Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome
Abstract
1. Introduction
2. Results
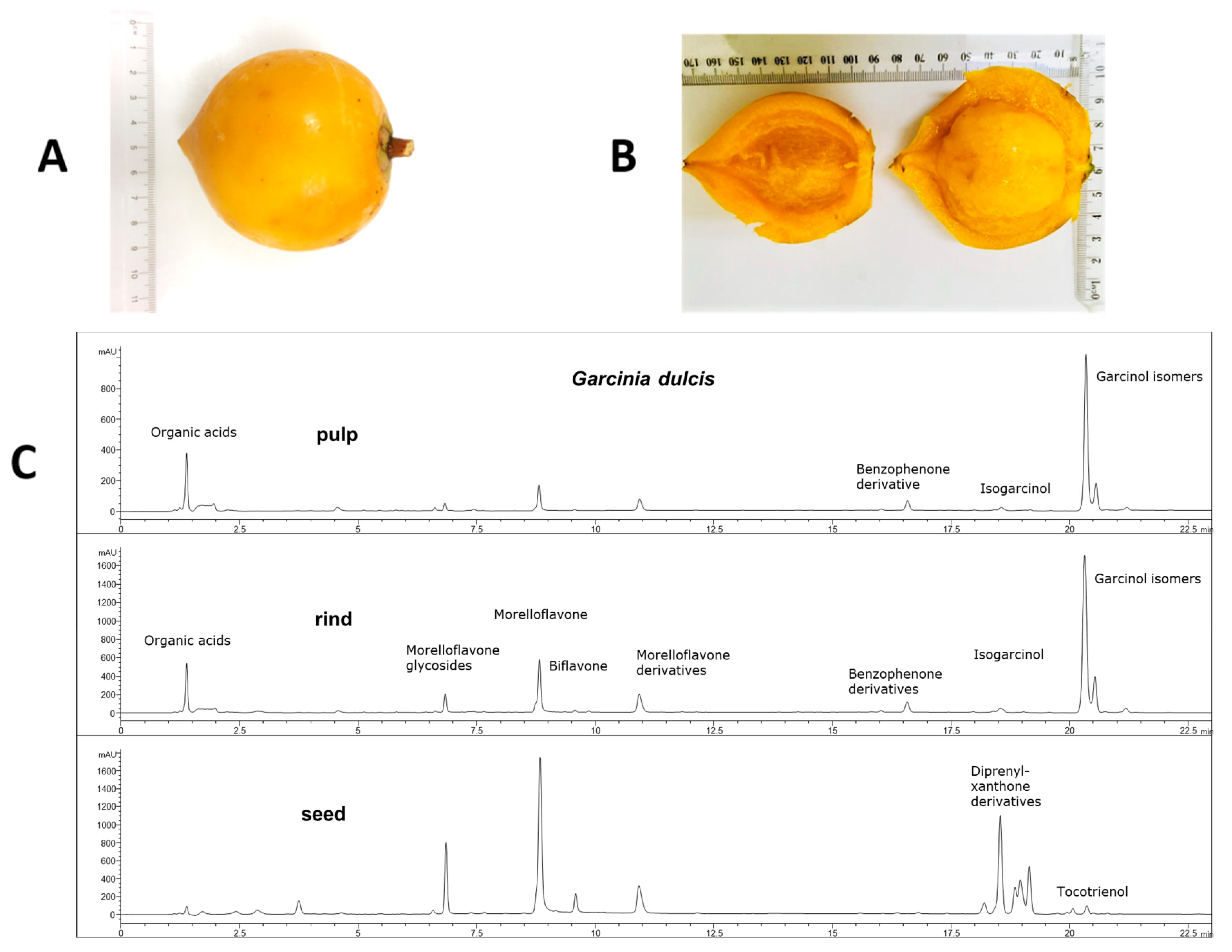
2.1. Weight and Phytochemical Analysis of G. dulcis Rind, Pulp and Seed
2.2. Metabolic Parameters
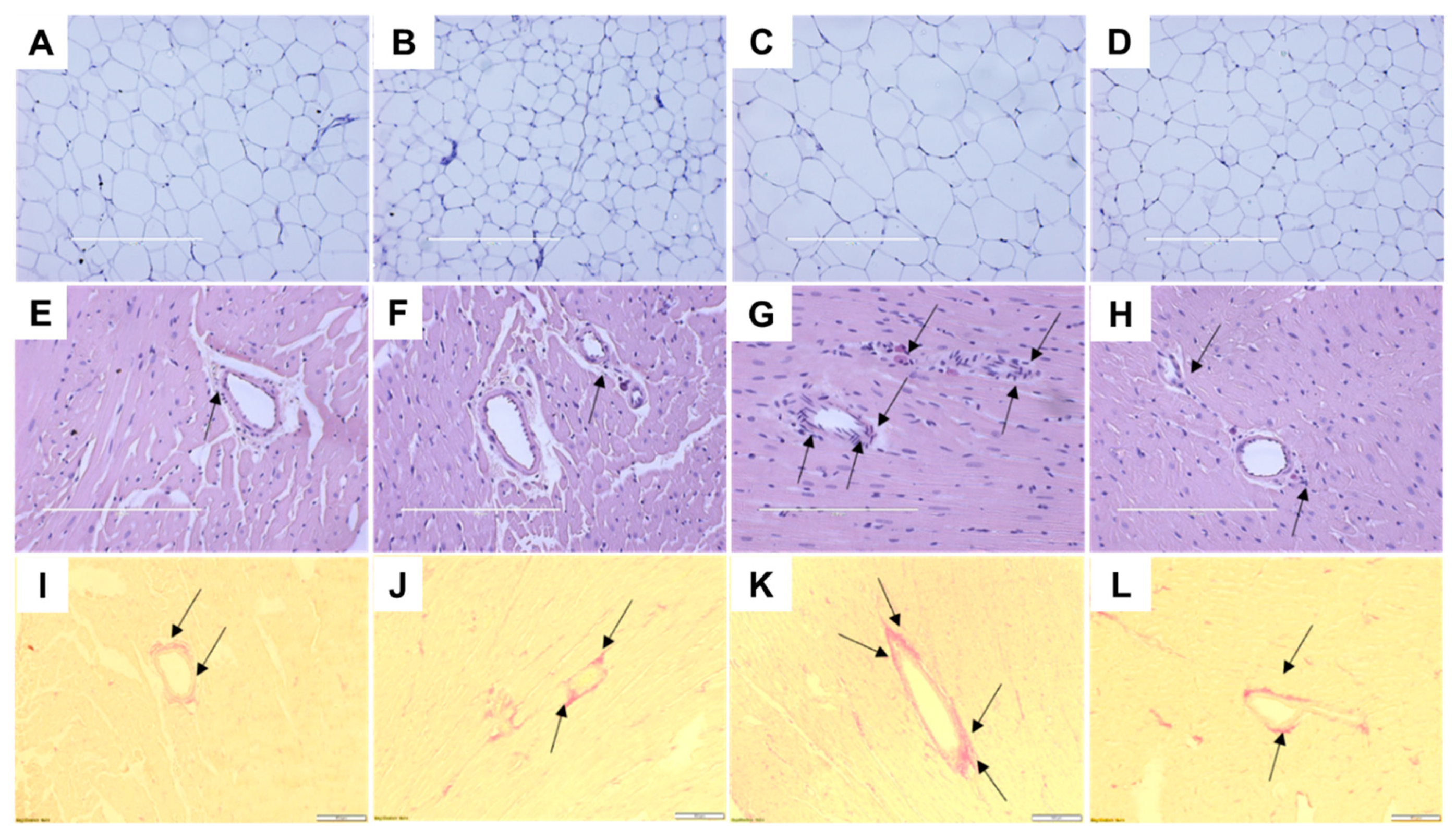
2.3. Cardiovascular Structure and Function
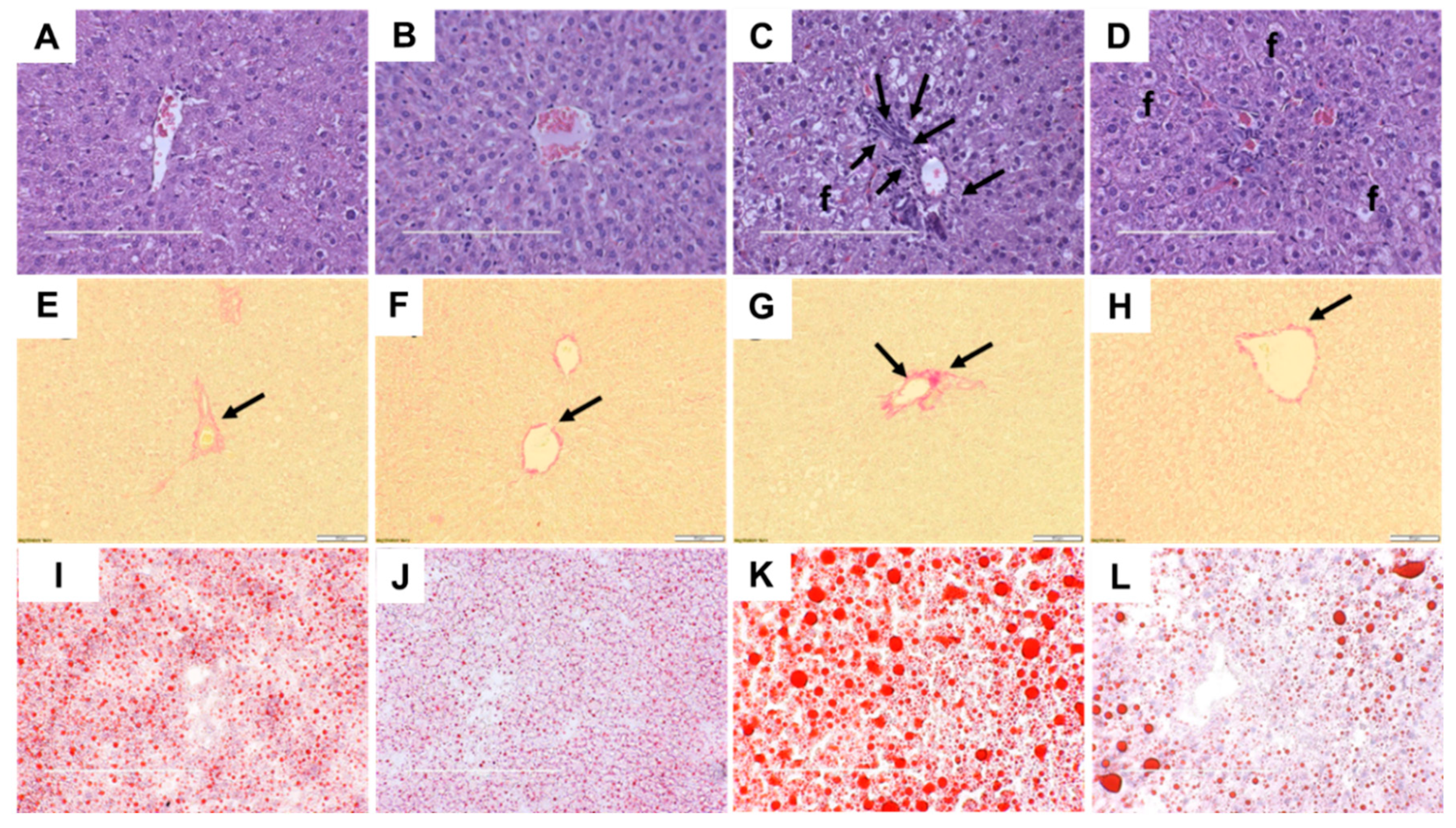
2.4. Liver Structure and Function
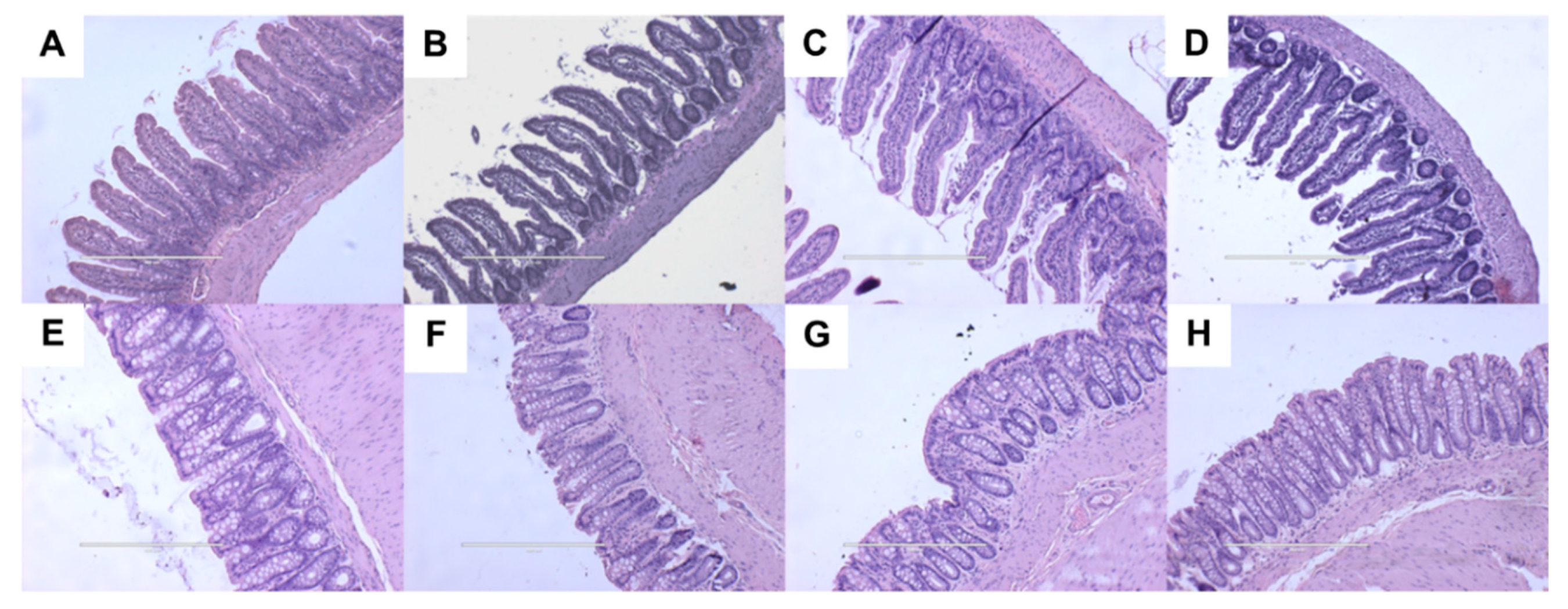
2.5. Gut Structure and Microbiota
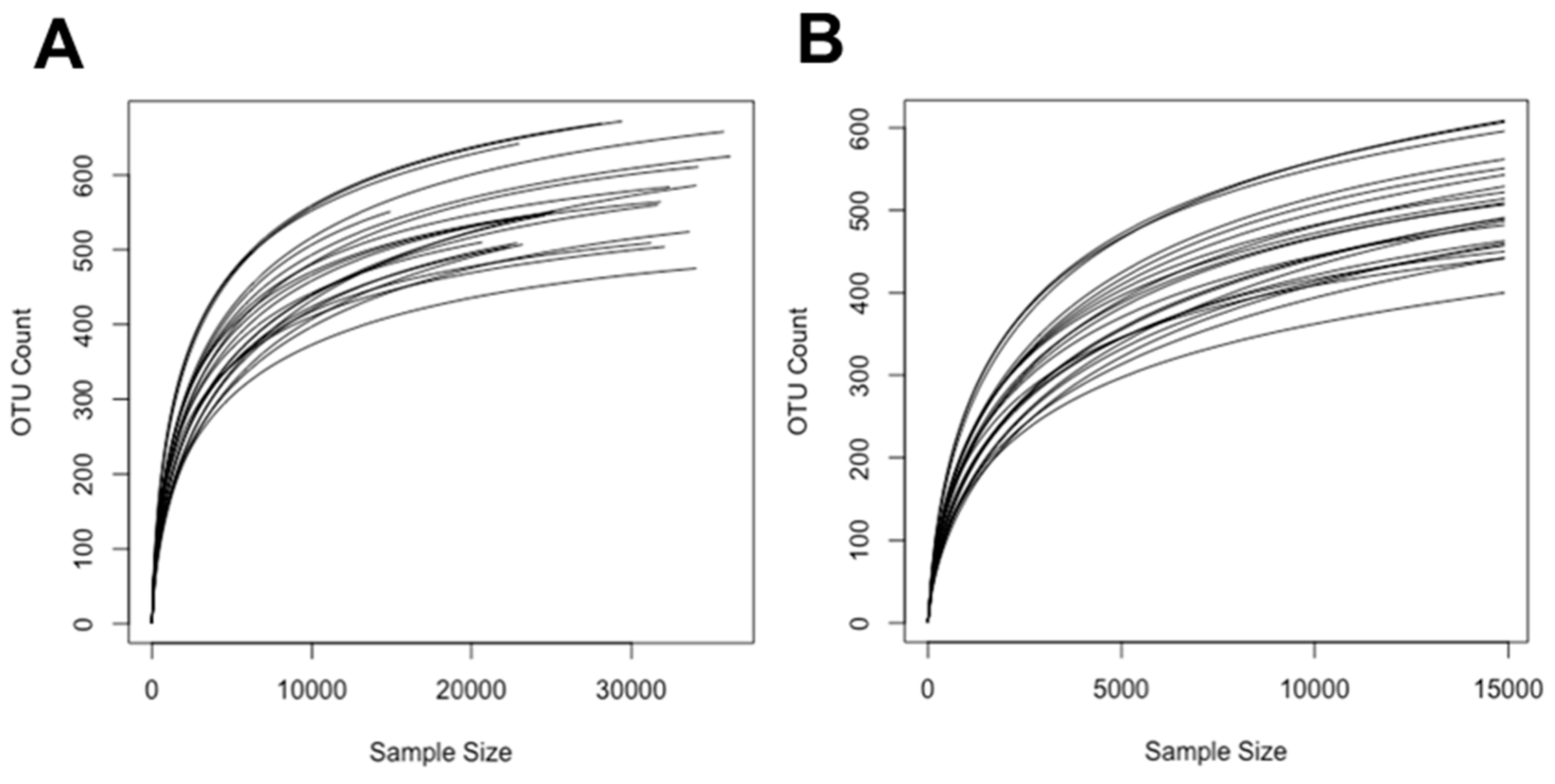
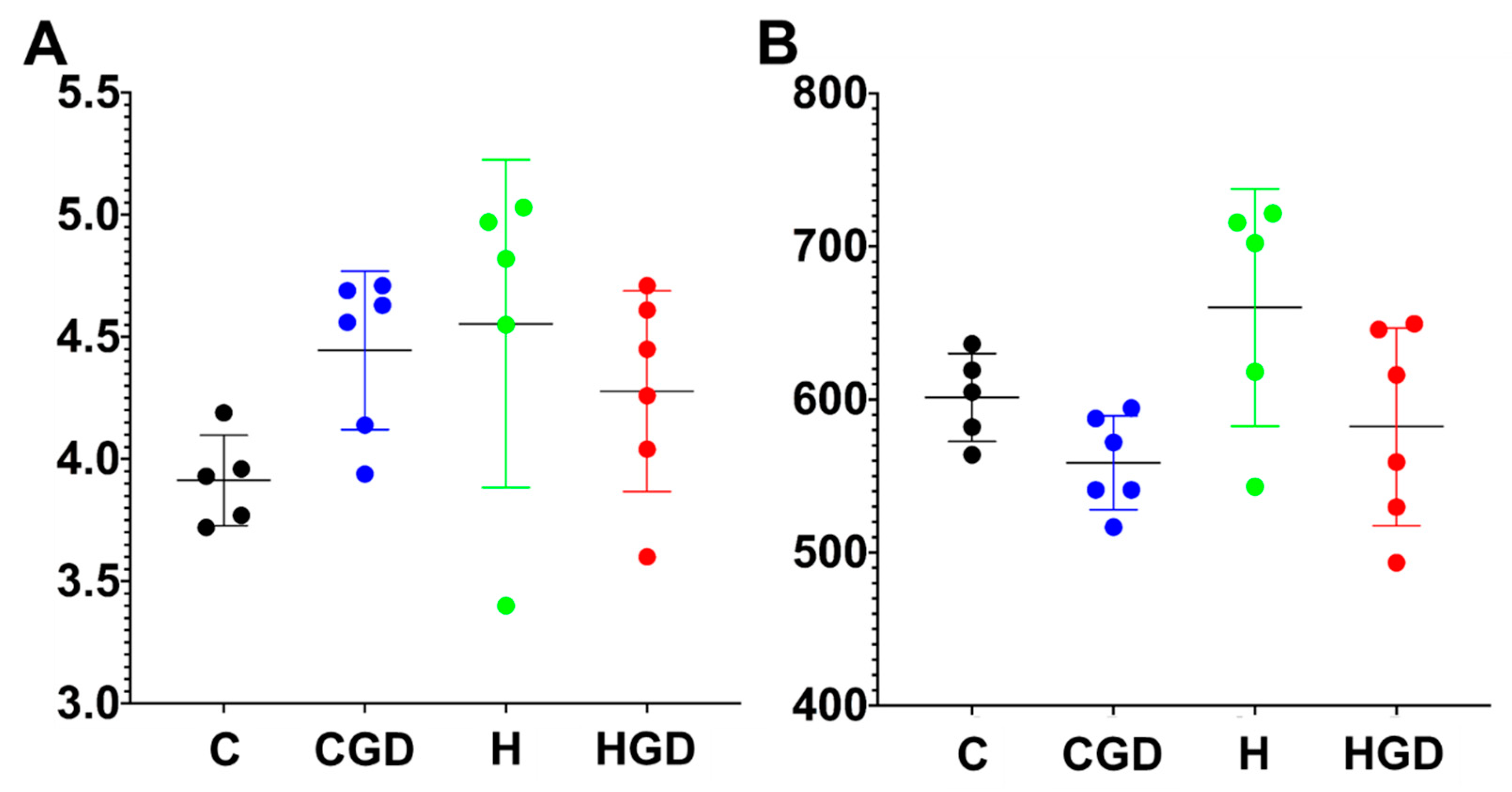
2.5.1. Alpha Diversity Measures (Shannon Diversity and Richness) of the Bacterial Communities
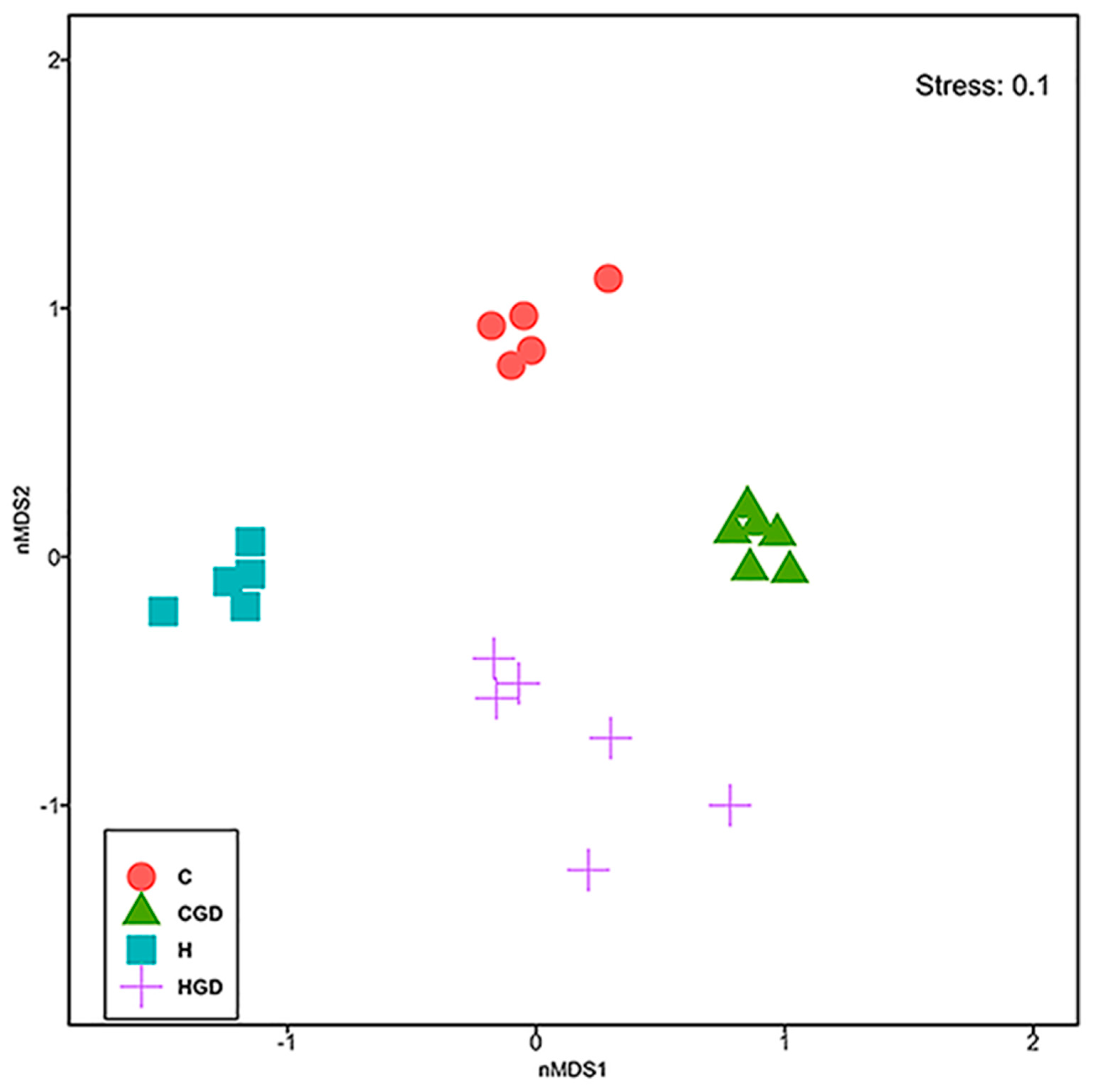
2.5.2. Bacterial Community Structure
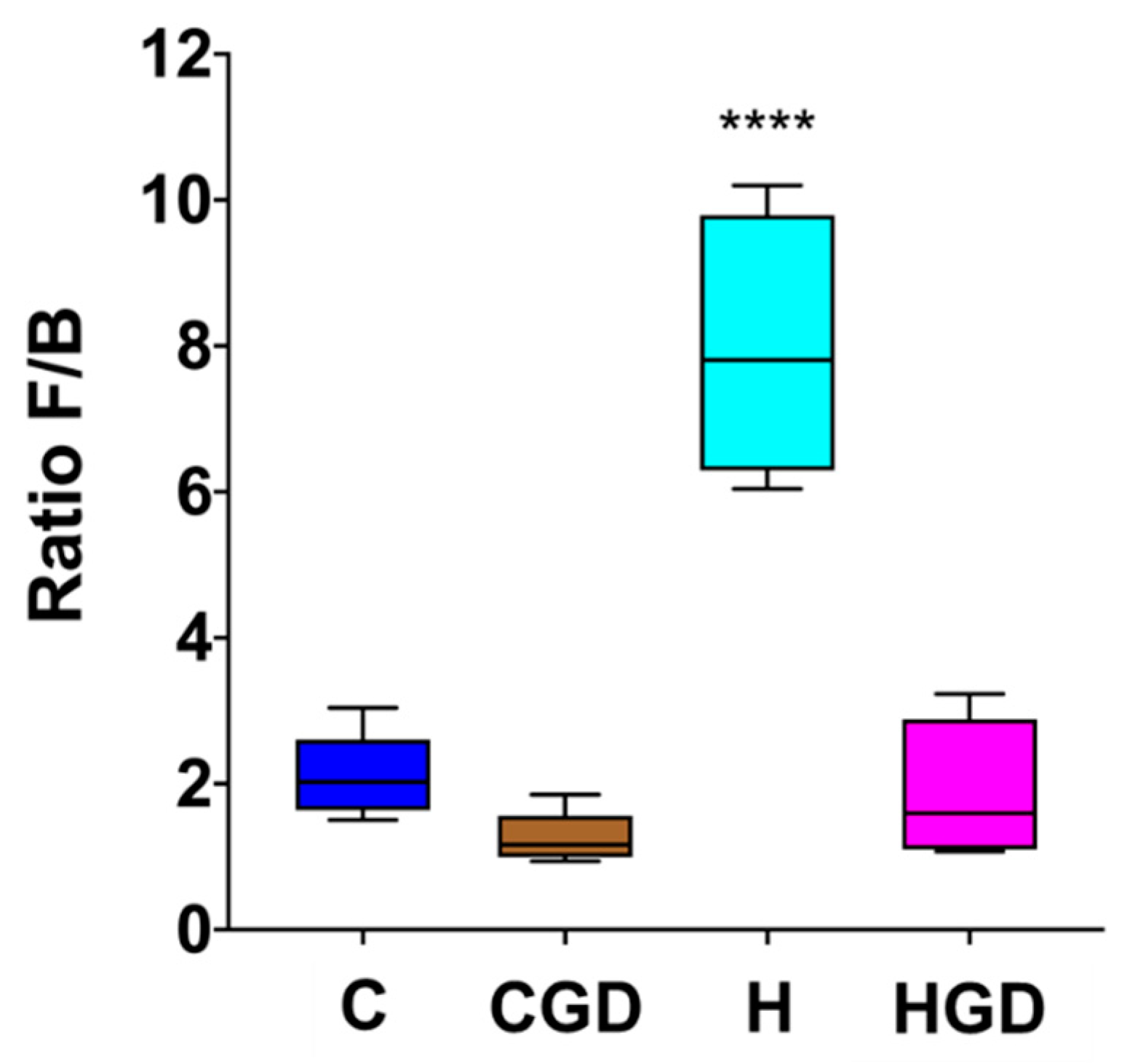
2.5.3. Firmicutes and Bacteroidetes Ratio as an Indicator for Obesity
2.5.4. Taxonomic Structure of the Bacterial Communities
2.5.5. Differentially Abundant zOTUs
2.5.6. Multivariate Analysis of Physiological Data
2.5.7. Correlation of Microbiota and Physiological Parameters
3. Discussion
4. Materials and Methods
4.1. Garcinia dulcis Rind, Pulp and Seed Preparation and Analyses
4.1.1. Citric Acid Analysis
4.1.2. Garcinol Analysis
4.1.3. Morelloflavone Analysis
4.2. Rats and Diets
4.3. Measurements in Living Rats
4.4. Measurements after Euthanasia
4.5. Gut Microbiota Analysis
4.5.1. DNA Extraction of Microbial Samples
4.5.2. 16S rRNA Gene Amplification and Sequencing
- 341F (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG)
- 785R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC)
4.5.3. 16S rRNA Gene Sequencing Analysis
4.6. Statistical Analysis
4.6.1. Physiological and Metabolic Parameters
4.6.2. Microbiota Community Analysis
4.6.3. Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Corn starch diet-fed rats |
| CGD | Corn starch diet-fed rats supplemented with Garcinia dulcis rind powder |
| F/B | Ratio of Firmicutes/Bacteriodetes |
| H | High-carbohydrate, high-fat diet-fed rats |
| HGD | High-carbohydrate, high-fat diet-fed rats supplemented with Garcinia dulcis rind powder |
| GLM | Generalised linear model |
| HPLC | High-performance liquid chromatography |
| MGLM | Multivariate generalised linear model |
| nMDS | Non-metric, multi-dimensional scaling |
| PERMANOVA | Permutational multivariate analysis of variance |
| SEM | Standard error of the mean |
| TMAO | Trimethylamine N-oxide |
| USP | United States Pharmacopeia |
| zOTU | Zero-radius operational taxonomic unit |
References
- John, O.D.; Brown, L.; Panchal, S.K. Garcinia fruits: Their potential to combat metabolic syndrome. In Nutraceuticals and Natural Product Derivatives: Disease Prevention & Drug Discovery; Ullah, M., Ahmad, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; Chapter 3; pp. 39–80. [Google Scholar] [CrossRef]
- Lim, T. Garcinia dulcis. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 2, pp. 35–40. [Google Scholar] [CrossRef]
- Cooper, W. A taxonomic revision of Garcinia L.(Clusiaceae) in Australia, including four new species from tropical Queensland. Austrobaileya 2013, 9, 1–29. [Google Scholar]
- Allen, G. Mangosteen and its relatives; Sub-Tropical Fruit Club of Qld Inc. 2004. Available online: http://stfc.org.au/mangosteen-and-its-relatives (accessed on 7 August 2019).
- Kraikruan, W.; Klaipook, W.; Thanumthat, R. Benefits of local humid tropical fruit trees in Thailand. Acta Hortic. 2017, 1186, 235–240. [Google Scholar] [CrossRef]
- Tuansulong, K.A.; Hutadilok-Towatana, N.; Mahabusarakam, W.; Pinkaew, D.; Fujise, K. Morelloflavone from Garcinia dulcis as a novel biflavonoid inhibitor of HMG-CoA reductase. Phytother. Res. 2011, 25, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.F.; Ahmad, N.E.; Suleiman, M.; Rahmat, A.; Isha, A. Garcinia dulcis fruit extract induced cytotoxicity and apoptosis in HepG2 liver cancer cell line. BioMed Res. Int. 2015, 2015, 916902. [Google Scholar] [CrossRef]
- Gogoi, N.; Gogoi, A.; Neog, B.; Baruah, D.; Singh, K.D. Evaluation of antioxidant and hepatoprotective activity of fruit rind extract of Garcinia dulcis (Roxburgh) Kurz. Pharmacogn. Res. 2017, 9, 266–272. [Google Scholar] [CrossRef]
- Khamthong, N.; Hutadilok-Towatana, N. Phytoconstituents and biological activities of Garcinia dulcis (Clusiaceae): A review. Nat. Prod. Commun. 2017, 12, 453–460. [Google Scholar] [CrossRef]
- Decha-Dier, U.; Hutadilok-Towatana, N.; Mahabusarakam, W.; Sawangjaroen, K.; Pinkaew, D. Anti-altherogenic effects of morelloflavone from Garcinia dulcis leaves in cholesterol fed rabbits. J. Nat. Remed. 2008, 8, 151–159. [Google Scholar] [CrossRef]
- Pinkaew, D.; Hutadilok-Towatana, N.; Teng, B.-B.; Mahabusarakam, W.; Fujise, K. Morelloflavone, a biflavonoid inhibitor of migration-related kinases, ameliorates atherosclerosis in mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H451–H458. [Google Scholar] [CrossRef]
- Liu, C.; Ho, P.C.-L.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sánchez, C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Muñoz-Quezada, S.; Fontana, L.; Abadía-Molina, F. Microbial population changes and their relationship with human health and disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Su, L.; Wang, J.; Duan, X.; Jiang, X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 2018, 21, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Sabaté, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: The adventist health study 2. Diabetes Care 2011, 34, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Arazo, M.; Bello, A.; Rastrelli, L.; Montelier, M.; Delgado, L.; Panfet, C. Antioxidant properties of pulp and peel of yellow mangosteenfruits. Emirates J. Food Agric. 2011, 23, 517–524. [Google Scholar]
- John, O.; Wanyonyi, S.; Mouatt, P.; Panchal, S.; Brown, L. Achacha (Garcinia humilis) rind improves cardiovascular function in rats with diet-induced metabolic syndrome. Nutrients 2018, 10, 1425. [Google Scholar] [CrossRef]
- Subhadrabandhu, S. Under-Utilized Tropical Fruits of Thailand. FAO Regional Office for Asia and the Pacific. 2001. Available online: http://www.fao.org/3/a-ab777e.pdf (accessed on 19 November 2018).
- Chaovanalikit, A.; Mingmuang, A.; Kitbunluewit, T.; Choldumrongkool, N.; Sondee, J.; Chupratum, S. Anthocyanin and total phenolics content of mangosteen and effect of processing on the quality of mangosteen products. Int. Food Res. J. 2012, 19, 1047–1053. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Deachathai, S.; Mahabusarakam, W.; Phongpaichit, S.; Taylor, W. Phenolic compounds from the fruit of Garcinia dulcis. Phytochemistry 2005, 66, 2368–2375. [Google Scholar] [CrossRef]
- Martin, F.W.; Campbell, C.W.; Ruberté, R.M. Perennial Edible Fruits of the Tropics: An Inventory; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1987; Volume 642. Available online: https://naldc.nal.usda.gov/download/CAT87886130/PDF (accessed on 15 October 2018).
- Jena, B.S.; Jayaprakasha, G.K.; Sakariah, K.K. Organic acids from leaves, fruits, and rinds of Garcinia cowa. J. Agric. Food Chem. 2002, 50, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Sunitha, K.; Sebasatin Santhosh, M.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.S.; Niranjana, S.R.; Girish, K.S. An overview on genus Garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. J. Obes. 2011, 2011, 509038. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Saadat, N.; Gupta, S.V. Potential role of garcinol as an anticancer agent. J. Oncol. 2012, 2012, 647206. [Google Scholar] [CrossRef]
- Behera, A.K.; Swamy, M.M.; Natesh, N.; Kundu, T.K. Garcinol and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 435–452. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Shimada, R.; Kagami, S.; Yamaguchi, F.; Kataoka, S.; Ariga, T.; Murakami, A.; Koshimizu, K.; Ohigashi, H. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis 2000, 21, 1183–1189. [Google Scholar] [CrossRef]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef]
- Mali, K.K.; Dias, R.J.; Havaldar, V.D.; Yadav, S.J. Antidiabetic effect of garcinol on streptozotocin-induced diabetic rats. Indian J. Pharm. Sci. 2017, 79, 463–468. [Google Scholar] [CrossRef]
- Setiawan, A.; Hanum, L.; Wardoyo, E.R.P. The effect of mundu fruit Garcinia dulcis Roxb Kurz methanol extract on lyphoprotein profile and trygliseride white rat Rattus norvegicus L. J. Biol. Res. 2015, 19, 74–77. [Google Scholar] [CrossRef]
- Liao, C.H.; Sang, S.; Liang, Y.C.; Ho, C.T.; Lin, J.K. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 in downregulating nuclear factor-kappa B pathway by garcinol. Mol. Carcinog. 2004, 41, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, K.; Naik, P.R. Modulatory effect of garcinol in streptozotocin-induced diabetic Wistar rats. Arch. Physiol. Biochem. 2017, 123, 322–329. [Google Scholar] [CrossRef]
- Hung, W.L.; Tsai, M.L.; Sun, P.P.; Tsai, C.Y.; Yang, C.C.; Ho, C.T.; Cheng, A.C.; Pan, M.H. Protective effects of garcinol on dimethylnitrosamine-induced liver fibrosis in rats. Food Funct. 2014, 5, 2883–2891. [Google Scholar] [CrossRef]
- Bodhankar, S.L.; Kushawaha, S.K.; Thakurdesai, P.; Mohan, V. Effect of cyclodextrin garcinol complex on isoproterenol-induced cardiotoxicity and cardiac hypertrophy in rats. Diabesity 2016, 2, 12–18. [Google Scholar] [CrossRef]
- Chan, E.C.; Dusting, G.J.; Guo, N.; Peshavariya, H.M.; Taylor, C.J.; Dilley, R.; Narumiya, S.; Jiang, F. Prostacyclin receptor suppresses cardiac fibrosis: Role of CREB phosphorylation. J. Mol. Cell. Cardiol. 2010, 49, 176–185. [Google Scholar] [CrossRef]
- Thongsepee, N.; Mahabusarakam, W.; Hiranyachattada, S. Diuretic and hypotensive effect of morelloflavone from Garcinia dulcis in two-kidneys-one-clip (2K1C) hypertensive rat. Sains Malays. 2017, 46, 1479–1490. [Google Scholar] [CrossRef]
- De Castro Moreira, M.E.; de Oliveira Araújo, F.; de Sousa, A.R.; Toledo, R.C.L.; dos Anjos Benjamin, L.; Veloso, M.P.; de Souza Reis, K.; dos Santos, M.H.; Martino, H.S.D. Bacupari peel extracts (Garcinia brasiliensis) reduces the biometry, lipogenesis and hepatic steatosis in obese rats. Food Res. Int. 2018, 114, 169–177. [Google Scholar] [CrossRef]
- Pinkaew, D.; Cho, S.G.; Hui, D.Y.; Wiktorowicz, J.E.; Hutadilok-Towatana, N.; Mahabusarakam, W.; Tonganunt, M.; Stafford, L.J.; Phongdara, A.; Liu, M.; et al. Morelloflavone blocks injury-induced neointimal formation by inhibiting vascular smooth muscle cell migration. Biochim. Biophys. Acta 2009, 1790, 31–39. [Google Scholar] [CrossRef]
- Lamai, J.; Mahabusarakam, W.; Ratithammatorn, T.; Hiranyachattada, S. Effects of morelloflavone from Garcinia dulcis on vasorelaxation of isolated rat thoracic aorta. J. Physiol. Biomed. Sci. 2013, 26, 13–17. [Google Scholar]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef]
- Lee, P.S.; Teng, C.Y.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Garcinol reduces obesity in high-fat-diet-fed mice by modulating gut microbiota composition. Mol. Nutr. Food Res. 2019, 63, e1800390. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.L.; Kallstrom, G.; Faith, J.J.; Reyes, A.; Moore, A.; Dantas, G.; Gordon, J.I. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc. Natl. Acad. Sci. USA 2011, 108, 6252–6257. [Google Scholar] [CrossRef] [PubMed]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Ren, H.; Gao, Y.F.; Lee, C.Y.; Li, S.F.; Zhang, F.; Li, L.; Chen, H. Dietary capsaicin improves glucose homeostasis and alters the gut microbiota in obese diabetic ob/ob mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Kasahara, K.; Tanoue, T.; Yamashita, T.; Yodoi, K.; Matsumoto, T.; Emoto, T.; Mizoguchi, T.; Hayashi, T.; Kitano, N.; Sasaki, N.; et al. Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflammation in atherosclerosis. J. Lipid Res. 2017, 58, 519–528. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Wong, V.W.; Tse, C.-H.; Lam, T.T.; Wong, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Law, P.T.; Kwan, H.-S.; Yu, J.; et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis—A longitudinal study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Q.; Li, H. Gut microbiota and nonalcoholic fatty liver disease: Insights on mechanisms and therapy. Nutrients 2017, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Citric Acid (CAS No: 77-92-9). In United Nations Environment Programme; UNEP Publications: Orlando, FL, USA, 2001. [Google Scholar]
- Majeed, M.; Bani, S.; Bhat, B.; Pandey, A.; Mundkur, L.; Neupane, P. Safety profile of 40% garcinol from Garcinia indica in experimental rodents. Toxicol. Rep. 2018, 5, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yi, T.; Yi, Z.; Cho, S.G.; Qu, W.; Pinkaew, D.; Fujise, K.; Liu, M. Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting rho GTPases and extracellular signal-regulated kinase signaling pathways. Cancer Res. 2009, 69, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.; Bagchi, D.; Bagchi, M.; Rao, C.; Dey, D.; Satyanarayana, S. Effects of a natural extract of (–)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX plus niacin-bound chromium and Gymnema sylvestre extract on weight loss. Diabetes Obes. Metab. 2004, 6, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Awe, S.O.; LeDay, A.M.; Opere, C.A.; Bagchi, D. Effect of hydroxycitric acid on serotonin release from isolated rat brain cortex. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 210–216. [Google Scholar]
- Ohia, S.E.; Opere, C.A.; LeDay, A.M.; Bagchi, M.; Bagchi, D.; Stohs, S.J. Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX). Mol. Cell. Biochem. 2002, 238, 89–103. [Google Scholar] [CrossRef]
- Walker, E.B. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci. 2007, 30, 1229–1234. [Google Scholar] [CrossRef]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Chapter Six—Quantifying size and number of adipocytes in adipose tissue. In Methods in Enzymology; Macdougald, O.A., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 537, pp. 93–122. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Good, C.A.; Kramer, H.; Somogyi, M. The determination of glycogen. J. Biol. Chem. 1933, 100, 485–491. [Google Scholar]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar]
- Illumina. MiSeq System User Guide; Illumina Inc.: Berlin, Germany, 2013. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wemheuer, B.; Wemheuer, F. Assessing Bacterial and Fungal Diversity in the Plant Endosphere. In Metagenomics: Methods and Protocols; Streit, W.R., Daniel, R., Eds.; Springer: New York, NY, USA, 2017; pp. 75–84. [Google Scholar] [CrossRef]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016, 081257. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.5-2. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 March 2019).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.4-2. 2017. Available online: https://cran.r-project.org/ (accessed on 30 March 2019).
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. mvabund—An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Asyifah, M.R.; Lu, K.; Ting, H.L.; Zhang, D. Hidden potential of tropical fruit waste components as a useful source of remedy for obesity. J. Agric. Food Chem. 2014, 62, 3505–3516. [Google Scholar] [CrossRef] [PubMed]

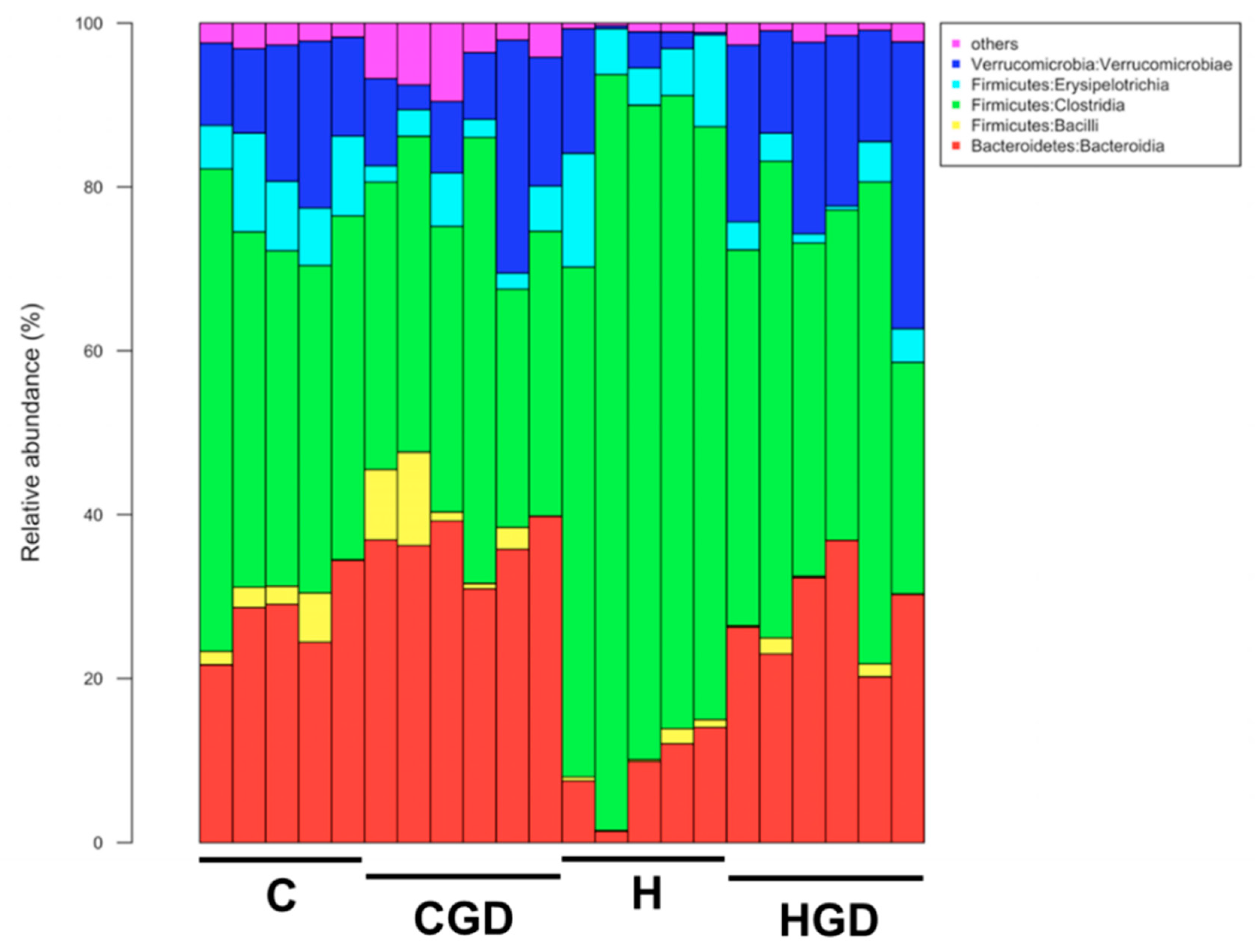
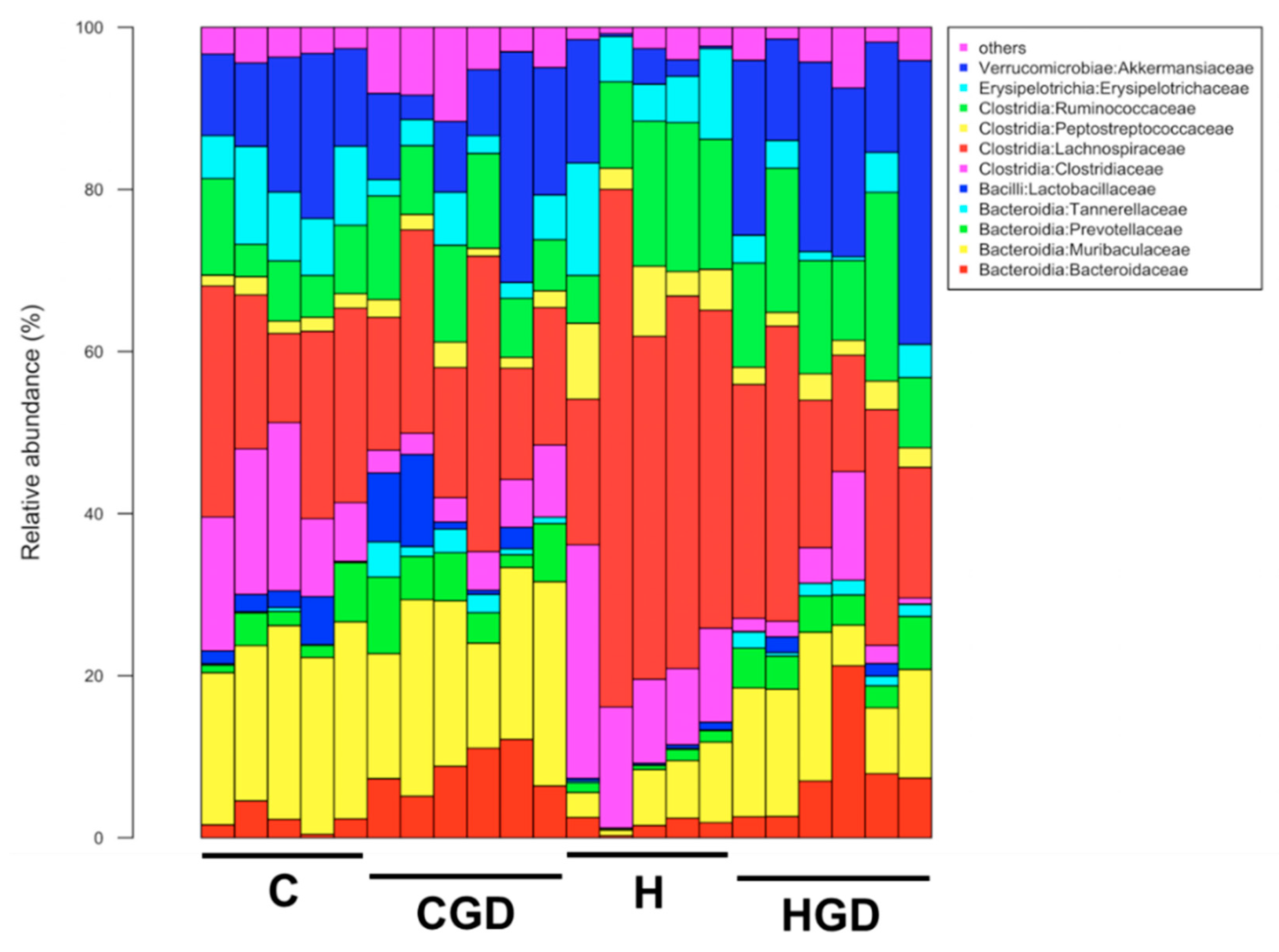
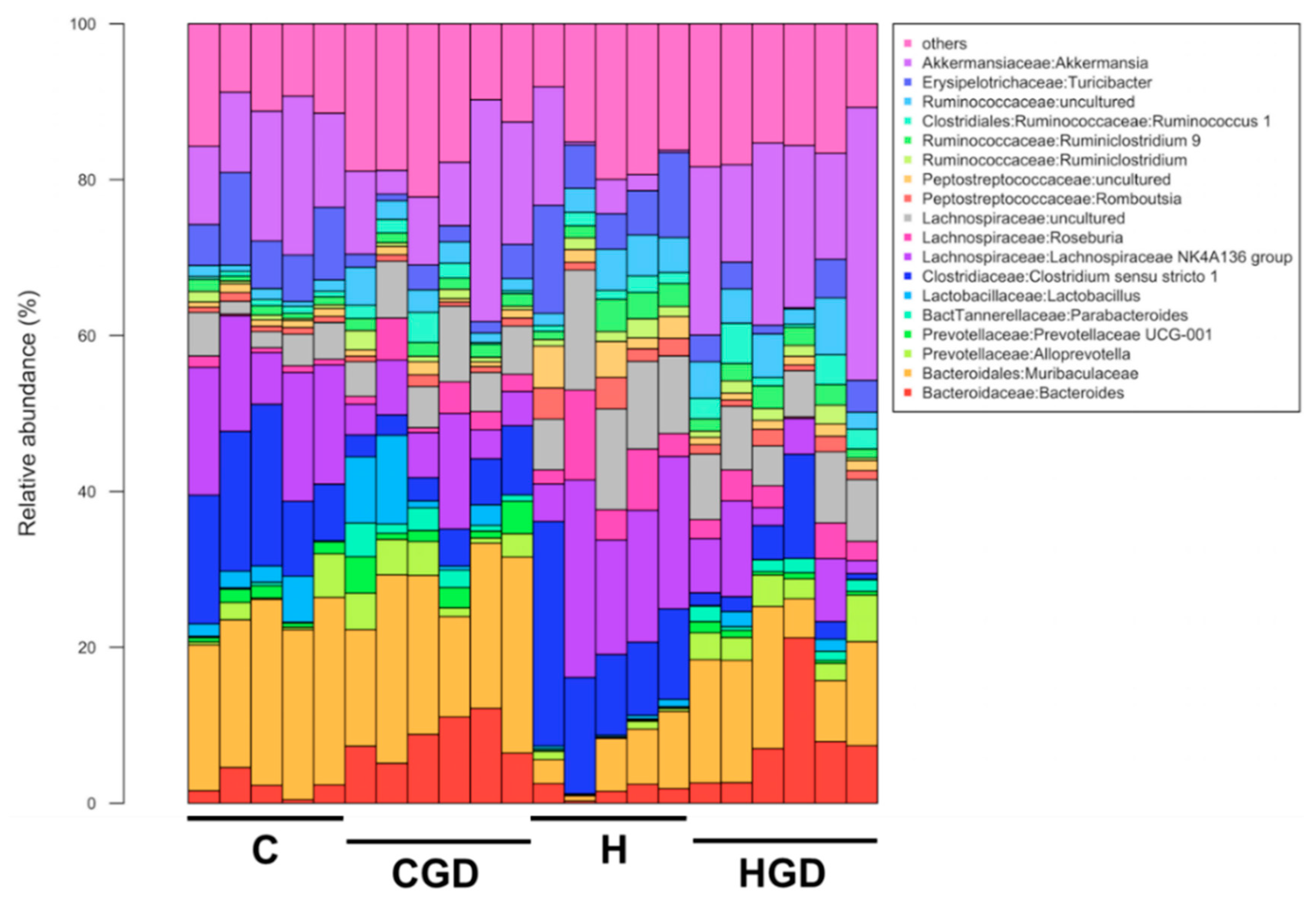
| Variables | C | CGD | H | HGD | p Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| Physiological Parameters | |||||||
| Initial body weight, g | 338 ± 0.9a | 339 ± 0.7a | 337 ± 0.7a | 337 ± 0.7a | 0.35 | 0.46 | 0.32 |
| 8-week body weight, g | 355 ± 6.6b | 362 ± 7.0b | 423 ± 6.4a | 422 ± 10a | <0.0001 | 0.75 | 0.61 |
| 16-week body weight, g | 404 ± 8b | 306 ± 6d | 508 ± 11a | 362 ± 10c | <0.0001 | <0.0001 | 0.011 |
| Food intake, g/day | 41.2 ± 2.3a | 26.3 ± 2.3b | 27.0 ± 2.5b | 20.1 ± 1.9b | 0.0001 | <0.0001 | 0.10 |
| Water intake, g/day | 23.6 ± 2.6a | 28.7 ± 2.9a | 28.6 ± 6.0a | 37.6 ± 4.5a | 0.10 | 0.09 | 0.64 |
| Garcinol intake, mg/kg/day | - | 210 ± 7 | - | 130 ± 8 | - | - | - |
| Citric acid intake, mg/kg/day | - | 780 ± 27 | - | 480 ± 23 | - | - | - |
| Morelloflavone intake, mg/kg/day | - | 7.3 ± 0.2 | - | 5.4 ± 0.1 | - | - | - |
| Energy intake, kJ/day | 462 ± 28b | 318 ± 10c | 603 ± 52a | 501 ± 12b | <0.0001 | 0.0002 | 0.49 |
| Feed efficiency (8–16 weeks), g/kJ | 0.10 ± 0.01a | −0.18 ± 0.02b | 0.15 ± 0.02a | −0.11 ± 0.02b | 0.002 | <0.0001 | 0.58 |
| Body weight gained (8–16 weeks), % | 13.7 ± 1.0b | −15.4 ± 0.8c | 20.1 ± 2.8a | −13.0 ± 2.2c | 0.024 | <0.0001 | 0.29 |
| Abdominal circumference 8 weeks, cm | 16.1 ± 0.2b | 16.6 ± 0.3b | 19.8 ± 0.5a | 20.4 ± 0.5a | <0.0001 | 0.18 | 0.91 |
| Abdominal circumference 16 weeks, cm | 18.4 ± 0.4b | 15.0 ± 0.2d | 22.6 ± 0.4a | 16.8 ± 0.4c | <0.0001 | <0.0001 | 0.001 |
| Whole body lean mass 8 weeks, g | 283 ± 12a | 300 ± 4a | 310 ± 6a | 302 ± 5a | 0.047 | 0.56 | 0.09 |
| Whole body lean mass 16 weeks, g | 289 ± 10a | 267 ± 5a | 297 ± 8a | 265 ± 13a | 0.71 | 0.007 | 0.62 |
| Whole body fat mass 8 weeks, g | 57 ± 5b | 51 ± 8b | 94 ± 8a | 105 ± 19a | 0.002 | 0.86 | 0.55 |
| Whole body fat mass 16 weeks, g | 96 ± 9b | 23 ± 3c | 203 ± 12a | 84 ± 6b | <0.0001 | <0.0001 | 0.012 |
| Bone mineral content 8 weeks, g | 11.0 ± 0.6a | 10.6 ± 0.5a | 11.8 ± 0.3a | 12.0 ± 0.6a | 0.025 | 0.83 | 0.81 |
| Bone mineral content 16 weeks, g | 12.4 ± 0.5b | 10.0 ± 0.2c | 16.1 ± 0.5a | 11.8 ± 0.3b | <0.0001 | <0.0001 | 0.019 |
| Bone mineral density 8 weeks, g/cm2 | 0.163 ± 0.004a | 0.160 ± 0.005a | 0.168 ± 0.004a | 0.164 ± 0.002a | 0.26 | 0.39 | 0.87 |
| Bone mineral density 16 weeks, g/cm2 | 0.173 ± 0.004b | 0.165 ± 0.003b | 0.185 ± 0.003a | 0.162 ± 0.003b | <0.0001 | 0.18 | 0.027 |
| Body mass index 16 weeks, g/cm2 | 0.63 ± 0.02b | 0.54 ± 0.02c | 0.77 ± 0.02a | 0.60 ± 0.02b | <0.0001 | <0.0001 | 0.052 |
| Retroperitoneal fat, mg/mm tibial length | 255 ± 22b | 71 ± 7d | 497 ± 33a | 160 ± 18c | <0.0001 | <0.0001 | 0.001 |
| Epididymal fat, mg/mm tibial length | 101 ± 16b | 39 ± 6c | 165 ± 18a | 49 ± 5c | 0.006 | <0.0001 | 0.040 |
| Omental fat, mg/mm tibial length | 159 ± 14b | 66 ± 4d | 260 ± 11a | 115 ± 8c | <0.0001 | <0.0001 | 0.015 |
| Total abdominal fat, mg/mm tibial length | 515 ± 45b | 176 ± 13d | 951 ± 49a | 325 ± 29c | <0.0001 | <0.0001 | 0.001 |
| Visceral adiposity index, % | 5.84 ± 0.43b | 2.60 ± 0.20d | 8.65 ± 0.39a | 4.24 ± 0.37c | <0.0001 | <0.0001 | 0.11 |
| Kidney wet weight, mg/mm tibial length | 50.2 ± 1.2b | 40.7 ± 1.2c | 56.0 ± 1.6a | 47.1 ± 1.3b | <0.0001 | <0.0001 | 0.82 |
| Heat production 16 weeks, kcal/hour | 4.06 ± 0.40a | 2.06 ± 0.14b | 4.20 ± 0.49a | 3.59 ± 0.31a | 0.029 | 0.003 | 0.06 |
| Heat production area under the curve 16 weeks, (kcal/hour) × minutes | 2878 ± 212ab | 1419 ± 76c | 3193 ± 97a | 2500 ± 201b | 0.002 | <0.0001 | 0.053 |
| Respiratory exchange ratio 16 weeks | 0.973 ± 0.010a | 0.986 ± 0.024a | 0.886 ± 0.017b | 0.898 ± 0.037b | 0.012 | 0.66 | 0.99 |
| Respiratory exchange ratio area under the curve 16 weeks | 700 ± 9a | 707 ± 16a | 618 ± 15b | 631 ± 18b | 0.0005 | 0.48 | 0.82 |
| Mean liver fat vacuoles area, µm2 | 14.1 ± 0.8c | 9.8 ± 0.5c | 150.1 ± 13.6a | 73.0 ± 8.8b | <0.0001 | <0.0001 | <0.0001 |
| Mean retroperitoneal adipocyte area, µm2 | 4299 ± 120b | 2290 ± 67c | 9966 ± 195a | 4115 ± 326b | <0.0001 | <0.0001 | <0.0001 |
| Plasma Biochemistry and Glucose Response | |||||||
| Plasma alanine transaminase activity, U/L | 28.4 ± 4.0b | 33.8 ± 2.7a | 42.3 ± 5.0a | 39.1 ± 5.1a | 0.025 | 0.89 | 0.28 |
| Plasma aspartate transaminase activity, U/L | 83.0 ± 8.0b | 86.5 ± 9.0b | 173.9 ± 24.0a | 91.4 ± 6.6b | 0.001 | 0.007 | 0.003 |
| Plasma total cholesterol, mmol/L | 1.37 ± 0.07a | 0.95 ± 0.06b | 1.66 ± 0.10a | 1.50 ± 0.12a | <0.0001 | 0.003 | 0.16 |
| Plasma triglycerides, mmol/L | 0.45 ± 0.05b | 0.25± 0.02b | 1.15 ± 0.17a | 0.35 ± 0.08b | 0.0008 | <0.0001 | 0.009 |
| Plasma non-esterified fatty acids, mmol/L | 0.87 ± 0.18b | 0.35 ± 0.04c | 3.25 ± 0.17a | 0.80 ± 0.16b | <0.0001 | <0.0001 | <0.0001 |
| Basal blood glucose 8 weeks, mmol/L | 2.4 ± 0.1b | 2.3 ± 0.1b | 3.1 ± 0.1a | 3.0 ± 0.1a | <0.0001 | 0.33 | 1.0 |
| Basal blood glucose 16 weeks, mmol/L | 2.6 ± 0.1ab | 2.4 ± 0.2b | 3.0 ± 0.1a | 2.8 ± 0.1ab | 0.003 | 0.13 | 1.0 |
| Blood glucose area under the curve 8 weeks, mmol/L × minutes | 488 ± 9b | 494 ± 11b | 596 ± 13a | 585 ± 7a | <0.0001 | 0.82 | 0.43 |
| Blood glucose area under the curve 16 weeks, mmol/L × minutes | 466 ± 17c | 401 ± 17d | 602 ± 24a | 544 ± 19b | <0.0001 | 0.003 | 0.86 |
| Insulin response area under the curve 8 weeks, mmol/L × minutes | 175 ± 23b | 200 ± 34b | 416 ± 55a | 413 ± 12a | <0.0001 | 0.78 | 0.72 |
| Insulin response area under the curve 16 weeks, mmol/L × minutes | 149 ± 19c | 154 ± 22c | 373 ± 24a | 281 ± 21b | <0.0001 | 0.028 | 0.017 |
| Liver wet weight, mg/mm tibial length | 231 ± 8b | 212 ± 7b | 358 ± 15a | 341 ± 13a | <0.0001 | 0.14 | 0.91 |
| Liver glycogen, mg/g | 12.9 ± 0.4a | 4.8 ± 0.5c | 13.9 ± 0.4a | 8.4 ± 1.2b | 0.002 | <0.0001 | 0.08 |
| Plasma catalase activity, kU/L | 39.0 ± 4.7b | 45.6 ± 6.0ab | 56.9 ± 8.1a | 56.9 ± 8.8a | 0.030 | 0.69 | 0.69 |
| Plasma C-reactive protein, µg/mL | 432 ± 5b | 355 ± 22c | 506 ± 8a | 376 ± 19c | 0.034 | 0.0002 | 0.19 |
| Faecal lipid content, mg/g | 21.8 ± 1.5b | 8.1 ± 0.4c | 43.3 ± 1.6a | 42.1 ± 5.4a | <0.0001 | 0.015 | 0.039 |
| Cardiovascular Variables | |||||||
| Systolic blood pressure 8 weeks, mmHg | 116 ± 3b | 121 ± 2b | 133 ± 4a | 132 ± 3a | <0.0001 | 0.45 | 0.24 |
| Systolic blood pressure 16 weeks, mmHg | 117 ± 2bc | 111 ± 3c | 135 ± 2a | 121 ± 5b | <0.0001 | 0.006 | 0.43 |
| Left ventricle + septum wet weight, mg/mm tibial length | 23.3 ± 1.4a | 18.9 ± 0.7a | 22.8 ± 1.4a | 18.5 ± 0.8a | 0.72 | 0.0005 | 0.99 |
| Right ventricle wet weight, mg/mm tibial length | 4.1 ± 0.2ab | 3.6 ± 0.3b | 4.7 ± 0.2a | 4.0 ± 0.3ab | 0.027 | 0.059 | 0.74 |
| Left ventricular diastolic stiffness (κ) | 21.6 ± 0.2b | 21.9 ± 0.9b | 26.7 ± 0.6a | 22.5 ± 0.6b | <0.0001 | 0.004 | 0.001 |
| Source | df | SS | MS | Pseudo-F | p (perm) | Unique Perms |
| Diet | 1 | 6986.2 | 6986.2 | 7.0877 | 0.0001 | 9879 |
| Treatment | 1 | 7162.9 | 7162.9 | 7.267 | 0.0001 | 9905 |
| Diet × treatment | 1 | 2735.8 | 2735.8 | 2.7756 | 0.0001 | 9859 |
| Res | 18 | 17,742 | 985.68 | |||
| Total | 21 | 34,411 | ||||
| PAIR-WISE TESTS | ||||||
| Groups | t | p (perm) | Unique Perms | |||
| C, CGD | 2.2169 | 0.0019 | 462 | |||
| C, H | 2.6651 | 0.0074 | 126 | |||
| C, HGD | 2.2135 | 0.0026 | 462 | |||
| CGD, H | 3.2027 | 0.0017 | 462 | |||
| CGD, HGD | 1.7959 | 0.0018 | 461 | |||
| H, HGD | 2.2624 | 0.0024 | 461 | |||
| PERMDISP (PAIRWISE COMPARISONS) | ||||||
| Groups | t | p (perm) | ||||
| C, CGD | 0.28079 | 0.8116 | ||||
| C, H | 1.6414 | 0.2101 | ||||
| C, HGD | 1.6237 | 0.2481 | ||||
| CGD, H | 2.4479 | 0.074 | ||||
| CGD, HGD | 1.7921 | 0.1642 | ||||
| H, HGD | 2.7484 | 0.0338 | ||||
| Global Test (GLMs) by Mvabund | ||
| Diet | p < 0.0001 | |
| Treatment | p < 0.0001 | |
| Diet × Treatment | p < 0.0001 | |
| Univariate Analysis by mvabund (p < 0.05) | ||
| Factor | Number of Differentially Abundant OTUs | % of Total Number of OTUs |
| Diet | 19 | 1.70% |
| Treatment | 35 | 3.14% |
| Diet × Treatment | 2 | 0.18% |
| Total (unique zOTUs affected by one or more factors) | 56 | 5.03% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, O.D.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 272. https://doi.org/10.3390/ijms21010272
John OD, Mouatt P, Majzoub ME, Thomas T, Panchal SK, Brown L. Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. International Journal of Molecular Sciences. 2020; 21(1):272. https://doi.org/10.3390/ijms21010272
Chicago/Turabian StyleJohn, Oliver D., Peter Mouatt, Marwan E. Majzoub, Torsten Thomas, Sunil K. Panchal, and Lindsay Brown. 2020. "Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome" International Journal of Molecular Sciences 21, no. 1: 272. https://doi.org/10.3390/ijms21010272
APA StyleJohn, O. D., Mouatt, P., Majzoub, M. E., Thomas, T., Panchal, S. K., & Brown, L. (2020). Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. International Journal of Molecular Sciences, 21(1), 272. https://doi.org/10.3390/ijms21010272







