Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study
Abstract
:1. Introduction
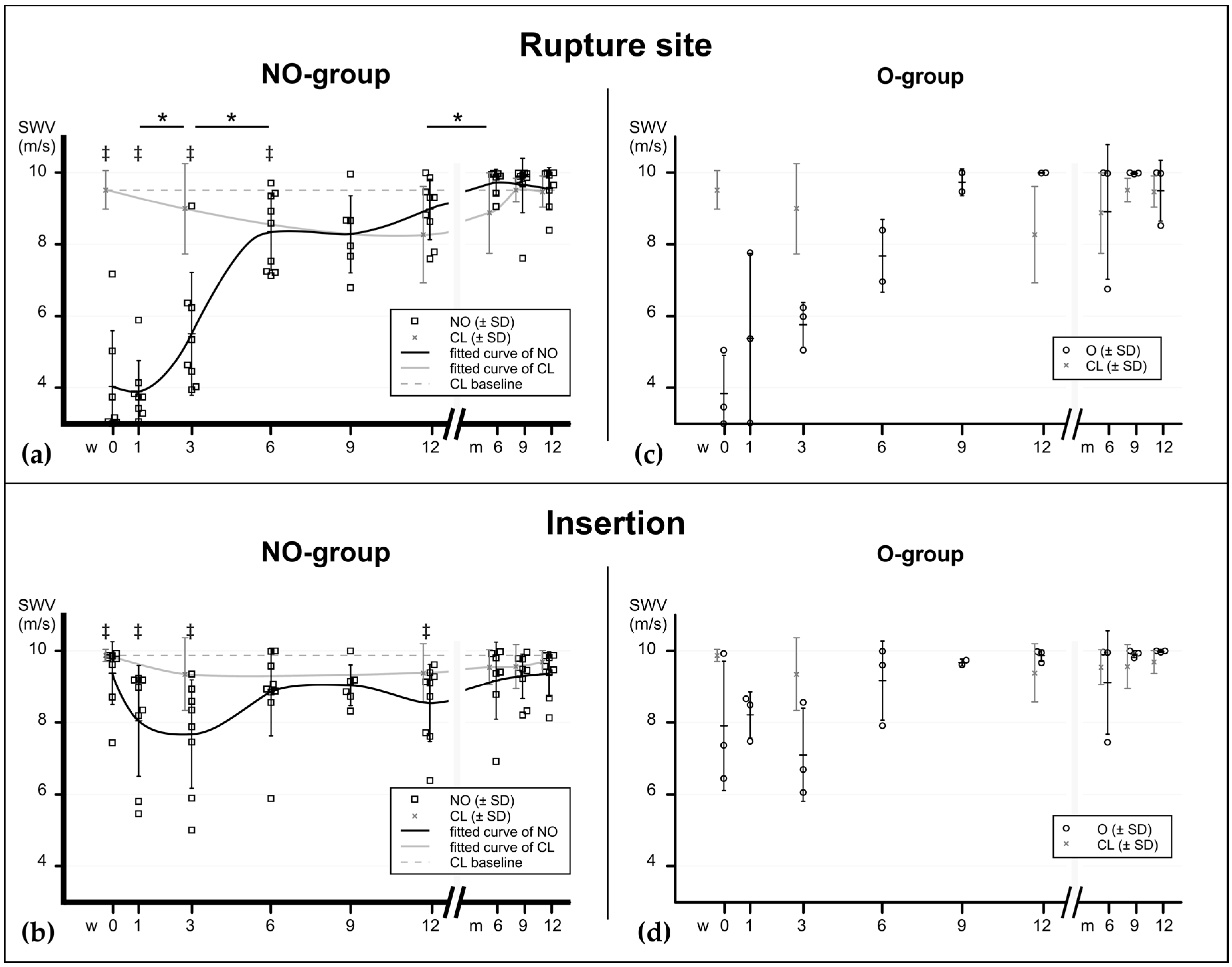
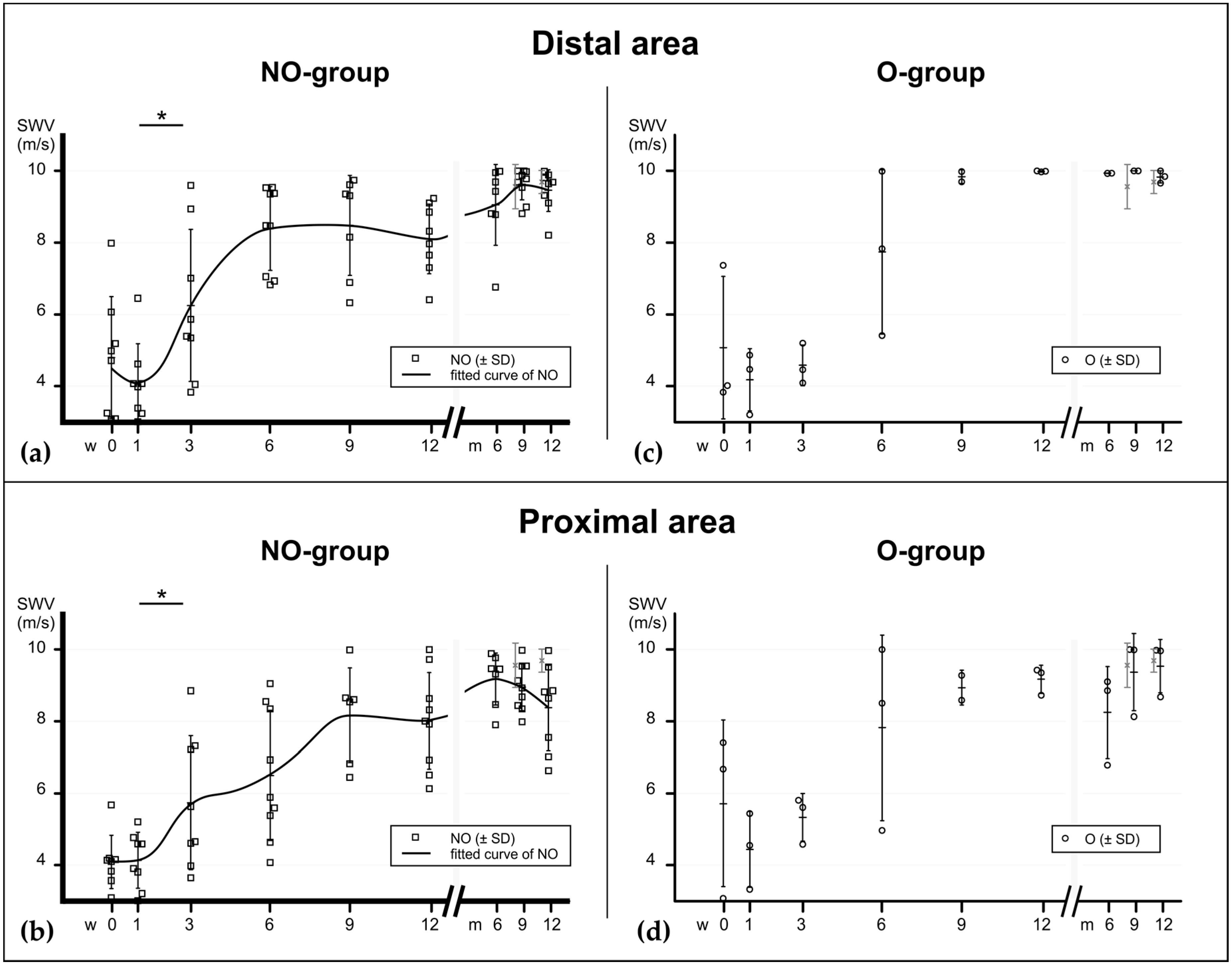
2. Results
2.1. Patients
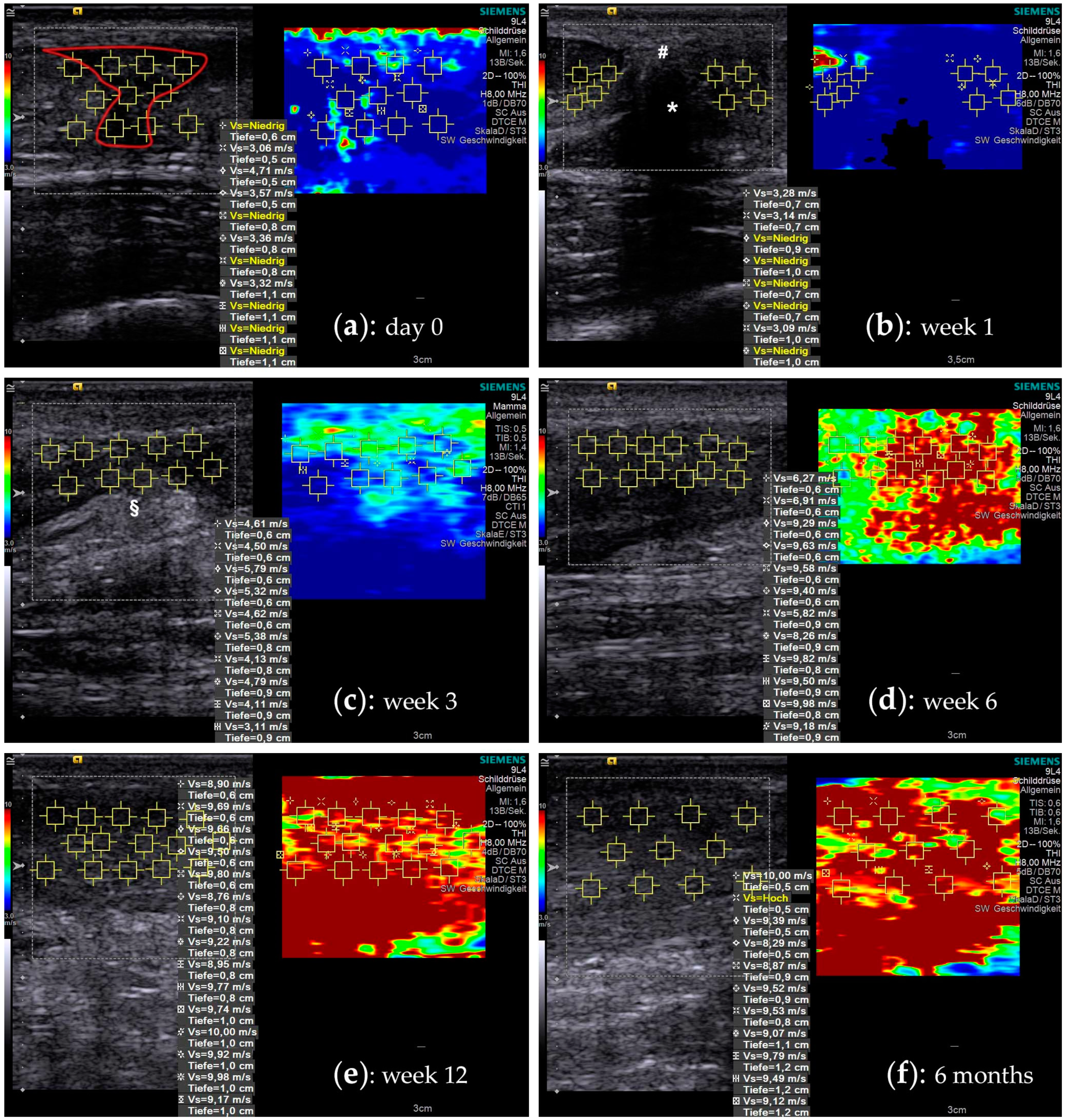
2.2. Shear Wave Elastography
2.3. Elastographic Behavior of Healing Tendons
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Rehabilitation Program
4.3. Ultrasound Examination
4.4. Data and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Achilles tendon rupture |
| CL | Contralateral tendons |
| ECM | Extracellulary matrix |
| ER | Emergency room |
| FOAS | Foot and Ankle Outcome Score |
| MRI | Magnetic resonance imaging |
| NO | Non-operatively treated patients |
| O | Operatively treated patients |
| PDS | Polydioxanone suture |
| ROM | Range of motion |
| SWE | Shear wave elastography |
| SWV | Shear wave velocity |
| VTIQ | Virtual Touch Imaging and Quantification mode |
Appendix A
References
- Lantto, I.; Heikkinen, J.; Flinkkilä, T.; Ohtonen, P.; Leppilahti, J. Epidemiology of Achilles tendon ruptures: Increasing incidence over a 33-year period. Scand. J. Med. Sci. Sports 2015, 25, e133–e138. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, T.T.; Kannus, P.; Rolf, C.; Felländer-Tsai, L.; Mattila, V.M. Acute Achilles Tendon Ruptures Incidence of Injury and Surgery in Sweden Between 2001 and 2012. Am. J. Sports Med. 2014, 42, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Sheth, U.; Wasserstein, D.; Jenkinson, R.; Moineddin, R.; Kreder, H.; Jaglal, S.B. The epidemiology and trends in management of acute Achilles tendon ruptures in Ontario, Canada: A population-based study of 27 607 patients. Bone Jt. J. 2017, 99-B, 78–86. [Google Scholar] [CrossRef]
- Soroceanu, A.; Sidhwa, F.; Aarabi, S.; Kaufman, A.; Glazebrook, M. Surgical Versus Nonsurgical Treatment of Acute Achilles Tendon Rupture: A Meta-Analysis of Randomized Trials. J. Bone Jt. Surg. Am. 2012, 94, 2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliasson, P.; Andersson, T.; Aspenberg, P. Rat Achilles tendon healing: Mechanical loading and gene expression. J. Appl. Physiol. 2009, 107, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomopoulos, S.; Zampiakis, E.; Das, R.; Silva, M.J.; Gelberman, R.H. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J. Orthop. Res. 2008, 26, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Mark-Christensen, T.; Troelsen, A.; Kallemose, T.; Barfod, K.W. Functional rehabilitation of patients with acute Achilles tendon rupture: A meta-analysis of current evidence. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1852–1859. [Google Scholar] [CrossRef]
- Suchak, A.A.; Bostick, G.P.; Beaupré, L.A.; Durand, D.C.; Jomha, N.M. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J. Bone Joint Surg. Am. 2008, 90, 1876–1883. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.L.; MacMillan, K.; Halliday, D.; Chester, R.; Shepstone, L.; Robinson, A.H.N.; Donell, S.T. Randomised controlled trials of immediate weight-bearing mobilisation for rupture of the tendo Achillis. J. Bone Joint Surg. Br. 2006, 88-B, 69–77. [Google Scholar] [CrossRef]
- Frankewycz, B.; Krutsch, W.; Weber, J.; Ernstberger, A.; Nerlich, M.; Pfeifer, C.G. Rehabilitation of Achilles tendon ruptures: Is early functional rehabilitation daily routine? Arch. Orthop. Trauma Surg. 2017, 137, 333–340. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.-C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- Bressel, E.; McNair, P.J. Biomechanical Behavior of the Plantar Flexor Muscle-Tendon Unit after an Achilles Tendon Rupture. Am. J. Sports Med. 2001, 29, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankewycz, B.; Penz, A.; Weber, J.; da Silva, N.P.; Freimoser, F.; Bell, R.; Nerlich, M.; Jung, E.M.; Docheva, D.; Pfeifer, C.G. Achilles tendon elastic properties remain decreased in long term after rupture. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2018, 26, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, T.; Lukas, C.; Merk, J.; Brauner, T.; Mündermann, A. Deficits 10-Years after Achilles Tendon Repair. Int. J. Sports Med. 2012, 33, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Guo, Q.; Li, B. Tendon Biomechanics and Mechanobiology—A Minireview of Basic Concepts and Recent Advancements. J. Hand Ther. 2012, 25, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Steiner, M. Biomechanics of tendon healing. J. Biomech. 1982, 15, 951–958. [Google Scholar] [CrossRef]
- Nagasawa, K.; Noguchi, M.; Ikoma, K.; Kubo, T. Static and dynamic biomechanical properties of the regenerating rabbit Achilles tendon. Clin. Biomech. 2008, 23, 832–838. [Google Scholar] [CrossRef]
- Hirsch, G. Tensile Properties during Tendon Healing: A Comparative Study of Intact and Sutured Rabbit Peroneus Brevis Tendons. Acta Orthop. Scand. 1974, 45, 1–145. [Google Scholar] [CrossRef] [Green Version]
- Jernberger, A. Measurement of Stability of Tibial Fractures: A Mechanical Method. Acta Orthop. Scand. 1970, 41, 1–88. [Google Scholar] [CrossRef]
- Burny, F.; Donkerwolcke, M.; Bourgois, R.; Domb, M.; Saric, O. Twenty Years Experience in Fracture Healing Measurement with Strain Gauges. Orthopedics 1984, 7, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Ulstrup, A.K. Biomechanical concepts of fracture healing in weight-bearing long bones. Acta Orthop. Belg. 2008, 74, 291–302. [Google Scholar] [PubMed]
- Kubiak, E.N.; Beebe, M.J.; North, K.; Hitchcock, R.; Potter, M.Q. Early Weight Bearing After Lower Extremity Fractures in Adults. JAAOS—J. Am. Acad. Orthop. Surg. 2013, 21, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, C.P. Shear wave measurements of the elasticity of the ground. Géotechnique 1981, 31, 91–104. [Google Scholar] [CrossRef]
- Scheibner, C.; Souslov, A.; Banerjee, D.; Surówka, P.; Irvine, W.T.M.; Vitelli, V. Odd elasticity. Nat. Phys. 2020, 1–6. [Google Scholar] [CrossRef]
- Bishop, J.; Poole, G.; Leitch, M.; Plewes, D.B. Magnetic resonance imaging of shear wave propagation in excised tissue. J. Magn. Reson. Imaging 1998, 8, 1257–1265. [Google Scholar] [CrossRef]
- Muthupillai, R.; Ehman, R.L. Magnetic resonance elastography. Nat. Med. 1996, 2, 601–603. [Google Scholar] [CrossRef] [Green Version]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Cosgrove, D.; Piscaglia, F.; Bamber, J.; Bojunga, J.; Correas, J.-M.; Gilja, O.; Klauser, A.; Sporea, I.; Calliada, F.; Cantisani, V.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 2: Clinical Applications. Ultraschall Med.—Eur. J. Ultrasound 2013, 34, 238–253. [Google Scholar] [CrossRef] [Green Version]
- Thiele, M.; Madsen, B.; Procopet, B.; Hansen, J.; Møller, L.; Detlefsen, S.; Berzigotti, A.; Krag, A. Reliability Criteria for Liver Stiffness Measurements with Real-Time 2D Shear Wave Elastography in Different Clinical Scenarios of Chronic Liver Disease. Ultraschall Med.—Eur. J. Ultrasound 2016. [Google Scholar] [CrossRef] [Green Version]
- Nierhoff, J.; Chávez Ortiz, A.A.; Herrmann, E.; Zeuzem, S.; Friedrich-Rust, M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: A meta-analysis. Eur. Radiol. 2013, 23, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- Rosskopf, A.B.; Bachmann, E.; Snedeker, J.G.; Pfirrmann, C.W.A.; Buck, F.M. Comparison of shear wave velocity measurements assessed with two different ultrasound systems in an ex-vivo tendon strain phantom. Skelet. Radiol. 2016, 45, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-L.; Kuo, P.-L.; Li, P.-C. Correlation between the shear wave speed in tendon and its elasticity properties. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Lingfield, UK, 1–4 October 2013; pp. 9–12. [Google Scholar]
- Martin, J.A.; Biedrzycki, A.H.; Lee, K.S.; DeWall, R.J.; Brounts, S.H.; Murphy, W.L.; Markel, M.D.; Thelen, D.G. In Vivo Measures of Shear Wave Speed as a Predictor of Tendon Elasticity and Strength. Ultrasound Med. Biol. 2015, 41, 2722–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.-L.; Kuo, P.-L.; Gennisson, J.-L.; Brum, J.; Tanter, M.; Li, P.-C. Shear-Wave Measurements for Evaluation of Tendon Diseases. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1. [Google Scholar] [CrossRef]
- Iida, N.; Taniguchi, K.; Watanabe, K.; Miyamoto, H.; Taniguchi, T.; Fujimiya, M.; Katayose, M. Relationship between shear modulus and passive tension of the posterior shoulder capsule using ultrasound shear wave elastography: A cadaveric study. J. Biomech. 2020, 99, 109498. [Google Scholar] [CrossRef]
- Haen, T.X.; Roux, A.; Soubeyrand, M.; Laporte, S. Shear waves elastography for assessment of human Achilles tendon’s biomechanical properties: An experimental study. J. Mech. Behav. Biomed. Mater. 2017, 69, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Busilacchi, A.; Olivieri, M.; Ulisse, S.; Gesuita, R.; Skrami, E.; Lording, T.; Fusini, F.; Gigante, A. Real-time sonoelastography as novel follow-up method in Achilles tendon surgery. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2014. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, W.; Wang, Y.; Jiao, Z.; Zhang, L.; Luo, Y.; Tang, P. Evaluation of Elastic Stiffness in Healing Achilles Tendon After Surgical Repair of a Tendon Rupture Using In Vivo Ultrasound Shear Wave Elastography. Med. Sci. Monit. 2016, 22, 1186–1191. [Google Scholar] [CrossRef] [Green Version]
- Coombes, B.K.; Tucker, K.; Vicenzino, B.; Vuvan, V.; Mellor, R.; Heales, L.; Nordez, A.; Hug, F. Achilles and patellar tendinopathy display opposite changes in elastic properties: A shear wave elastography study. Scand. J. Med. Sci. Sports 2018, 28, 1201–1208. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Maganaris, C.N.; Narici, M.V.; Maffulli, N. Biomechanics of the Achilles tendon. Disabil. Rehabil. 2008, 30, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Searle, G.F.C. Elementary Theory of Elasticity. In Experimental Elasticity; Cambridge University Press: Cambridge, UK, 2013; pp. 2–16. ISBN 978-1-107-66422-7. [Google Scholar]
- Chen, X.-M.; Cui, L.-G.; He, P.; Shen, W.-W.; Qian, Y.-J.; Wang, J.-R. Shear wave elastographic characterization of normal and torn achilles tendons a pilot study. J. Ultrasound Med. 2013, 32, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Cheung, D.T.; DiCesare, P.; Benya, P.D.; Libaw, E.; Nimni, M.E. The presence of intermolecular disulfide cross-links in type III collagen. J. Biol. Chem. 1983, 258, 7774–7778. [Google Scholar] [PubMed]
- Liu, S.H.; Yang, R.S.; Al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing. A current review. Clin. Orthop. 1995, 265–278. [Google Scholar]
- Tillman, L.; Chansan, N. Properties of dense connective tissue and wound healing. In Management of Common Musculoskeletal Disorders; Hertling, D., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; p. 22. [Google Scholar]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015, 52, 1–13. [Google Scholar] [CrossRef] [Green Version]
- DeWall, R.J.; Slane, L.C.; Lee, K.S.; Thelen, D.G. Spatial variations in Achilles tendon shear wave speed. J. Biomech. 2014, 47, 2685–2692. [Google Scholar] [CrossRef] [Green Version]
- Gimbel, J.A.; Van Kleunen, J.P.; Mehta, S.; Perry, S.M.; Williams, G.R.; Soslowsky, L.J. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J. Biomech. 2004, 37, 739–749. [Google Scholar] [CrossRef]
- Payne, C.; Watt, P.; Webborn, N. Shear Wave Elastography Measures of the Achilles Tendon: Influence of Time of Day, Leg Dominance and the Impact of an Acute 30-Minute Bout of Running. Appl. Sci. 2018, 8, 1170. [Google Scholar] [CrossRef] [Green Version]
- Siu, W.; Chan, C.; Lam, C.; Lee, C.; Ying, M. Sonographic evaluation of the effect of long-term exercise on Achilles tendon stiffness using shear wave elastography. J. Sci. Med. Sport 2016, 19, 883–887. [Google Scholar] [CrossRef]
- Chen, T.M.; Rozen, W.M.; Pan, W.; Ashton, M.W.; Richardson, M.D.; Taylor, G.I. The arterial anatomy of the Achilles tendon: Anatomical study and clinical implications. Clin. Anat. 2009, 22, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, T.; Gaulke, R.; Imrecke, J.; Krettek, C.; Stübig, T. Konservativ-funktionelle Behandlung der Achillessehnenruptur. Unfallchirurg 2010, 113, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Hertel, G.; Götz, J.; Grifka, J.; Willers, J. Achillessehnenruptur. Der Orthopäde 2016, 45, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Managaris, C.N.; Narici, M.V. Mechanical Properties of Tendons. In Tendon Injuries: Basic Science and Clinical Medicine; Maffuli, N., Renstrom, P., Leadbetter, W., Eds.; Springer Science & Business Media: London, UK, 2005; pp. 15–24. [Google Scholar]
- Braunstein, M.; Baumbach, S.F.; Boecker, W.; Carmont, M.R.; Polzer, H. Development of an accelerated functional rehabilitation protocol following minimal invasive Achilles tendon repair. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 846–853. [Google Scholar] [CrossRef]
- Schepull, T.; Aspenberg, P. Early controlled tension improves the material properties of healing human achilles tendons after ruptures: A randomized trial. Am. J. Sports Med. 2013, 41, 2550–2557. [Google Scholar] [CrossRef]
- Fu, S.; Cui, L.; He, X.; Sun, Y. Elastic Characteristics of the Normal Achilles Tendon Assessed by Virtual Touch Imaging Quantification Shear Wave Elastography. J. Ultrasound Med. 2016, 35, 1881–1887. [Google Scholar] [CrossRef] [Green Version]
- Beach, Z.M.; Gittings, D.J.; Soslowsky, L.J. Tendon Biomechanics. In Muscle and Tendon Injuries: Evaluation and Management; Canata, G.L., d’Hooghe, P., Hunt, K.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 15–22. ISBN 978-3-662-54184-5. [Google Scholar]
- Slane, L.C.; Martin, J.; DeWall, R.; Thelen, D.; Lee, K. Quantitative ultrasound mapping of regional variations in shear wave speeds of the aging Achilles tendon. Eur. Radiol. 2016. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. RadioGraphics 2017, 37, 855–870. [Google Scholar] [CrossRef] [Green Version]
- Amlang, M.H.; Zwipp, H.; Friedrich, A.; Peaden, A.; Bunk, A.; Rammelt, S. Ultrasonographic Classification of Achilles Tendon Ruptures as a Rationale for Individual Treatment Selection. ISRN Orthop. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- van Bergen, C.J.A.; Sierevelt, I.N.; Hoogervorst, P.; Waizy, H.; van Dijk, C.N.; Becher, C. Translation and validation of the German version of the foot and ankle outcome score. Arch. Orthop. Trauma Surg. 2014, 134, 897–901. [Google Scholar] [CrossRef]
- Duenwald, S.; Kobayashi, H.; Frisch, K.; Lakes, R.; Vanderby, R. Ultrasound echo is related to stress and strain in tendon. J. Biomech. 2011, 44, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankewycz, B.; Henssler, L.; Weber, J.; Silva, N.P.B.d.; Koch, M.; Jung, E.M.; Docheva, D.; Alt, V.; Pfeifer, C.G. Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study. Int. J. Mol. Sci. 2020, 21, 3427. https://doi.org/10.3390/ijms21103427
Frankewycz B, Henssler L, Weber J, Silva NPBd, Koch M, Jung EM, Docheva D, Alt V, Pfeifer CG. Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study. International Journal of Molecular Sciences. 2020; 21(10):3427. https://doi.org/10.3390/ijms21103427
Chicago/Turabian StyleFrankewycz, Borys, Leopold Henssler, Johannes Weber, Natascha Platz Batista da Silva, Matthias Koch, Ernst Michael Jung, Denitsa Docheva, Volker Alt, and Christian G. Pfeifer. 2020. "Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study" International Journal of Molecular Sciences 21, no. 10: 3427. https://doi.org/10.3390/ijms21103427
APA StyleFrankewycz, B., Henssler, L., Weber, J., Silva, N. P. B. d., Koch, M., Jung, E. M., Docheva, D., Alt, V., & Pfeifer, C. G. (2020). Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study. International Journal of Molecular Sciences, 21(10), 3427. https://doi.org/10.3390/ijms21103427







