All reagents were purchased from Merck (Darmstadt, Germany), J.T. Baker, and Sigma and Aldrich Chemical Co. and they were used without purification. 1H NMR and 13C NMR were recorded at 400 and 100 MHz, respectively, on a Bruker Ultrashield-400 spectrometer. The chemical shifts (δ) are reported in parts per million (ppm), using CDCl3 as solvent with TMS as an internal standard. The coupling constant (J) are reported in Hertz (Hz). A FT-IR Bruker Tensor 27 spectrophotometer coupled to Bruker platinum ATR cell was used to obtain IR spectra. Mass spectra were recorded using a Bruker Daltonics ESI-IT Amazon X spectrometer with direct injection, operating in full scan at 300 °C and 4500 V in the capillary. High-resolution mass spectrometry ESI-MS analyses were conducted in a high-resolution hybrid quadrupole (Q) and orthogonal time-of-flight (TOF) mass spectrometer (Waters/Micromass Q-TOF micro, Manchester, UK) with a constant nebulizer temperature of 100 °C. The elemental analysis of the different compounds was performed in ThermoScientific CHNS-O analyzer equipment (Model Flash 2000, Thermo Scientific, Waltham, MA, USA). Melting points (uncorrected) were determined on an Electrothermal IA9100 melting point apparatus. The progress of reaction was monitored by TLC, purification of compounds was performed by column chromatography using silica gel as support. Solvents employed were of analytical grade.
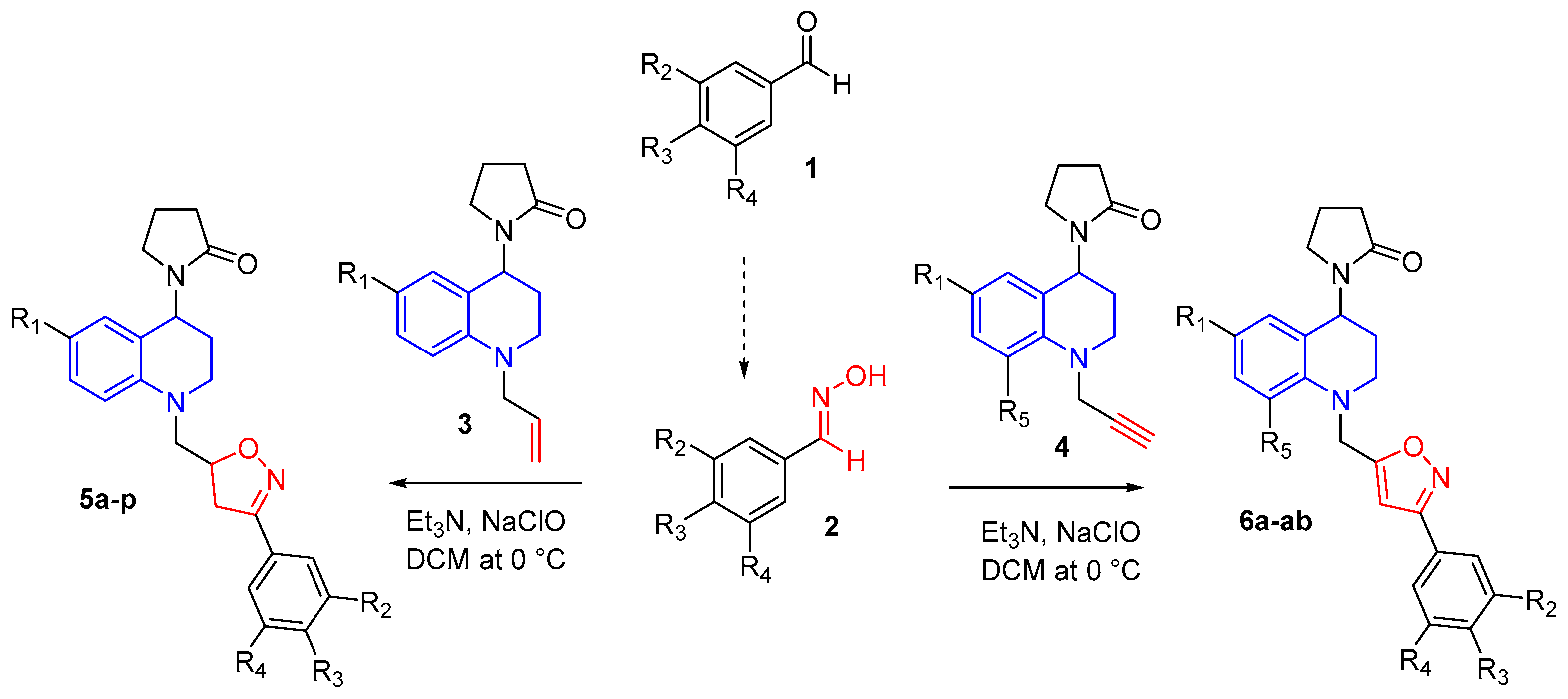
3.1.2. General Procedure for Isoxazole/Isoxazoline Synthesis
A mixture of
N-Allyl (3) or
N-propargyl (4) THQ (1 mmol) and the appropriate aldoxime 2 was dissolved in 8 mL of DCM and stirred for 10 min. Triethylamine (1 mmol) was added and the reaction was cooled to 0 °C and stirred. 8 mL of NaClO was added dropwise. The reaction mixture was stirred 3–4 h at room temperature. Water was added and ethyl acetate was used to extract. The organic layer was separated and dried with NaSO
4. The solvent was removed and the final product purified by column chromatography using appropriate ethyl acetate and petroleum ether mixture to provide isoxazolines 5 and isoxazoles 6 compounds. NMR spectra of compounds can be found in the
Supplementary Materials (Figures S3–S22).
3-phenyl-5-[(4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5 dihydroisoxazole (5a): 74% yield; Yellow oil; IR (ATR): 1664, 1605, 1502, 1357 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 77.63–7.60 (8H, m), 7.40–7.37 (8H, m), 7.18–7.15 (2H, m), 5.41 (2H, q, J = 7.2 Hz, 4′-H), 4.80–4.73 (2H, m), 3.54–3.44 (6H, m), 3.32–3.28 (4H, m), 3.24–3.16 (4H, m), 3.05–2.99 (2H, m), 2.45–2.39 (4H, m), 2.01–1.91 (4H, m), 1.49–1.47 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 174.75, 156.73, 146.21, 138.31, 131.56, 130.18, 129.60, 129.34, 128.78 (2C), 126.88 (2C), 126.50, 126.30, 79.96, 57.88, 57.51, 48.32, 42.43, 38.53, 31.41, 17.96, 16.48. Diastereomer β: 174.75, 156.68, 146.21, 138.07, 131.56, 130.16, 129,61, 129.30, 128.78 (2C), 126.88 (2C), 126.48, 126.27, 79,96, 57.86, 57.50, 48.28, 42.41, 38.53, 31.41, 17.96, 16.42. ESI-MS (m/z): 376 [M + H]+, 398 [M + Na]+, 414 [M + K]+, 763 [2M + Na]+, 291 [(M + H)-C4H6NO]+, 160 [(M + H)-C13H15N2O]+. Anal. Calcd. for C23H25N3O2: C, 73.57; H, 6.71; N, 11.19%. Found: C, 72.45; H, 6.59; N, 10.89%.
3-(4-methoxyphenyl)-5-[4-((2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5b): 64% yield; Yellow oil; IR (ATR): 1668, 1608, 1512, 1355 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.63–7.58 (4H, m), 7.24–7.20 (2H, m), 7.04 (2H, m), 6.93–6.87 (4H, m), 6.76 (2H, m), 6.58 (2H, m), 5.34 (2H, q, J = 7.5 Hz), 5.21–5.11 (2H, m), 3.82 (3H, sα), 3.81 (3H, sβ), 3.60–3.23 (14H, m), 3.10–3.01 (2H, m), 2.51–2.42 (4H, m), 2.08–1.97 (4H, m), 1.58–1.52 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 174.74, 156.94, 145.45, 130.82, 129.86, 128.04, 128.32 (2C), 127.49, 126.50, 122.12, 114.27 (2C), 113.19, 81.87, 57.29, 55.45, 48.22, 47.0, 43.37, 38.78, 31.38, 18.17, 16.88. Diastereomer β: 174.67, 156.58, 144.93, 130.60, 129.77, 128.01, 128.32 (2C), 127.45, 126.16, 121.98, 114.27 (2C), 113.02, 81.70, 56.79, 55.45, 47.33, 46.3, 42.83, 38.11, 31.17, 17.99, 16.75. ESI-MS (m/z): 406 [M + H]+, 428 [M + Na]+, 444 [M + K]+, 833 [2M + Na]+, 321 [(M + H)-C4H6NO]+, 190 [(M + H)-C13H15N2O]+. Anal. Calcd. for C24H27N3O3: C, 71.09; H, 6.71; N, 10.36%. Found: C, 72.08; H, 6.62; N, 10.08%.
3-(3,4-dimethoxyphenyl)-5-[4-((2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5c): 65% yield; Yellow oil; IR (ATR): 1666, 1602, 1514, 1366 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.37 (1H, sα), 7.35 (1H, sβ), 7.23–7.14 (2H, m), 7.02–6.97 (2H, m), 6.85–6.91 (4H, m), 6.75–6.69 (4H, m), 5.40 (2H, q, J = 7.4 Hz), 5.08–4.99 (2H, m), 3.90 (6H, sα), 3.89 (6H, sβ), 3.56–3.37 (10H, m), 3.25–3.01 (6H, m), 2.42–2.38 (4H, m), 2.03–1.90 (4H, m), 1.47–1.44 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 174.71, 156.86, 151.00, 149.19, 147.20, 129.68, 128.29, 128.22, 122.38, 120.50, 118.60, 113.17, 110.53, 108.66, 79.76, 56.02 (2C), 48.54, 48.27, 47.18, 42.27, 38.25, 31.68, 17.94, 16.49. Diastereomer β: 174.42, 156.47, 150.83, 149.12, 147.20, 129.35, 128.29, 128.35, 122.10, 120.50, 118.60, 113.17, 110.45, 108.60, 79.42, 56.02 (2C), 48.54, 48.17, 47.18, 42.21, 38.25, 31.40, 17.90, 16.38. ESI-MS (m/z): 436 [M + H]+, 458 [M + Na]+, 474 [M + K]+, 893 [2M + Na]+, 351 [(M + H)-C4H6NO]+, 220 [(M + H)-C13H15N2O]+. Anal. Calcd. for C25H29N3O4: C, 68.95; H, 6.71; N, 9.65%. Found: C, 68.39; H, 6.59; N, 9.89%.
3-(3,4,5-trimethoxyphenyl)-5-[4-((2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5d): 65% yield; Red oil; IR (ATR): 1666, 1606, 1514, 1357 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.36–7.30 (2H, m), 7.14 (2H, m), 7.01–6.99 (2H, m), 6.88 (2H, sα), 6.86 (2H, sβ), 6.63 (2H, m), 5.37 (2H, q, J = 6.5 Hz, 4′-H), 4.94–4.80 (2H, m, 5-H), 3.89 (6H, s), 3.85 (6H, s), 3.84 (6H, s), 3.60–3.40 (8H, m), 3.39–3.23 (6H, m), 3.05–2.97 (2H, m), 2.46–2.35 (4H, m), 2.16–1.99 (4H, m), 1.53–1.49 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.48, 155.32, 153.50 (2C), 144.87, 140.12, 128.05, 127.12, 124.94, 121.65, 121.45, 112.58, 104.19 (2C), 79.88, 61.32, 56.55 (2C), 54.74, 48.90, 47.88, 43.12, 38.42, 31.19, 18.34, 16.94. Diastereomer β: 175.39, 155.32, 153.42 (2C), 144.75, 139.95, 127.94, 127.08, 124.87, 121.58, 121.38, 112.45, 104.12 (2C), 79.64, 61.29, 56.50 (2C), 54.70, 48.75, 47.80, 42.95, 38.40, 31.12, 18.29, 16.78. ESI-MS (m/z): 466 [M + H]+, 488 [M + Na]+, 504 [M + K]+, 953 [2M + Na]+, 381 [(M + H)-C4H6NO]+, 250 [(M + H)-C13H15N2O]+. Anal. Calcd. for C26H31N3O5: C, 68.95; H, 6.71; N, 9.65%. Found: C, 68.45; H, 6.65; N, 9.82%.
5-[(6-methyl-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-phenyl-4,5-dihydroisoxazole (5e): 65% yield; Orange oil; IR (ATR): 1664, 1600, 1496, 1357 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.68–764 (6H, m), 7.42–7.39 (4H, m), 6.93 (1H, ddα, J = 8.4, 2.0 Hz), 6.91 (1H, ddβ, J = 8.4, 2.0 Hz), 6.72 (1H, dα, J = 2.0 Hz), 6.67 (1H, dβ, J = 2.0 Hz), 6.54 (2H, d, J = 8.4 Hz), 5.37(1H, ddα, J = 9.4, 5.8 Hz), 5.31(1H, ddβ, J = 9.4, 5.8 Hz), 5.09–5.01 (2H, m), 3.56–3.37 (10H, m), 3.25–3.01 (6H, m), 2.51–2.46 (4H, m), 2.20 (3H, sα), 2.19 (3H, sβ), 2.14–2.05 (4H, m), 1.98–1.90 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.62, 156.71, 144.02, 130.35, 129.46, 129.38, 128.87 (2C), 128.84, 126.77 (2C), 126.05, 119.73, 111.47, 79.86, 55.35, 48.96, 48.07, 44.39, 38.23, 31.63, 26.97, 20.40, 18.43. Diastereomer β: 175.51, 156.70, 143.50, 130.28, 129.33, 128.99, 128.84 (2C), 128.51, 126.77 (2C), 125.90, 119.51, 111.27, 79.43, 54.96, 48.79, 47.95, 43.67, 37.92, 31.58, 26.71, 20.38, 18.34. ESI-MS (m/z): 390 [M + H]+, 412 [M + Na]+, 428 [M + K]+, 801 [2M + Na]+, 305 [(M + H)-C4H6NO]+, 243 [(M + H)-C9H8NO]+, 160 [(M + H)-C14H17N2O]+. Anal. Calcd. for C24H27N3O2: C, 74.01; H, 6.99; N, 10.79%. Found: C, 73.76; H, 6.87; N, 10.54%.
3-(4-methoxyphenyl)-5-[(6-methyl-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5f): 70% yield; Orange oil; IR (ATR): 1676, 1606, 1514, 1359 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.62 (2H, dα, J = 8.9 Hz), 7.61 (2H, dβ, J = 8.9 Hz), 6.93 (2H, dα, J = 8.8 Hz), 6.91 (2H, dβ, J = 8.9 Hz), 6.72 (2H, dd, J = 8.6, 2.7 Hz), 6.63(1H, dα, J = 2.7 Hz), 6.61 (1H, dβ, J = 2.7 Hz), 6.50 (1H, dα, J = 8.6 Hz), 6.49 (1H, dβ, J = 8.6 Hz), 5.39 (1H, ddα, J = 9.2, 5.6 Hz), 5.34 (1H, ddβ, J = 9.8, 6.5 Hz), 5.08–4.99 (2H, m), 3.89 (3H, sα), 3.83 (3H, sβ), 3.58–3.35 (10H, m), 3.24–3.01 (6H, m), 2.51–2.45 (4H, m), 2.22 (3H, sα), 2.21 (3H, sβ), 2.11–2.03 (4H, m), 2.01–1.92 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 174.78, 155.10, 150.90, 144.195, 129.44, 128.94 (2C), 128.44, 127.37, 122.12, 121.16, 114.41 (2C), 113.88, 82.01, 57.12, 55.52, 47.20, 46.77, 43.55, 38.72, 31.34, 27.80, 19.30, 18.37. Diastereomer β: 174.56, 154.88, 150.90, 143.75, 129.23, 128.94 (2C), 128.34, 126.88, 122.07, 121.16, 114.18 (2C), 113.88, 81.79, 56.46, 55.47, 47.20, 46.77, 42.61, 37.68, 31.18, 27.80, 19.30, 17.75. ESI-MS (m/z): 420 [M + H]+, 442 [M + Na]+, 458 [M + K]+, 861 [2M + Na]+, 335 [(M + H)-C4H6NO]+, 243 [(M + H)-C10H10NO2]+, 190 [(M + H)-C14H17N2O]+. Anal. Calcd. for C25H29N3O3: C, 71.57; H, 6.97; N, 10.02%. Found: C, 72.06; H, 6.83; N, 10.29%.
3-(3,4-dimethoxyphenyl)-5-[(6-methyl-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5g): 63% yield; Orange oil; IR (ATR): 1666, 1598, 1508, 1365 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.40 (2H, s), 7.07–7.00 (4H, m), 6.92 (1H, ddα, J = 8.6, 2.0 Hz), 6.90 (1H, ddβ, J = 8.6, 2.0 Hz), 6.70 (1H, dα, J = 2.0Hz), 6.66 (1H, dβ, J = 2.0 Hz), 6.53 (2H, d, J = 8.6 Hz), 5.34 (1H, ddα, J = 9.5, 5.8 Hz), 5.30 (1H, ddβ, J = 7.4, 5.4 Hz), 5.04–4.99 (2H, m, 5-H), 3.90 (6H, sα), 3.89 (6H, sβ), 3.50–3.38 (10H, m), 3.24–3.03 (6H, m), 2.50–2.47 (4H, m), 2.19 (3H, sα), 2.18 (3H, sβ), 2.11–2.04 (4H, m), 1.99–1.94 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.68, 156.47, 150.98, 149.20, 143.98, 129.32, 128.93, 127.48, 126.00, 120.46, 119.68, 111.51, 110.51, 108.61, 79.65, 56.09 (2C), 48.90, 48.10, 47.19, 43.70, 38.09, 31.61, 26.94, 20.38, 18.34. Diastereomer β: 175.56, 156.47, 150.93, 149.18, 143.47, 129.19, 128.44, 127.35, 125.84, 120.43, 119.45, 111.27, 110.47, 108.61, 79.26, 56.02 (2C), 48.77, 47.99, 47.14, 43.54, 37.84, 31.56, 26.68, 20.36, 18.30. ESI-MS (m/z): 450 [M + H]+, 472 [M + Na]+, 488 [M + K]+, 921 [2M + Na]+, 365 [(M + H)-C4H6NO]+, 243 [(M + H)-C11H12NO3]+, 220 [(M + H)-C14H17N2O]+. Anal. Calcd. for C26H31N3O4: C, 69.47; H, 6.95; N, 9.35%. Found: C, 70.12; H, 6.79; N, 9.61%.
5-[(6-methyl-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-(3,4,5-trimethoxyphenyl)-4,5-dihydroisoxazole (5h): 68% yield; Orange oil; IR (ATR): 1664, 1598, 1508, 1369 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 6.92 (2H, sα), 6.90 (2H, sβ), 6.73 (1H, dα, J = 2.6 Hz), 6.71 (1H, dβ, J = 2.6 Hz), 6.64 (1H, ddα, J = 8.8, 2.6 Hz), 6.63 (1H, ddβ, J = 8.8, 2.6 Hz), 6.55 (2H, dd, J = 8.8 Hz), 5.39 (1H, ddα, J = 9.6, 6.0 Hz), 5.33 (1H, ddβ, J = 7.4, 5.6 Hz), 5.05–4.98 (2H, m, 5-H), 3.90 (6H, s), 3.89 (6H, s), 3.87 (6H, s), 3.58–3.35 (10H, m), 3.23–3.00 (6H, m), 2.53–2.45 (4H, m), 2.23 (3H, sα), 2.21 (3H, sβ), 2.14–2.06 (4H, m), 2.01–1.94 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.88, 155.40, 153.56 (2C), 144.10, 139.40, 129.70, 128.74, 125.65, 124.22, 121.41, 111.54, 104.12 (2C), 79.94, 61.08, 56.43 (2C), 55.77, 47.63, 47.20, 43.60, 38.02, 31.58, 26.46, 20.65, 18.41. Diastereomer β: 175.62, 155.40, 153.49 (2C), 143.69, 139.40, 129.70, 128.74, 125.65, 124.22, 121.41, 111.54, 104.06 (2C), 78.22, 61.04, 56.38 (2C), 54.31, 47.58, 46.75, 42.88, 37.52, 31.23, 26.46, 20.65, 17.85. ESI-MS (m/z): 480 [M + H]+, 502 [M + Na]+, 518 [M + K]+, 981 [2M + Na]+, 395 [(M + H)-C4H6NO]+, 250 [(M + H)-C14H17N2O]+. Anal. Calcd. for C27H33N3O5: C, 67.62; H, 6.94; N, 8.76%. Found: C, 68.23; H, 6.83; N, 8.54%.
5-[(6-methoxy-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-phenyl-4,5-dihydroisoxazole (5i): 77% yield; Red oil; IR (ATR): 1668, 1606, 1502, 1357 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.67–7.64 (4H, m), 7.41–7.37 (6H, m), 6.73 (1H, ddα, J = 8.7, 2.6 Hz), 6.71 (1H, ddβ, J = 8.7, 2.6 Hz), 6.59 (2H, d, J = 8.7 Hz), 6.51 (1H, dα, J = 2.6 Hz), 6.46 (1H, dβ, J = 2.6 Hz), 5.37 (1H, ddα, J = 9.6, 6.0 Hz), 5.33 (1H, ddβ, J = 8.4, 5.6 Hz), 5.06–4.99 (2H, m), 3.70 (3H, sα), 3.69 (3H, sβ), 3.53–3.35 (10H, m), 3.25–3.05 (6H, m), 2.49–2.44 (4H, m), 2.17–2.05 (4H, m), 1.98- 1.90 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.48, 156.65, 151.37, 140.30, 130.22, 129.33, 128.78 (2C), 126.69 (2C), 120.95, 114.16, 113.57, 112.55, 79.47, 55.86, 55.52, 48.84, 48.09, 43.41, 37.93, 31.44, 26.60, 18.28. Diastereomer β: 175.48, 156.65, 151.37, 140.30, 130.22, 129.33, 128.78 (2C), 126.69 (2C), 120.95, 114.16, 113.57, 112.55, 79.47, 55.86, 55.52, 48.84, 48.09, 43.41, 37.93, 31.44, 26.60, 18.28. ESI-MS (m/z): 406 [M + H]+, 428 [M + Na]+, 444 [M + K]+, 833 [2M + Na]+, 321 [(M + H)-C4H6NO]+, 259 [(M + H)-C9H8NO]+, 160 [(M + H)-C14H17N2O2]+. Anal. Calcd. for C24H27N3O3: C, 71.09; H, 6.71; N, 10.36%. Found: C, 70.78; H, 6.67; N, 10.12%.
3-(4-methoxyphenyl)-5-[(6-methoxy-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5j): 70% yield; Red oil; IR (ATR): 1664, 1602, 1508, 1369 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.62 (4H, d, J = 8.8 Hz), 6.91 (2H, dα, J = 8.8 Hz), 6.90 (2H, dβ, J = 8.8 Hz), 6.72 (1H, ddα, J = 8.9, 2.6 Hz), 6.70 (1H, ddβ, J = 8.9, 2.6 Hz), 6.59 (1H, dα, J = 2.6 Hz), 6.57 (1H, dβ, J = 2.6 Hz), 6.50 (1H, dα, J = 8.9 Hz), 6.49 (1H, dβ, J = 8.9 Hz), 5.39 (1H, ddα, J = 9.6, 6.0 Hz), 5.33 (1H, ddβ, J = 7.4, 5.6 Hz), 5.05–4.97 (2H, m, 5-H), 3.83 (3H, sα), 3.82 (3H, sβ), 3.70 (3H, sα), 3.69 (3H, sβ), 3.50–3.38 (10H, m), 3.22–3.09 (6H, m), 2.49–2.46 (4H, m), 2.16–2.07 (4H, m), 1.98–1.94 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.64, 156.63, 155.08, 151.49, 140.84, 128.32 (2C), 122.11, 121.25, 115.99, 114.21 (2C), 112.81, 111.97, 79.52, 55.94, 55.61, 55.22, 48.94, 47.39, 43.40, 38.50, 31.51, 26.89, 18.42. Diastereomer β: 175.54, 156.28, 154.93, 151.37, 140.32, 128.30 (2C), 122.02, 120.96, 115.76, 113.63 (2C), 112.59, 111.64, 79.19, 55.79, 55.46, 54.13, 48.27, 46.94, 42.79, 38.26, 31.29, 26.66, 18.34. ESI-MS (m/z): 436 [M + H]+, 458 [M + Na]+, 474 [M + K]+, 893 [2M + Na]+, 351 [(M + H)-C4H6NO]+, 259 [(M + H)-C10H10NO2]+, 190 [(M + H)-C14H17N2O2]+. Anal. Calcd. for C25H29N3O4: C, 68.95; H, 6.71; N, 9.65%. Found: C, 68.78; H, 6.54; N, 9.41%.
3-(3,4-dimethoxyphenyl)-5-[(6-methoxy-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5k): 76% yield; Red oil; IR (ATR): 1666, 1602, 1502, 1357 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.39 (2H, d, J = 2.0 Hz), 7.02 (1H, ddα, J =8.3, 2.0 Hz), 7.00 (1H, ddβ, J = 8.3, 2.0 Hz), 6.85 (2H, dα, J = 8.3 Hz), 6.83 (2H, dβ, J = 8.3 Hz), 6.73 (1H, ddα, J = 9.0, 2.8 Hz), 6.71 (1H, ddβ, J = 9.0, 2.8 Hz), 6.58 (2H, d, J = 9.0 Hz), 6.50 (1H, d, J = 2.8 Hz), 6.46 (1H, dβ, J = 2.8 Hz), 5.39 (1H, dd, J = 9.6, 6.0 Hz), 5.33 (1H, dd, J = 8.4, 5.6 Hz),5.05–4.97 (2H, m), 3.90 (12H, s), 3.70 (3H, sα), 3.69 (3H, sβ), 3.54–3.36 (10H, m), 3.27–3.04 (6H, m), 2.50–2.45 (4H, m), 2.17–2.05 (4H, m), 1.99–1.92 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.68, 156.49, 151.53, 151.00, 149.22, 140.81, 122.26, 121.31, 120.47, 114.46, 113.97, 112.84, 110.53, 108.63, 79.67, 56.07, 55.95, 55.86, 49.06, 48.29, 44.18, 38.40, 31.56, 26.89, 21.18, 18.43. Diastereomer β: 175.58, 156.48, 151.42, 150.96, 149.22, 140.35, 122.19, 121.00, 120.44, 114.23, 113.60, 112.60, 110.49, 108.62, 79.37, 56.03, 55.94, 55.64, 48.92, 48.18, 43.51, 38.18, 31.52, 26.66, 20.91, 18.37. ESI-MS (m/z): 466 [M + H]+, 488 [M + Na]+, 504 [M + K]+, 953 [2M + Na]+, 381 [(M + H)-C4H6NO]+, 259 [(M + H) C11H12NO3]+, 220 [(M + H)-C14H17N2O2]+. Anal. Calcd. for C26H31N3O5: C, 67.08; H, 6.71; N, 9.03%. Found: C, 67.41; H, 6.59; N, 9.29%.
5-[(6-methoxy-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-(3,4,5-trimethoxyphenyl)-4,5-dihydroisoxazole (5l): 68% yield; Red oil; IR (ATR): 1670, 1597, 1504, 1369 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 6.88 (2H, sα), 6.86 (2H, sβ),6.72 (1H, ddα, J = 8.7, 2.8 Hz), 6.71 (1H, ddβ, J = 8.7, 2.8 Hz), 6.59 (1H, dα, J = 8.7 Hz), 6.57 (1H, dβ, J = 8.7 Hz), 6.50 (1H, dα, J = 2.8 Hz), 6.47 (1H, dβ, J = 2.8 Hz), 5.40 (1H, ddα, J = 9.6, 5.9 Hz), 5.33 (1H, ddβ, J = 8.6, 5.6 Hz), 5.07–5.00 (2H, m), 3.87 (12H, s), 3.86 (3H, sα), 3.85 (3H, sβ), 3.70 (3H, sα), 3.69 (3H, sβ), 3.54–3.37(10H, m), 3.25–3.05 (6H, m), 2.50–2.45 (4H, m), 2.17–2.07 (4H, m), 2.01–1.97 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.69, 156.59, 153.43 (2C), 151.57, 140.77, 139.96, 124.91, 121.40, 114.43, 113.95, 112.87, 104.02 (2C), 79.94, 61.08, 56.38 (2C), 55.95, 55.84, 49.06, 48.27, 44.15, 38.35, 31.58, 26.87, 18.44. Diastereomer β: 175.57, 156.59, 153.39 (2C), 151.47, 140.33, 139.90, 124.85, 121.10, 114.20, 113.56, 112.58, 104.02 (2C), 79.68, 61.08, 56.38 (2C), 55.92, 55.62, 48.93, 48.18, 43.46, 38.14, 31.48, 26.65, 18.35. ESI-MS (m/z): 496 [M + H]+, 518 [M + Na]+, 534 [M + K]+, 1013 [2M + Na]+, 411 [(M + H)-C4H6NO]+, 259 [(M + H)-C12H14NO4]+, 250 [(M + H)-C14H17N2O2]+. Anal. Calcd. for C27H33N3O6: C, 65.44; H, 6.71; N, 8.48%. Found: C, 65.21; H, 6.60; N, 8.29%.
5-[(6-chloro-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-phenyl-4,5-dihydroisoxazole (5m): 74% yield; Yellow oil; IR (ATR): 1676, 1597, 1497, 1356 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.67–7.62 (4H, m), 7.42–7.38 (6H, m), 7.04 (1H, ddα, J = 8.9, 2.6 Hz), 7.03 (1H, ddβ, J = 8.9, 2.6 Hz), 6.83 (1H, dα, J = 2.6 Hz), 6.79 (1H, dβ, J = 2.6 Hz), 6.55 (1H, dα, J = 8.9 Hz), 6.53 (1H, dβ, J = 8.9 Hz), 5.34 (1H, ddα, J = 9.8, 5.7 Hz), 5.29 (1H, ddβ, J = 8.8, 5.4 Hz), 5.05–4.98 (2H, m), 3.58–3.37 (10H, m), 3.27–3.01 (6H, m), 2.52–2.44 (4H, m), 2.16–2.04 (4H, m), 1.98–1.91 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.64, 156.67, 144.64, 130.39, 129.22, 128.84 (2C), 128.47, 127.57, 126.69 (2C), 121.50, 121.32, 112.51, 79.45, 55.17, 48.80, 47.85, 43.90, 38.13, 31.36, 26.28, 18.30. Diastereomer β: 175.57, 156.67, 144.15, 130.33, 129.16, 128.82 (2C), 128.38, 127.22, 126.69 (2C), 121.36, 121.12, 112.23, 79.07, 54.87, 48.78, 47.77, 43.32, 37.85, 31.31, 26.14, 18.23. ESI-MS (m/z): 410 [M + H]+, 432 [M + Na]+, 448 [M + K]+, 841 [2M + Na]+, 325 [(M + H)-C4H6NO]+, 160 [(M + H)-C13H14ClN2O]+. Anal. Calcd. for C23H24ClN3O2: C, 67.39; H, 5.90; N, 10.25%. Found: C, 66.98; H, 5.84; N, 9.99%.
5-[(6-chloro-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-(4-methoxyphenyl)-4,5-dihydroisoxazole (5n): 64% yield; Yellow oil; IR (ATR): 1673, 1604, 1497, 1356 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.59 (2H, dα, J = 9.0 Hz), 7.58 (2H, dβ, J = 9.0 Hz), 7.04 (1H, ddα, J = 8.8, 2.6 Hz), 7.03 (1H, ddβ, J = 8.8, 2.6 Hz), 6.92 (2H, dα, J = 9.0 Hz), 6.90 (2H, dβ, J = 9.0 Hz), 6.83 (1H, dα, J = 2.6 Hz), 6.79(1H, dβ, J = 2.6 Hz), 6.55 (1H, dα, J = 8.8 Hz), 6.53 (1H, dβ, J = 8.8 Hz), 5.35 (1H, ddα, J = 9.7, 5.6 Hz), 5.30 (1H, ddβ, J = 8.7, 5.2 Hz), 5.02–4.95 (2H, m), 3.83 (3H, sα), 3.82 (3H, sβ), 3.58–3.33 (10H, m), 3.27–3.00 (6H, m), 2.53–2.45 (4H, m), 2.16–2.04 (4H, m), 2.00–1.94 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.63, 156.22, 144.80, 128.53, 128.50 (2C), 128.45, 127.57, 121.76, 121.45, 121.25, 114.22 (2C), 112.51, 79.11, 55.49, 55.23, 48.85, 47.95, 43.99, 38.46, 31.46, 26.37, 18.39. Diastereomer β: 175.56, 156.22, 144.17, 128.53, 128.50 (2C), 128.34, 127.22, 121.69, 121.34, 121.09, 114.17 (2C), 112.19, 78.73, 55.49, 54.95, 48.85, 47.85, 43.42, 38.21, 31.40, 26.20, 18.32. ESI-MS (m/z): 440 [M + H]+, 462 [M + Na]+, 478 [M + K]+, 901 [2M + Na]+, 355 [(M + H)-C4H6NO]+, 190 [(M + H)-C13H14ClN2O]+. Anal. Calcd. for C24H26ClN3O3: C, 65.52; H, 5.96; N, 9.55%. Found: C, 65.98; H, 5.88; N, 9.41%.
3-(4,5-dimethoxyphenyl)-5-[(6-chloro-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-4,5-dihydroisoxazole (5o): 75% yield; Yellow oil; IR (ATR): 1674, 1599, 1499, 1369 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.38 (2H, s), 7.07–6.98 (4H, m), 6.85 (1H, ddα, J = 8.6, 2.6 Hz), 6.83 (1H, ddβ, J = 8.6, 2.6 Hz), 6.83 (1H, dα, J = 2.6 Hz), 6.79 (1H, dβ, J = 2.6 Hz), 6.55 (1H, dα, J = 8.6 Hz), 6.53 (1H, dβ, J = 8.6 Hz), 5.35 (1H, ddα, J = 9.7, 5.6 Hz), 5.29 (1H, ddβ, J = 8.8, 5.3 Hz), 5.03–4.96 (2H, m), 3.90 (6H, sα), 3.89 (6H, sβ), 3.57–3.39 (10H, m), 3.24–3.02 (6H, m), 2.53–2.45 (4H, m), 2.19–2.05 (4H, m), 1.99–1.94 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.74, 156.49, 151.10, 149.26, 144.70, 128.54, 127.65, 122.09, 121.56, 121.38, 120.50, 112.60, 110.56, 108.62, 79.33, 56.07 (2C), 55.24, 48.86, 47.96, 43.99, 38.34, 31.44, 26.36, 18.38. Diastereomer β: 175.66, 156.49, 151.06, 149.26, 144.21, 128.45, 127.28, 122.02, 121.43, 121.19, 120.47, 112.28, 110.53, 108.62, 78.98, 56.04 (2C), 54.98, 48.86, 47.87, 43.43, 38.10, 31.39, 26.21, 18.33. ESI-MS (m/z): 470 [M + H]+, 492 [M + Na]+, 508 [M + K]+, 961 [2M + Na]+, 385 [(M + H)-C4H6NO]+, 220 [(M + H)-C13H14ClN2O]+. Anal. Calcd. for C25H28ClN3O4: C, 63.89; H, 6.01; N, 8.94%. Found: C, 64.18; H, 6.13; N, 9.11%.
5-[(6-chloro-4-(2′-oxopyrrolidin-1′-yl)-3,4-dihydroquinolin-1(2H)-yl)-methyl]-3-(3,4,5-trimethoxyphenyl)-4,5-dihydroisoxazole (5p): 75% yield; Yellow oil; IR (ATR): 1674, 1597, 1501, 1370 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.05 (1H, ddα, J = 8.9, 2.6 Hz), 7.03 (1H, ddβ, J = 8.9, 2.6 Hz), 6.88 (2H, sα), 6.86 (2H, sβ), 6.83 (1H, dα, J = 2.6 Hz), 6.79 (1H, dβ, J = 2.6 Hz), 6.55 (2H, d, J = 8.9 Hz), 5.37 (1H, ddα, J = 9.8, 5.4 Hz), 5.30 (1H, ddβ, J = 9.2, 5.2 Hz), 5.07–4.99 (2H, m), 3.88 (6H, s), 3.87 (6H, s), 3.86 (6H, s), 3.57–3.40 (10H, m), 3.22–3.02 (6H, m), 2.52–2.46 (4H, m), 2.19–2.04 (4H, m), 2.01–1.97 (4H, m). 13C NMR (100 MHz, CDCl3) δ (ppm). Diastereomer α: 175.76, 156.60, 153.49 (2C), 144.66, 140.11, 128.55, 127.64, 124.75, 121.64, 121.47, 112.61, 104.07 (2C), 79.64, 61.13, 56.39 (2C), 55.26, 48.93, 47.97, 43.96, 38.31, 31.46, 26.36, 18.40. Diastereomer β: 175.65, 156.60, 153.49 (2C), 144.16, 139.99, 128.44, 127.25, 124.67, 121.55, 121.29, 112.27, 104.07 (2C), 79.33, 60.54, 54.39 (2C), 54.95, 48.87, 47.85, 43.37, 38.09, 31.37, 26.20, 18.32. ESI-MS (m/z): 500 [M + H]+, 522 [M + Na]+, 538 [M + K]+, 1021 [2M + Na]+, 415 [(M + H)-C4H6NO]+, 250 [(M + H)-C13H14ClN2O]+. Anal. Calcd. for C26H30ClN3O5: C, 62.46; H, 6.05; N, 8.40%. Found: C, 62.01; H, 6.18; N, 8.56%.
3-phenyl-5-((4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6a): 55% yield; Orange oil; IR (ATR): 3465, 2931, 1670, 1497, 1438, 1167 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.76–7.72 (2H, m), 7.43–7.40 (3H, m), 7.10 (1H, tdd,
J = 7.8, 1.6, 0.7 Hz), 6.89 (1H, td,
J = 7.4, 1.1 Hz), 6.70 (1H, dd,
J = 7.4, 1.0 Hz), 6.66 (1H, d,
J = 8.6 Hz), 6.37 (1H, s), 5.41 (1H, dd,
J = 9.2, 5.4 Hz), 4.62 (1H, d, J = 17.5 Hz), 4.56 (1H, d, J = 17.5 Hz), 3.60–3.39 (2H, m), 3.27–3.10 (2H, m), 2.51–2.47 (2H, m), 2.23–2.05 (2H, m), 2.05–1.95 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.72, 170.00, 162.51, 144.97, 130.15, 128.97 (2C), 128.83, 128.80, 127.91, 126.87 (2C), 120.11, 117.11, 111.96, 100.38, 48.20, 47.97, 47.71, 43.83, 31.5, 26.70, 18.35. ESI-MS (m/z): 289.1 [M-C
4H
8NO]
+, 374.1 [M + H]
+, 396.1 [M + Na]
+, 769.1 [2M + Na]
+. Anal. Calcd. for C
23H
23N
3O
2: C, 73.97; H, 6.21; N, 11.25%. Found: C, 73.49; H, 6.34; N, 11.25%. The NMR and ESI-MS data match the previously reported data [
21].
3-(4-methoxyphenyl)-5-((4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6b): 65% yield; Red oil; IR (ATR): 2961, 1672, 1422, 1250, 1017 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.67 (2H, d, J = 8.9 Hz), 7.09 (2H, td, J = 7.4, 1.2 Hz), 6.92 (2H, d, J = 8.9 Hz), 6.88 (1H, td, J = 7.7, 1.4 Hz), 6.68 (1H, dd, J = 7.4, 0.9 Hz), 6.64 (1H, d, J = 8.5 Hz), 6.32 (1H, s), 5.40 (1H, dd, J = 9.4, 5.6 Hz), 4.59 (1H, d, J = 17.7 Hz), 4.53 (1H, d, J = 17.7 Hz), 3.81 (3H, s), 3.59–3.37 (2H, m), 3.27–3.09 (2H, m), 2.48 (2H, td, J = 7.7, 1.0 Hz), 2.21–2.02 (2H, m), 2.08–1.91 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.70, 169.68, 162.02, 161.01, 144.97, 128.67, 128.24 (2C), 127.82, 121.27, 120.04, 117.60, 114.18 (2C), 111.93, 100.10, 55.38, 48.14, 47.94, 47.64, 43.79, 31.47, 26.65, 18.31. ESI-MS (m/z): 426.1 [M + Na]+, 829.1 [2M + Na]+. Anal. Calcd. for C24H25N3O3: C, 71.44; H, 6.25; N, 10.41%. Found: C, 72.01; H, 6.12; N, 10.18%.
3-(3,4,5-trimethoxyphenyl)-5-((4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6c): 75% yield; Brown oil; IR (ATR): 3437, 2939, 1668, 1421, 1236, 1124 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.31–7.27 (1H, m), 7.06–6.92 (2H, m), 6.85–6.81 (1H, m), 6.64 (1H, d,
J = 10.1 Hz), 6.56 (1H, d,
J = 7.1 Hz), 6.32 (1H, s), 5.47–5.34 (1H, m), 4.64 (1H, d,
J = 16.7 Hz), 4.51 (1H, d,
J = 16.7 Hz), 3.91 (6H, s), 3.87 (3H, s), 3.70–3.36 (2H, m), 3.35–3.12 (2H, m), 2.56–2.44 (2H, m), 2.25–1.89 (4H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 174.98, 169.49, 162.41, 153.65 (2C), 143.46, 139.65, 128.61, 127.12, 124.11, 122.55, 121.96, 113.17, 104.05 (2C), 100.49, 61.02, 56.44 (2C), 49.98, 48.41, 47.82, 43.53, 31.24, 26.38, 18.33. ESI-EM (m/z): 486.0 [M + Na]
+, 520.1 [M + Cl + Na]
+. Anal. Calcd for C
26H
29N
3O
5: C, 67.37; H, 6.31; N, 9.07%. Found: C, 67.78; H, 6.17; N, 8.81%. The NMR and ESI-MS data match the previously reported data [
21].
5-((6′-methyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-phenylisoxazole (6d): 72% yield; Red oil; IR (ATR): 2952, 1667, 1421, 1285, 1093 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.76–7.72 (2H, m), 7.43–7.40 (3H, m), 6.91 (1H, dd,
J = 8.4, 2.1 Hz), 6.71 (1H, d,
J = 1.8 Hz), 6.58 (1H, d,
J = 8.4 Hz), 6.36 (1H, s), 5.38 (1H, dd,
J = 9.0, 5.4 Hz), 4.59 (1H, d,
J = 17.4 Hz), 4.54 (1H, d,
J = 17.4 Hz), 3.56–3.36 (2H, m), 3.27–3.10 (2H, m), 2.52–2.48 (2H, m), 2.19 (3H, s), 2.24–2.15 (2H, m), 2.10–1.95 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.66, 170.18, 162.52, 142.82, 130.15, 129.37, 128.98 (2C), 128.90, 128.51, 127.04, 126.90 (2C), 120.18, 112.23, 100.41, 48.23, 47.83, 47.87, 43.93, 31.56, 26.98, 20.46, 18.41. ESI-MS (m/z): 410.1 [M + Na]
+, 797.2 [2M + Na]
+. Anal. Calcd for C
24H
25N
3O
2: C, 74.39; H, 6.50; N, 10.84%. Found: C, 74.78; H, 6.37; N, 10.61%. The NMR and ESI-MS data match the previously reported data [
21].
3-(4-methoxyphenyl)-5-((6′-methyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6e): 64% yield; Red oil; IR (ATR): 2957, 1664, 1425, 1251, 1176 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.72 (2H, dd,
J = 6.1, 2.1 Hz), 7.10 (1H, d,
J = 1.9 Hz), 6.94 (2H, dd,
J = 6.1, 1.9 Hz), 6.89–6.85 (1H, m), 6.71 (1H, d,
J = 1.8 Hz), 6.59 (1H, s), 6.32 (1H, s), 5.36 (1H, dd,
J = 9.2, 7.2 Hz), 4.38 (1H, d,
J = 16.5 Hz), 4.31 (1H, d,
J = 16.5 Hz), 3.84 (3H, s), 3.39–3.22 (2H, m), 3.20–2.96 (2H, m), 2.54–2.43 (2H, m), 2.21 (2H, s), 2.12–1.81 (4H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.84, 171.14, 162.22, 161.99, 142.23, 133.44, 130.68, 129.08, 128.23 (2C), 127.71, 126.88, 121.46, 114.31 (2C), 100.75, 55.39, 48.69, 47.70, 46.96, 43.03, 31.21, 26.33, 20.60, 18.16. ESI-MS (m/z): 474.1 [M + Cl + Na]+, 925.1 [2M + 2Cl + Na]+. Anal. Calcd for C
25H
27N
3O
3: C, 71.92; H, 6.52; N, 10.06%. Found: C, 73.72; H, 6.68; N, 9.86%. The NMR and ESI-MS data match the previously reported data [
20].
5-((8′-chloro-6′-methyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4-dimethoxyphenyl)isoxazole (6f): 58% yield; Beige solid; IR (ATR): 2966, 1656, 1429, 1255, 1143, 1016 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.40 (1H, d, J = 8.3 Hz), 7.30 (1H, dd, J = 8.3, 2.0 Hz), 7.11 (1H, d, J = 2.1 Hz), 6.91 (1H, d, J = 8.3 Hz), 6.73 (1H, t, J = 1.0 Hz), 6.63 (1H, s), 5.38 (1H, dd, J = 8.8, 7.1 Hz), 4.38 (1H, d, J = 16.3 Hz), 4.33 (1H, d, J = 16.3 Hz), 3.94 (3H, s), 3.91 (3H, s), 3.31–3.21 (2H, m), 3.24–3.00 (2H, m), 2.51–2.45 (2H, m), 2.23 (3H, s), 2.06–1.95 (2H, m), 1.94–1.84 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.75, 171.34, 162.42, 150.62, 149.30, 142.30, 133.53, 130.73, 129.18, 127.78, 126.96, 121.75, 120.03, 111.05, 109.24, 100.78, 56.09, 56.02, 49.82, 47.70, 46.98, 43.05, 31.28, 21.41, 20.67, 18.24. ESI-MS (m/z): 482.0 [M + H]+, 504.1 [M + Na]+, 985.1 [2M + Na]+. Anal. Calcd for C26H28ClN3O4: C, 64.79; H, 5.86; N, 8.72%. Found: C, 64.58; H, 5.75; N, 8.61%
3-(3,4,5-trimethoxyphenyl)-5-((6′-methyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6g): 63% yield; Red oil; IR (ATR): 2935, 1668, 1421, 1124, 1001 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 6.96 (2H, s), 6.91 (1H, dd,
J = 8.4, 2.0 Hz), 6.7 (1H, d,
J = 2.0 Hz), 6.56 (1H, d,
J = 8.4 Hz), 6.32 (1H, s), 5.40 (1H, dd,
J = 9.2, 5.6 Hz), 4.62 (1H, d,
J = 17.6 Hz), 4.51 (1H, d,
J = 17.6 Hz), 3.90 (6H, s), 3.86 (3H, s), 3.58–3.37 (2H, m), 3.28–3.12 (2H, m), 2.55–2.47 (2H, m), 2.19 (3H, s), 2.23–2.06 (2H,m), 2.05–1.95 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.75, 170.33, 162.43, 153.65 (2C), 142.81, 139.64, 133.64, 129.39, 128.34, 124.57, 120.19, 112.16, 104.08 (2C), 100.29, 61.06, 56.42 (2C), 48.37, 48.00, 47.99, 43.83, 31.55, 26.92, 20.48, 18.42. ESI-MS (m/z): 391.1 [M-C
4H
7NO]
+, 478.3 [M + H]
+, 500.2 [M + Na]
+, 539.1 [M+C
2H
8CONa]
+. Anal. Calcd for C
27H
31N
3O
5: C, 67.91; H, 6.54; N, 8.80%. Found: C, 68.33; H, 6.69; N, 9.04%. The NMR and ESI-MS data match the previously reported data [
21].
5-((6′-methoxy-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-phenylisoxazole (6h): 42% yield; Brown oil; IR (ATR): 2949, 1661, 1502, 1421, 1285, 1039 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.74–7.72 (2H, m), 7.45–7.39 (3H, m), 6.69 (1H, dd,
J = 2.9, 0.7 Hz), 6.61 (1H, d,
J = 9.0 Hz), 6.49 (1H, dd,
J = 2.9, 0.7 Hz), 6.35 (1H, s), 5.39 (1H, dd,
J = 9.3, 5.6 Hz), 4.57 (1H, d,
J = 17.4 Hz), 4.49 (1H, d, J = 17.4 Hz), 3.68 (3H, s), 3.51–3.32 (2H, m), 3.26–3.10 (2H, m), 2.50–2.45 (2H, m), 2.19–2.06 (2H, m), 2.06–1.93 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.69, 170.16, 162.46, 152.08, 139.43, 130.12, 128.95 (2C), 128.84, 126.85 (2C), 121.77, 114.12, 113.68, 113.48, 100.45, 55.78, 48.70, 48.35, 48.16, 43.68, 31.45, 26.85, 18.36. ESI-MS (m/z): 426.1 [M + Na]
+, 829.1 [2M + Na]
+. Anal. Calcd. for C
24H
25N
3O
3 (403.48 g/mol). Anal. Calcd for C
24H
25N
3O
3: C, 71.44; H, 6.25; N, 10.41%. Found: C, 71.08; H, 6.39; N, 10.13%. The NMR and ESI-MS data match the previously reported data [
21].
3-(4-methoxyphenyl)-5-((6′-methoxy-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6i): 74% yield; Red oil; IR (ATR): 2937, 1662, 1427, 1249, 1174 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.67 (2H, dd, J = 6.8, 2.1 Hz), 6.92 (2H, dd, J = 6.8, 1.9 Hz), 6.70 (1H, dd, J = 8.9, 2.7 Hz) 6.61 (1H, d, J = 8.9 Hz), 6.49 (1H, d, J = 0.9 Hz), 6.29 (1H, s), 5.36 (1H, dd, J = 8.9, 6.5 Hz), 4.56 (1H, d, J = 17.6 Hz), 4.48 (1H, d, J = 17.6 Hz), 3.82 (3H, s), 3.69 (3H, s), 3.51–3.32 (2H, m), 3.18–3.00 (2H, m), 2.50–2.45 (2H, m), 2.20–2.04 (2H, m), 2.09–1.91 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.72, 169.80, 161.65, 152.08, 138.32, 130.40, 128.98, 128.20 (2C), 121.23, 114.32 (2C), 114.14, 113.51, 111.72, 100.22, 55.77, 55.42, 48.17, 47.99, 47.00, 43.98, 31.23, 26.87, 18.38. ESI-MS (m/z): 456.1 [M + Na]+, 889.2 [2M + Na]+. Anal. Calcd for C25H27N3O4: C, 69.27; H, 6.28; N, 9.69%. Found: C, 68.87; H, 6.15; N, 9.44%.
5-((8′-chloro-6′-methoxy-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4-dimethoxyphenyl)isoxazole (6j): 86% yield; Beige solid; IR (ATR): 2943, 2839, 1670, 1429, 1261 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.40 (1H, d, J = 2.0 Hz), 7.29 (1H, dd, J = 8.5, 2.0 Hz), 6.91 (1H, d, J = 8.5 Hz), 6.90 (1H, dd, J = 2.8, 0.8 Hz), 6.62 (1H, s), 6.50 (1H, dd, J = 2.9, 0.9 Hz), 5.39 (1H, t, J = 8.2 Hz), 4.34 (1H, d, J = 16.3 Hz), 4.28 (1H, d, J = 16.3 Hz), 3.93 (3H, s), 3.91 (3H, s), 3.72 (3H, s), 3.40–3.19 (2H, m), 3.27–3.01 (2H, m), 2.49–2.45 (2H, m), 2.15–1.96 (2H, m), 1.95–1.83 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.71, 171.32, 162.42, 155.47, 150.63, 149.31, 138.32, 130.42, 128.98, 121.74, 120.03, 115.91, 111.73, 111.06, 109.25, 100.78, 56.09, 56.02, 55.77 49.86, 47.96, 46.95, 42.97, 31.23, 21.58, 18.28. ESI-MS (m/z): 486.2 [[M-Cl]+Na]+, 520.1 [M + Na]+, 1017.2 [2M + Na]+. Anal. Calcd for C26H28ClN3O5: C, 62.71; H, 5.67; N, 8.44%. Found: C, 62.54; H, 5.57; N, 8.67%.
3-(3,4,5-trimethoxyphenyl)-5-((6′-methoxy-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6k): 74% yield; Red oil; IR (ATR): 2937, 1664, 1421, 1238, 1124 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 6.97 (2H, s), 6.71 (1H, dd,
J = 9.0, 2.9 Hz), 6.61 (1H, d,
J = 9.0 Hz), 6.50 (1H, dd,
J = 2.9, 0.8 Hz), 6.31 (1H, s), 5.43 (1H, dd,
J = 9.6, 5.7 Hz), 4.62 (1H, d,
J = 17.4 Hz), 4.48 (1H, d,
J = 17.4 Hz), 3.90 (6H, s), 3.87 (3H, s), 3.70 (3H, s), 3.56–3.35 (2H, m), 3.28–3.14 (2H, m), 2.53–2.48 (2H, m), 2.21–2.08 (2H,m), 2.07–1.97 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.77, 170.34, 162.44, 153.68 (2C), 152.18, 139.67, 139.43, 124.38, 121.88, 114.12, 113.63, 113.44, 104.10 (2C), 100.38, 61.09, 56.44 (2C), 55.86, 48.57, 48.35, 48.19, 43.64, 31.51, 26.86, 18.44. ESI-MS (m/z): 516.1 [M + Na]
+, 1009.2 [2M + Na]
+. Anal. Calcd for C
27H
31N
3O
6: C, 65.71; H, 6.33; N, 8.51%. Found: C, 65.49; H, 6.21; N, 8.30%. The NMR and ESI-MS data match the previously reported data [
21].
5-((6′-chloro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-phenylisoxazole (6l): 55% yield; Red oil; IR (ATR): 2930, 1657, 1496, 1127 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.77–7.72 (2H, m), 7.44–7.41 (3H, m), 7.04 (1H, dd,
J = 8.9, 2.5 Hz), 6.83 (1H, dd,
J = 2.5, 1.0 Hz), 6.57 (1H, d,
J = 8.9 Hz), 6.37 (1H, s), 5.37 (1H, dd,
J = 9.6, 5.9 Hz), 4.60 (1H, d,
J = 17.5 Hz), 4.52 (1H, d,
J = 17.5 Hz), 3.60–3.39 (2H, m), 3.28–3.11 (2H, m), 2.55–2.44 (2H, m), 2.21–2.03 (2H, m), 2.09–1.98 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.77, 169.43, 162.55, 143.55, 130.25, 129.01 (2C), 128.69, 128.60, 127.28, 126.87 (2C), 122.56, 121.90, 113.23, 100.47, 48.24, 47.75, 47.69, 43.54, 31.15, 26.41, 18.34. ESI-MS (m/z): 430.0 [M + Na]
+, 837.1 [2M + Na]
+. Anal. Calcd for C
23H
22ClN
3O
2: C, 67.73; H, 5.44; N, 10.30%. Found: C, 67.36; H, 5.58; N, 10.09%. The NMR and ESI-MS data match the previously reported data [
21].
5-((6′-chloro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(4-methoxyphenyl)isoxazole (6m): 51% yield; Orange oil; IR (ATR): 2976, 1678, 1431, 1253, 1178 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.67 (2H, dd,
J = 6.1, 2.2 Hz), 7.03 (1H, ddd,
J = 8.9, 2.6, 0.8 Hz), 6.93 (2H, dd,
J = 6.1, 2.2 Hz), 6.83 (1H, dd,
J = 2.6, 1.0 Hz), 6.57 (1H, d,
J = 8.9 Hz), 6.31 (1H, s), 5.37 (1H, dd,
J = 9.6, 5.4 Hz), 4.58 (1H, d,
J = 17.6 Hz), 4.50 (1H, d,
J = 17.6 Hz), 3.82 (3H, s), 3.60–3.39 (2H, m), 3.28–3.12 (2H, m), 2.56–2.46 (2H, m), 2.19–2.06 (2H, m), 2.09–2.01 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.94, 169.14, 162.16, 161.14, 143.60, 128.64, 128.30 (2C), 127.30, 122.54, 121.82, 121.19, 114.37 (2C), 113.27, 100.25, 55.44, 48.21, 47.83, 47.70, 43.68, 31.36, 26.43, 18.16. ESI-MS (m/z): 460.1 [M + Na]
+, 897.1 [2M + Na]
+. Anal. Calcd for C
24H
24ClN
3O
3: C, 65.83; H, 5.52; N, 9.60%. Found: C, 67.47; H, 5.66; N, 9.48%. The NMR and ESI-MS data match the previously reported data [
20].
5-((6′-chloro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4-dimethoxyphenyl)isoxazole (6n): 68% yield; Orange oil; IR (ATR): 2935, 1665, 1420, 1257, 1022 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.34 (1H, d, J = 2.0 Hz), 7.22 (1H, dd, J = 8.3, 2.2 Hz), 7.02 (1H, dd, J = 8.8, 2.5 Hz), 6.87 (1H, d, J = 8.3 Hz), 6.82 (1H, dd, J = 2.5, 1.0 Hz), 6.56 (1H, d, J = 8.8 Hz), 6.31 (1H, s), 5.36 (1H, dd, J = 9.7, 5.3 Hz), 4.59 (1H, d, J = 17.6 Hz), 4.49 (1H, d, J = 17.6 Hz), 3.91 (3H, s), 3.89 (3H, s), 3.60–3.36 (2H, m), 3.27–3.11 (2H, m), 2.55–2.44 (2H, m), 2.21–2.10 (2H, m), 2.09–1.98 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.76, 169.20, 162.24, 150.68, 149.27, 143.54, 128.57, 127.19, 122.50, 121.87, 121.36, 120.05, 113.20, 110.97, 109.16, 100.22, 56.07, 55.98, 48.25, 47.75, 47.68, 43.56, 31.32, 26.36, 18.34. ESI-MS (m/z): 456.1 [[M-Cl]+H]+, 490.1 [M + Na]+, 597.1 [2M + Na]+. Anal. Calcd for C25H26ClN3O4: C, 64.17; H, 5.60; N, 8.98%. Found: C, 63.91; H, 5.49; N, 8.81%.
5-((6′,8′-dichloro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4-dimethoxyphenyl)isoxazole (6o): 88% yield; Orange solid; IR (ATR): 2954, 1662, 1427, 1249 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.40 (1H, d, J = 2.0 Hz), 7.30 (1H, d, J = 0.8 Hz), 7.29 (1H, dd, J = 8.3, 2.0 Hz), 6.92 (1H, d, J = 0.8 Hz), 6.91 (1H, d, J = 8.4 Hz), 6.61 (1H, s), 5.39 (1H, t, J = 8.3 Hz), 4.43 (1H, d, J = 16.4 Hz), 4.36 (1H, d, J = 16.4 Hz), 3.94 (3H, s), 3.92 (3H, s), 3.32–3.24 (2H, m), 3.25–3.01 (2H, m), 2.55–2.42 (2H, m), 2.06–1.97 (2H, m), 1.96–1.85 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.83, 170.79, 162.46, 150.73, 149.37, 143.62, 130.42, 129.95, 128.61, 128.25, 126.29, 121.63, 120.08, 111.08, 109.24, 100.93, 56.13, 56.06, 49.44, 47.80, 47.27, 42.96, 31.13, 21.41, 18.28. ESI-MS (m/z): 490.1 [[M-Cl] + Na]+, 524.0 [M + Na]+, 1027.0 [2M + Na]+. Anal. Calcd for C25H25Cl2N3O4: C, 59.77; H, 5.02; N, 8.36%. Found: C, 59.52; H, 4.91; N, 8.46%.
5-((6′-chloro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4,5-trimethoxyphenyl)isoxazole (6p): 85% yield; Brown oil; IR (ATR): 2936, 1667, 1582, 1420, 1122 cm
−1.
1H NMR (400 MHz, CDCl
3) δ (ppm): 7.05 (1H, dd,
J = 8.8, 2.6 Hz), 6.96 (2H, s), 6.84 (1H, dd,
J = 2.5, 0.9 Hz), 6.57 (1H, d,
J = 8.8 Hz), 6.31 (1H, s), 5.40 (1H, dd,
J = 9.9, 5.4 Hz), 4.64 (1H, d,
J = 17.6 Hz), 4.51 (1H, d,
J = 17.6 Hz), 3.90 (6H, s), 3.87 (3H, s), 3.64–3.40 (2H, m), 3.30–3.14 (2H, m), 2.59–2.44 (2H, m), 2.23–2.05 (2H, m), 2.16–1.97 (2H, m).
13C NMR (100 MHz, CDCl
3) δ (ppm): 175.82, 169.55, 160.50, 153.72 (2C), 143.55, 139.80, 128.67, 127.23, 124.20, 122.69, 122.02, 113.22, 104.14 (2C), 100.39, 61.08, 56.45 (2C), 48.44, 47.86, 47.84, 43.54, 31.38, 26.40, 18.40. ESI-MS (m/z): 520.1 [M + Na]
+, 1017.2 [2M + Na]+, 1513.0 [3M + Na]
+. Anal. Calcd for C
26H
28ClN
3O
5: C, 62.71; H, 5.67; N, 8.44%. Found: C, 63.12; H, 5.53; N, 8.68%. The NMR and ESI-MS data match the previously reported data [
21].
5-((6′-ethyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-phenylisoxazole (6q): 40% yield; Brown oil; IR (ATR): 2863, 2363, 1681, 1510, 1335, 1169 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.75 (2H, d, J = 4.8 Hz), 7.42 (3H, br), 6.95 (1H, d, J = 8.3 Hz), 6.74 (1H, s), 6.61 (1H, d, J = 8.3 Hz), 6.38 (1H, s), 5.44–5.38 (1H, m), 4.63–4.52 (2H, m), 3.53 (1H, t, J = 10,1 Hz), 3.44–3.37 (1H, m), 3.24 (1H, dd, J = 16.0, 8.0 Hz), 3.13 (1H, dd, J = 14.6, 8.0 Hz), 2.52 (4H, bs), 2.24–2.06 (2H, m), 2.03–1.93 (2H, m), 1.15 (3H, t, J = 7.5 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.91, 170.61, 162.88, 143.43, 134.01, 130.47, 129.32 (2C), 128.99, 128.49, 127.79, 127.26 (2C), 120.56, 112.57, 100.75, 48.59, 48.37, 48.29, 44.33, 31.95, 28.24, 27.39, 18.87, 16.31. HR-ESI-MS (m/z): 402.2156 [M + H]+, 420.2031 [M + Na]+, 440.1745 [M + K]+. Anal. Calcd. for C25H27N3O2 (401.2103 g/mol).
5-((6′-ethyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(4-methoxyphenyl)isoxazole (6r): 60% yield; Orange oil; IR (ATR): 2961, 2358, 1963, 1679, 1507, 1436, 1255, 1027 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.79 (2H, d, J = 8.1 Hz), 7.36 (1H, s), 7.04 (2H, d, J = 8.2 Hz), 6.84 (1H, s), 6.71 (1H, d, J = 8.2 Hz), 6.42 (1H, s), 5.54–5.48 (1H, m), 4.66 (2H, s), 3.94 (3H, s), 3.63 (1H, t, J = 10.1 Hz), 3.53–3.46 (1H, m), 3.34 (1H, dd, J = 16.1, 8.0 Hz), 3.23 (1H, dd, J = 13.9, 8.7 Hz), 2.64–2.54 (4H, m), 2.33–2.23 (2H, m), 2.13–2.03 (2H, m), 1.25 (3H, t, J = 7.5 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.98, 170.32, 162.50, 161.47, 143.48, 133.98, 128.68 (2C), 128.51, 127.79, 121.85, 120.52, 114.73 (2C), 112.60, 100.52, 55.81, 48.57, 48.42, 48.30, 44.39, 31.97, 28.25, 27.40, 18.88, 16.32. HR-ESI-MS (m/z): 432.2271 [M + H]+, 346.0085 [M-C4H7NO]+. Anal. Calcd. for C26H29N3O3 (431.2209 g/mol).
5-((8′-chloro-6′-ethyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4-dimethoxyphenyl)isoxazole (6s): 73% yield; Beige solid; IR (ATR): 2966, 2360, 1679, 1470, 1267, 1019 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.41 (1H, s), 7.30 (1H, d, J = 8.2 Hz), 7.15 (1H, s), 6.92 (1H, d, J = 8.2 Hz), 6.77 (1H, s), 6.62 (1H, s), 5.42 (1H, m), 4.45–4.33 (2H, m), 3.95 (3H, s), 3.92 (3H, s), 3.34–3.23 (2H, m), 3.22–2.99 (2H, m), 2.58–2.51 (2H, m), 2.50–2.45 (2H, m), 2.05–1.95 (2H, m), 1.95–1.88 (2H, m), 1.18 (3H, t, J = 7.5 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 176.00, 171.73, 162.79, 151.07, 149.77, 142.88, 140.25, 129.93, 129.64, 128.21, 126.25, 122.23, 120.41, 111.53, 109.78, 101.14, 56.49, 56.41, 50.21, 48.21, 47.42, 43.47, 31.68, 28.39, 21.94, 18.74, 15.94. HR-ESI-MS (m/z): 496.2019 [M + H]+, 518.1739 [M + Na]+, 534.1592 [M + K]+. Anal. Calcd. for C27H30ClN3O4 (495.1925 g/mol).
5-((6′-ethyl-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4,5- trimethoxyphenyl)isoxazole (6t): 72% yield; Orange oil; IR (ATR): 2961, 2929, 1679, 1581, 1230, 1127 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.13 (2H, br), 7.10 (1H, d, J = 7.7 Hz), 6.88 (1H, s), 6.76 (1H, d, J = 8.4 Hz), 6.52 (1H, s), 5.59–5.53 (1H, m), 4.76 (1H, d, J = 17.4 Hz), 4.68 (1H, d, J = 17.5 Hz), 4.05 (6H, s), 4.02 (3H, s), 3.75–3.52 (2H, m), 3.45–3.26 (2H, m), 2.70–2.62 (4H, m), 2.50–2.32 (2H, m), 2.28–2.21 (2H, m), 1.30 (3H, t, J = 7.5 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 176.16, 170.61, 162.61, 153.87 (2C), 143.27, 139.97, 133.85, 128.38, 127.45, 124.58, 120.28, 112.43, 104.45 (2C), 100.57, 61.22, 56.63 (2C), 48.49, 48.38, 48.17, 44.22, 31.76, 28.08, 27.14, 18.68, 16.17. HR-ESI-MS (m/z): 492.2448 [M + H]+. Anal. Calcd. for C28H33N3O5 (491.2420 g/mol).
5-((6′-fluor-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-phenylisoxazole (6u): 89% yield; Orange oil; IR (ATR): 2941, 2870, 1665, 1499, 1281, 1155 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.75 (2H, d, J = 2.7 Hz), 7.43 (3H, br), 6.83 (1H, t, J = 8.4 Hz), 6.67–6.58 (2H, m), 6.37 (1H, s), 5.45–5.39 (1H, m), 4.61 (1H, d, J = 17.4 Hz), 4.53 (1H, d, J = 17.4 Hz), 3.56 (1H, t, J = 10.7 Hz), 3.44–3.37 (1H, m), 3.27 (1H, dd, J = 16.2, 8.1 Hz), 3.15 (1H, dd, J = 14.6, 7.8 Hz), 2.55–2.46 (2H, m), 2.23–2.07 (2H, m), 2.06–2.00 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.56, 169.65, 162.46, 156.94, 154.59, 141.44, 130.11, 128.91 (2C), 126.81 (2C), 122.02, 115.64 (JC-F = 22.2), 114. 52 (JC-F = 22.2), 113.10 (JC-F = 7.2), 100.38, 48.38, 48.07, 47.86, 43.38, 31.26, 26.49, 18.27. HR-ESI-MS (m/z): 392.1917 [M + H]+, 414.1691 [M + Na]+, 430.1480 [M + K]+. Anal. Calcd. for C23H22FN3O2 (391.1696 g/mol).
5-((6′-fluoro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(4-methoxyphenyl)isoxazole (6v): 77% yield; Orange oil; IR (ATR): 2954, 2836, 1669, 1608, 1429, 1252, 1027 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.69 (d, J = 7.9 Hz, 1H), 6.94 (d, J = 7.9 Hz, 1H), 6.82 (t, J = 8.4 Hz, 1H), 6.66–6.57 (m, 1H), 6.30 (s, 1H), 5.45–5.37 (m, 1H), 4.58 (d, J = 17.4 Hz, 1H), 4.51 (d, J = 17.3 Hz, 1H), 3.84 (s, 1H), 3.59–3.36 (m, 1H), 3.31–3.11 (m, 1H), 2.58–2.44 (m, 1H), 2.23–2.06 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.57, 169.36, 162.06, 161.07, 156.92, 141.47, 128.21 (2C), 122.00, 121.25, 115.18 (JC-F = 19.3), 114.30 (2C), 114.05 (JC-F = 22.6), 113.12 (JC-F = 7.3), 100.13, 55.36, 48.35, 48.05, 47.87, 43.39, 31.26, 26.48, 18.27. HR-ESI-MS (m/z): 422.1874 [M + H]+, 444.1710 [M + Na]+. Anal. Calcd. for C24H24FN3O3 (421.1802 g/mol).
5-((6′-fluoro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4-dimethoxyphenyl)isoxazole (6w): 75% yield; Orange oil; IR (ATR): 2934, 2834, 1679, 1502, 1264, 1024 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.40 (1H, s), 7.29 (1H, d, J = 5.8 Hz), 6.93 (1H, d, J = 8.2 Hz), 6.86 (1H, t, J = 8.4 Hz), 6.65 (2H, m), 6.35 (1H, s), 5.49–5.42 (1H, m), 4.63 (1H, d, J = 17.4 Hz), 4.54 (1H, d, J = 17.4 Hz), 3.97 (3H, s), 3.95 (3H, s), 3.59 (1H, t, J = 10.7 Hz), 3.47–3.40 (1H, m), 3.30 (1H, dd, J = 16.1, 8.1 Hz), 3.19 (1H, dd, J = 14.7, 7.6 Hz), 2.54 (2H, m), 2.27–2.10 (2H, m), 2.10–2.04 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 176.05, 169.90, 162.63, 157.38, 155.02, 151.16, 149.77, 141.88, 121.91, 120.44, 115,62 (JC-F = 22.1), 114.46 (JC-F = 22.6), 113.55 (JC-F = 7.4), 111.48, 109.73, 100.61, 56.50, 56.40, 48.84, 48.52, 48.34, 43.81, 31.70, 26.90, 18.71. HR-ESI-MS (m/z): 452.1956 [M + H]+, 474.1831 [M + Na]+. Anal. Calcd. for C25H26FN3O4 (451.1907 g/mol).
5-((6′-fluoro-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4,5- trimethoxyphenyl)isoxazole (6x): 87% yield; Orange oil; IR (ATR): 2935, 2357, 1661, 1504, 1254, 1126 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 6.97 (2H, s), 6.82 (1H, t, J = 8.4 Hz), 6.63 (1H, d, J = 9.0 Hz), 6.59 (1H, dd, J = 8.8, 4.2 Hz), 6.31 (1H, s), 5.43 (1H, m), 4.63 (1H, d, J = 17.5 Hz), 4.50 (1H, d, J = 17.4 Hz), 3.91 (6H, s), 3.88 (3H, s), 3.58 (1H, t, J = 10.8 Hz), 3.44–3.37 (1H, m), 3.28 (1H, dd, J = 16.1, 8.1 Hz), 3.17 (1H, dd, J = 14.7, 7.8 Hz), 2.54–2.47 (2H, m), 2.25–2.08 (2H, m), 2.06–2.00 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 176.07, 170.19, 162.80, 157.40, 155.05, 154.06, 141.82, 140.25, 124.59, 122.45, 115.62 (JC-F = 22.1), 114.40 (JC-F = 22.7), 113.49 (JC-F = 7.3), 104.60 (2C), 100.72, 61.40, 56.81 (2C), 48.93, 48.56, 48.38, 43.76, 31.69, 26.86, 18.71. HR-ESI-MS (m/z): 482.2097 [M + H]+, 504.1880 [M + Na]+, 520.1732 [M + K]+. Anal. Calcd. for C26H28FN3O5 (481.2013 g/mol).
5-((6′-bromo-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-phenylisoxazole (6y): 95% yield; Beige solid; IR (ATR): 2949, 2877, 1676, 1497, 1277 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.74 (2H, d, J = 1.6 Hz), 7.42 (3H, br), 7.17 (1H, d, J = 8.7 Hz), 6.97 (1H, s), 6.53 (1H, d, J = 8.7 Hz), 6.37 (1H, s), 5.42–5.33 (1H, m), 4.59 (1H, d, J = 17.4 Hz), 4.53 (1H, d, J = 17.4 Hz), 3.61–3.38 (2H, m), 3.28–3.11 (2H, m), 2.56–2.42 (2H, m), 2.23–2.05 (2H, m), 2.05–1.95 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.29, 169.05, 162.24, 143.71, 131.18, 129.91 (2C), 128.69 (2C), 128.44, 126.57 (2C), 122.13, 113.35, 109.36, 100.15, 47.88, 47.39, 47.33, 43.33, 31.04, 26.14, 18.08. HR-ESI-MS (m/z): 452.0906 [M + H]+, 368.8798 [M-C4H7NO]+. Anal. Calcd. for C23H22BrN3O2 (451.0895 g/mol).
3-(4-methoxyphenyl)-5-((6′-bromo-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6z): 67% yield; Beige solid; IR (ATR): 2928, 2039, 1680, 1252, 1023 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.68 (2H, d, J = 7.4 Hz), 7.17 (1H, d, J = 8.8 Hz), 6.97 (1H, s), 6.94 (2H, d, J = 7.4 Hz), 6.53 (1H, d, J = 8.4 Hz), 6.30 (1H, s), 5.41–5.34 (1H, m), 4.57 (1H, d, J = 17.5 Hz), 4.51 (1H, d, J = 17.5 Hz), 3.83 (3H, s), 3.60–3.38 (2H, m), 3.28–3.10 (2H, m), 2.57–2.41 (2H, m), 2.22–2.06 (2H, m), 2.05–1.99 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.33, 168.76, 161.86, 160.87, 143.76, 131.20, 129.91, 128.00, 122.10, 120.95, 114.09, 113.39, 109.34, 99.92, 55.14, 47.88, 47.42, 47.35, 43.37, 31.06, 26.17, 18.10. HR-ESI-MS (m/z): 482.1091 [M + H]+, 504.0896 [M + Na]+, 520.0751 [M + K]+. Anal. Calcd. for C24H24BrN3O3 (481.1001 g/mol).
3-(3,4-dimethoxyphenyl)-5-((6′-bromo-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)isoxazole (6aa): 70% yield; Orange oil; IR (ATR): 2936, 2832, 1677, 1497, 1264, 1019 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.35 (1H, s), 7.23 (1H, d, J = 8.2 Hz), 7.17 (1H, d, J = 8.7 Hz), 6.97 (1H, s), 6.88 (1H, d, J = 8.2 Hz), 6.53 (1H, d, J = 8.7 Hz), 6.31 (1H, s), 5.40–5.35 (1H, m), 4.59 (1H, d, J = 17.4 Hz), 4.50 (1H, d, J = 17.4 Hz), 3.92 (3H, s), 3.90 (3H, s), 3.60–3.38 (2H, m), 3.28–3.11 (2H, m), 2.55–2.44 (2H, m), 2.22–2.05 (2H, m), 2.04–2.00 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 175.38, 168.84, 161.97, 150.50, 149.09, 143.71, 131.19, 129.85, 122.08, 121.15, 119.79, 113.36, 110.81, 109.34, 109.05, 99.94, 55.82, 55.72, 47.91, 47.44, 47.35, 43.34, 31.04, 26.12, 18.08. HR-ESI-MS (m/z): 512.1180 [M + H]+, 534.0969 [M + Na]+, 550.0728 [M + K]+. Anal. Calcd. for C25H26BrN3O4 (511.1107 g/mol).
5-((6′-bromo-4′-(2′’-oxopyrrolidin-1′’-yl)-3′,4′-dihydroquinolin-1′(2′H)-yl)methyl)-3-(3,4,5- trimethoxyphenyl)isoxazole (6ab): 43% yield; Orange oil; IR (ATR): 2939, 2830, 1680, 1419, 1125, 1000 cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.18 (1H, d, J = 8.7 Hz), 6.99–6.94 (3H, br.s.), 6.52 (1H, d, J = 8.8 Hz), 6.31 (1H, s), 5.42–5.36 (1H, m), 4.62 (1H, d, J = 17.5 Hz), 4.51 (1H, d, J = 17.5 Hz), 3.90 (6H, s), 3.87 (3H, s), 3.63–3.40 (2H, m), 3.29–3.12 (2H, m), 2.55–2.45 (2H, m), 2.24–2.07 (2H, m), 2.06–2.02 (2H, m). 13C NMR (100 MHz, CDCl3) δ (ppm): 176.04, 169.78, 162.81, 154.05, 144.31, 140.24, 131.87, 130.45, 124.50, 122.79, 113.97, 110.06, 104.58, 100.70, 77.16, 61.38, 56.79, 48.67, 48.13, 48.07, 43.93, 31.69, 26.74, 18.75. HR-ESI-MS (m/z): 542.1245 [M + H]+. Anal. Calcd. for C26H28BrN3O5 (541.1212 g/mol).