Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms
Abstract
1. Introduction
1.1. Well-Functioning Endometrium Is Essential to Ensure Embryo Implantation: General Aspects
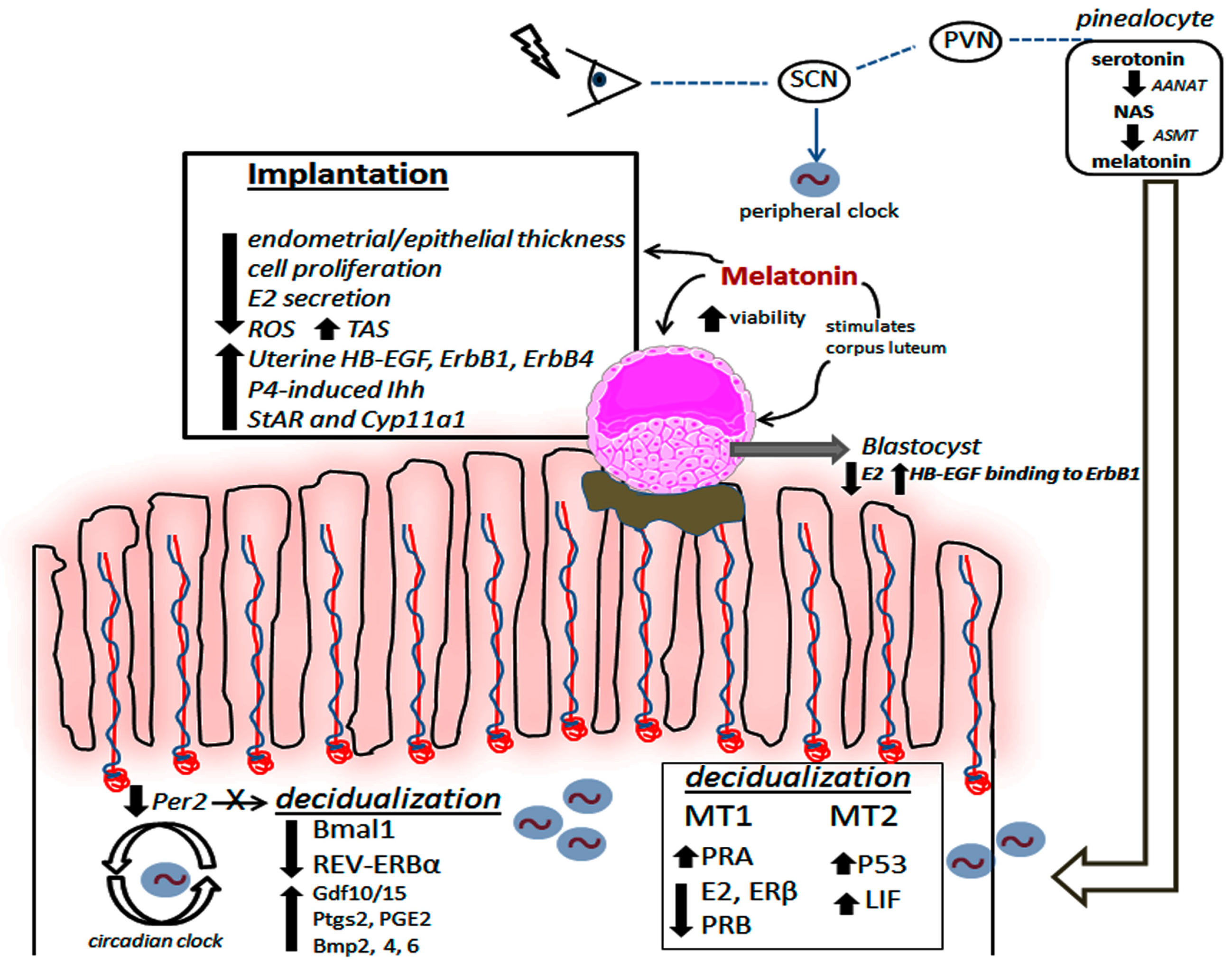
1.2. The Uterine Biological Rhythm as a Target for Melatonin’s Action
1.3. Melatonin Actively Participates during Decidualization and Implantation Process
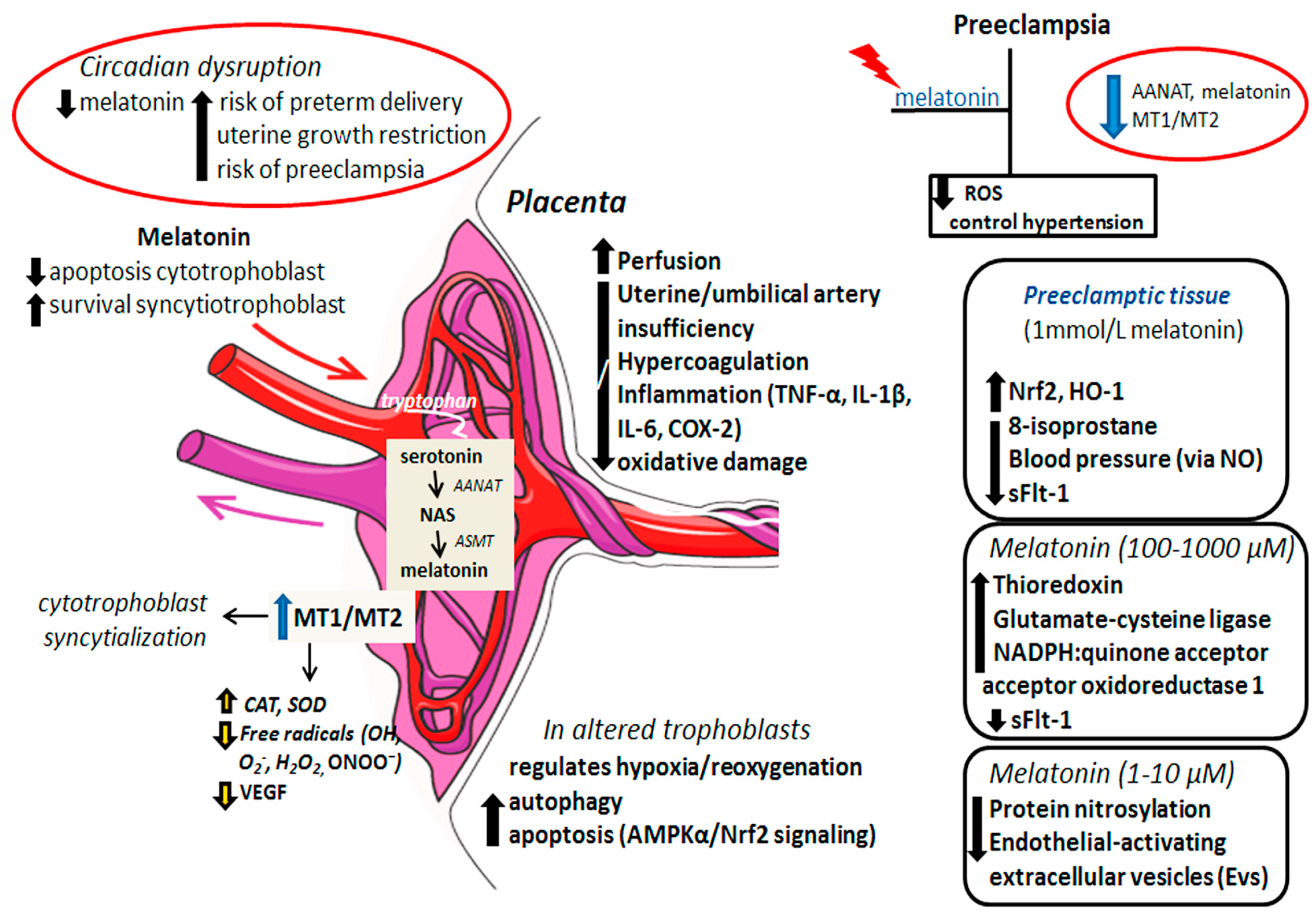
1.4. The Beneficial Effects of Melatonin on Placental Tissue: A Link to Preeclampsia
1.5. Melatonin’s Action on the Immune Microenvironment at the Maternal–Fetal Interface: A Brief Overview
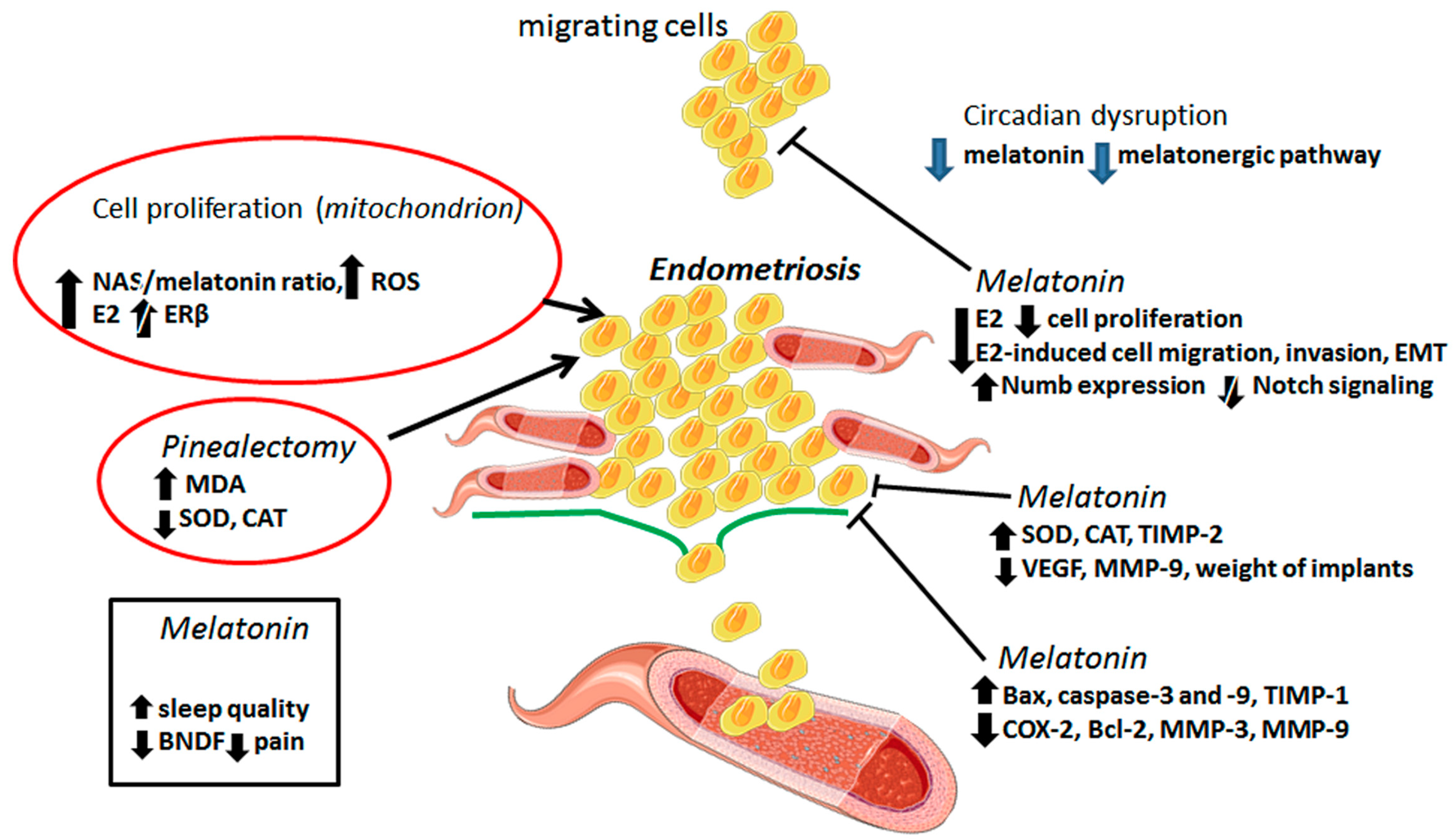
2. The Benefits of Melatonin in the Treatment of Endometriosis
3. Concluding Remarks and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AANAT | Arylalkylamine-N-acetyltransferase |
| AMPKα | 5’Adenosine monophosphate-activated protein kinase |
| aPL | Antiphospholipid antibodies |
| BDNF | Brain-derived neurotrophic factor |
| BMP-2 | Bone morphogenetic protein 2 |
| BMPs | Bone morphogenetic proteins |
| CAT | Catalase |
| CRY | Cryptochrome |
| DD | Constant darkness |
| NK | Natural killer cell |
| E12 | Embryonic day 12 |
| E2 | 17β-Estradiol |
| E22 | Embryonic day 22 |
| EMS | Endometriosis |
| ERα | Estrogen receptor alpha |
| Gdf | Growth/differentiation factor |
| H/R | Hypoxia/reoxygenation |
| HB-EGF | Heparin binding-epidermal growth factor |
| hMTNR1B | Melatonin receptor 1B |
| Hox | Homeobox genes |
| Ihhi | P4-Regulated Indian Hedgehog |
| IL-11 | Interleukin 11 |
| IUGR | Intrauterine growth restriction |
| LD | Light–dark cycle |
| LIF | Leukemia inhibitory factor |
| MDA | Malondialdehyde |
| MMPs | Metalloproteinases |
| MR1A | Melatonin receptor 1A |
| MR1B | Melatonin receptor 1B |
| MT1 | Melatonin receptor 1 |
| MT2 | Melatonin receptor 2 |
| MT3 | Melatonin receptor 3 |
| NAS | N-acetylserotonin |
| NF-kB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| P4 | Progesterone |
| PER | Period |
| Per1-luc | Period1-luciferase |
| PG | Prostaglandin |
| PI | Placental insufficiency |
| PRA | Progesterone receptor A |
| Ptgs2 | Prostaglandin G/H synthase 2 |
| ROCK1 | Coiled-coil containing protein kinases 1 |
| SCID | Severe combined immunodeficiency |
| SCN | Suprachiasmatic nucleus |
| sFlt-1 | Fms-like tyrosine kinase 1 |
| SOD | Superoxide dismutase |
| TGF-β | Transforming growth factor beta |
| UESCs | Uterus endometrial stromal cells |
| UP | Uterine peristalsis |
| VEGF | Vascular endothelial growth factor |
References
- Madero, S.; Rodriguez, A.; Vassena, R.; Vernaeve, V. Endometrial preparation: Effect of estrogen dose and administration route on reproductive outcomes in oocyte donation cycles with fresh embryo transfer. Hum. Reprod. 2016, 31, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil. Steril. 2019, 111, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, J.W.; Wang, J.; Ma, L.; Martin, J.F.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. Bmp2 is critical for the murine uterine decidual response. Mol. Cell Biol. 2007, 27, 5468–5478. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Arici, A.; Olive, D.; Igarashi, P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Investig. 1998, 101, 1379–1384. [Google Scholar] [CrossRef]
- Taylor, H.S.; Igarashi, P.; Olive, D.L.; Arici, A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J. Clin. Endocrinol. Metab. 1999, 84, 1129–1135. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef]
- Jensen, A.L.; Collins, J.; Shipman, E.P.; Wira, C.R.; Guyre, P.M.; Pioli, P.A. A subset of human uterine endometrial macrophages is alternatively activated. Am. J. Reprod. Immunol. 2012, 68, 374–386. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Wang, C.; Umesaki, N.; Nakamura, H.; Tanaka, T.; Nakatani, K.; Sakaguchi, I.; Ogita, S.; Kaneda, K. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000, 300, 285–293. [Google Scholar]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kuijsters, N.P.M.; Methorst, W.G.; Kortenhorst, M.S.Q.; Rabotti, C.; Mischi, M.; Schoot, B.C. Uterine peristalsis and fertility: Current knowledge and future perspectives: A review and meta-analysis. Reprod. Biomed. Online 2017, 35, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. The mammalian circadian clock: A network of gene expression. Front. Biosci. 2004, 9, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Reddy, A.B.; Garabette, M.; King, V.M.; Chahad-Ehlers, S.; O’Brien, J.; Maywood, E.S. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Found. Symp. 2003, 253, 203–217. [Google Scholar] [PubMed]
- Maywood, E.S.; O’Neill, J.S.; Chesham, J.E.; Hastings, M.H. Minireview: The circadian clockwork of the suprachiasmatic nuclei—Analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology 2007, 148, 5624–5634. [Google Scholar] [CrossRef]
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythm. 2005, 20, 391–403. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian clocks during embryonic and fetal development. Birth Defects Res. C Embryo Today 2007, 81, 204–214. [Google Scholar] [CrossRef]
- Akiyama, S.; Ohta, H.; Watanabe, S.; Moriya, T.; Hariu, A.; Nakahata, N.; Chisaka, H.; Matsuda, T.; Kimura, Y.; Tsuchiya, S.; et al. The uterus sustains stable biological clock during pregnancy. Tohoku J. Exp. Med. 2010, 221, 287–298. [Google Scholar] [CrossRef]
- Landgraf, D.; Achten, C.; Dallmann, F.; Oster, H. Embryonic development and maternal regulation of murine circadian clock function. Chronobiol. Int. 2015, 32, 416–427. [Google Scholar] [CrossRef]
- Muter, J.; Lucas, E.S.; Chan, Y.W.; Brighton, P.J.; Moore, J.D.; Lacey, L.; Quenby, S.; Lam, E.W.; Brosens, J.J. The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells. FASEB J. 2015, 29, 1603–1614. [Google Scholar] [CrossRef]
- Tasaki, H.; Zhao, L.; Isayama, K.; Chen, H.; Yamauchi, N.; Shigeyoshi, Y.; Hashimoto, S.; Hattori, M.A. Inhibitory role of REV-ERBα in the expression of bone morphogenetic protein gene family in rat uterus endometrium stromal cells. Am. J. Physiol. Cell Physiol. 2015, 308, C528–C538. [Google Scholar] [CrossRef] [PubMed]
- Isayama, K.; Zhao, L.; Chen, H.; Yamauchi, N.; Shigeyoshi, Y.; Hashimoto, S.; Hattori, M.A. Removal of Rev-erbα inhibition contributes to the prostaglandin G/H synthase 2 expression in rat endometrial stromal cells. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E650–E661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Isayama, K.; Chen, H.; Yamauchi, N.; Shigeyoshi, Y.; Hashimoto, S.; Hattori, M.A. The nuclear receptor REV-ERBα represses the transcription of growth/differentiation factor 10 and 15 genes in rat endometrium stromal cells. Physiol. Rep. 2016, 4, e12663. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Reiter, R.J.; Piekarski, C. Light, timing of biological rhythms, and chronodisruption in man. Naturwissenschaften 2003, 90, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a hormone: New physiological and clinical insights. Endocr Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Silva, J.; Cipolla-Neto, J.; Peliciari-Garcia, R.A. The in vitro maintenance of clock genes expression within the rat pineal gland under standard and norepinephrine-synchronized stimulation. Neurosci. Res. 2014, 81-82, 1–10. [Google Scholar] [CrossRef]
- Slominski, A.T.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 2003, 270, 3335–3344. [Google Scholar] [CrossRef]
- Slominski, A.T.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Reiter, R.J.; Ahmad, N. Sirtuins, melatonin and circadian rhythms: Building a bridge between aging and cancer. J. Pineal Res. 2010, 48, 9–19. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Seiva, F.R.F.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Lupi, L.A. Clock genes and the role of melatonin in cancer cells: An overview. Melatonin Res. 2019, 2, 133–157. [Google Scholar] [CrossRef]
- Chuffa, L.G.; Lupi Júnior, L.A.; Seiva, F.R.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.; Dos Santos, L.D.; Martinez, F.E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Chuffa, L.G.; Seiva, F.R.F.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Lupi, L.A. Mitochondrial functions and melatonin: A tour of the reproductive cancers. Cell. Mol. Life Sci. 2019, 76, 837–863. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy-and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Jetten, A.M. RORα is not a receptor for melatonin. Bioessays 2016, 38, 1193–1194. [Google Scholar] [CrossRef]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczynski, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Carvalho, K.C.; Maganhin, C.C.; Paiotti, A.P.; Oshima, C.T.; Simões, M.J.; Baracat, E.C.; Soares, J.M., Jr. Does melatonin influence the apoptosis in rat uterus of animals exposed to continuous light? Apoptosis 2016, 21, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Rocco, V.; Monsó, C.; Valenzuela, F.J.; Campino, C.; Germain, A.; Torrealba, F.; Valenzuela, G.J.; Seron-Ferre, M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 2006, 147, 4618–4626. [Google Scholar] [CrossRef]
- Serón-Ferré, M.; Mendez, N.; Abarzua-Catalan, L.; Vilches, N.; Valenzuela, F.J.; Reynolds, H.E.; Llanos, A.J.; Rojas, A.; Valenzuela, G.J.; Torres-Farfan, C. Circadian rhythms in the fetus. Mol. Cell Endocrinol. 2012, 349, 68–75. [Google Scholar] [CrossRef]
- Mosher, A.A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.K.; Leyland, N.A.; Foster, W.G. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef]
- Sharkey, J.T.; Puttaramu, R.; Word, R.A.; Olcese, J. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 2009, 94, 421–427. [Google Scholar] [CrossRef]
- Olcese, J.; Beesley, S. Clinical significance of melatonin receptors in the human myometrium. Fertil. Steril. 2014, 102, 329–335. [Google Scholar] [CrossRef]
- Beesley, S.; Lee, J.; Olcese, J. Circadian clock regulation of melatonin MTNR1B receptor expression in human myometrial smooth muscle cells. Mol. Hum. Reprod. 2015, 21, 662–671. [Google Scholar] [CrossRef]
- Zhao, H.; Pang, S.F.; Poon, A.M. Variations of mt1 melatonin receptor density in the rat uterus during decidualization, the estrous cycle and in response to exogenous steroid treatment. J. Pineal Res. 2002, 33, 140–145. [Google Scholar] [CrossRef]
- Zhao, H.; Pang, S.F.; Poon, A.M. mt(1) Receptor-mediated antiproliferative effects of melatonin on the rat uterine antimesometrial stromal cells. Mol. Reprod. Dev. 2002, 61, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Dair, E.L.; Simoes, R.S.; Simões, M.J.; Romeu, L.R.; Oliveira-Filho, R.M.; Haidar, M.A.; Baracat, E.C.; Soares, J.M., Jr. Effects of melatonin on the endometrial morphology and embryo implantation in rats. Fertil. Steril. 2008, 89, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Dardes, R.C.; Baracat, E.C.; Simões, M.J. Modulation of estrous cycle and LH, FSH and melatonin levels by pinealectomy and sham-pinealectomy in female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2000, 24, 441–453. [Google Scholar] [CrossRef]
- Stavreus-Evers, A.; Mandelin, E.; Koistinen, R.; Aghajanova, L.; Hovatta, O.; Seppälä, M. Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil. Steril. 2006, 85, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, Z.; Teresky, A.K.; Levine, A.J. p53 regulates maternal reproduction through LIF. Nature 2007, 450, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Song, Y.; Liu, G. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef]
- Lv, X.; Cai, Z.; Li, S. Increased apoptosis rate of human decidual cells and cytotrophoblasts in patients with recurrent spontaneous abortion as a result of abnormal expression of CDKN1A and Bax. Exp. Ther. Med. 2016, 12, 2865–2868. [Google Scholar] [CrossRef]
- Saat, N.; Risvanli, A.; Dogan, H.; Onalan, E.; Akpolat, N.; Seker, I.; Sahna, E. Effect of melatonin on torsion and reperfusion induced pathogenesis of rat uterus. Biotech. Histochem. 2019, 94, 533–539. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Arjmand, F.; Khanmohammadi, M.; Arasteh, S.; Mohammadzadeh, A.; Kazemnejad, S.; Akhondi, M.M. Extended culture of encapsulated human blastocysts in alginate hydrogel containing decidualized endometrial stromal cells in the presence of melatonin. Mol. Biotechnol. 2016, 58, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Moghani-Ghoroghi, F.; Moshkdanian, G.; Sehat, M.; Nematollahi-Mahani, S.N.; Ragerdi-Kashani, I.; Pasbakhsh, P. Melatonin pretreated blastocysts along with calcitonin administration improved implantation by upregulation of heparin binding-epidermal growth factor expression in murine endometrium. Cell J. 2018, 19, 599–606. [Google Scholar] [PubMed]
- Guan, S.; Xie, L.; Ma, T.; Lv, D.; Jing, W.; Tian, X.; Song, Y.; Liu, Z.; Xiao, X.; Liu, G. Effects of melatonin on early pregnancy in mouse: Involving the regulation of StAR, Cyp11a1, and Ihh expression. Int. J. Mol. Sci. 2017, 18, E1637. [Google Scholar] [CrossRef] [PubMed]
- Moshkdanian, G.; Moghani-Ghoroghi, F.; Pasbakhsh, P.; Nematollahi-Mahani, S.N.; Najafi, A.; Kashani, S.R. Melatonin upregulates ErbB1 and ErbB4, two primary implantation receptors, in pre-implantation mouse embryos. Iran. J. Basic Med. Sci. 2017, 20, 655–661. [Google Scholar] [PubMed]
- Xie, H.; Wang, H.; Tranguch, S.; Iwamoto, R.; Mekada, E.; Demayo, F.J.; Lydon, J.P.; Das, S.K.; Dey, S.K. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 18315–18320. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, F.; Tian, X.; Ji, P.; Liu, G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 2017, 15, 78. [Google Scholar] [CrossRef]
- Groothuis, P.G.; Dassen, H.H.; Romano, A.; Punyadeera, C. Estrogen and the endometrium: Lessons learned from gene expression profiling in rodents and human. Hum. Reprod. Update 2007, 13, 405–417. [Google Scholar] [CrossRef]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A., Jr.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef]
- Tan, J.; Paria, B.C.; Dey, S.K.; Das, S.K. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999, 140, 5310–5321. [Google Scholar] [CrossRef]
- Abd-Allah, A.R.; El-Sayed, E.S.M.; Abdel-Wahab, M.H.; Hamada, F.M. Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol. Res. 2003, 47, 349–354. [Google Scholar] [CrossRef]
- Chuffa, L.G.; Seiva, F.R.; Fávaro, W.J.; Teixeira, G.R.; Amorim, J.P.; Mendes, L.O.; Fioruci, B.A.; Pinheiro, P.F.; Fernandes, A.A.; Franci, J.A.; et al. Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 2011, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Morioka, N.; Hayashi, K. Changes in nocturnal pineal melatonin synthesis during the perimenopausal period: Relation to estrogen levels in female rats. J. Pineal Res. 1999, 27, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.Y. Melatonin and the circadian system: Contributions to successful female reproduction. Fertil. Steril. 2014, 102, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From implantation to birth: Insight into molecular melatonin functions. Int. J. Mol. Sci. 2018, 19, E2802. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Nakazawa, K.; Sakai, J.; Kometani, K.; Iwashita, M.; Yoshimura, Y.; Maruyama, T. Melatonin as a local regulator of human placental function. J. Pineal Res. 2005, 39, 261–265. [Google Scholar] [CrossRef]
- Lanoix, D.; Guérin, P.; Vaillancourt, C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: New insights into the role of this hormone in pregnancy. J. Pineal Res. 2012, 53, 417–425. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Tamura, H.; Cruz, M.H.; Fuentes-Broto, L. Clinical relevance of melatonin in ovarian and placental physiology: A review. Gynecol. Endocrinol. 2014, 30, 83–89. [Google Scholar] [CrossRef]
- Waddell, B.J.; Wharfe, M.D.; Crew, R.C.; Mark, P.J. A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta 2012, 33, 533–539. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Soliman, A.; Vaillancourt, C. Maternal and placental melatonin: Actions and implication for successful pregnancies. Minerva Ginecol. 2014, 66, 251–266. [Google Scholar]
- Valenzuela, F.J.; Vera, J.; Venegas, C.; Pino, F.; Lagunas, C. Circadian system and melatonin hormone: Risk factors for complications during pregnancy. Obstet. Gynecol. Int. 2015, 2015, 825802. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Li, S.; Shin, N.E.; Na, Q.; Dong, J.; Jia, B.; Jones-Beatty, K.; McLane, M.W.; Ozen, M.; Lei, J.; et al. Melatonin for prevention of placental malperfusion and fetal compromise associated with intrauterine inflammation-induced oxidative stress in a mouse model. J. Pineal Res. 2019, 67, e12591. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Song, H.; Dash, O.; Park, M.; Shin, N.E.; McLane, M.W.; Lei, J.; Hwang, J.Y.; Burd, I. Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am. J. Reprod. Immunol. 2019, 82, e13151. [Google Scholar] [CrossRef] [PubMed]
- Berbets, A.; Koval, H.; Barbe, A.; Albota, O.; Yuzko, O. Melatonin decreases and cytokines increase in women with placental insufficiency. J. Matern Fetal Neonatal Med. 2019, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- Zeng, K.; Gao, Y.; Wan, J.; Tong, M.; Lee, A.C.; Zhao, M.; Chen, Q. The reduction in circulating levels of melatonin may be associated with the development of preeclampsia. J. Hum. Hypertens. 2016, 30, 666–671. [Google Scholar] [CrossRef]
- Soliman, A.; Lacasse, A.A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 2015, 59, 38–46. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Assunção Salustiano, E.M.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Bienvenue-Pariseault, J.; Vaillancourt, C. Melatonin: The smart molecule that differentially modulates autophagy in tumor and normal placental cells. PLoS ONE 2019, 14, e0202458. [Google Scholar] [CrossRef]
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508. [Google Scholar] [CrossRef] [PubMed]
- Uzun, M.; Gencer, M.; Turkon, H.; Oztopuz, R.O.; Demir, U.; Ovali, M.A. Effects of melatonin on blood pressure, oxidative stress and placental expressions of TNFα, IL-6, VEGF and sFlt-1 in RUPP rat model of preeclampsia. Arch. Med. Res. 2017, 48, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Binder, N.K.; Beard, S.; Nguyen, T.V.; Kaitu’u-Lino, T.J.; Tong, S. Melatonin enhances antioxidant molecules in the placenta, reduces secretion of soluble fms-like tyrosine kinase 1 (sFLT) from primary trophoblast but does not rescue endothelial dysfunction: An evaluation of its potential to treat preeclampsia. PLoS ONE 2018, 13, e0187082. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Y.; Xu, L.; Hickey, A.; Groom, K.; Stone, P.R.; Chamley, L.W.; Chen, Q. Melatonin prevents preeclamptic sera and antiphospholipid antibodies inducing the production of reactive nitrogen species and extrusion of toxic trophoblastic debris from first trimester placentae. Placenta 2017, 58, 17–24. [Google Scholar] [CrossRef]
- Ireland, K.E.; Maloyan, A.; Myatt, L. Melatonin improves mitochondrial respiration in syncytiotrophoblasts from placentas of obese women. Reprod. Sci. 2018, 25, 120–130. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The regulations and role of circadian clock and melatonin in uterine receptivity and pregnancy—An immunological perspective. Am. J. Reprod. Immunol. 2017, 78. [Google Scholar] [CrossRef]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Malkani, G.; Shi, X.; Meyer, M.; Cunningham-Runddles, S.; Ma, X.; Sun, Z.S. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect. Immun. 2006, 74, 4750–4756. [Google Scholar] [CrossRef]
- Galazka, K.; Wicherek, L.; Pitynski, K.; Kijowski, J.; Zajac, K.; Bednarek, W.; Dutsch-Wicherek, M.; Rytlewski, K.; Kalinka, J.; Basta, A.; et al. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7-H4 macrophages—A preliminary report. Am. J. Reprod. Immunol. 2009, 61, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. Cytokine network at the feto-maternal interface. J. Reprod. Immunol. 2000, 47, 87–103. [Google Scholar] [CrossRef]
- Lissoni, P.; Rovelli, F.; Brivio, F.; Brivio, O.; Fumagalli, L. Circadian secretions of IL-2, IL-12, IL-6 and IL-10 in relation to the light/dark rhythm of the pineal hormone melatonin in healthy humans. Nat. Immun. 1998, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef]
- Sanchez, S.; Paredes, S.D.; Sanchez, C.L.; Barriga, C.; Reiter, R.J.; Rodriguez, A.B. Tryptophan administration in rats enhances phagocytic function and reduces oxidative metabolism. Neuro Endocrinol. Lett. 2008, 29, 1026–1032. [Google Scholar]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef]
- Kuklina, E.M.; Glebezdina, N.S.; Nekrasova, I.V. Role of melatonin in the regulation of differentiation of T cells producing interleukin-17 (Th17). Bull. Exp. Biol. Med. 2016, 160, 656–658. [Google Scholar] [CrossRef]
- Glebezdina, N.S.; Olina, A.A.; Nekrasova, I.V.; Kuklina, E.M. Role of endogenous melatonin in the regulation of Th17/Treg balance during pregnancy. Bull. Exp. Biol. Med. 2018, 164, 462–465. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Reprint of: Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2019, 112, e137–e152. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Reprint of: Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2019, 112, e153–e161. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Marino, J.L.; Holt, V.L.; Chen, C.; Davis, S. Shift work, hCLOCK T3111C polymorphism, and endometriosis risk. Epidemiology. 2008, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Koc, O.; Gunduz, B.; Topcuoglu, A.; Bugdayci, G.; Yilmaz, F.; Duran, B. Effects of pinealectomy and melatonin supplementation on endometrial explants in a rat model. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 153, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, G.; Attar, R.; Ozkan, F.; Kumbak, B.; Ficicioglu, C.; Yesildaglar, N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: A preliminary study. Fertil. Steril. 2010, 93, 1787–1792. [Google Scholar] [CrossRef]
- Yesildaglar, N.; Yildirim, G.; Yildirim, O.K.; Attar, R.; Ozkan, F.; Akkaya, H.; Yilmaz, B. The effects of melatonin on endometriotic lesions induced by implanting human endometriotic cells in the first SCID-mouse endometriosis-model developed in Turkey. Clin. Exp. Obstet. Gynecol. 2016, 43, 25–30. [Google Scholar]
- Cetinkaya, N.; Attar, R.; Yildirim, G.; Ficicioglu, C.; Ozkan, F.; Yilmaz, B.; Yesildaglar, N. The effects of different doses of melatonin treatment on endometrial implants in an oophorectomized rat endometriosis model. Arch. Gynecol. Obstet. 2015, 291, 591–598. [Google Scholar] [CrossRef]
- Güney, M.; Oral, B.; Karahan, N.; Mungan, T. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil. Steril. 2008, 89, 934–942. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kilic, S.; Aksakal, O.; Ertas, I.E.; Tanrisever, G.G.; Aksoy, Y.; Lortlar, N.; Kelekci, S.; Gungor, T. Melatonin causes regression of endometriotic implants in rats by modulating angiogenesis, tissue levels of antioxidants and matrix metalloproteinases. Arch. Gynecol. Obstet. 2015, 292, 209–216. [Google Scholar] [CrossRef]
- Spuijbroek, M.D.; Dunselman, G.A.; Menheere, P.P.; Evers, J.L. Early endometriosis invades the extracellular matrix. Fertil. Steril. 1992, 58, 929–933. [Google Scholar] [CrossRef]
- Paul, S.; Bhattacharya, P.; Das Mahapatra, P.; Swarnakar, S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J. Pineal Res. 2010, 49, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sharma, A.V.; Mahapatra, P.D.; Bhattacharya, P.; Reiter, R.J.; Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Y.; Hu, C.; Wang, Y.; Yan, Z.; Li, Z.; Wu, R. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic. Biol. Med. 2019, 136, 22–34. [Google Scholar] [CrossRef]
- Liao, T.L.; Lee, Y.C.; Tzeng, C.R.; Wang, Y.P.; Chang, H.Y.; Lin, Y.F.; Kao, S.H. Mitochondrial translocation of estrogen receptor β affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free Radic. Biol. Med. 2019, 134, 359–373. [Google Scholar] [CrossRef]
- Anderson, G. Endometriosis pathoetiology and pathophysiology: Roles of vitamin A, estrogen, immunity, adipocytes, gut microbiome and melatonergic pathway on mitochondria regulation. Biomol. Concepts 2019, 10, 133–149. [Google Scholar] [CrossRef]
- Qi, S.; Yan, L.; Liu, Z.; Mu, Y.L.; Li, M.; Zhao, X.; Chen, Z.J.; Zhang, H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod. Biol. Endocrinol. 2018, 16, 62. [Google Scholar] [CrossRef]
- Schwertner, A.; Conceição Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef]
- Yang, H.L.; Zhou, W.J.; Gu, C.J.; Meng, Y.H.; Shao, J.; Li, D.J.; Li, M.Q. Pleiotropic roles of melatonin in endometriosis, recurrent spontaneous abortion, and polycystic ovary syndrome. Am. J. Reprod. Immunol. 2018, 80, e12839. [Google Scholar] [CrossRef]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tong, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Melatonin concentration in follicular fluid is correlated with antral follicle count (AFC) and in vitro fertilization (IVF) outcomes in women undergoing assisted reproductive technology (ART) procedures. Gynecol. Endocrinol. 2018, 34, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, F.; Akbari Asbagh, F.; Azmoodeh, O.; Bakhtiyari, M.; Almasi-Hashiani, A. Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: A randomized clinical trial. Int. J. Fertil. Steril. 2019, 13, 225–229. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida Chuffa, L.G.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 300. https://doi.org/10.3390/ijms21010300
de Almeida Chuffa LG, Lupi LA, Cucielo MS, Silveira HS, Reiter RJ, Seiva FRF. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. International Journal of Molecular Sciences. 2020; 21(1):300. https://doi.org/10.3390/ijms21010300
Chicago/Turabian Stylede Almeida Chuffa, Luiz Gustavo, Luiz Antonio Lupi, Maira Smaniotto Cucielo, Henrique Spaulonci Silveira, Russel J. Reiter, and Fábio Rodrigues Ferreira Seiva. 2020. "Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms" International Journal of Molecular Sciences 21, no. 1: 300. https://doi.org/10.3390/ijms21010300
APA Stylede Almeida Chuffa, L. G., Lupi, L. A., Cucielo, M. S., Silveira, H. S., Reiter, R. J., & Seiva, F. R. F. (2020). Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. International Journal of Molecular Sciences, 21(1), 300. https://doi.org/10.3390/ijms21010300







