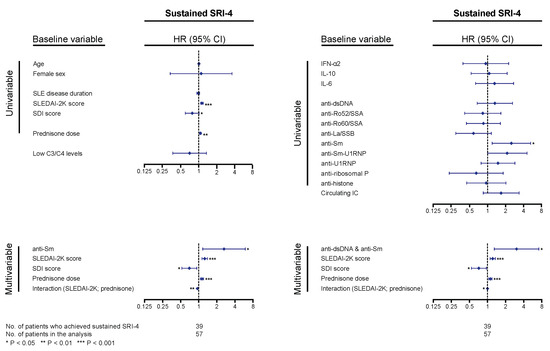
Figure 5.
Associations with systemic lupus erythematosus responder index 4 (SRI-4) response. The forest plots illustrate results from proportional hazards regression (Cox regression) models created to explore potential associations between baseline levels of cytokines, autoantibodies, or circulating immune complexes (IC) and achievement of sustained SRI-4 response (fulfilment of the SRI-4 conditions at two consecutive follow-up visits, at least 3 months apart). Demographic and disease-specific factors were also analyzed for the purpose of adjustments for confounding potentiality. Time in the models represents the time from baseline to the first follow-up visit when sustained SRI-4 was achieved for patients who achieved the outcome, and total follow-up time for patients who did not. All baseline variables were first tested in univariable (simple) models, and variables showing significant associations with attainment (or non-attainment) of SRI-4 were next included in a multivariable model. Next, we substituted anti-Smith antigen (Sm) positivity with double positivity for anti-double stranded (ds)DNA and anti-Sm antibodies to assess whether this would improve the model. Asterisks indicate statistically significant associations. SRI-4: systemic lupus erythematosus responder index 4; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; SDI: Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index; HR: hazard ratio; CI: confidence interval.
For analysis with regard to clinical remission, at least two follow-up visits and SLEDAI-2K and prednisone dose data availability were required. Of a total of 66 patients qualifying for analysis, 27 patients (40.9%) achieved sustained clinical version of SLEDAI-2K (cSLEDAI-2K) = 0 during follow-up, after a median time of 6.6 (IQR: 5.8–12.2) months; 39 patients (59.1%) did not achieve the outcome after having been followed for a median time of 13.1 (IQR: 9.3–36.1) months. No demographic (age, sex) or disease-associated (disease activity, organ damage, disease duration, glucocorticoid dose, C3/C4 status) characteristics and no cytokine, autoantibody specificity, or IC positivity showed any association with attainment of cSLEDAI-2K = 0 in Cox regression analysis. This was also the case when the glucocorticoid restriction (prednisone equivalent dose ≤ 7.5 mg/day) was added in the definition criteria (Figure S1). Sustained cSLEDAI-2K = 0 and prednisone equivalent dose ≤ 7.5 mg/day was achieved by 22 patients (33.3%) after a median time of 7.8 (IQR: 6.1–17.5) months, whereas 44 patients (66.7%) did not meet the criteria of this composite outcome during follow-up, that is, a median time of 13.4 (IQR: 9.7–35.7) months.
Similarly, no significant associations were found with regard to attainment of sustained Lupus Low Disease Activity State (LLDAS; Figure S1). A total of 35/66 patients (53.0%) achieved sustained LLDAS after a median time of 7.9 (IQR: 5.0–16.4) months; 31 patients (47.0%) did not attain sustained LLDAS after a total median follow-up of 11.5 (IQR: 9.3–23.9) months.
Numbers and proportions of patients with undetectable cytokine levels or negative autoantibody or IC levels at baseline who achieved sustained SRI-4, cSLEDAI-2K = 0, cSLEDAI-2K = 0 and prednisone equivalent dose ≤ 7.5 mg/day, or LLDAS are presented in Table S2.
2.5. Early Changes in Cytokine, Autoantibody, and IC Levels as Predictors of Response
Early changes in cytokine, autoantibody, and IC levels from baseline to month 3 (Table S3) and from baseline to month 6 (Table S4) were next evaluated as predictors of response to belimumab therapy. Early decline in IL-6 levels from baseline to month 3 was found to be consistently associated with attainment of sustained SRI-4, cSLEDAI-2K = 0, and cSLEDAI-2K = 0 and prednisone equivalent dose ≤ 7.5 mg/day (Table S3). In a subanalysis of patients who had detectable levels of IL-6 at baseline or month 3, IL-6 levels declined from baseline to month 3 in patients who later attained sustained cSLEDAI-2K = 0 and prednisone equivalent dose ≤ 7.5 mg/day (median change: -5.9 pg/mL; 25th percentile: -6.8 pg/mL; 75th percentile: -4.6 pg/mL), but increased in patients who did not attain this outcome (median change: 3.2 pg/mL; 25th percentile: -0.4 pg/mL; 75th percentile: 18.1 pg/mL), yielding a significant difference (p = 0.029).
3. Discussion
In the present study, we demonstrated that IFN-α2, IL-6, IL-10, circulating IC levels, and levels of multiple autoantibodies against nuclear components decreased during anti-BAFF treatment with belimumab, albeit differently in terms of intensity and/or sustainability. To the best of our knowledge, this is the first report to assess the performance of positive status regarding multiple nuclear antigen autoantibody specificities as predictors of response to belimumab treatment. Interestingly, anti-Sm antibody positivity at baseline and early decline of IL-6 levels were associated with favorable response to belimumab treatment.
Serum levels of IL-10 showed the most rapid and prominent changes, being significant from month 3 and throughout the entire follow-up period. Serum levels of IFN-α2 and IL-6 also decreased, however in a more moderate and slower fashion. The decreases in IL-10 and IL-6 levels most likely reflect the overall abatement of inflammatory activity, following the decreases in clinical markers of disease activity, as shown herein and in previous observational studies [2,4,5,17,18,19,20,21] and clinical trials [22,23,24,25]. Although the principal functions of IL-10 were initially coupled with suppression of cytokine secretion and termination of inflammatory responses [26,27], there is evidence that IL-10 may induce and amplify autoantibody production in autoimmune conditions [28,29,30], and IL-10 levels may therefore reflect the inflammatory state in such conditions. Importantly, early decline in IL-6 levels within 3 months of belimumab treatment was associated with belimumab efficacy, that is, attainment of sustained SRI-4 and clinical remission. The consistency in these associations advocate for the role of IL-6 as a useful marker of inflammation in patients with SLE, as also implied in our recent study of rituximab therapy [31]. In patients with detectable IL-6 levels at baseline, early declines could hence signify suitability for treatment continuation.
IFN-α signaling is known to be aberrant in SLE [32]; type I IFNs derived from plasmacytoid dendritic cells (pDCs) are important in proinflammatory cytokine production, including B cell differentiation and survival factors BAFF and APRIL [33]. The mechanistic explanation underlying the decrease of IFN-α levels is not totally clear, but could be traced to the overall decrease of autoantibody levels and circulating IC, presumably resulting in reduced Fcγ receptor stimulation on pDCs towards production of type I IFNs [32]. Thus, belimumab treatment might have indirect effects on the BAFF/APRIL pathway apart from the direct binding to soluble BAFF. In light of the recent clinical trials of anti-IFN-α (rontalizumab [34], sifalimumab [35]) and anti-IFN α/β receptor (IFNAR; antifrolumab [36,37]) agents in SLE, it is of particular importance to demonstrate how a currently available lupus therapy indirectly impacts on this pathway. Nonetheless, it is important to place emphasis on the fact that IFN-α2 only represents a fraction of the type I IFN activity; studies of larger cohorts and analyses of other cytokines of the same family are therefore necessary in order to more accurately evaluate the impact of belimumab treatment on the type I IFN pathway. It is worth noting that only one patient had detectable levels of IL-17A at baseline, which contrasts with a previous report of higher proportions (12%) in SLE patients from Sweden [38], and might depend on the different assays used to measure cytokine levels in the two studies, or patient selection. Supportive of the latter is a previous report from our group that demonstrated elevated IL-17 levels in SLE patients with nephritis [39]. In the same lupus nephritis cohort, the proportion of patients with detectable INF-α levels was also higher (66%) [40] compared with the findings in the present study (15%).
Serum levels of autoantibodies belonging to the Ro/La-system, that is, anti-Ro52/SSA, anti-Ro60/SSA, and anti-La/SSB, did not display substantial changes throughout the follow-up period. In SLE, levels of these antibodies have not been shown to have a value in monitoring disease activity [41], in line with their inertia to change in our study. Serum levels of anti-dsDNA and anti-Sm antibodies showed profound, rapid, and sustainable decreases throughout the follow-up. In a similar manner, levels of anti-U1RNP and anti-Sm-U1RNP antibodies as well as IC levels decreased rapidly and remained decreased during follow-up. Ratios of anti-dsDNA, anti-ribosomal P, and anti-PCNA to total IgG also decreased, pointing to a specific effect of belimumab treatment on these specificities rather than reflection of the general effect of the drug on total IgG levels. The observation that only two patients had positive levels of anti-PCNA at baseline is worth noting in light of our recent report showing profound enrichment of this particular specificity in IC [9], pointing to high binding affinity of anti-PCNA to its autoantigen.
An important finding was that, in conformity with previous observations following B cell depletion with the anti-CD20 antibody rituximab [42], the presence of anti-Sm but not anti-dsDNA antibodies was associated with a higher probability and/or shorter time to achieve clinical improvement following belimumab treatment, irrespective of disease activity, glucocorticoid dose, and organ damage degree. These findings contrast with observations from early clinical trials of belimumab in which proportions of responders did not differ across patient subsets positive for different autoantibody subtypes at baseline, including anti-dsDNA, anti-Sm, and anti-Ro [43], and also with later studies of belimumab in which anti-dsDNA positivity was implicated to be a predictor of SRI-4 response [6], LLDAS [44], and cSLEDAI-2K = 0 and prednisone dose ≤ 7.5 mg/day [8] in different reports. The lack of association between baseline autoantibody profiles and LLDAS or clinical remission in the present study may have been due to the stringency of these outcomes along with the relatively low number of patients; as previously shown, SRI-4 is sensitive to change, whereas attainment of LLDAS and clinical remission is less frequent and occurs at later time points [4,44,45,46]. Double positivity for anti-dsDNA and anti-Sm antibodies yielded a slightly stronger association with clinical improvement compared with anti-Sm positivity alone, but because a lower number of patients is expected to be positive for both antibodies, the clinical value of this stringency augmentation is questionable. Further investigation of the clinical usefulness of anti-Sm positivity in the selection of patients for belimumab treatment is merited.
The low number of patients and, as a result, the low frequencies of detectable cytokine levels at baseline limited us from performing certain analyses. For example, stratification into clinical manifestations would be of relevance; from a clinical point of view, the therapy is steered by organ involvement, and identification of predictors within different disease subsets is needed to facilitate individualized management approaches. For the same reason, we were unable to analyze concomitant and previous medication other than glucocorticoids. The observational design may also be considered a drawback; decisions steered by the treating physician and not the purposes of the study may, for instance, impede standardisation of the background therapy. However, the patient cases represent real-life scenarios and the follow-up represents current clinical practice, both of which may also be regarded as strengths of the study. In contrast to clinical trial settings, no exclusion criteria were applied in the present study; all patients who were initiated at belimumab treatment were asked to be included in our prospective follow-up program, increasing the heterogeneity of the study cohort, and minimizing the selection bias.
The prospective collection of detailed clinical data along with regular serum sampling may be acknowledged as a strength. It is also worth noting that the study was conducted at tertiary referral centers of the tax-financed health system of Sweden, limiting potential bias imposed by private health insurance in other systems. Investigation of multiple nuclear antigen autoantibody specificities in the context of belimumab treatment was a novelty, and the consistent level reductions of the majority of them has to be seen in light of previous knowledge that belimumab treatment alters the B cell constitution of SLE patients towards declining numbers and proportions of naïve and autoreactive B cells over time on therapy [3,16].
4. Materials and Methods
A total of 78 patients with SLE from the Karolinska (n = 45), Skåne (n = 23), and Linköping (n = 10) University Hospitals who were initiated at belimumab treatment between September 2011 and October 2018 were included in the present real-life observational study. All patients met the 1982 ACR [47] and/or SLICC [48] criteria for classification of SLE. Belimumab was given as intravenous infusions at a dose of 10 mg/kg at baseline, week 2, week 4, and every fourth week thereafter. Baseline characteristics of the patients are summarized in Table 1.

Table 1.
Patient characteristics.
The study complied with the ethical principles of the Declaration of Helsinki, and all study participants signed informed consent forms prior to recruitment. The study protocol was reviewed and approved by the respective regional ethics review boards in Stockholm, Lund, and Linköping.
4.1. Determination of Levels of Cytokines, Autoantibodies and IC
Serum samples obtained at baseline and after 3, 6, 12, and 24 months of treatment were stored at −80 °C until analysis. All samples were obtained prior to belimumab infusion. Data from clinical and laboratory evaluations from the same time points were incorporated in the dataset.
Serum cytokine levels, that is, IFN-α2, IL-6, IL-10, and IL-17A, were measured using Luminex xMAP technology (Milliplex Map kit HCYTOMAG-60K-04; EMD Millipore Corp., Billerica, USA). The corresponding serum concentrations were expressed in pg/mL. The lower detection limit of the assay was 1.5 pg/mL for IFN-α2, 0.5 pg/mL for IL-6, 0.6 pg/mL for IL-10, and 0.4 pg/mL for IL-17A. Non-detectable levels were set to half the lower detection limit for the purpose of statistical analysis.
Serum levels of nuclear antigen autoantibodies against dsDNA, tripartite motif-containing protein 21 (TRIM21, or Ro52/SSA), Ro60/SSA, La/SSB, Smith antigen (Sm), the Sm-U1RNP complex, U1RNP, histone, ribosomal P, and proliferating cell nuclear antigen (PCNA), were determined by addressable laser bead immunoassay (ALBIA) using the Connective Profile MX117 FIDISTM kit (Theradiag, Croissy Beaubourg, Marne La Vallée, France). Serum levels of antibodies were expressed in arbitrary units per mL (AU/mL), except for anti-dsDNA levels which were expressed in international units per mL (IU/mL). Data evaluation was performed using the Theradiag Solinium software. Levels over 40 AU/mL or IU/mL were considered positive, as recommended by the manufacturer. Serum levels of total IgG were determined using an in-house enzyme-linked immunosorbent assay (ELISA), as described in previous studies [9,50,51], and expressed as mg/mL.
Serum concentrations of C1q-binding IC were measured using Quanta Lite ELISA (INOVA Diagnostics, San Diego, CA, USA). Serum IC concentrations were expressed as microgram equivalents per milliliter (μg Eq/mL), and levels over 10.8 μg Eq/mL were considered positive. Complement C3 (reference range 0.67–1.29 g/L) and C4 (reference range 0.13–0.32 g/L) levels were determined using nephelometry at the local laboratory of each one of the centers.
4.2. Clinical Evaluation
Clinical assessment was performed at baseline, month 3, 6, 12, 24, and thereafter once yearly until month 72, or at clinical indication. The total follow-up time for each one of the patients is visualized in Figure 1. Reasons for observations at early time points only included adverse events and recent recruitment. Reasons for discontinuation at late time points included inadequate effect, adverse events, pregnancy plans, and disease quiescence, as previously reported for the majority of patients [4].
We assessed global SLE disease activity using the SLEDAI-2K [52], and organ damage using the SLICC/ACR Damage Index (SDI) [53]. For SLEDAI-2K scores, laboratory and serological items were calculated on the basis of routine test results at the local laboratories; for the anti-dsDNA item, the Crithidia luciliae substrate-based immunofluorescence technique (CLIFT) [54] was used.
We defined response to treatment as attainment of the SLE Responder Index (SRI)-4 criteria [22,23], that is, reduction of ≥4 points in the SLEDAI-2K score, no new A, and no more than one new B in the British Isles Lupus Assessment Group (BILAG) index [55], and no worsening in the physician’s global assessment (PGA) by ≥30% compared with the baseline evaluation. We defined clinical remission as a zero score in the clinical version of SLEDAI-2K (cSLEDAI-2K), in which the serological items (anti-dsDNA and complement levels) are excluded [56]. Finally, we defined low disease activity in accordance with the Lupus Low Disease Activity State (LLDAS) [57], requiring a SLEDAI-2K score ≤4 with no activity in major organ systems (renal activity, central nervous system involvement, cardiopulmonary activity, vasculitis, fever), no signs of haemolytic anaemia or gastrointestinal activity, no new features of SLE disease activity, a physician’s global assessment (PGA) score ≤1 (on a scale 0–3), a prednisone or prednisone equivalent dose of ≤ 7.5 mg/day, and well-tolerated doses of immunosuppressive drugs and/or approved biologic agents.
According to recent treat-to-target recommendations, treatment should aim for sustained clinical remission or low disease activity with the lowest glucocorticoid dose possible [58]. In order to incorporate the latter in the definition of clinical remission used here, we next calculated a composite clinical remission score in which a glucocorticoid dose restriction was added to cSLEDAI-2K = 0, that is, a prednisone or prednisone equivalent dose of ≤ 7.5 mg/day, as used in previous studies [8,45]. For each one of the aforementioned definitions, we further required that the patient met the respective definition criteria at the evaluation of at least two consecutive follow-up visits, at least 3 months apart, and therefore termed the definitions “sustained SRI-4”, “sustained cSLEDAI-2K = 0”, “sustained cSLEDAI-2K = 0 and prednisone equivalent dose ≤ 7.5 mg/day”, and “sustained LLDAS”. For the purpose of proportional hazards (Cox) regression analysis, time from belimumab treatment initiation until achievement of each one of the sustained definitions was registered.
4.3. Statistics
The IBM SPSS Statistics 25 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Comparisons between baseline and follow-up visits were conducted using the non-parametric paired Wilcoxon signed rank test. Proportional hazards (Cox) regression models were created in order to evaluate the potential associations of baseline cytokine and autoantibody or IC levels (detectable and positive levels, respectively) with time-varying achievement of predefined outcomes (sustained SRI-4, clinical remission, and LLDAS). Demographic (age, sex) and disease-specific (disease activity, organ damage, disease duration, C3/C4 status) characteristics were included in multivariable models together with cytokines and autoantibodies under investigation, in case they reached statistical significance in initial univariable models. Associations between early changes in cytokine, autoantibody, or IC levels and predefined outcomes were evaluated using the non-parametric Mann–Whitney U test. p values < 0.05 were considered statistically significant. Graphs were constructed using the GraphPad Prism 7 software.
5. Conclusions
In our cohort, belimumab treatment lowered IFN-α2, IL-6, IL-10, and circulating IC levels, as well as levels of multiple autoantibodies against nuclear components, however, after different time spans from baseline. Notably, anti-Sm antibody positivity at baseline and early decline of IL-6 levels were associated with favorable response to belimumab treatment independently of other factors. Further investigation of these associations is merited.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/10/3463/s1.
Author Contributions
I.P. and I.G. were responsible for conceptualization, design, and coordination of the project; I.P., E.Å., C.S., A.S., A.J., A.G., M.F., A.Z., A.A.B., J.R., and I.G. were responsible for acquisition of data; I.P., E.Å., and A.G. were responsible for the statistical analysis; I.P., E.Å., C.S., A.S., A.J., A.G., M.F., A.Z., A.A.B., J.R., and I.G. contributed to the interpretation of the results; I.P., E.Å., and A.G. drafted the manuscript. All authors read and critically revised the manuscript for intellectual content, approved its final version prior to submission, and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Swedish Research Council; Swedish Rheumatism Association (R-859621; R-862161; R-844801); Professor Nanna Svartz Foundation (2018-00250); Ulla and Roland Gustafsson Foundation (2019-12); King Gustaf V’s 80-year Foundation; Swedish Society of Medicine; Ingegerd Johansson Donation; King Gustaf V and Queen Victoria’s Foundation of Freemasons; Region Östergötland (ALF grants); Region Stockholm; Region Uppsala; Region Skåne; Faculty of Medicine, Lund University; Alfred Österlund’s Foundation; Anna-Greta Crafoord Foundation; Greta and Johan Kock’s Foundation; Skåne University Hospital; and the Karolinska Institutet Foundations.
Acknowledgments
We would like to express our gratitude to Eva Jemseby, Julia Norkko, Sonia Möller and Eva Malmquist (Karolinska), Maria Andersson (Lund), and Marianne Peterson (Linköping), as well as all participating patients.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Iaccarino, L.; Bettio, S.; Reggia, R.; Zen, M.; Frassi, M.; Andreoli, L.; Gatto, M.; Piantoni, S.; Nalotto, L.; Franceschini, F.; et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. 2017, 69, 115–123. [Google Scholar] [CrossRef]
- Stohl, W.; Hiepe, F.; Latinis, K.M.; Thomas, M.; Scheinberg, M.A.; Clarke, A.; Aranow, C.; Wellborne, F.R.; Abud-Mendoza, C.; Hough, D.R.; et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Parodis, I.; Sjöwall, C.; Jönsen, A.; Ramsköld, D.; Zickert, A.; Frodlund, M.; Sohrabian, A.; Arnaud, L.; Rönnelid, J.; Malmström, V.; et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun. Rev. 2017, 16, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Adamichou, C.; Koutsoviti, S.; Panopoulos, S.; Staveri, C.; Klagou, A.; Tsalapaki, C.; Pantazi, L.; Konsta, S.; Mavragani, C.P.; et al. Low disease activity-irrespective of serologic status at baseline-associated with reduction of corticosteroid dose and number of flares in patients with systemic lupus erythematosus treated with belimumab: A real-life observational study. Semin. Arthritis Rheum. 2018, 48, 467–474. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F.; Petri, M.A.; Cervera, R.; Roth, D.A.; Ji, B.N.; Kleoudis, C.S.; Zhong, Z.J.; Freimuth, W. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann. Rheum. Dis. 2012, 71, 1343–1349. [Google Scholar] [CrossRef]
- Parodis, I.; Gomez, A.; Frodlund, M.; Jönsen, A.; Zickert, A.; Sjöwall, C.; Bengtsson, A.A.; Gunnarsson, I. Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin. Biol. Ther. 2018, 18, 911–920. [Google Scholar] [CrossRef]
- Parodis, I.; Johansson, P.; Gomez, A.; Soukka, S.; Emamikia, S.; Chatzidionysiou, K. Predictors of low disease activity and clinical remission following belimumab treatment in systemic lupus erythematosus. Rheumatology 2019, 58, 2170–2176. [Google Scholar] [CrossRef]
- Sohrabian, A.; Parodis, I.; Carlströmer-Berthen, N.; Frodlund, M.; Jönsen, A.; Zickert, A.; Sjöwall, C.; Bengtsson, A.A.; Gunnarsson, I.; Rönnelid, J. Increased levels of anti-dsDNA antibodies in immune complexes before treatment with belimumab associate with clinical response in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2019, 21, 259. [Google Scholar] [CrossRef]
- Chan, O.T.; Hannum, L.G.; Haberman, A.M.; Madaio, M.P.; Shlomchik, M.J. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999, 189, 1639–1648. [Google Scholar] [CrossRef]
- Mamula, M.J.; Fatenejad, S.; Craft, J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J. Immunol. 1994, 152, 1453–1461. [Google Scholar] [PubMed]
- Renaudineau, Y.; Pers, J.O.; Bendaoud, B.; Jamin, C.; Youinou, P. Dysfunctional B cells in systemic lupus erythematosus. Autoimmun. Rev. 2004, 3, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Arendt, B.K.; Darce, J.R.; Wu, X.; Jelinek, D.F. A role for BLyS in the activation of innate immune cells. Blood 2006, 108, 2687–2694. [Google Scholar] [CrossRef]
- Wang, H.; Marsters, S.A.; Baker, T.; Chan, B.; Lee, W.P.; Fu, L.; Tumas, D.; Yan, M.; Dixit, V.M.; Ashkenazi, A.; et al. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat. Immunol. 2001, 2, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Sepúlveda, J.I.; Bolin, K.; Mofors, J.; Leonard, D.; Svenungsson, E.; Jonsen, A.; Bengtsson, C.; Nordmark, G.; Rantapää Dahlqvist, S.; Bengtsson, A.A.; et al. Sex differences in clinical presentation of systemic lupus erythematosus. Biol. Sex Differ. 2019, 10, 60. [Google Scholar] [CrossRef]
- Ramsköld, D.; Parodis, I.; Lakshmikanth, T.; Sippl, N.; Khademi, M.; Chen, Y.; Zickert, A.; Mikes, J.; Achour, A.; Amara, K.; et al. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine 2019, 40, 517–527. [Google Scholar] [CrossRef]
- Collins, C.E.; Dall’Era, M.; Kan, H.; Macahilig, C.; Molta, C.; Koscielny, V.; Chang, D.J. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci. Med. 2016, 3, e000118. [Google Scholar] [CrossRef]
- Schwarting, A.; Schroeder, J.O.; Alexander, T.; Schmalzing, M.; Fiehn, C.; Specker, C.; Perna, A.; Cholmakow-Bodechtel, C.; Koscielny, V.B.; Carnarius, H. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: Results from the OBSErve Germany study. Rheumatol. Ther. 2016, 3, 271–290. [Google Scholar] [CrossRef]
- Cortes, J.; Andreu, J.L.; Calvo, J.; Garcia-Aparicio, A.M.; Coronell, C.G.; Diaz-Cerezo, S. Evaluation of use of Belimumab In clinical practice settings (observe study) in Spain: Health resource utilization and labour absenteeism. Value Health 2014, 17, A534. [Google Scholar] [CrossRef]
- Touma, Z.; Sayani, A.; Pineau, C.A.; Fortin, I.; Matsos, M.; Ecker, G.A.; Chow, A.; Iczkovitz, S. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada Study. Rheumatol. Int. 2017, 37, 865–873. [Google Scholar] [CrossRef]
- von Kempis, J.; Duetsch, S.; Reuschling, N.; Villiger, R.; Villiger, P.M.; Vallelian, F.; Schaer, D.J.; Mueller, R.B. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med. Wkly 2019, 149, w20022. [Google Scholar] [CrossRef] [PubMed]
- Navarra, S.V.; Guzman, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.; Thomas, M.; Kim, H.Y.; Leon, M.G.; et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 721–731. [Google Scholar] [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzova, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef]
- Zhang, F.; Bae, S.C.; Bass, D.; Chu, M.; Egginton, S.; Gordon, D.; Roth, D.A.; Zheng, J.; Tanaka, Y. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann. Rheum. Dis. 2018, 77, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Stohl, W.; Schwarting, A.; Okada, M.; Scheinberg, M.; van Vollenhoven, R.; Hammer, A.E.; Groark, J.; Bass, D.; Fox, N.L.; et al. Efficacy and safety of subcutaneous belimumab in anti-double-stranded DNA-positive, hypocomplementemic patients with systemic lupus erythematosus. Arthritis Rheumatol. 2018, 70, 1256–1264. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Rousset, F.; Garcia, E.; Defrance, T.; Peronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef]
- Rönnelid, J.; Tejde, A.; Mathsson, L.; Nilsson-Ekdahl, K.; Nilsson, B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann. Rheum. Dis. 2003, 62, 37–42. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Yang, S.; Wang, S.; Wei, X.; Song, S. IL-10-expressing Th2 cells contribute to the elevated antibody production in rheumatoid arthritis. Inflammation 2016, 39, 1017–1024. [Google Scholar] [CrossRef]
- Parodis, I.; Söder, F.; Faustini, F.; Kasza, Z.; Samuelsson, I.; Zickert, A.; Svenungsson, E.; van Vollenhoven, R.F.; Malmström, V.; Wermeling, F.; et al. Rituximab-mediated late-onset neutropenia in systemic lupus erythematosus - distinct roles of BAFF and APRIL. Lupus 2018, 27, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Rönnblom, L.; Alm, G.V.; Eloranta, M.L. Type I interferon and lupus. Curr. Opin. Rheumatol. 2009, 21, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, M.L.; Alm, G.V.; Rönnblom, L. Disease mechanisms in rheumatology--tools and pathways: plasmacytoid dendritic cells and their role in autoimmune rheumatic diseases. Arthritis Rheum. 2013, 65, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Kalunian, K.C.; Merrill, J.T.; Maciuca, R.; McBride, J.M.; Townsend, M.J.; Wei, X.; Davis, J.C., Jr.; Kennedy, W.P. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 2016, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W.; CD1067 study investigators. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016, 75, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.A.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Kalunian, K.C.; Vital, E.M.; Lawrence Ford, T.; Gupta, R.; Hiepe, F.; Santiago, M.; et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): A randomised, controlled, phase 3 trial. Lancet Rheumatology 2019, 1, e208–e219. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Investigators, trial of Anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Oke, V.; Brauner, S.; Larsson, A.; Gustafsson, J.; Zickert, A.; Gunnarsson, I.; Svenungsson, E. IFN-lambda1 with Th17 axis cytokines and IFN-alpha define different subsets in systemic lupus erythematosus (SLE). Arthritis Res. Ther. 2017, 19, 139. [Google Scholar] [CrossRef]
- Zickert, A.; Amoudruz, P.; Sundström, Y.; Rönnelid, J.; Malmström, V.; Gunnarsson, I. IL-17 and IL-23 in lupus nephritis—Association to histopathology and response to treatment. BMC Immunol. 2015, 16, 7. [Google Scholar] [CrossRef]
- Zickert, A.; Oke, V.; Parodis, I.; Svenungsson, E.; Sundström, Y.; Gunnarsson, I. Interferon (IFN)-lambda is a potential mediator in lupus nephritis. Lupus Sci. Med. 2016, 3, e000170. [Google Scholar] [CrossRef]
- Hassan, A.B.; Lundberg, I.E.; Isenberg, D.; Wahren-Herlenius, M. Serial analysis of Ro/SSA and La/SSB antibody levels and correlation with clinical disease activity in patients with systemic lupus erythematosus. Scand J. Rheumatol. 2002, 31, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, G.; Isenberg, D.A.; Edwards, J.C.; Leandro, M.J.; Migone, T.S.; Teodorescu, M.; Stohl, W. B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann. Rheum. Dis. 2008, 67, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.A.; Petri, M.A.; Wallace, D.J.; Ginzler, E.M.; Merrill, J.T.; Stohl, W.; Chatham, W.W.; Strand, V.; Weinstein, A.; Chevrier, M.R.; et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 2009, 61, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Oon, S.; Huq, M.; Golder, V.; Ong, P.X.; Morand, E.F.; Nikpour, M. Lupus low disease activity state (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of belimumab in systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Parodis, I.; Emamikia, S.; Gomez, A.; Gunnarsson, I.; van Vollenhoven, R.F.; Chatzidionysiou, K. Clinical SLEDAI-2K zero may be a pragmatic outcome measure in SLE studies. Expert Opin. Biol. Ther. 2019, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Parodis, I.; Emamikia, S.; Gomez, A.; Gentline, C.; Arkema, E.V.; Chatzidionysiou, K.; van Vollenhoven, R.F. Definitions of remission in systemic lupus erythematosus: a post-hoc analysis of two randomised clinical trials. Lancet Rheumatology 2019, 1, e163–e173. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Sjöwall, C.; Hjorth, M.; Eriksson, P. Successful treatment of refractory systemic lupus erythematosus using proteasome inhibitor bortezomib followed by belimumab: description of two cases. Lupus 2017, 26, 1333–1338. [Google Scholar] [CrossRef]
- Åhlin, E.; Mathsson, L.; Eloranta, M.L.; Jonsdottir, T.; Gunnarsson, I.; Rönnblom, L.; Rönnelid, J. Autoantibodies associated with RNA are more enriched than anti-dsDNA antibodies in circulating immune complexes in SLE. Lupus 2012, 21, 586–595. [Google Scholar] [CrossRef]
- Mathsson, L.; Lampa, J.; Mullazehi, M.; Rönnelid, J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res. Ther. 2006, 8, R64. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Ibanez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar] [PubMed]
- Gladman, D.; Ginzler, E.; Goldsmith, C.; Fortin, P.; Liang, M.; Urowitz, M.; Bacon, P.; Bombardieri, S.; Hanly, J.; Hay, E.; et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996, 39, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Aarden, L.A.; de Groot, E.R.; Feltkamp, T.E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann. NY Acad. Sci. 1975, 254, 505–515. [Google Scholar] [CrossRef]
- Hay, E.M.; Bacon, P.A.; Gordon, C.; Isenberg, D.A.; Maddison, P.; Snaith, M.L.; Symmons, D.P.; Viner, N.; Zoma, A. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q. J. Med. 1993, 86, 447–458. [Google Scholar]
- Uribe, A.G.; Vila, L.M.; McGwin, G., Jr.; Sanchez, M.L.; Reveille, J.D.; Alarcon, G.S. The systemic lupus activity measure-revised, the Mexican systemic lupus erythematosus disease activity index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J. Rheumatol. 2004, 31, 1934–1940. [Google Scholar]
- Franklyn, K.; Lau, C.S.; Navarra, S.V.; Louthrenoo, W.; Lateef, A.; Hamijoyo, L.; Wahono, C.S.; Chen, S.L.; Jin, O.; Morton, S.; et al. Asia-pacific lupus, definition and initial validation of a lupus low disease activity state (LLDAS). Ann. Rheum. Dis. 2016, 75, 1615–1621. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Mosca, M.; Bertsias, G.; Isenberg, D.; Kuhn, A.; Lerstrom, K.; Aringer, M.; Bootsma, H.; Boumpas, D.; Bruce, I.N.; et al. Treat-to-target in systemic lupus erythematosus: Recommendations from an international task force. Ann. Rheum. Dis. 2014, 73, 958–967. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).