Global Landscapes of the Na+/H+ Antiporter (NHX) Family Members Uncover their Potential Roles in Regulating the Rapeseed Resistance to Salt Stress
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Identification of NHXs in Plant Species
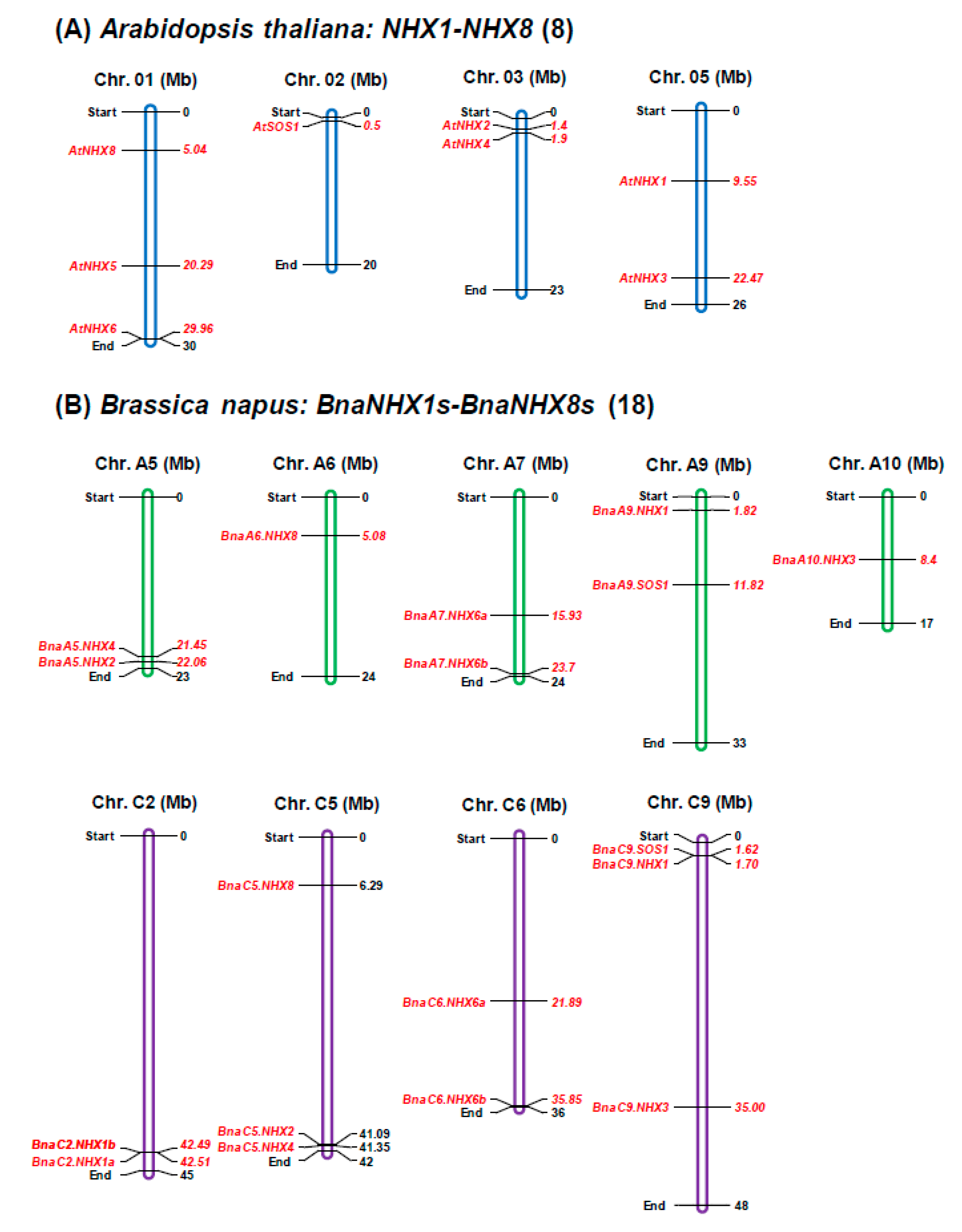
2.2. Genomic Distribution and Expansion Patterns of BnaNHXs
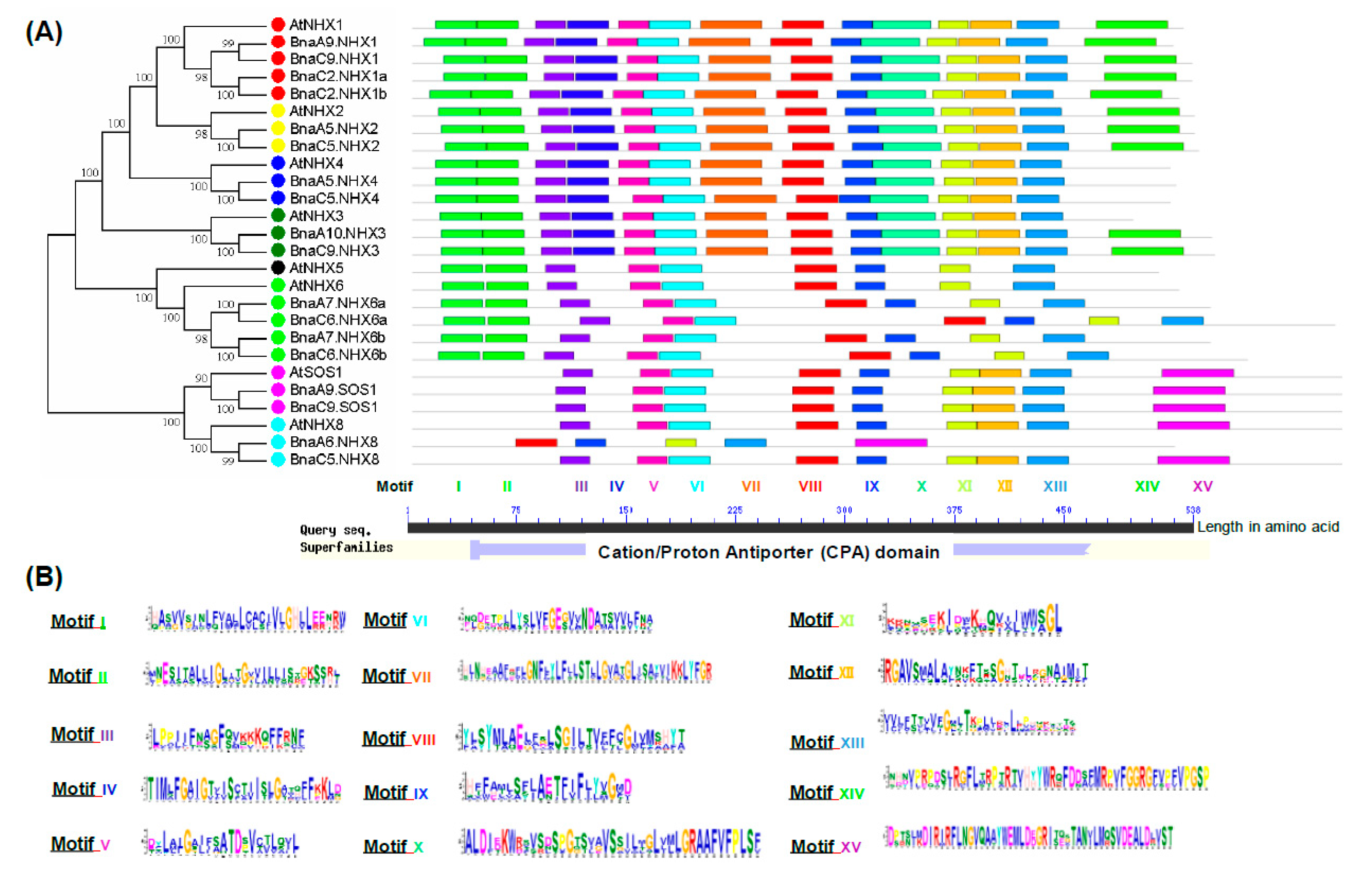
2.3. Phylogeny Analysis of BnaNHXs
2.4. Molecular Characterization of BnaNHXs
2.5. Identification of Evolutionary Selection Pressure on BnaNHXs
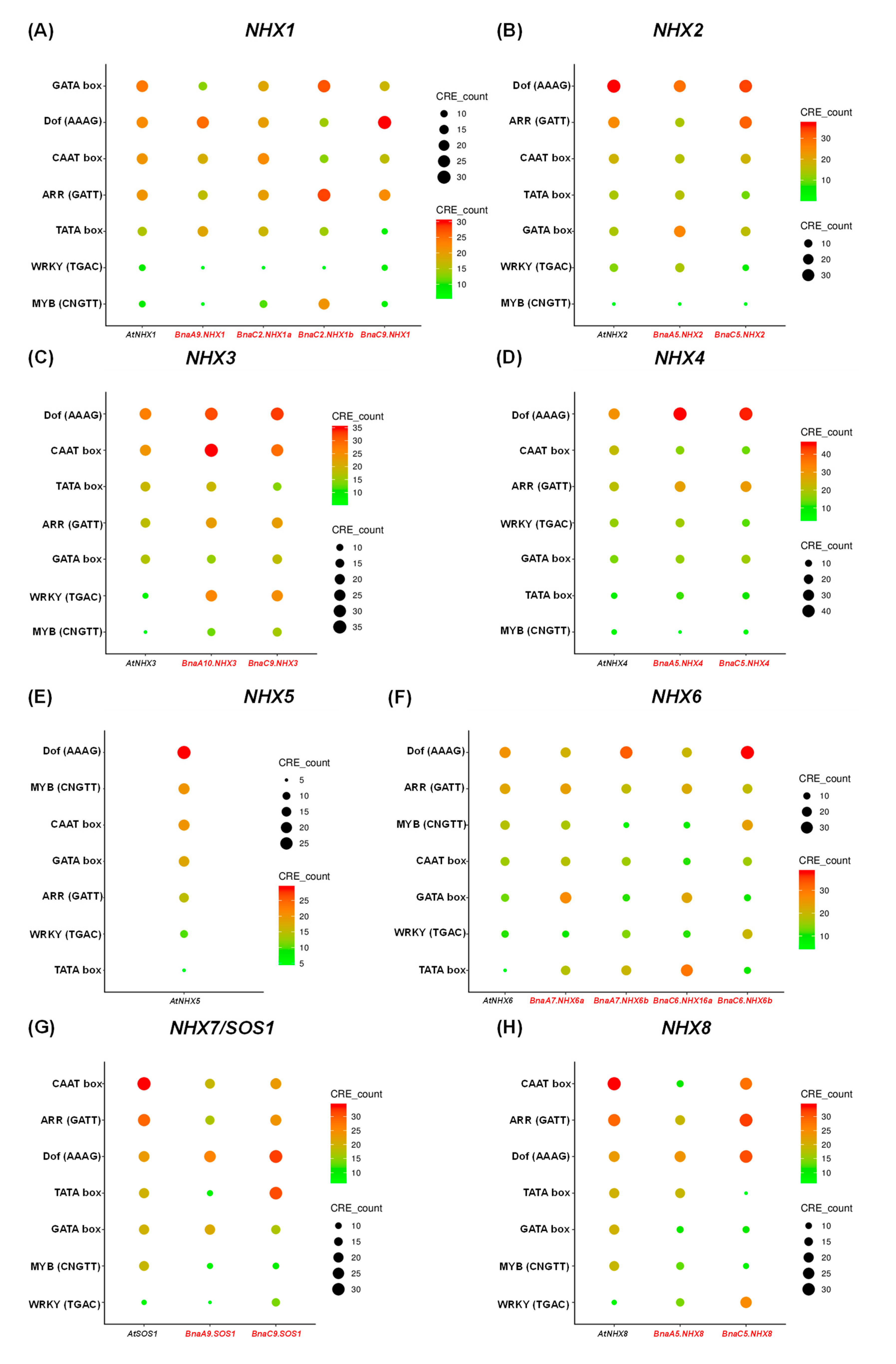
2.6. Conserved Domain, Gene Structure, Protein Interaction and Transcriptional Regulatory Analysis
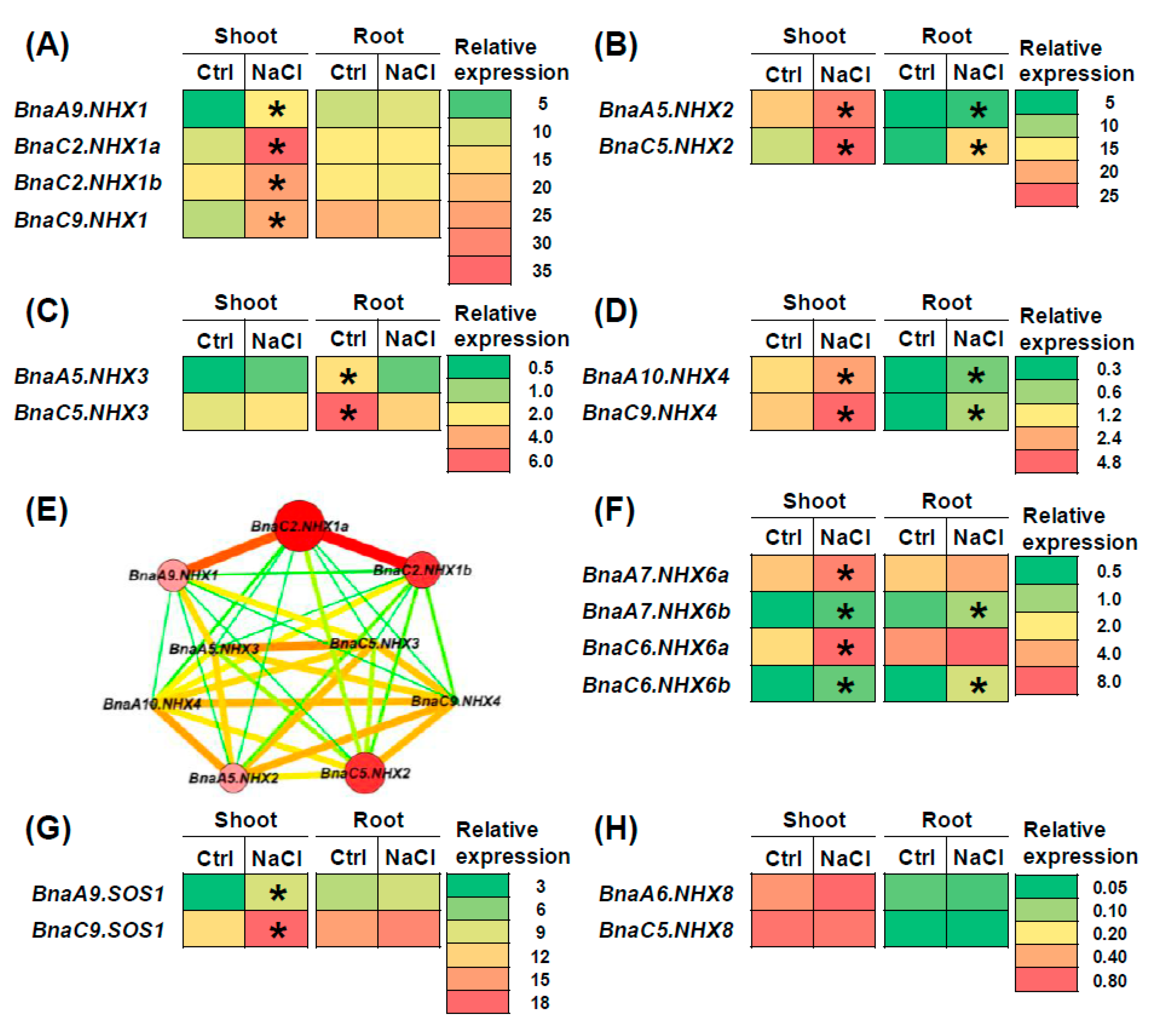
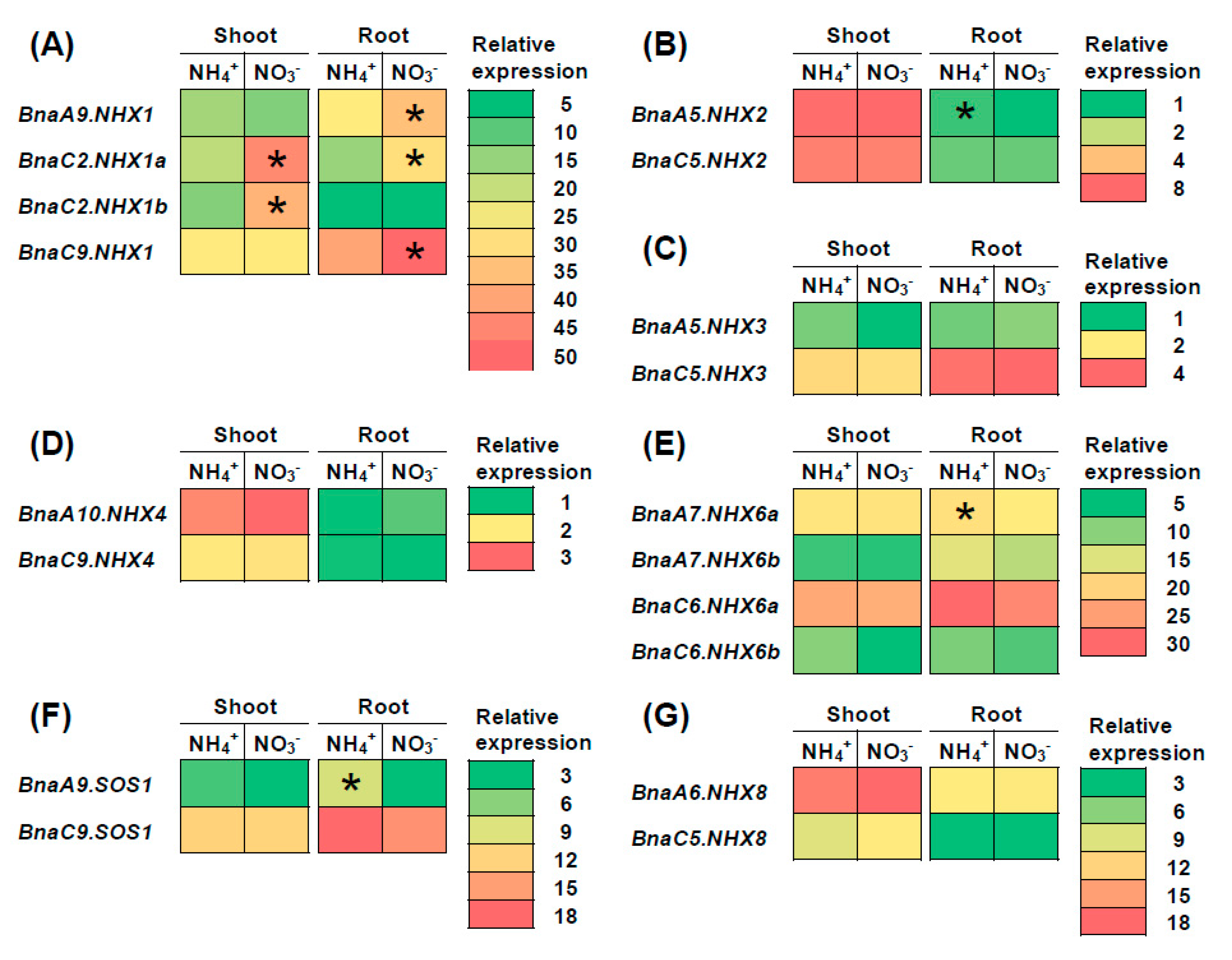
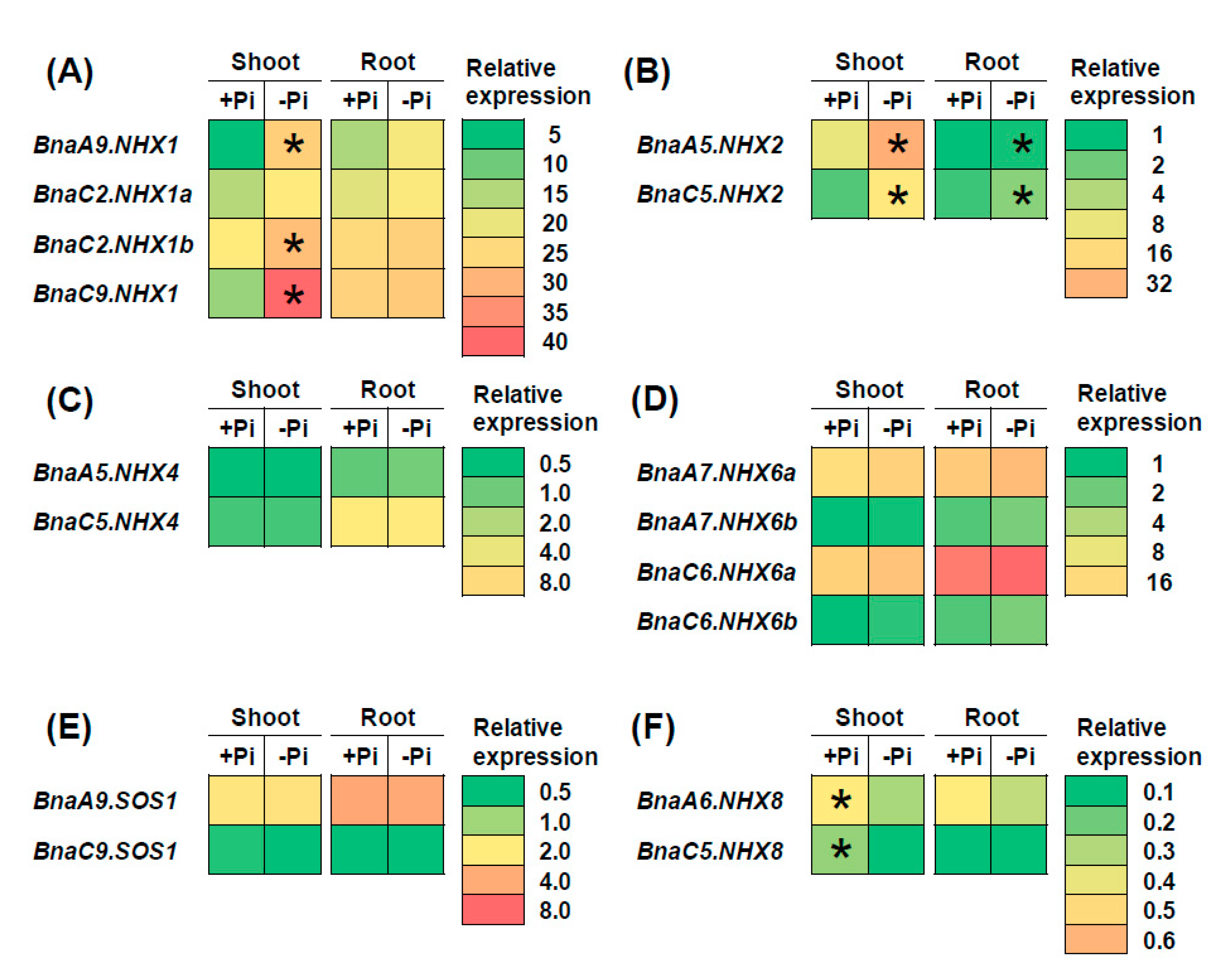
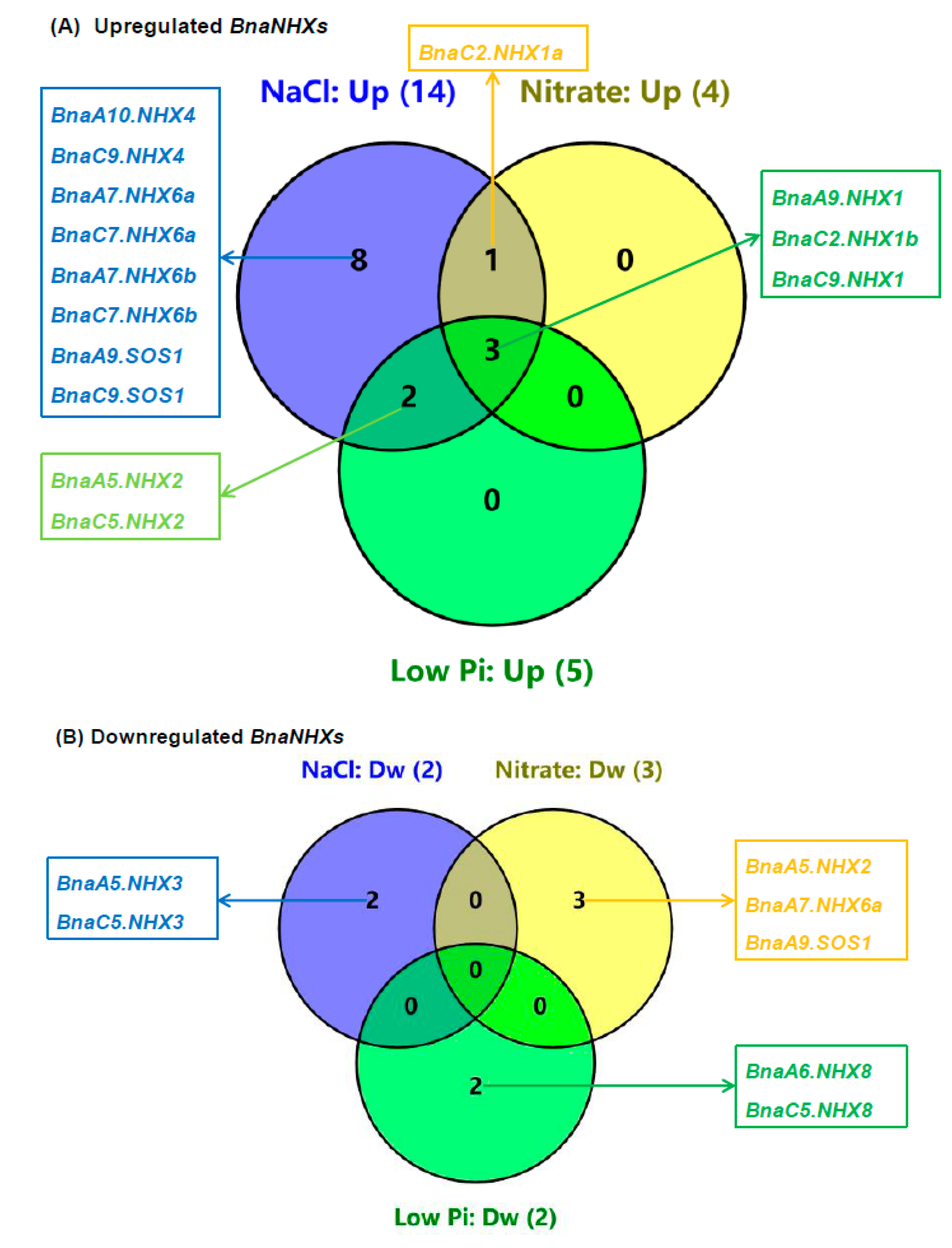
2.7. Transcriptional Analysis of BnaNHXs Under Diverse Nutrient Stresses
3. Discussion
3.1. An Integrated Bioinformatics Analysis Provided Comprehensive Insights into the Molecular Features of BnaNHXs
3.2. Transcriptional Analysis Revealed Differential Responses and Core Regulators of BnaNHXs Under Salt Stress
3.3. BnaNHX1s and BnaNHX2s Might be Involved in the Nitrate- and Low Phosphate-Mediated Salt Stress Resistance Enhancement
4. Materials and methods
4.1. Retrieval of NHX Sequences
4.2. Gene Nomenclature of NHXs in B. napus
4.3. Physical Mapping and Family Expansion Analysis of BnaNHXs
4.4. Sequence Alignment and Phylogeny Analysis of BnaNHXs
4.5. Analysis of Evolutionary Selection Pressure and Functional Divergence of BnaNHXs
4.6. Molecular Characterization of BnaNHXs
4.7. Conserved Motif Identification of BnaNHXs
4.8. Elucidation of Gene Structure and Promoter Regulatory Elements of BnaNHXs
4.9. Transcriptional Characterization of BnaNHXs Under Diverse Nutrient Conditions
4.10. Quantitative Reverse-Transcription PCR Assays
Abbreviation
| At | Arabidopsis thaliana |
| Bna | Brasssica napus |
| Bol | Brassica oleracea |
| Bra | Brassica rapa |
| BRAD | Brassica Database |
| CDS | Coding sequence |
| CRE | cis-acting regulatory element |
| DEGs | differentially expressed genes |
| gDNA | genomic DNA |
| MEME | Multiple expectation maximization for motif elicitation |
| MW | Molecular weight |
| Na+ | sodium ion |
| N | nitrogen |
| NCBI | National Center for Biotechnology Information |
| NH4+ | ammonium |
| NHX | Na+/H+ antiporter |
| NO3 - | nitrate |
| PM | Plasma membrane |
| PPI | Protein-protein interaction |
| Pi | phosphate |
| qRT-PCR | quantitative reverse-transcription polymerase chain reaction |
| SOS | Salt overly sensitive |
| SSR | salt stress resistance |
| TAIR | The Arabidopsis Information Resource |
| TF | transcription factor |
| TM | Transmembrane |
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na⁺ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A basic helix-loop-helix transcription factor, PtrbHLH, of poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.; Wang, T.; Chen, S.; Dai, S. Proteomics-based investigation of salt-responsive mechanisms in plant roots. J. Proteom. 2013, 82, 230–253. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Pehlivan, N.; Sun, L.; Jarrett, P.; Yang, X.; Mishra, N.; Chen, L.; Kadioglu, A.; Shen, G.; Zhang, H. Co-overexpressing a plasma membrane and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic Arabidopsis plants. Plant Cell Physiol. 2016, 57, 1069–1084. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef]
- Bao, A.K.; Wang, S.M.; Wu, G.Q.; Xi, J.J.; Zhang, J.L.; Wang, C.M. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240. [Google Scholar] [CrossRef]
- Aharon, G.S.; Apse, M.P.; Duan, S.; Hua, X.; Blumwald, E. Characterization of a family of vacuolar Na+/H+ antiporters Arabidopsis thaliana. Plant Soil 2003, 253, 245–256. [Google Scholar] [CrossRef]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.A.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Apse, M.P.; Sottosanto, J.B.; Blumwald, E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 2003, 36, 229–239. [Google Scholar] [CrossRef]
- Ohnishi, M.; Fukada-Tanaka, S.; Hoshino, A.; Takada, J.; Inagaki, Y.; Iida, S. Characterization of a novel Na+/H+ antiporter gene InNHX2 and comparison of InNHX2 with InNHX1, which is responsible for blue flower coloration by increasing the vacuolar pH in the Japanese morning glory. Plant Cell Physiol. 2005, 46, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Leidi, E.O.; Pardo, J.M. How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal. Behav. 2010, 5, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [PubMed]
- Huertas, R.; Rubio, L.; Cagnac, O.; García-Sánchez, M.J.; Alché-Jde, D.; Venema, K.; Fernández, J.A.; Rodríguez-Rosales, M.P. The K+/H+ antiporter LeNHX2 increases salt tolerance by improving K+ homeostasis in transgenic tomato. Plant Cell Environ. 2013, 36, 2135–2149. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.S. Plant and yeast NHX antiporters: Roles in membrane trafficking. J. Integr. Plant Biol. 2012, 54, 66–72. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, M.P.; Gálvez, F.J.; Huertas, R.; Aranda, M.N.; Baghour, M.; Cagnac, O.; Venema, K. Plant NHX cation/proton antiporters. Plant Signal. Behav. 2009, 4, 265–276. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmoticstress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Adler, G.; Blumwald, E.; Bar-Zvi, D. The sugar beet gene encoding the sodium/proton exchanger 1 (BvNHX1) is regulated by a MYB transcription factor. Planta 2010, 232, 187–195. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Zhang, H.X.; Blumwald, E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol. 2001, 19, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhao, J.R.; Guo, D.D.; Ma, X.N.; Xu, F.C.; Yang, W.W.; Gao, W. Identification of NHXs in Gossypium species and the positive role of GhNHX1 in salt tolerance. BMC Plant Biol. 2020, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Mushke, R.; Yarra, R.; Kirti, P.B. Improved salinity tolerance and growth performance in transgenic sunflower plants via ectopic expression of a wheat antiporter gene (TaNHX2). Mol. Biol. Rep. 2019, 46, 5941–5953. [Google Scholar] [CrossRef]
- Wu, X.X.; Li, J.; Wu, X.D.; Liu, Q.; Wang, Z.K.; Liu, S.S.; Li, S.N.; Ma, Y.L.; Sun, J.; Zhao, L.; et al. Ectopic expression of Arabidopsis thaliana Na+(K+)/H+ antiporter gene, AtNHX5, enhances soybean salt tolerance. Genet. Mol. Res. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Blackshaw, R.; Johnson, E.; Gan, Y.T.; May, W.; McAndrew, D.; Barthet, V.; McDonald, T.; Wispinski, D. Alternative oilseed crops for biodiesel feedstock on the Canadian prairies. Can. J. Plant Sci. 2001, 91, 889–896. [Google Scholar] [CrossRef]
- Bayer, P.E.; Hurgobin, B.; Golicz, A.A.; Chan, C.-K.K.; Yuan, Y.; Lee, H.; Renton, M.; Meng, J.; Li, R.; Zou, J.; et al. Assembly and comparison of two closely related Brassica napus genomes. Plant Biotechnol. J. 2017, 15, 1602–1610. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Corréa, M.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Liu, W.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef]
- Shokri-Gharelo, R.; Noparvar, P.M. Molecular response of canola to salt stress: Insights on tolerance mechanisms. PeerJ 2018, 6, e4822. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Schmidt, R.; Acarkan, A.; Boivin, K. Comparative structural genomics in the Brassicaceae family. Plant Physiol. Biochem. 2001, 39, 253–262. [Google Scholar] [CrossRef]
- Schranz, M.E.; Lysak, M.A.; Mitchell-Olds, T. The ABC’s of comparative genomics in the Brassicaceae: Building blocks of crucifer genomes. Trends Plant Sci. 2006, 11, 535–542. [Google Scholar] [CrossRef]
- Ding, X.; Li, J.; Pan, Y.; Zhang, Y.; Ni, L.; Wang, Y.; Zhang, X. Genome-wide identification and expression analysis of the UGlcAE gene family in tomato. Int. J. Mol. Sci. 2018, 19, 1583. [Google Scholar] [CrossRef]
- Verma, D.; Lakhanpal, N.; Singh, K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genom. 2019, 20, 227. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Ali, A.; Maggio, A.; Bressan, R.A.; Yun, D.J. Role and functional differences of HKT1-type transporters in plants under salt stress. Int. J. Mol. Sci. 2019, 20, 1059. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Primo, C.; Yang, J.; Kandel, R.; Gaxiola, R.A.; Hirschi, K.D. Arabidopsis the flip side of the type I proton-pumping pyrophosphatase (AVP1): Using a transmembrane H gradient to synthesize pyrophosphate. J. Biol. Chem. 2019, 294, 1290–1299. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Kalay, G. Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 2011, 13, 59–69. [Google Scholar] [CrossRef]
- Gao, L.; Liu, M.; Wang, M.; Shen, Q.; Guo, S. Enhanced salt tolerance under nitrate nutrition is associated with apoplast Na+ content in Canola (Brassica. napus L.) and Rice (Oryza sativa L.) Plants. Plant Cell Physiol. 2016, 57, 2323–2333. [Google Scholar] [CrossRef]
- Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y. Phosphorus limitation improved salt tolerance in Maize through tissue tass density increase, osmolytes accumulation, and Na+ uptake inhibition. Front. Plant Sci. 2019, 10, 856. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, K.; Wu, W.; Song, J.; Sun, X.; Lin, J.; Li, X.; Tang, K. Molecular cloning and characterization of a new Na+/H+ antiporter gene from Brassica napus. DNA Seq. 2003, 14, 351–358. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Wu, B.; Yuan, X.; Zhang, H.; Cui, X.; Liu, X. Whole-genome identification and expression analysis of K+ efflux antiporter (KEA) and Na+/H+ antiporter (NHX) families under abiotic stress in soybean. J. Integr. Agric. 2015, 14, 1171–1183. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Ma, B.; Du, C.; Zheng, L.; Wang, Y. Expression of a Na+ /H+ antiporter RtNHX1 from recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana. J. Plant Physiol. 2017, 218, 109–120. [Google Scholar] [CrossRef]
- Wu, H. Plant salt tolerance and Na+ sensing and transport. Crop. J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Liang, J.; Cheng, F.; Wang, X. Brassica database (BRAD) version 2.0: Integrating and mining Brassicaceae species genomic resources. Database (Oxf.) 2015, 2015, bav093. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, M.; Wang, X.; Tong, C.; Huang, S.; Tehrim, S.; Liu, Y.; Hua, W.; Liu, S. Bolbase: A comprehensive genomics database for Brassica oleracea. BMC Genom. 2013, 14, 664. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Ostergaard, L.; King, G.J. Standardized gene nomenclature for the Brassica genus. Plant Methods 2008, 4, 10. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Zhang, L.; Li, X.; Liu, Y.; Wu, Z.; Dong, F.; Wan, L.; Liu, K.; Hong, D.; et al. BnaC9.SMG7b functions as a positive regulator of the number of seeds per silique in Brassica napus by regulating the formation of functional female gametophytes. Plant Physiol. 2015, 169, 2744–2760. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Song, J.; Zhao, B.; Guo, C.; Wang, B.; Zhang, Q.; Wang, J.; King, G.J.; Liu, K. An auxin signaling gene BnaA3.IAA7 contributes to improved plant architecture and yield heterosis in rapeseed. New Phytol. 2019, 222, 837–851. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Sievers, F.; Wilm, A.; Dineen, D.G.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Wang, D.P.; Zhang, Y.B.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Guruprasad, K.; Reddy, B.V.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990, 4, 155–161. [Google Scholar] [CrossRef]
- Hamburger, D.; Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Harrison, R.G.; Bagajewicz, M.J. Predicting the solubility of recombinant proteins in Escherichia coli. Methods Mol. Biol. 2015, 1258, 403–408. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef]
- Hua, Y.P.; Zhou, T.; Song, H.X.; Guan, C.Y.; Zhang, Z.H. Integrated genomic and transcriptomic insights into the two-component high-affinity nitrate transporters in allotetraploid rapeseed. Plant Soil 2018, 427, 245–268. [Google Scholar] [CrossRef]
- Maillard, A.; Etienne, P.; Diquélou, S.; Trouverie, J.; Billard, V.; Yvin, J.C.; Ourry, A. Nutrient deficiencies in Brassica napus modify the ionomic composition of plant tissues: A focus on cross-talk between molybdenum and other nutrients. J. Exp. Bot. 2016, 67, 5631–5641. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, J.; Huang, S.; Guo, T.; Deng, L.; Hua, W. Selection and evaluation of novel reference genes for quantitative reverse transcription PCR (qRT-PCR) based on genome and transcriptome data in Brassica napus L. Gene 2014, 538, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
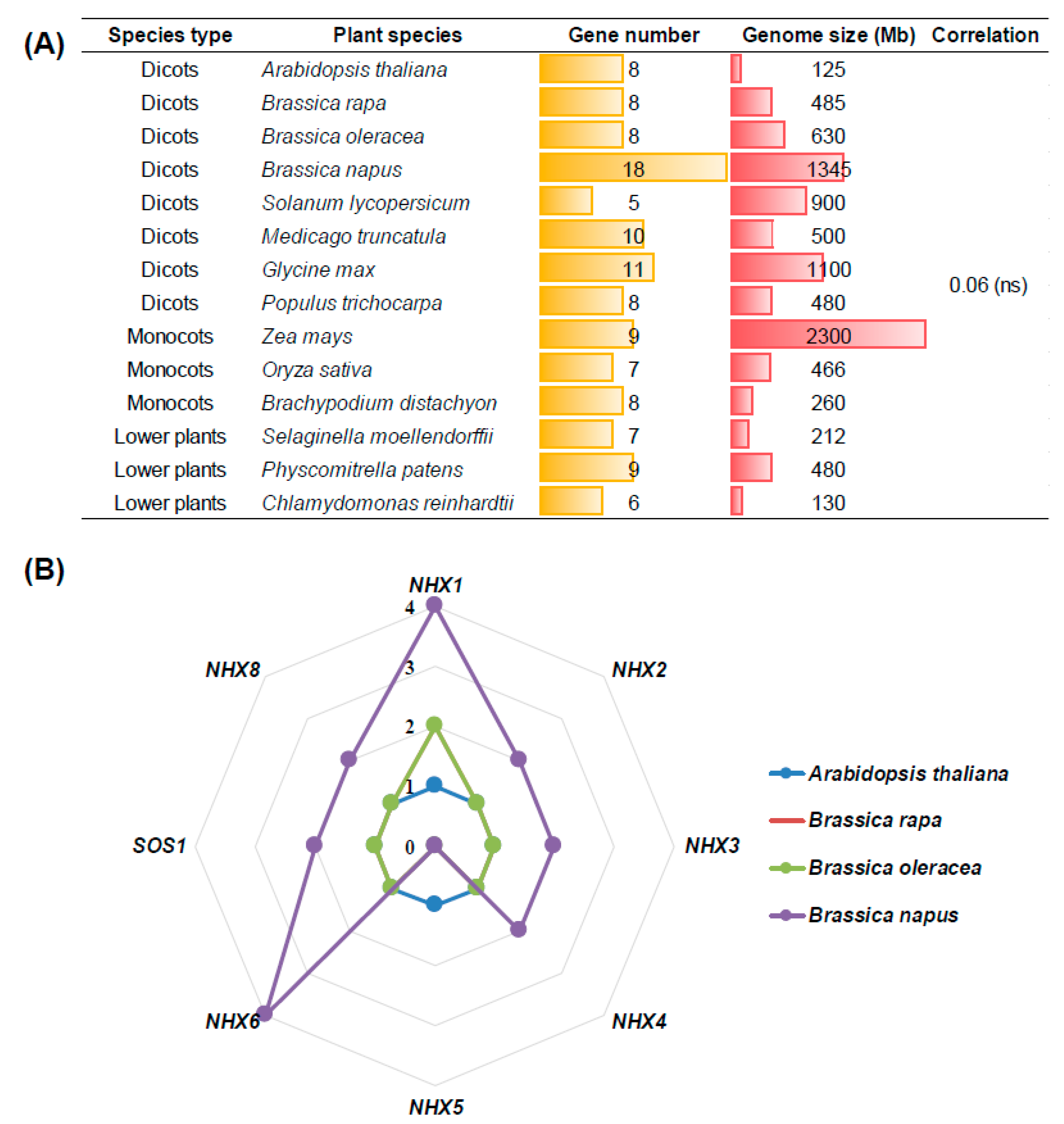



| Gene Name | Arabidopsis thaliana (125 Mb) | Brassica rapa (465 Mb) | Brassica oleracea (485 Mb) | Brassica napus (1130 Mb) |
|---|---|---|---|---|
| NHX1 | 1 | 2 | 2 | 4 |
| NHX2 | 1 | 1 | 1 | 2 |
| NHX3 | 1 | 1 | 1 | 2 |
| NHX4 | 1 | 1 | 1 | 2 |
| NHX5 | 1 | 0 | 0 | 0 |
| NHX6 | 1 | 1 | 1 | 4 |
| SOS1/NHX7 | 1 | 1 | 1 | 2 |
| NHX8 | 1 | 1 | 1 | 2 |
| Total | 8 | 8 | 8 | 18 |
| Gene ID | Gene Name | Block | CDS (bp) | Exon/ Intron | Amino Acid (aa) | Ka | Ks | Ka/Ks | Divergent Time (Mya) |
|---|---|---|---|---|---|---|---|---|---|
| At5g27150 | AtNHX1 | Q | 1617 | 13/12 | 538 | ||||
| BnaA09g03600D | BnaA9.NHX1 | Q | 1596 | 13/12 | 531 | 0.0357 | 0.4543 | 0.0787 | 13.10 |
| BnaC02g39630D | BnaC2.NHX1a | Q | 1635 | 13/12 | 544 | 0.0338 | 0.3803 | 0.089 | 13.29 |
| BnaC02g39600D | BnaC2.NHX1b | Q | 1635 | 13/12 | 544 | 0.0366 | 0.4048 | 0.0903 | 13.48 |
| BnaC09g02990D | BnaC9.NHX1 | Q | 1608 | 13/12 | 535 | 0.0423 | 0.4996 | 0.0848 | 12.49 |
| At3g05030 | AtNHX2 | F | 1641 | 14/13 | 546 | ||||
| BnaA05g32130D | BnaA5.NHX2 | F | 1641 | 14/13 | 546 | 0.0381 | 0.4029 | 0.0946 | 15.69 |
| BnaC05g46790D | BnaC5.NHX2 | F | 1650 | 14/13 | 549 | 0.0357 | 0.4697 | 0.076 | 16.76 |
| At5g55470 | AtNHX3 | W | 1590 | 14/13 | 529 | ||||
| BnaA10g09580D | BnaA10.NHX3 | W | 1590 | 14/13 | 529 | 0.044 | 0.3738 | 0.1178 | 15.59 |
| BnaC09g31970D | BnaC9.NHX3 | W | 1602 | 14/13 | 533 | 0.0482 | 0.3807 | 0.1265 | 15.96 |
| At3g06370 | AtNHX4 | F | 1512 | 13/12 | 503 | ||||
| BnaA05g30980D | BnaA5.NHX4 | F | 1677 | 13/12 | 558 | 0.0751 | 0.4801 | 0.1564 | 13.15 |
| BnaC05g45460D | BnaC5.NHX4 | F | 1683 | 13/12 | 560 | 0.0728 | 0.476 | 0.1529 | 12.88 |
| At1g54370 | AtNHX5 | C | 1566 | 21/20 | 521 | ||||
| At1g79610 | AtNHX6 | E | 1608 | 22/21 | 535 | ||||
| BnaA07g20250D | BnaA7.NHX6a | E | 1674 | 21/20 | 557 | 0.0449 | 0.3825 | 0.1174 | 18.26 |
| BnaA07g35030D | BnaA7. NHX6b | E | 1935 | 22/21 | 644 | 0.037 | 0.3676 | 0.1007 | 15.22 |
| BnaC06g19720D | BnaC6. NHX6a | E | 1674 | 21/20 | 557 | 0.0439 | 0.384 | 0.1144 | 17.99 |
| BnaC06g39970D | BnaC6. NHX6b | E | 1752 | 24/23 | 583 | 0.0472 | 0.3961 | 0.1191 | 14.56 |
| At2g01980 | AtSOS1 | K | 3441 | 23/22 | 1146 | ||||
| BnaA09g18880D | BnaA9.SOS1 | K | 3420 | 23/22 | 1139 | 0.0864 | 0.3656 | 0.2364 | 16.55 |
| BnaC09g52290D | BnaC9.SOS1 | K | 3420 | 23/22 | 1139 | 0.0909 | 0.3756 | 0.242 | 18.16 |
| At1g14660 | AtNHX8 | A | 2271 | 20/19 | 756 | ||||
| BnaA06g09480D | BnaA6.NHX8 | A | 2232 | 19/18 | 743 | 0.0837 | 0.2542 | 0.3291 | 11.81 |
| BnaC05g10850D | BnaC5.NHX8 | A | 1599 | 14/13 | 532 | 0.0801 | 0.2814 | 0.2847 | 17.52 |
| Gene ID | Gene Name | pI | MW (kDa) | II | GRAVY | TM Domains | Subcellular Localization | |
|---|---|---|---|---|---|---|---|---|
| At5g27150 | AtNHX1 | 6.73 | 59.5 | 32.71 | 0.458 | 12 | Vac | |
| BnaA09g03600D | BnaA9.NHX1 | 7.76 | 58.9 | 35.14 | 0.4543 | 12 | Vac | |
| BnaC02g39630D | BnaC2.NHX1a | 7.50 | 60.3 | 34.95 | 0.3803 | 12 | Vac | |
| BnaC02g39600D | BnaC2.NHX1b | 7.50 | 60.4 | 34.89 | 0.4048 | 12 | Vac | |
| BnaC09g02990D | BnaC9.NHX1 | 7.50 | 59.3 | 34.44 | 0.4996 | 12 | Vac | |
| At3g05030 | AtNHX2 | 8.14 | 60.5 | 36.74 | 0.465 | 12 | Vac | |
| BnaA05g32130D | BnaA5.NHX2 | 7.47 | 60.6 | 38.24 | 0.4029 | 12 | Vac | |
| BnaC05g46790D | BnaC5.NHX2 | 7.49 | 61.0 | 37.62 | 0.4697 | 11 | Vac | |
| At5g55470 | AtNHX3 | 8.17 | 58.9 | 32.12 | 0.485 | 11 | Vac | |
| BnaA10g09580D | BnaA10.NHX3 | 7.09 | 59.0 | 34.00 | 0.3738 | 11 | Vac | |
| BnaC09g31970D | BnaC9.NHX3 | 7.07 | 58.7 | 34.11 | 0.3807 | 11 | Vac | |
| At3g06370 | AtNHX4 | 6.92 | 55.6 | 39.49 | 0.594 | 10 | Vac | |
| BnaA05g30980D | BnaA5.NHX4 | 7.07 | 61.8 | 38.63 | 0.4801 | 12 | Vac | |
| BnaC05g45460D | BnaC5.NHX4 | 7.07 | 62.2 | 39.76 | 0.476 | 12 | Vac | |
| At1g54370 | AtNHX5 | 4.98 | 57.3 | 42.99 | 0.444 | 10 | Endo | |
| At1g79610 | AtNHX6 | 5.68 | 59.3 | 40.30 | 0.377 | 9 | Endo | |
| BnaA07g20250D | BnaA7.NHX6a | 5.62 | 62.3 | 41.65 | 0.3825 | 10 | Endo | |
| BnaA07g35030D | BnaA7.NHX6b | 6.68 | 72.4 | 39.36 | 0.3676 | 12 | Endo | |
| BnaC06g19720D | BnaC6.NHX6a | 5.83 | 62.4 | 42.95 | 0.384 | 10 | Endo | |
| BnaC06g39970D | BnaC6.NHX6b | 6.91 | 65.5 | 38.43 | 0.3961 | 10 | Endo | |
| At2g01980 | AtSOS1 | 7.62 | 127.2 | 38.60 | 0.098 | 9 | PM | |
| BnaA09g18880D | BnaA9.SOS1 | 7.02 | 125.8 | 34.99 | 0.3656 | 12 | PM | |
| BnaC09g52290D | BnaC9.SOS1 | 7.00 | 125.8 | 35.07 | 0.3756 | 12 | PM | |
| At1g14660 | AtNHX8 | 6.58 | 83.5 | 26.66 | 0.273 | 12 | PM | |
| BnaA06g09480D | BnaA6.NHX8 | 7.53 | 60.0 | 24.53 | 0.2542 | 8 | PM | |
| BnaC05g10850D | BnaC5.NHX8 | 7.34 | 82.5 | 26.00 | 0.2814 | 9 | PM | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.-q.; Hua, Y.-p.; Zhou, T.; Liu, Y.; Huang, J.-y.; Yue, C.-p. Global Landscapes of the Na+/H+ Antiporter (NHX) Family Members Uncover their Potential Roles in Regulating the Rapeseed Resistance to Salt Stress. Int. J. Mol. Sci. 2020, 21, 3429. https://doi.org/10.3390/ijms21103429
Cui J-q, Hua Y-p, Zhou T, Liu Y, Huang J-y, Yue C-p. Global Landscapes of the Na+/H+ Antiporter (NHX) Family Members Uncover their Potential Roles in Regulating the Rapeseed Resistance to Salt Stress. International Journal of Molecular Sciences. 2020; 21(10):3429. https://doi.org/10.3390/ijms21103429
Chicago/Turabian StyleCui, Jia-qian, Ying-peng Hua, Ting Zhou, Ying Liu, Jin-yong Huang, and Cai-peng Yue. 2020. "Global Landscapes of the Na+/H+ Antiporter (NHX) Family Members Uncover their Potential Roles in Regulating the Rapeseed Resistance to Salt Stress" International Journal of Molecular Sciences 21, no. 10: 3429. https://doi.org/10.3390/ijms21103429
APA StyleCui, J.-q., Hua, Y.-p., Zhou, T., Liu, Y., Huang, J.-y., & Yue, C.-p. (2020). Global Landscapes of the Na+/H+ Antiporter (NHX) Family Members Uncover their Potential Roles in Regulating the Rapeseed Resistance to Salt Stress. International Journal of Molecular Sciences, 21(10), 3429. https://doi.org/10.3390/ijms21103429




