Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage
Abstract
1. Introduction
2. Results
2.1. Melatonin Stimulates PER1 Clock Gene in Normal Human Dermal Fibroblast (NHDF) and in Normal Human Epidermal Keratinocytes (NHEK)
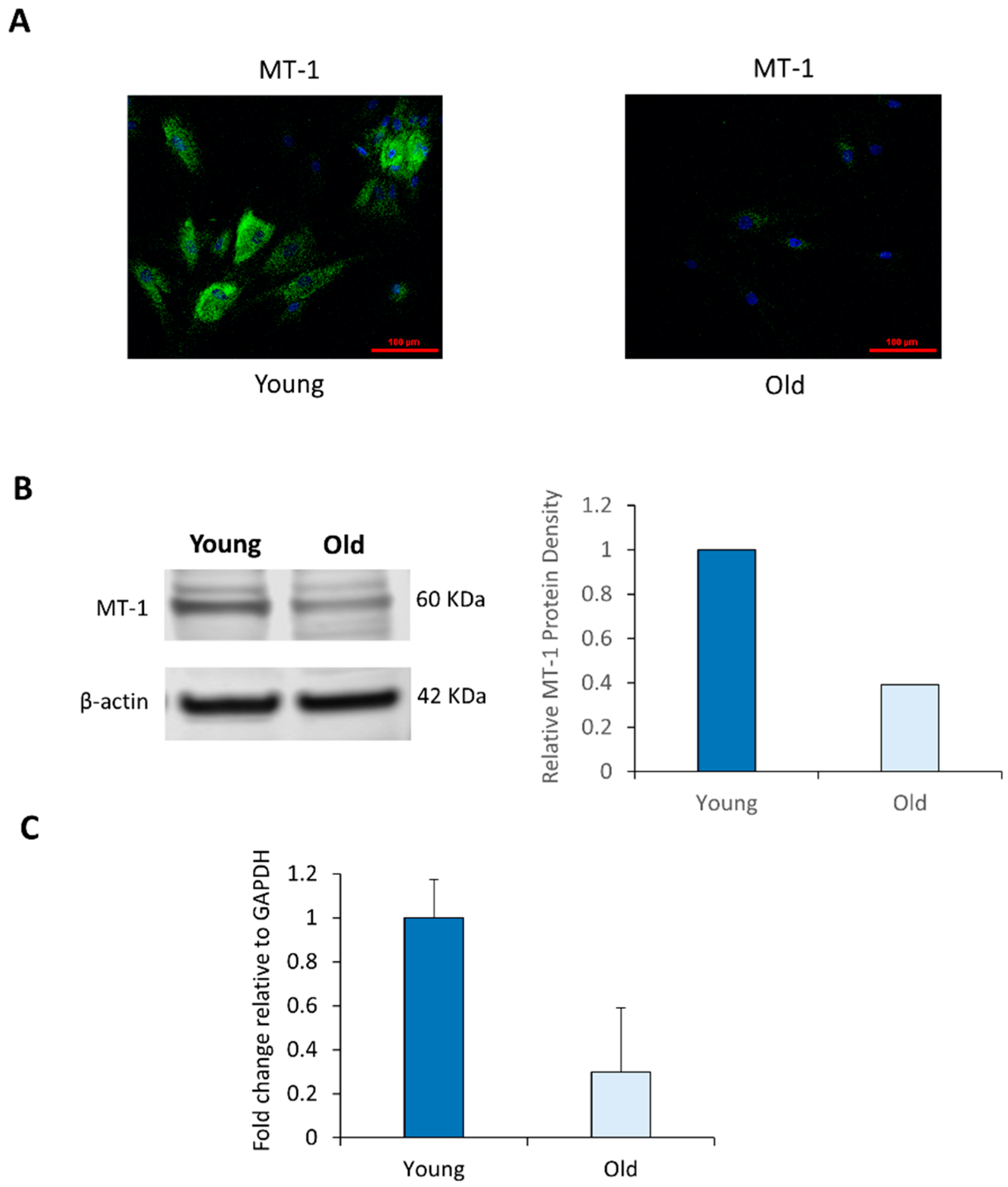
2.2. NHDF Express MT-1 Receptor and Its Level Is Decreased with Age
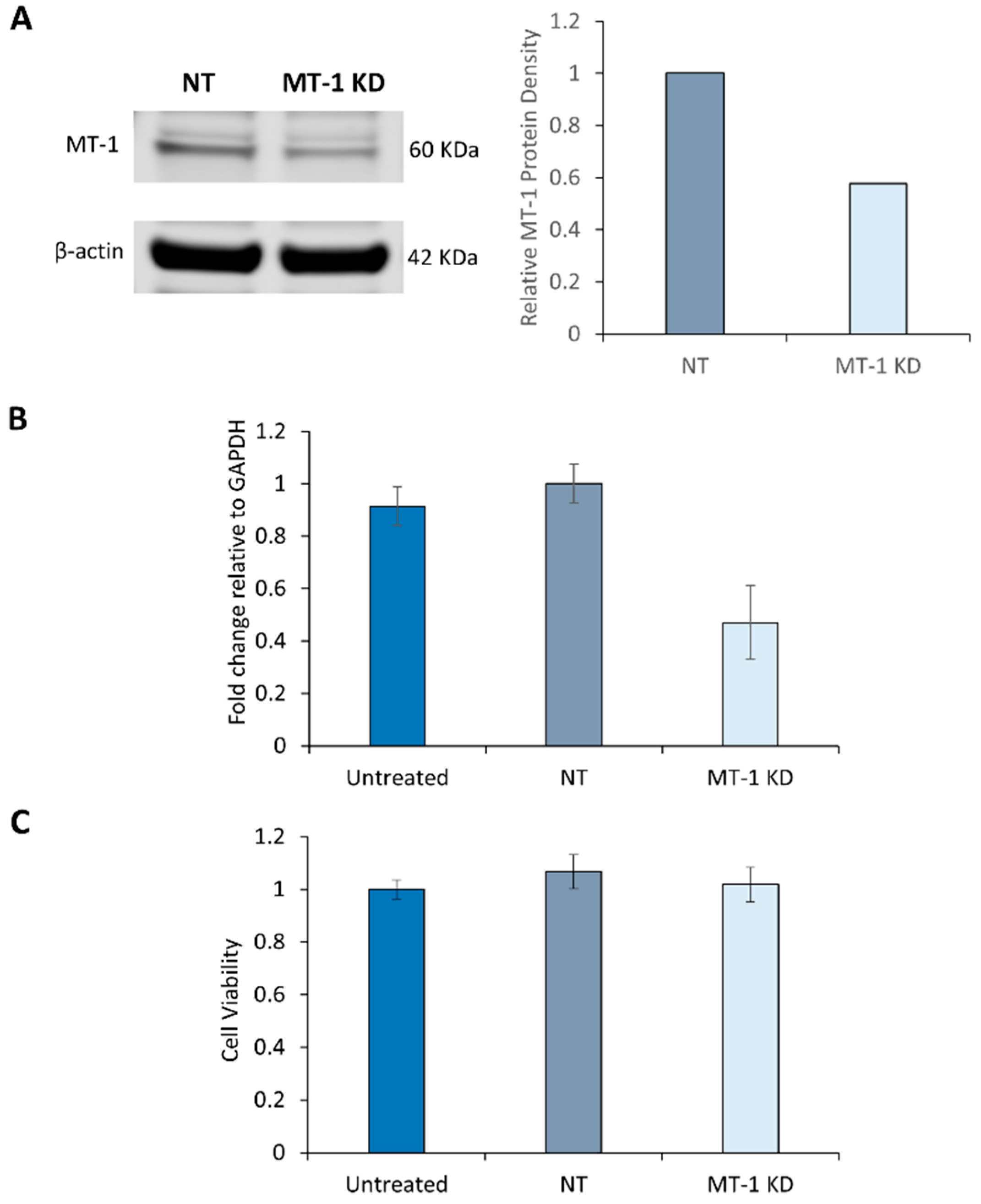
2.3. Characterization of MT-1 Receptor Knockdown Using siRNA Technology
2.4. Melatonin Receptor Knockdown Does not affect the Cell Viability of NHDF
2.5. MT-1 Receptor Knockdown Increases the Sensitivity of NHDF to UV Irradiation
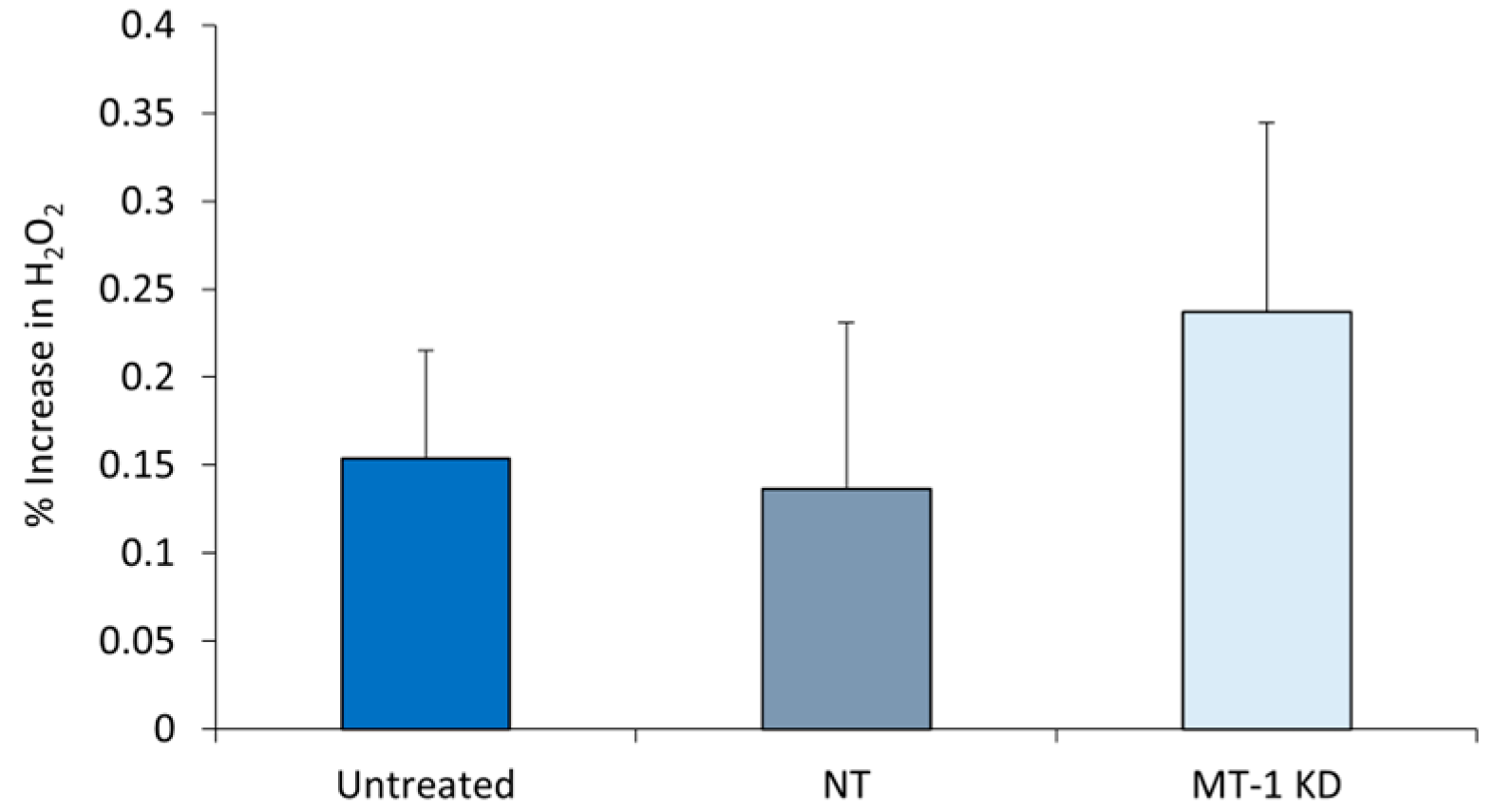
2.6. Increased Oxidative Stress in MT-1 Receptor Knockdown NHDF
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. PER1 Gene Expression
4.3. MT-1 Melatonin Receptor Immunohistochemistry
4.4. Western Blotting
4.5. Electroporation of siRNA
4.6. Real-Time PCR
4.7. UV Exposure
4.8. Comet Assay (DNA Damage)
4.9. Cell Viability Measurement
4.10. H2O2 Measurement in UV-irradiated NHDF
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NHDF | Normal Human Dermal Fibroblasts |
| NHEK | Normal Human Epidermal Keratinocytes |
| ROS | Reactive Oxygen Species |
| ANOVA | Analysis of Variance |
| SEM | Standard Error of the Mean |
References
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The antioxidant network of the stratum corneum. In Oxidants and Antioxidants in Cutaneous Biology; Karger Publishers: Basel, Switzerland, 2001; Volume 29, pp. 26–42. [Google Scholar]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venerol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Pillai, S.; Yatskayer, M.; Puccetti, G.; McDaniel, D. Antioxidants and Skin Aging: A Review. Cosmet. Dermatol. 2009, 22, 563–570. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2007, 19, 17–24. [Google Scholar] [CrossRef]
- Ndiaye, M.A.; Nihal, M.; Wood, G.S.; Ahmad, N. Skin reactive oxygen species and circadian clocks. Antioxid. Redox Signal 2014, 20, 2982–2996. [Google Scholar] [CrossRef]
- Le Fur, I.; Reinberg, A.; Lopez, S.; Morizot, F.; Mechkouri, M.; Tschachler, E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J. Investig. Dermatol. 2001, 117, 718–724. [Google Scholar] [CrossRef]
- Sporl, F.; Korge, S.; Jurchott, K.; Wunderskirchner, M.; Schellenberg, K.; Heins, S.; Specht, A.; Stoll, C.; Klemz, R.; Maier, B.; et al. Krüppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 10903–10908. [Google Scholar] [CrossRef]
- Luber, A.J.; Ensanyat, S.H.; Zeichner, J.A. Therapeutic implications of the circadian clock on skin function. J. Drugs Dermatol. 2014, 13, 130–134. [Google Scholar]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: Transepidermal water loss, stratum corneum hydration, skin surface pH and skin temperature. J. Investig. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef]
- Desotelle, J.A.; Wilking, M.J.; Ahmad, N. The circadian control of skin and cutaneous photodamage. Photochem. Photobiol. 2012, 88, 1037–1047. [Google Scholar] [CrossRef]
- Sandu, C.; Liu, T.; Malan, A.; Challet, E.; Pévet, P.; Felder-Schmittbuhl, M.P. Felder-Schmittbuhl, Circadian clocks in rat skin and dermal fibroblasts: Differential effects of aging, temperature and melatonin. Cell. Mol. Life Sci. 2015, 72, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Kleszczynski, K.; Fischer, T.W. Melatonin and human skin aging. Dermato-Endocrinology 2012, 4, 245–252. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, T.D.; Kim, H.J.; Jung, Y.; Lee, D.; Lee, K.H.; Kim, D.Y.; Woo, K.C.; Kim, K.T. Heterogeneous ribonucleoprotein R regulates arylalkylamine N-acetyltransferase synthesis via internal ribosomal entry site-mediated translation in a circadian manner. J. Pineal Res. 2015, 59, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Pelle, E.; McCarthy, J.; Dong, K.; Layman, D.; Zamfir, R.; Yarosh, D.B.; Pernodet, N. Clock gene activity in keratinocytes measured with a per1-promoter construct. J. Investig. Dermatol. 2012, 132, 60–61. [Google Scholar]
- Sardo, F.L.; Muti, P.; Blandino, G.; Strano, S. Melatonin and Hippo Pathway: Is There Existing Cross-Talk? Int. J. Mol. Sci. 2017, 18, 1913. [Google Scholar] [CrossRef]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the role of melatonin in skin physiology and pathology. Endocrine 2005, 27, 137–148. [Google Scholar] [CrossRef]
- Santoro, R.; Mori, F.; Marani, M.; Grasso, G.; Cambria, M.A.; Blandino, G.; Muti, P.; Strano, S. Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis 2013, 34, 1051–1061. [Google Scholar] [CrossRef]
- Ryoo, Y.W.; Suh, S.I.; Mun, K.C.; Kim, B.C.; Lee, K.S. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J. Dermatol. Sci. 2001, 27, 162–169. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102. [Google Scholar] [CrossRef]
- Kim, T.; Kleszczyński, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef]
- Skobowiat, C.; Brożyna, A.; Janjetovic, Z.; Jeayeng, S.; Oak, A.S.W.; Kim, T.; Panich, U.; Reiter, R.J.; Slominski, A.T. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J. Pineal Res. 2018, 65, 12501. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. 2017, 7, 1274. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes. Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Kandalepas, P.C.; Mitchell, J.W.; Gillette, M.U. Melatonin Signal Transduction Pathways Require E-Box-Mediated Transcription of Per1 and Per2 to Reset the SCN Clock at Dusk. PLoS ONE 2016, 11, e0157824. [Google Scholar] [CrossRef] [PubMed]
- Brydon, L.; Roka, F.; Petit, L.; de Coppet, P.; Tissot, M.; Barrett, P.; Morgan, P.J.; Nanoff, C.; Strosberg, A.D.; Jockers, R. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3) and G(q/11) proteins. Mol. Endocrinol. 1999, 13, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A. Neuroendocrine activity of the melanocyte. Exp. Dermatol. 2009, 18, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Kleszczynski, K.; Hardkop, L.H.; Fisher, T.W. Differential effects of melatonin as a broad range UV-damage preventive dermato-endocrine regulator. Dermato-Endocrinology 2011, 3, 27–31. [Google Scholar] [CrossRef]
- Tamaru, T.; Hattori, M.; Ninomiya, Y.; Kawamura, G.; Vares, G.; Honda, K.; Mishra, D.P.; Wang, B.; Benjamin, I.; Sassone-Corsi, P.; et al. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS ONE 2013, 12, e82006. [Google Scholar] [CrossRef]
- Matsui, M.S.; Pelle, E.; Dong, K.; Pernodet, N. Biological Rhythms in the Skin. Int. J. Mol. Sci. 2016, 17, 801. [Google Scholar] [CrossRef]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Zwicker, S.; Tukaj, S.; Kasperkiewicz, M.; Zillikens, D.; Wolf, R.; Fischer, T.W. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J. Pineal Res. 2015, 58, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Tukaj, S.; Kruse, N.; Zillikens, D.; Fischer, T.W. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J. Pineal Res. 2013, 54, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Izykowska, I.; Gebarowska, E.; Cegielski, M.; Podhorska-Okolow, M.; Piotrowska, A.; Zabel, M.; Dziegiel, P. Effect of melatonin on melanoma cells subjected to UVA and UVB radiation in In vitro studies. In Vivo 2009, 23, 733–738. [Google Scholar]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Cutaneous oxidative stress. J. Cosmet. Dermatol. 2012, 11, 55–64. [Google Scholar] [CrossRef]
- Fischer, T.W.; Sweatman, T.W.; Semak, I.; Sayre, R.M.; Wortsman, J.; Slominski, A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006, 20, 1564–1566. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Fischer, T.W.; Kleszczynski, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Zillikens, D.; Fischer, T.W. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin and ubiquitin: what’s the connection? Cell. Mol. Life Sci. 2014, 71, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Pelle, E.; Yarosh, D.B.; Pernodet, N. Sirtuin 4 identification in normal human epidermal keratinocytes and its relation to sirtuin 3 and energy metabolism under normal conditions and UVB-induced stress. Exp. Dermatol. 2012, 21, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Bruls, W.A.; van Weelden, H.; van der Leun, J.C. Transmission of UV-radiation through human epidermal layers as a factor influencing the minimal erythema dose. Photochem. Photobiol. 1984, 39, 63–67. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int. J. Mol. Sci. 2020, 21, 326. https://doi.org/10.3390/ijms21010326
Dong K, Goyarts E, Rella A, Pelle E, Wong YH, Pernodet N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. International Journal of Molecular Sciences. 2020; 21(1):326. https://doi.org/10.3390/ijms21010326
Chicago/Turabian StyleDong, Kelly, Earl Goyarts, Antonella Rella, Edward Pelle, Yung Hou Wong, and Nadine Pernodet. 2020. "Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage" International Journal of Molecular Sciences 21, no. 1: 326. https://doi.org/10.3390/ijms21010326
APA StyleDong, K., Goyarts, E., Rella, A., Pelle, E., Wong, Y. H., & Pernodet, N. (2020). Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. International Journal of Molecular Sciences, 21(1), 326. https://doi.org/10.3390/ijms21010326



