Interactive Actions of Aldosterone and Insulin on Epithelial Na+ Channel Trafficking
Abstract
1. Introduction
2. Results
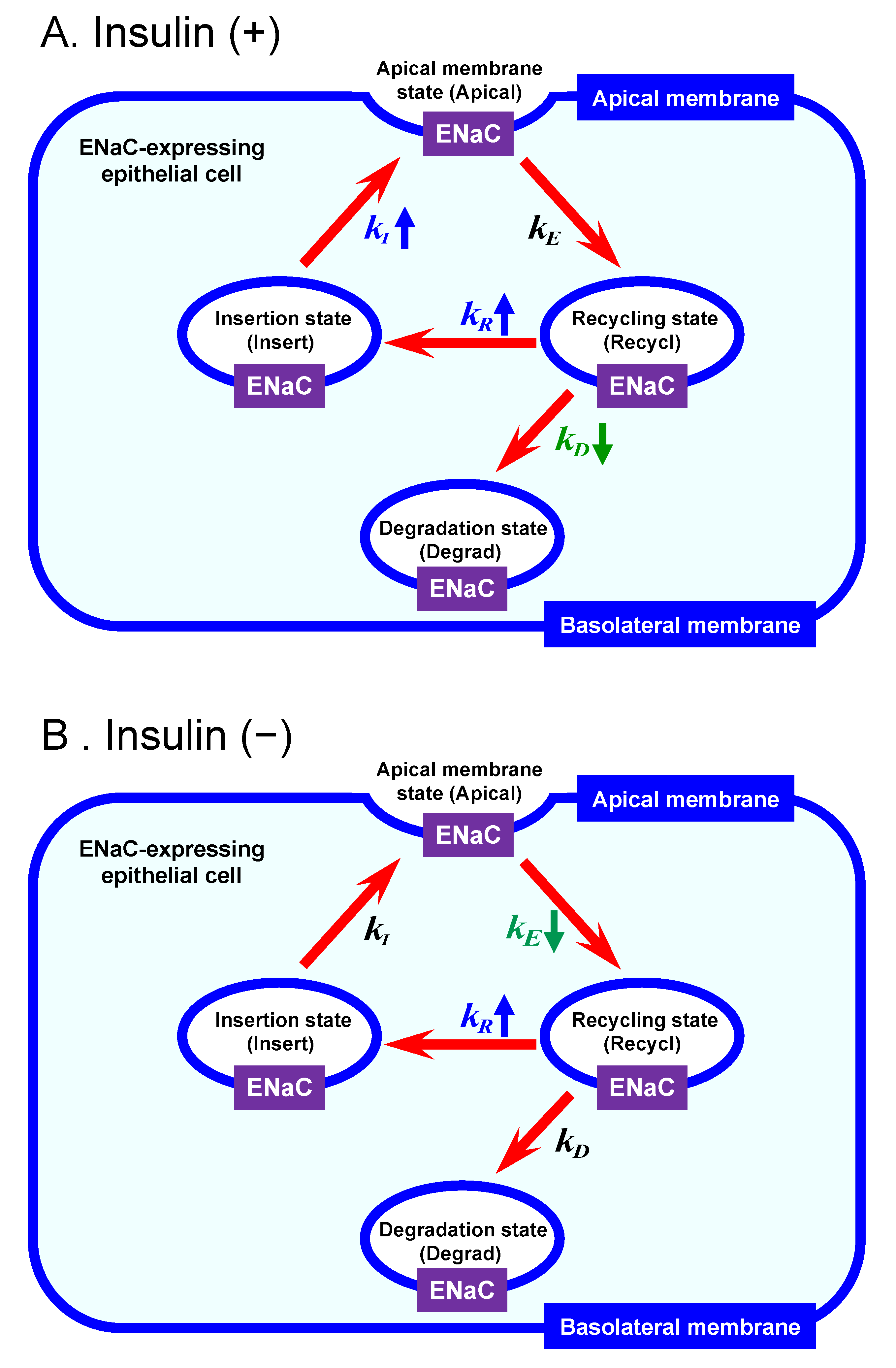
2.1. Mathematical Model of Intracellular ENaC Trafficking
2.2. The Short-Circuit Current (ISC) Was Sensitive to 10 µM Benzamil Under the Insulin-Stimulated and -Unsitmulated Conditions in Cells Treated with and without 1 µM Aldosterone for 20 h
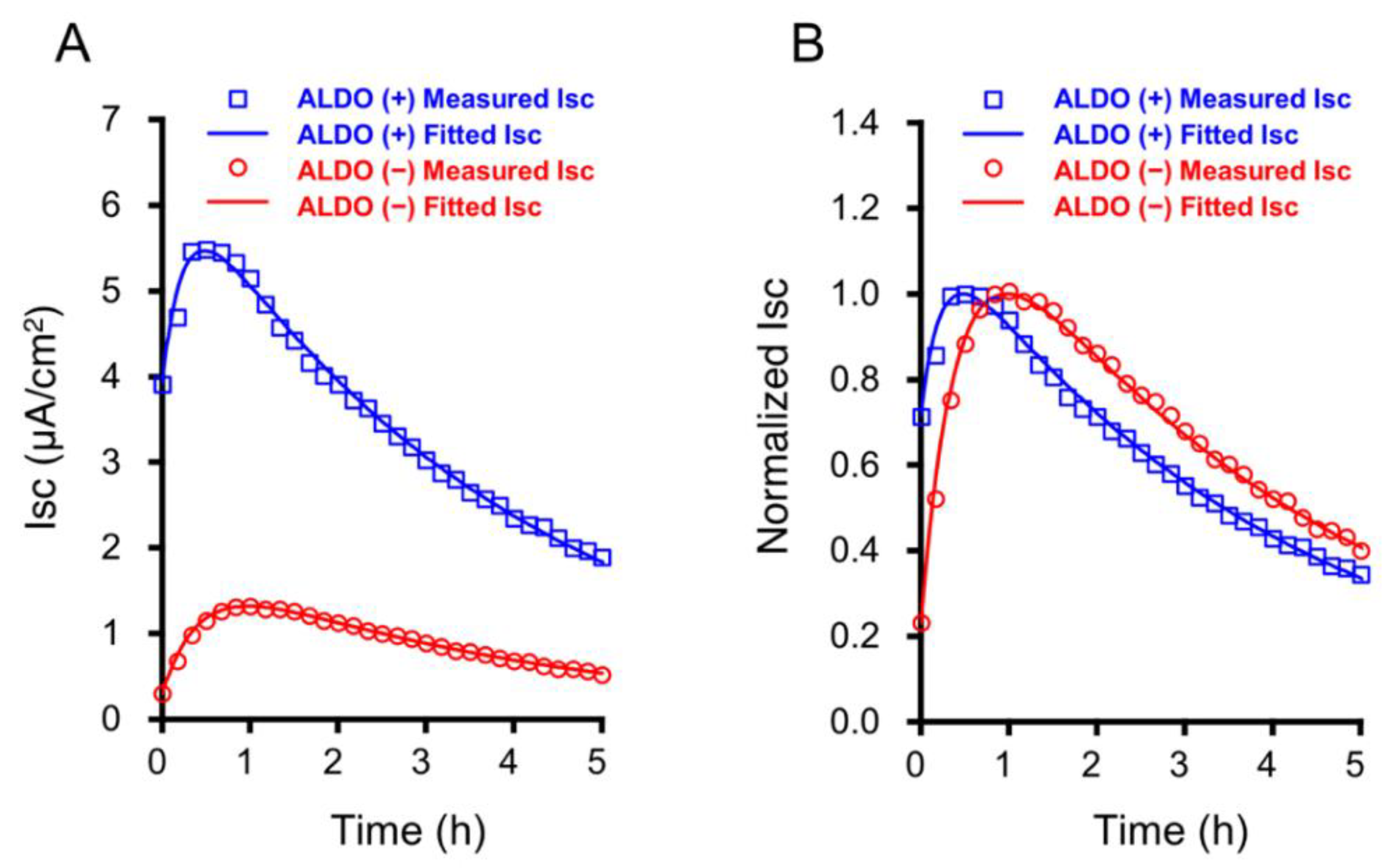
2.3. The Time Course of the Insulin-Stimulated ISC in Cells Treated with and without 1 µM Aldosterone for 20 h
2.4. Aldosterone Action on , , and Under the Insulin-Stimulated and -Unstimulated Conditions
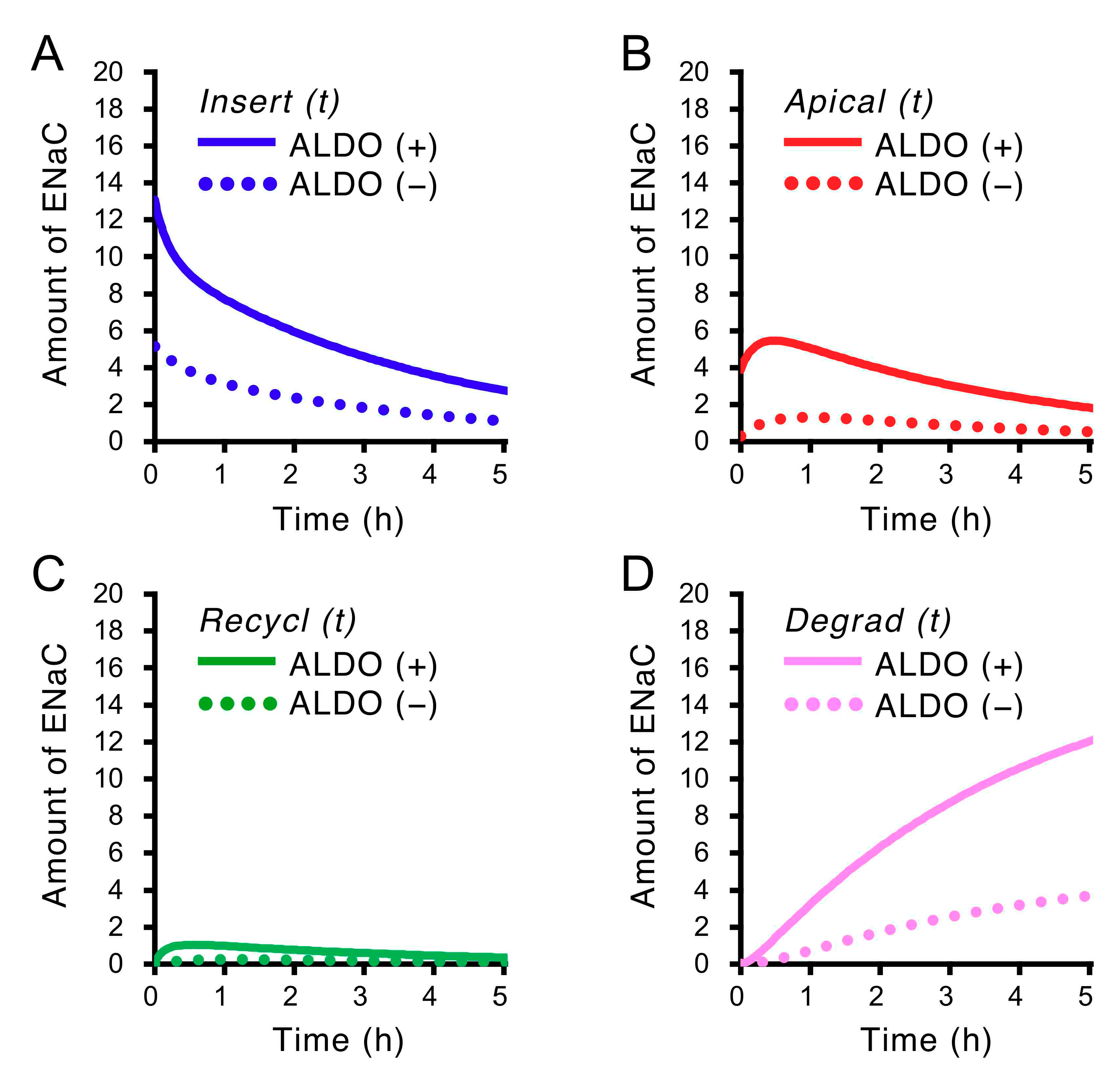
2.5. Insulin-Induced Time-Dependent Changes in the Amounts of ENaC Localized in Four States, Insert, Apical, Recycl and Degrad, Shown in Figure 1 in Cells Treated with and without Aldosterone (ALDO, 1 µM) for 20 h
2.6. Recycling Ratio of Endocytotic ENaC to the Apical Membrane, , Under the Insulin-Stimulated and -Unstimulated Conditions
2.7. Aldosterone Action on Relocation Number of ENaC to the Apical Membrane State, (= /), Under the Insulin-Stimulated and -Unstimulated Conditions
2.8. Aldosterone Action on Cumulative Na+ Absorption (ISC) () Under the Insulin-Stimulated and -Unstimulated Conditions
2.9. Total Amount of ENaC () in Cells with and without Aldosterone Treatment (1 µM for 20 h)
2.10. Aldosterone Action on the Residency Time How Long an Individual ENaC Stays in the Apical Membrane Each Time After the Insertion of ENaC into the Apical Membrane () Under the Insulin-Stimulated and -Unstimulated Conditions
2.11. Aldosterone Action on the Cumulative Time How Long an Individual ENaC Stays in the Apical Membrane During Its Whole Life-Time Period Before Degradation ( Under the Insulin-Stimulated and -Unstimulated Conditions
2.12. Aldosterone Action on Whole Life-Time of ENaC after the Initial Insertion to the Apical Membrane; , Under the Insulin-Stimulated and -Unstimulated Conditions
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Solutions
4.3. Cell Culture
4.4. Measurements of Short-Circuit Current (ISC) in Cells Treated with or without 1 µM Aldosterone for 20 h
4.5. Application of Insulin
4.6. Temperature
4.7. Data Presentation and Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALDO | Aldosterone |
| ENaC | Epithelial Na+ channel |
| ISC | Short-circuit current |
| Po | Open probability |
| PI3K | Phosphatidylinositide 3’-kinase |
| PI(4)P5K | Phosphatidylinositol-4-phosphate 5-kinase |
| SGK1 | Serum- and glucocorticoid-induced kinase 1 |
| The insertion rate of ENaC into the apical membrane for the intracellular store site | |
| The endocytotic rate of ENaC from the apical membrane to the intracellular recycling site | |
| The recycling rate of ENaC from the recycling state to the insertion state | |
| The degradation rate of ENaC from the recycling state to the degradation state | |
| Relocation number of ENaC to the apical membrane state | |
| The recycling rate of ENaC from the recycling state to the insertion state | |
| The cumulative Na+ absorption (ISC) | |
| The total amount of ENaC | |
| The residency time of ENaC in the apical membrane how long an individual ENaC stays in the apical membrane each time after the insertion of ENaC into the apical membrane | |
| The cumulative time how long an individual ENaC stays in the apical membrane during its whole life-time period before degradation | |
| The whole life-time after the first insertion to the apical membrane | |
| Insert | Insert state |
| Apical | Apical state |
| Recycl | Recycle state |
| Degrad | Degradation state |
| The amount of ENaC in the insertion state at time = after insulin application | |
| The amount of ENaC in the apical membrane state at time = after insulin application | |
| The amount of ENaC in the recycling state at time = after insulin application | |
| The amount of ENaC in the degradation state at time = after insulin application | |
References
- Verrey, F.; Loffing, J.; Zecevic, M.; Heitzmann, D.; Staub, O. SGK1: Aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell. Physiol. Biochem. 2003, 13, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Meneton, P.; Loffing, J.; Warnock, D.G. Sodium and potassium handling by the aldosterone-sensitive distal nephron: The pivotal role of the distal and connecting tubule. Am. J. Physiol. Renal Physiol. 2004, 287, F593–F601. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Lang, F. New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure. Curr. Opin. Nephrol. Hypertens. 2005, 14, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Niisato, N.; Sawabe, Y.; Miyazaki, H.; Marunaka, Y. Aldosterone-induced abnormal regulation of ENaC and SGK1 in Dahl salt-sensitive rat. Biochem. Biophys. Res. Commun. 2006, 341, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Niisato, N.; Sawabe, Y.; Miyazaki, H.; Tokuda, S.; Nishio, K.; Yoshikawa, T.; Marunaka, Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell. Biol. Int. 2007, 31, 1288–1291. [Google Scholar] [CrossRef]
- Mironova, E.; Bugaj, V.; Roos, K.P.; Kohan, D.E.; Stockand, J.D. Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc. Natl. Acad. Sci. USA 2012, 109, 10095–10100. [Google Scholar] [CrossRef]
- Stockand, J.D. The role of the epithelial Na+ channel (ENaC) in high AVP but low aldosterone states. Front. Physiol. 2012, 3, 304. [Google Scholar] [CrossRef]
- Marunaka, Y. Importance of expression and function of angiotensin II receptor type 1 in pulmonary epithelial cells. Respir. Physiol. Neurobiol. 2014, 196, 39–42. [Google Scholar] [CrossRef]
- Marunaka, Y. Characteristics and pharmacological regulation of epithelial Na+ channel (ENaC) and epithelial Na+ transport. J. Pharmacol. Sci. 2014, 126, 21–36. [Google Scholar] [CrossRef]
- Mironova, E.; Chen, Y.; Pao, A.C.; Roos, K.P.; Kohan, D.E.; Bugaj, V.; Stockand, J.D. Activation of ENaC by AVP contributes to the urinary concentrating mechanism and dilution of plasma. Am. J. Physiol. Renal. Physiol. 2015, 308, F237–F243. [Google Scholar] [CrossRef]
- Salker, M.S.; Hosseinzadeh, Z.; Alowayed, N.; Zeng, N.; Umbach, A.T.; Webster, Z.; Singh, Y.; Brosens, J.J.; Lang, F. LEFTYA activates theeEpithelial Na+ channel (ENaC) in endometrial cells via serum and glucocorticoid inducible kinase SGK1. Cell. Physiol. Biochem. 2016, 39, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Salker, M.S.; Steel, J.H.; Hosseinzadeh, Z.; Nautiyal, J.; Webster, Z.; Singh, Y.; Brucker, S.; Lang, F.; Brosens, J.J. Activation of SGK1 in endometrial epithelial cells in response to PI3K/AKT inhibition impairs embryo implantation. Cell. Physiol. Biochem. 2016, 39, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, R.; Zhao, X.; Matthay, M.A.; Nie, H.G.; Ji, H.L. ENaCs as Both Effectors and Regulators of MiRNAs in Lung Epithelial Development and Regeneration. Cell Physiol. Biochem. 2017, 44, 1120–1132. [Google Scholar] [CrossRef]
- Niisato, N.; Marunaka, Y. Activation of the Na+-K+ pump by hyposmolality through tyrosine kinase-dependent Cl- conductance in Xenopus renal epithelial A6 cells. J. Physiol. 1999, 518, 417–432. [Google Scholar] [CrossRef]
- Butterworth, M.B.; Edinger, R.S.; Johnson, J.P.; Frizzell, R.A. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J. Gen. Physiol. 2005, 125, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Jans, D.; Simaels, J.; Cucu, D.; Zeiske, W.; Van Driessche, W. Effects of extracellular Mg2+ on transepithelial capacitance and Na+ transport in A6 cells under different osmotic conditions. Pflugers Arch. 2000, 439, 504–512. [Google Scholar] [CrossRef]
- Marunaka, Y.; Eaton, D.C. Effects of vasopressin and cAMP on single amiloride-blockable Na channels. Am. J. Physiol. Cell Physiol. 1991, 260, C1071–C1084. [Google Scholar] [CrossRef]
- Marunaka, Y.; Hagiwara, N.; Tohda, H. Insulin activates single amiloride-blockable Na channels in a distal nephron cell line (A6). Am. J. Physiol. Renal Physiol. 1992, 263, F392–F400. [Google Scholar] [CrossRef]
- Niisato, N.; Van Driessche, W.; Liu, M.; Marunaka, Y. Involvement of protein tyrosine kinase in osmoregulation of Na+ transport and membrane capacitance in renal A6 cells. J. Membr. Biol. 2000, 175, 63–77. [Google Scholar] [CrossRef]
- Blass, G.; Klemens, C.A.; Brands, M.W.; Palygin, O.; Staruschenko, A. Postprandial Effects on ENaC-Mediated Sodium Absorption. Sci. Rep. 2019, 9, 4296. [Google Scholar] [CrossRef]
- Bubien, J.K. Epithelial Na+ channel (ENaC), hormones, and hypertension. J. Biol. Chem. 2010, 285, 23527–23531. [Google Scholar] [CrossRef] [PubMed]
- Niisato, N.; Taruno, A.; Marunaka, Y. Aldosterone-induced modification of osmoregulated ENaC trafficking. Biochem. Biophys. Res. Commun. 2007, 361, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, R.; Taruno, A.; Yamamoto, T.; Kanamura, N.; Marunaka, Y. Action of Protein Tyrosine Kinase Inhibitors on the Hypotonicity-Stimulated Trafficking Kinetics of Epithelial Na+ Channels (ENaC) in Renal Epithelial Cells: Analysis Using a Mathematical Model. Cell. Physiol. Biochem. 2018, 50, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, R.; Marunaka, Y. Aldosterone action on epithelial Na+ channel trafficking under the insulin-stimulated condition. J. Physiol. Sci. 2019, 69, S252. [Google Scholar]
- Sun, H.; Niisato, N.; Inui, T.; Marunaka, Y. Insulin is involved in transcriptional regulation of NKCC and the CFTR Cl− channel through PI3K activation and ERK inactivation in renal epithelial cells. J. Physiol. Sci. 2014, 64, 433–443. [Google Scholar] [CrossRef]
- Kleyman, T.R.; Cragoe, E.J., Jr. Cation transport probes: The amiloride series. Methods Enzymol. 1990, 191, 739–755. [Google Scholar]
- Blazer-Yost, B.L.; Esterman, M.A.; Vlahos, C.J. Insulin-stimulated trafficking of ENaC in renal cells requires PI3-kinase activity. Am. J. Physiol. Cell Physiol. 2003, 284, C1645–C1653. [Google Scholar] [CrossRef]
- Marunaka, Y.; Tohda, H.; Hagiwara, N.; Nakahari, T. Antidiuretic hormone-responding nonselective cation channel in distal nephron epithelium (A6). Am. J. Physiol. 1994, 266, C1513–C1522. [Google Scholar] [CrossRef]
- Weisz, O.A.; Wang, J.M.; Edinger, R.S.; Johnson, J.P. Non-coordinate regulation of endogenous epithelial sodium channel (ENaC) subunit expression at the apical membrane of A6 cells in response to various transporting conditions. J. Biol. Chem. 2000, 275, 39886–39893. [Google Scholar] [CrossRef]
- Taruno, A.; Marunaka, Y. Analysis of blocker-labeled channels reveals the dependence of recycling rates of ENaC on the total amount of recycled channels. Cell. Physiol. Biochem. 2010, 26, 925–934. [Google Scholar] [CrossRef]
- Palmer, L.G.; Frindt, G. Conductance and gating of epithelial Na channels from rat cortical collecting tubule. Effects of luminal Na and Li. J. Gen. Physiol. 1988, 92, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.G.; Frindt, G. Gating of Na channels in the rat cortical collecting tubule: Effects of voltage and membrane stretch. J. Gen. Physiol. 1996, 107, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Montelongo, R.; Barros, F.; Alvarez de la Rosa, D.; Giraldez, T. Plasma membrane insertion of epithelial sodium channels occurs with dual kinetics. Pflugers Arch. 2016, 468, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Valinsky, W.C.; Touyz, R.M.; Shrier, A. Aldosterone, SGK1, and ion channels in the kidney. Clin. Sci. (Lond) 2018, 132, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D. SGK1 regulation of epithelial sodium transport. Cell. Physiol. Biochem. 2003, 13, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Staub, O. NEDD4-2 and salt-sensitive hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 111–116. [Google Scholar] [CrossRef]
- Ware, A.W.; Rasulov, S.R.; Cheung, T.T.; Lott, J.S.; McDonald, F.J. Membrane trafficking pathways regulating the epithelial Na+ channel. Am. J. Physiol. Renal Physiol. 2020, 318, F1–F13. [Google Scholar] [CrossRef]
- Butterworth, M.B.; Edinger, R.S.; Frizzell, R.A.; Johnson, J.P. Regulation of the epithelial sodium channel by membrane trafficking. Am. J. Physiol. Renal Physiol. 2009, 296, F10–F24. [Google Scholar] [CrossRef]
- Verrey, F.; Fakitsas, P.; Adam, G.; Staub, O. Early transcriptional control of ENaC (de)ubiquitylation by aldosterone. Kidney Int. 2008, 73, 691–696. [Google Scholar] [CrossRef]
- Shibata, S.; Fujita, T. The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension. Curr. Hypertens. Rep. 2011, 13, 109–115. [Google Scholar] [CrossRef]
- Lang, F.; Shumilina, E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Record, R.D.; Froelich, L.L.; Vlahos, C.J.; Blazer-Yost, B.L. Phosphatidylinositol 3-kinase activation is required for insulin-stimulated sodium transport in A6 cells. Am. J. Physiol. Endocrinol. Metab. 1998, 274, E611–E617. [Google Scholar] [CrossRef] [PubMed]
- Blazer-Yost, B.L.; Vahle, J.C.; Byars, J.M.; Bacallao, R.L. Real-time three-dimensional imaging of lipid signal transduction: Apical membrane insertion of epithelial Na+ channels. Am. J. Physiol. Cell Physiol. 2004, 287, C1569–C1576. [Google Scholar] [CrossRef] [PubMed]
- Blazer-Yost, B.L.; Liu, X.; Helman, S.I. Hormonal regulation of ENaCs: Insulin and aldosterone. Am. J. Physiol. Cell Physiol. 1998, 274, C1373–C1379. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Nakamura, M.; Suzuki, M.; Suzuki, A.; Seki, G.; Horita, S. Roles of Akt and SGK1 in the Regulation of Renal Tubular Transport. Biomed. Res. Int. 2015, 2015, 971697. [Google Scholar] [CrossRef]
- Soundararajan, R.; Pearce, D.; Hughey, R.P.; Kleyman, T.R. Role of epithelial sodium channels and their regulators in hypertension. J. Biol. Chem. 2010, 285, 30363–30369. [Google Scholar] [CrossRef]
- Rotin, D.; Staub, O. Role of the ubiquitin system in regulating ion transport. Pflugers Arch. 2011, 461, 1–21. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Medina, J.; Gamper, N.; Genth, H.; Stockand, J.D.; Staruschenko, A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J. Biol. Chem. 2006, 281, 26520–26527. [Google Scholar] [CrossRef]
- Holz, R.W.; Hlubek, M.D.; Sorensen, S.D.; Fisher, S.K.; Balla, T.; Ozaki, S.; Prestwich, G.D.; Stuenkel, E.L.; Bittner, M.A. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 2000, 275, 17878–17885. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Callera, G.E.; Friederich-Persson, M.; Sanchez, A.; Dulak-Lis, M.G.; Tsiropoulou, S.; Montezano, A.C.; He, Y.; Briones, A.M.; Jaisser, F.; et al. Vascular dysfunction in obese diabetic db/db mice involves the interplay between aldosterone/mineralocorticoid receptor and Rho kinase signaling. Sci. Rep. 2018, 8, 2952. [Google Scholar] [CrossRef]
- Sasamoto, K.; Marunaka, R.; Niisato, N.; Sun, H.; Taruno, A.; Pezzotti, G.; Yamamoto, T.; Kanamura, N.; Zhu, W.; Nishio, K.; et al. Analysis of aprotinin, a protease inhibitor, action on the trafficking of epithelial Na+ Channels (ENaC) in renal epithelial cells using a mathematical model. Cell. Physiol. Biochem. 2017, 41, 1865–1880. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, K.; Niisato, N.; Marunaka, Y. Simulation of Cl− secretion in epithelial tissues: New methodology estimating activity of electroneutral Cl− transporter. J. Physiol. Sci. 2016, 66, S89. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Niisato, N.; Nishio, K.; Hamilton, K.L.; Marunaka, Y. Distinct action of flavonoids, myricetin and quercetin, on epithelial Cl− secretion: Useful tools as regulators of Cl− secretion. BioMed. Res. Int. 2014, 2014, 902735. [Google Scholar] [CrossRef] [PubMed]

| Insulin | ALDO | Insertion (h−1) | Endocytosis (h−1) | Recycling (h−1) | Degradation (h−1) |
|---|---|---|---|---|---|
| (+) | (+) | 1.57 ± 0.06 # | 2.12 ± 0.20 NS | 6.42 ± 0.99 ## | 3.16 ± 0.12 # |
| (−) | 0.47 ± 0.08 | 1.99 ± 0.74 | 3.23 ± 0.75 | 4.59 ± 0.40 | |
| (−) | (+) | 0.24 ± 0.02 NS | 2.45 ± 0.42 * | 3.62 ± 0.10 * | 5.20 ± 0.46 NS |
| (−) | 0.21 ± 0.01 | 5.22 ± 0.45 | 2.50 ± 0.13 | 5.62 ± 0.19 |
| Insulin | ALDO | Recycling Ratio, (= ) (%) | Relocation Number of ENaC to the Apical Membrane State, (= /) |
|---|---|---|---|
| (+) | (+) | 65.86 ± 2.75 # | 2.01 ± 0.26 # |
| (−) | 39.48 ± 6.87 | 0.74 ± 0.20 | |
| (−) | (+) | 41.86 ± 2.39 * | 0.71 ± 0.07 * |
| (−) | 30.83 ± 0.46 | 0.45 ± 0.01 |
| Insulin | ALDO | Cumulative Na+ Absorption (ISC) () (μC/cm2/day) | Total Amount of ENaC () |
|---|---|---|---|
| (+) | (+) | 91,154 ± 2334 # | 7.76 ± 0.82 # |
| (−) | 23,947 ± 1777 | 8.21 ± 0.75 | |
| (−) | (+) | 50,345 ± 3727 * | 17.06 ± 0.65 * |
| (−) | 8111 ± 2548 | 8.41 ± 0.95 |
| Insulin | ALDO | (h) | (h) | (h) |
|---|---|---|---|---|
| (+) | (+) | 0.49 ± 0.05 NS | 1.44 ± 0.08 # | 3.35 ± 0.16 NS |
| (−) | 0.53 ± 0.04 | 0.88 ± 0.11 | 2.80 ± 0.32 | |
| (−) | (+) | 0.45 ± 0.08 * | 0.75 ± 0.11 * | 4.18 ± 0.41 ** |
| (−) | 0.19 ± 0.02 | 0.28 ± 0.02 | 2.82 ± 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marunaka, R.; Marunaka, Y. Interactive Actions of Aldosterone and Insulin on Epithelial Na+ Channel Trafficking. Int. J. Mol. Sci. 2020, 21, 3407. https://doi.org/10.3390/ijms21103407
Marunaka R, Marunaka Y. Interactive Actions of Aldosterone and Insulin on Epithelial Na+ Channel Trafficking. International Journal of Molecular Sciences. 2020; 21(10):3407. https://doi.org/10.3390/ijms21103407
Chicago/Turabian StyleMarunaka, Rie, and Yoshinori Marunaka. 2020. "Interactive Actions of Aldosterone and Insulin on Epithelial Na+ Channel Trafficking" International Journal of Molecular Sciences 21, no. 10: 3407. https://doi.org/10.3390/ijms21103407
APA StyleMarunaka, R., & Marunaka, Y. (2020). Interactive Actions of Aldosterone and Insulin on Epithelial Na+ Channel Trafficking. International Journal of Molecular Sciences, 21(10), 3407. https://doi.org/10.3390/ijms21103407







