Therapeutic Potential of Hericium erinaceus for Depressive Disorder
Abstract
1. Introduction
2. Pathophysiology of Depression
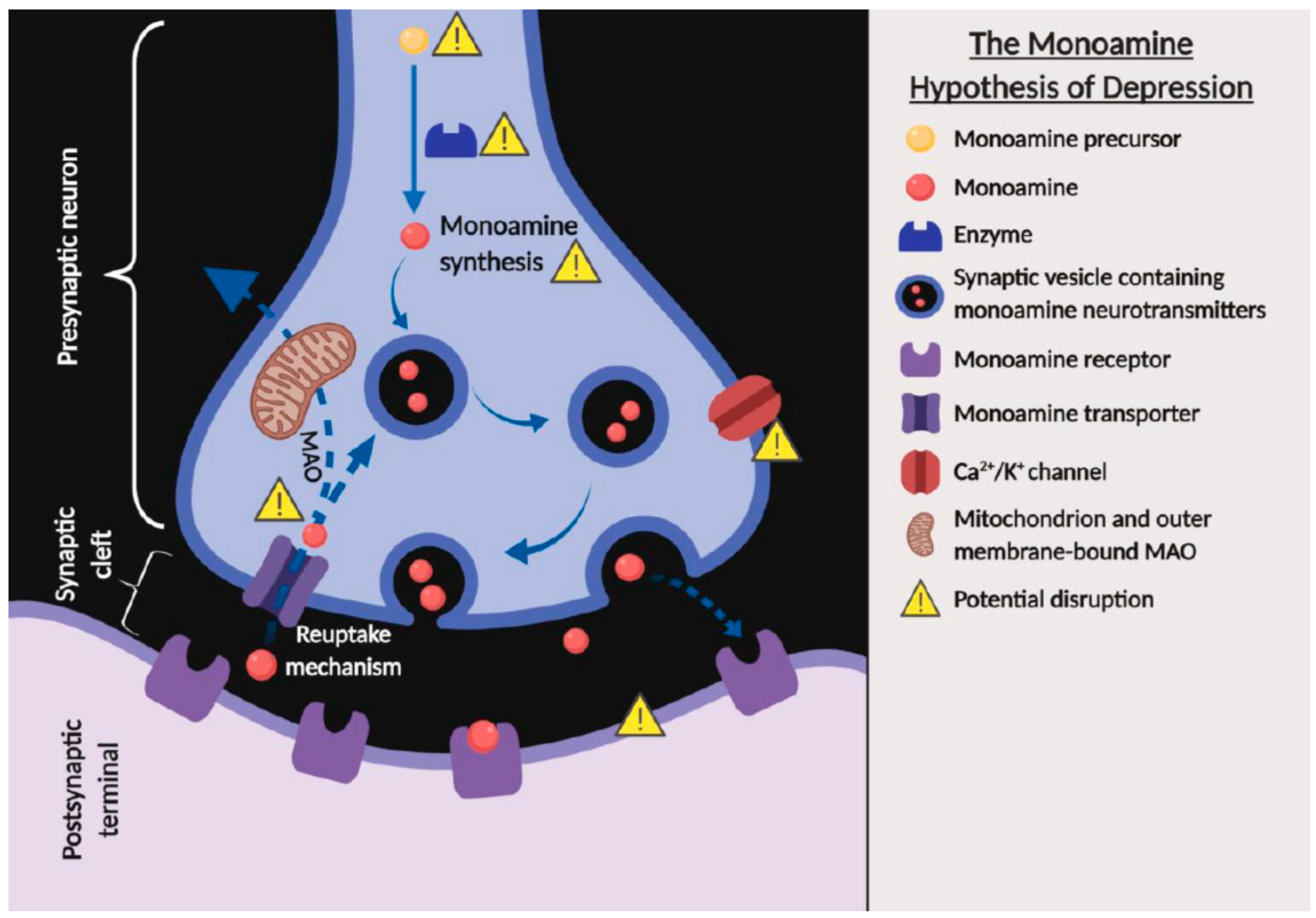
2.1. Monoamine Hypothesis
2.2. Neurotrophic/Neurogenic Hypothesis
2.3. Inflammatory Hypothesis
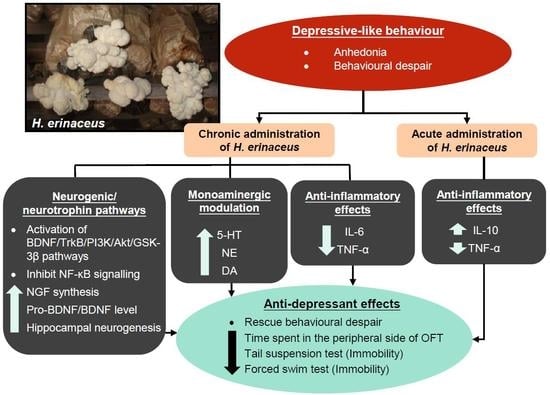
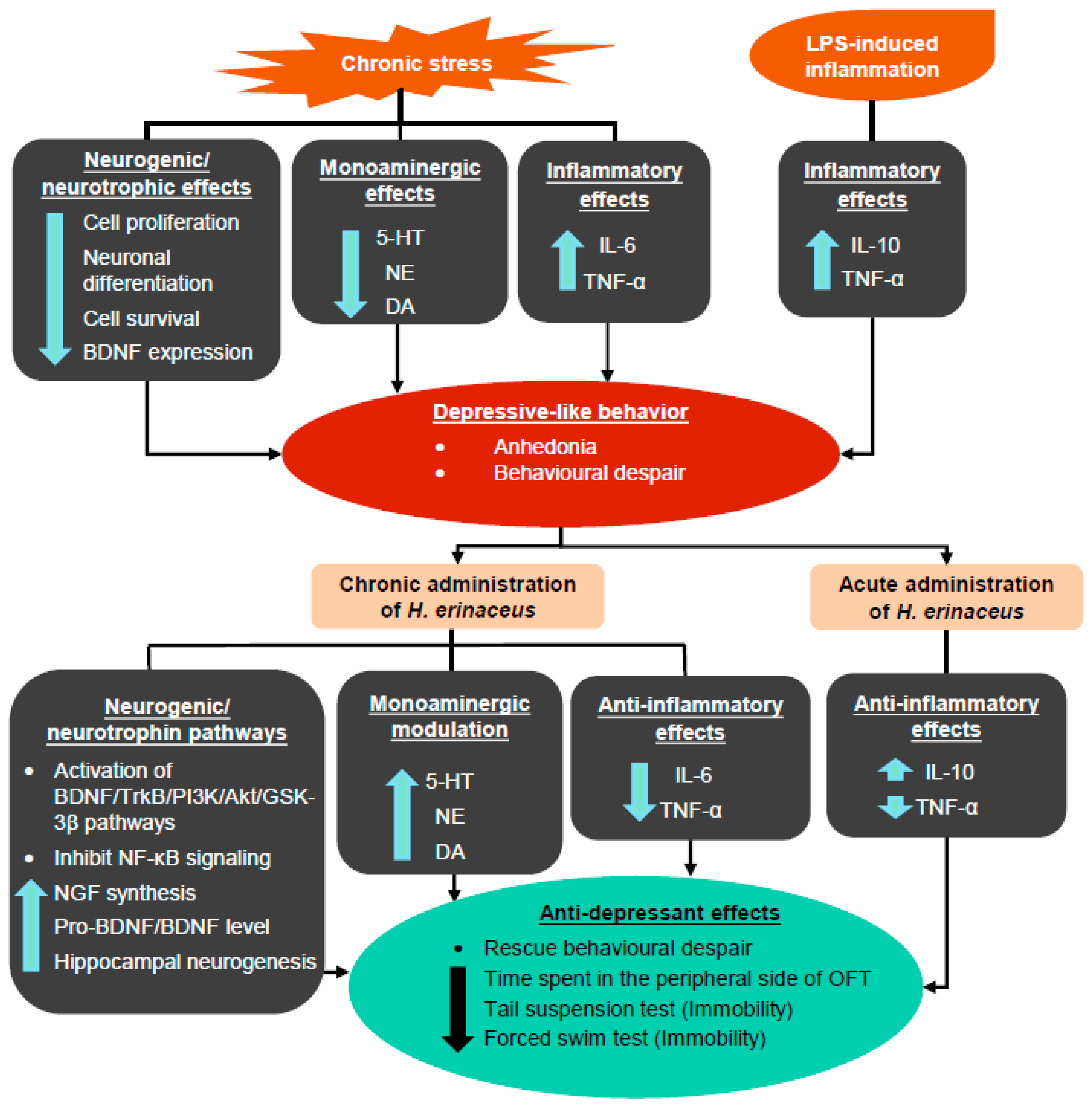
3. Hericium erinaceus Ameliorates Depressive-Like Behaviors
3.1. Pre-Clinical Studies
| Types of Study | Authors | Material Studied | Method of Extraction | Dose and Dosage | Research Model | Behavioural Effects | Physiological Effects/Mechanism |
|---|---|---|---|---|---|---|---|
| Pre-clinical | Yao et al., 2015 [33] | Amycenone®, H. erinaceus fruiting body extract (0.5% hericenones and 6% amyloban) | Patented extraction | 50, 100, or 200 * mg/kg amycenone (Amyloban® 3399), administered 60 min prior to 0.5 mg/kg LPS injection; P.O. | Male C57BL/6N mus musculus (LPS-induced inflammation model of depression) | Anti-inflammatory and antidepressant-like effects |
|
| Ryu et al., 2017 [32] | H. erinaceus | Ethanolic extract | 10, 60 * mg/kg daily for 4 weeks; P.O. | Male C57BL/6 mus musculus | Antidepressant-like and anxiolytic effects |
| |
| Chiu et al., 2018 [31] | Erinacine A enriched H. erinaceus mycelium | Ethanolic extract | 100, 200 *, and 400 * mg/kg daily for 4 weeks; P.O. | 50 (10/group) male ICR mus musculus (14 days restraint stress induced model of depression) | Antidepressant-like effects |
| |
| Clinical | Nagano et al., 2010 [77] | H. erinaceus fruiting body | Water extract | 500 * mg powdered fruiting body of H. erinaceus (Aso Biotech Inc) per cookie, 4 cookies daily for 4 weeks; P.O. | 30 female participants | Alleviate symptoms of depression and anxiety |
|
| Inanaga, 2014 [78] | Amycenone®, H. erinaceus fruiting body extract (0.5% hericenones and 6% amyloban) | Patented extraction | 1950 mg/tablet (Amyloban® 3399) 6 tablets, divided into 2 or 3 doses /day for 6 months; P.O. | 1 male patient | Improve neurocognitive impairment |
| |
| Okamura et al., 2015 [79] | Amycenone®, H. erinaceus fruiting body extract (0.5% hericenones and 6% amyloban) | Patented extraction | 1950 mg/tablet (Amyloban® 3399) 6 tablets, divided into 2 or 3 doses /day for 4 weeks; P.O. | 8 female healthy participants | Alleviate symptoms of depression and anxiety Alleviate sleep disorders |
| |
| Vigna et al., 2019 [80] | H. erinaceus (80% mycelia and 20% fruiting body) | Water and ethanolic extract | 1200 * mg per capsules (A.V.D. Reform s.r.l.), 3 capsules/day for 8 weeks; P.O. | 62 females and 15 males overweight or obese participants | Alleviate symptoms of depression and anxiety Alleviate sleep disorders |
|
3.2. Clinical Studies
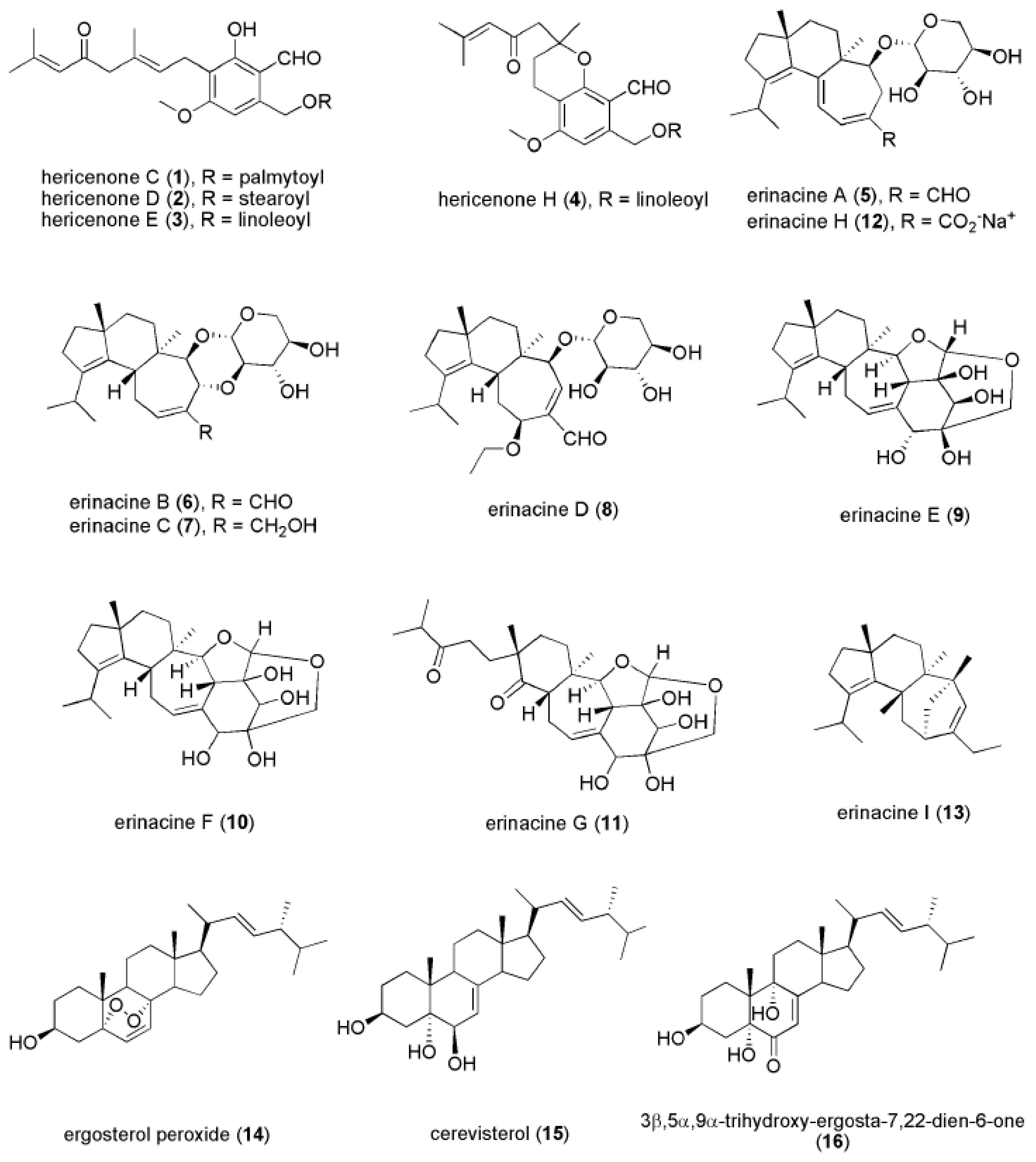
4. Bioactive Compounds of H. erinaceus that Contribute to Antidepressant-Like Activities
4.1. Hericenones
4.2. Erinacines
4.3. Novel Compounds
5. Mechanism of Action
5.1. Stimulation of NGF and Proliferative Activities
5.2. Monoaminergic Modulation
5.3. Anti-Inflammatory Pathway
5.4. BDNF Pathway
6. Future Perspectives of H. erinaceus Research in Depressive Disorder
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Depression 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 10 July 2019).
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Leopardi, R. Stress and depression: Preclinical research and clinical implications. PLoS ONE 2009, 4, e4265. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.W.; Busch, A.M.; Weeks, C.E.; Landes, S.J. The nature of clinical depression: Symptoms, syndromes, and behavior analysis. Behav. Anal. 2008, 31, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McMahon, F.J.; Buervenich, S.; Charney, D.; Lipsky, R.; Rush, A.J.; Wilson, A.F.; Sorant, A.J.M.; Papanicolaou, G.J.; Laje, G.; Fava, M.; et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am. J. Hum. Genet. 2006, 78, 804–814. [Google Scholar] [CrossRef]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef]
- Santarsieri, D.; Schwartz, T.L. Antidepressant efficacy and side-effect burden: A quick guide for clinicians. Drugs Context 2015, 4, 1–12. [Google Scholar] [CrossRef]
- InformedHealth.org. Depression: How Effective are Antidepressants? Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2015. [Google Scholar]
- Thase, M.E. Introduction: Defining remission in patients treated with antidepressants. J. Clin. Psychiatry 1999, 60, 3–6. [Google Scholar]
- Ashton, A.K.; Jamerson, B.D.; Weinstein, W.L.; Wagoner, C. Antidepressant-related adverse effects impacting treatment compliance: Results of a patient survey. Curr. Ther. Res. 2005, 66, 96–106. [Google Scholar] [CrossRef]
- Hodgson, K.; Tansey, K.E.; Uher, R.; Dernovšek, M.Z.; Mors, O.; Hauser, J.; Souery, D.; Maier, W.; Henigsberg, N.; Rietschel, M.; et al. Exploring the role of drug-metabolising enzymes in antidepressant side effects. Psychopharmacology 2015, 232, 2609–2617. [Google Scholar] [CrossRef]
- Rheker, J.; Winkler, A.; Doering, B.K.; Rief, W. Learning to experience side effects after antidepressant intake—Results from a randomized, controlled, double-blind study. Psychopharmacology 2017, 234, 329–338. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P. Active Substances for Preventing Hearing Deterioration, the Composition Containing the Active Substances, and the Preparation Method Thereof. U.S. Patent 10,405,504, 10 September 2019. [Google Scholar]
- Qureshi, N.A.; Al-Bedah, A.M. Mood disorders and complementary and alternative medicine: A literature review. Neuropsychiatr. Dis. Treat. 2013, 9, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.-K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.-F.; et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Tian, J.M.; Wei, J.; Gao, J.M. Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceum. Nat. Prod. Res. 2014, 28, 1288–1292. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Inhibitory effect on in vitro LDL oxidation and HMG Co-A reductase activity of the liquid-liquid partitioned fractions of Hericium erinaceus (Bull.) Persoon (Lion’s mane mushroom). BioMed Res. Int. 2014, 2014, 828149. [Google Scholar] [CrossRef]
- Yi, Z.; Shao-Long, Y.; Ai-Hong, W.; Zhi-Chun, S.; Ya-Fen, Z.; Ye-Ting, X.; Yu-Ling, H. Protective effect of ethanol extracts of Hericium erinaceus on alloxan-induced diabetic neuropathic pain in rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 595480. [Google Scholar] [CrossRef]
- Wang, J.C.; Hu, S.H.; Su, C.H.; Lee, T.M. Antitumor and immunoenhancing activities of polysaccharide from culture broth of Hericium spp. Kaohsiung J. Med Sci. 2001, 17, 461–467. [Google Scholar]
- Zhang, Z.; Liu, R.N.; Tang, Q.J.; Zhang, J.S.; Yang, Y.; Shang, X.D. A new diterpene from the fungal mycelia of Hericium erinaceus. Phytochem. Lett. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Mori, K.; Ouchi, K.; Hirasawa, N. The anti-inflammatory effects of lion’s mane culinary-medicinal mushroom, Hericium erinaceus (higher basidiomycetes) in a coculture system of 3T3-L1 adipocytes and RAW264 macrophages. Int. J. Med. Mushrooms 2015, 17, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.K.; Park, J.B.; Song, C.H. Hypolipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci. Biotechnol. Biochem. 2003, 67, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef]
- Kuo, H.C.; Lu, C.C.; Shen, C.H.; Tung, S.Y.; Hsieh, M.C.; Lee, K.C.; Lee, L.Y.; Chen, C.C.; Teng, C.C.; Huang, W.S.; et al. Hericium erinaceus mycelium and its isolated erinacine A protection from MPTP-induced neurotoxicity through the ER stress, triggering an apoptosis cascade. J. Transl. Med. 2016, 14, 78. [Google Scholar] [CrossRef]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, J.L.; Lin, W.H.; Mong, M.C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.O. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain. J. Med. Food 2018, 21, 174–180. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.-C.; Dong, C.; Zhuang, C.; Hirota, S.; Inanaga, K.; Hashimoto, K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015, 136, 7–12. [Google Scholar] [CrossRef]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialog. Clin. Neurosci. 2002, 4, 7–20. [Google Scholar]
- Coppen, A. The biochemistry of affective disorders. Br. J. Psychiatry 1967, 113, 1237–1264. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.J. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am. J. Psychiatry 1965, 122, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Bunney, E.W.; Davis, J.M. Norepinephrine in depressive reactions. A review. Arch. Gen. Psychiatry 1965, 13, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, A.A.; Hawkins, M.F.; Uzelac, S.M. The myth of reserpine-induced depression: Role in the historical development of the monoamine hypothesis. J. Hist. Neurosci. 2003, 12, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Freis, E.D. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N. Engl. J. Med. 1954, 251, 1006–1008. [Google Scholar] [CrossRef]
- Ikram, H.; Haleem, D.J. Repeated treatment with reserpine as a progressive animal model of depression. Pak. J. Pharm. Sci. 2017, 30, 897–902. [Google Scholar]
- Leith, N.J.; Barrett, R.J. Effects of chronic amphetamine or reserpine on self-stimulation responding: Animal model of depression? Psychopharmacology 1980, 72, 9–15. [Google Scholar] [CrossRef]
- Loomer, H.P.; Saunders, J.C.; Kline, N.S. A clinical and pharmacodynamic evaluation of iproniazid as a psychic energizer. Psychiatr. Res. Rep. 1957, 8, 129–141. [Google Scholar]
- Drevets, W.C. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 2001, 11, 240–249. [Google Scholar] [CrossRef]
- Hastings, R.S.; Parsey, R.V.; Oquendo, M.A.; Arango, V.; Mann, J.J. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 2004, 29, 952–959. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.M.; Campbell, S.; McEwen, B.S.; Macdonald, K.; Amano, S.; Joffe, R.T.; Nahmias, C.; Young, L.T. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA 2003, 100, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Vermetten, E.; Vythilingam, M.; Southwick, S.M.; Charney, D.S.; Bremner, J.D. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry 2003, 54, 693–702. [Google Scholar] [CrossRef]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol. Disord. Drug Targets 2008, 7, 46–62. [Google Scholar] [CrossRef]
- Frodl, T.; Schüle, C.; Schmitt, G.; Born, C.; Baghai, T.; Zill, P.; Bottlender, R.; Rupprecht, R.; Bondy, B.; Reiser, M.; et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch. Gen. Psychiatry 2007, 64, 410. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, J.M.; Lee, J.Y.; Kim, S.Y.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Kim, H.R.; Shin, M.G.; Yoon, J.S. BDNF promoter methylation and suicidal behavior in depressive patients. J. Affect. Disord. 2013, 151, 679–685. [Google Scholar] [CrossRef]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- MacQueen, G.M.; Ramakrishman, K.; Croll, S.D.; Siuciak, J.A.; Yu, G.; Young, T.; Fahnestock, M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav. Neurosci. 2001, 115, 1145–1153. [Google Scholar] [CrossRef]
- Karege, F.; Bondolfi, G.; Gervasoni, N.; Schwald, M.; Aubry, J.-M.; Bertschy, G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 2005, 57, 1068–1072. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.; Park, S.H.; Kim, Y.K. Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 2007, 101, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E. Neurotrophic effects of antidepressant drugs. Curr. Opin. Pharmacol. 2004, 4, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ohira, K.; Hayashi, M. A new aspect of the TrkB signaling pathway in neural plasticity. Curr. Neuropharmacol. 2009, 7, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Luborsky, L.; McKay, J.R.; Rosenthal, R.; Houldin, A.; Tax, A.; McCorkle, R.; Seligman, D.A.; Schmidt, K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav. Immun. 2001, 15, 199–226. [Google Scholar] [CrossRef]
- Maes, M. Cytokines in major depression. Biol. Psychiatry 1994, 36, 498–499. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Talarowska, M.; Bobińska, K.; Zajączkowska, M.; Su, K.P.; Maes, M.; Gałecki, P. Impact of oxidative/nitrosative stress and inflammation on cognitive functions in patients with recurrent depressive disorders. Med. Sci. Monit. 2014, 20, 110–115. [Google Scholar]
- Kubera, M.; Symbirtsev, A.; Basta-Kaim, A.; Borycz, J.; Roman, A.; Papp, M.; Claesson, M. Effect of chronic treatment with imipramine on interleukin 1 and interleukin 2 production by splenocytes obtained from rats subjected to a chronic mild stress model of depression. Pol. J. Pharmacol. 1996, 48, 503–506. [Google Scholar]
- Yirmiya, R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996, 711, 163–174. [Google Scholar] [CrossRef]
- Sakic, B.; Gauldie, J.; Denburg, J.A.; Szechtman, H. Behavioral effects of infection with IL-6 adenovector. Brain Behav. Immun. 2001, 15, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H.; Kokkinidis, L.; Merali, Z. Further evidence for the depressive effects of cytokines: Anhedonia and neurochemical changes. Brain Behav. Immun. 2002, 16, 544–556. [Google Scholar] [CrossRef]
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmächer, T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 2001, 58, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Bonsall, D.R.; Kim, H.; Tocci, C.; Ndiaye, A.; Petronzio, A.; McKay-Corkum, G.; Molyneux, P.C.; Scammell, T.E.; Harrington, M.E. Suppression of locomotor activity in female C57Bl/6J mice treated with interleukin-1β: Investigating a method for the study of fatigue in laboratory animals. PLoS ONE 2015, 10, e0140678. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Berkman, E.T.; Inagaki, T.K.; Rameson, L.T.; Mashal, N.M.; Irwin, M.R. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 2010, 68, 748–754. [Google Scholar] [CrossRef]
- Bossù, P.; Cutuli, D.; Palladino, I.; Caporali, P.; Angelucci, F.; Laricchiuta, D.; Gelfo, F.; De Bartolo, P.; Caltagirone, C.; Petrosini, L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J. Neuroinflamm. 2012, 9, 101. [Google Scholar] [CrossRef]
- Talarowska, M.; Szemraj, J.; Gałecki, P. The role of interleukin genes in the course of depression. Open Med. 2016, 11, 41–48. [Google Scholar] [CrossRef]
- Berthold-Losleben, M.; Himmerich, H. The TNF-α system: Functional aspects in depression, narcolepsy and psychopharmacology. Curr. Neuropharmacol. 2008, 6, 193–202. [Google Scholar] [CrossRef]
- Catena-Dell’Osso, M.; Bellantuono, C.; Consoli, G.; Baroni, S.; Rotella, F.; Marazziti, D. Inflammatory and neurodegenerative pathways in depression: A new avenue for antidepressant development? Curr. Med. Chem. 2011, 18, 245–255. [Google Scholar] [CrossRef]
- Mushroon Wisdom. Amyloban® 3399 from Lion’s Mane. 2019. Available online: http://www.mushroomwisdom.com/products_detail.php?product_id=37&productCat=amyloban&maitake_id= (accessed on 5 August 2019).
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef]
- Chu, X.; Zhou, Y.; Hu, Z.; Lou, J.; Song, W.; Li, J.; Liang, X.; Chen, C.; Wang, S.; Yang, B.; et al. 24-hour-restraint stress induces long-term depressive-like phenotypes in mice. Sci. Rep. 2016, 6, 32935. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: An analysis in human postmortem brain. Clin. Med. Insights Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Inanaga, K. Marked improvement of neurocognitive impairment after treatment with compounds from Hericium erinaceum: A case study of recurrent depressive disorder. Pers. Med. Universe 2014, 3, 46–48. [Google Scholar] [CrossRef]
- Okamura, H.; Anno, N.; Tsuda, A.; Inokuchi, T.; Uchimura, N.; Inanaga, K. The effects of Hericium erinaceus (Amyloban® 3399) on sleep quality and subjective well-being among female undergraduate students: A pilot study. Pers. Med. Universe 2015, 4, 76–78. [Google Scholar] [CrossRef]
- Vigna, L.; Morelli, F.; Agnelli, G.M.; Napolitano, F.; Ratto, D.; Occhinegro, A.; Di Iorio, C.; Savino, E.; Girometta, C.; Brandalise, F.; et al. Hericium erinaceus improves mood and sleep disorders in patients affected by overweight or obesity: Could circulating pro-BDNF and BDNF be potential biomarkers? Evid. Based Complement. Altern. Med. 2019, 2019, 7861297. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25, 617–623. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Shinba, K.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Chromans, hericenones F, G and H from the mushroom Hericium erinaceum. Phytochemistry 1992, 32, 175–178. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996, 37, 7399–7402. [Google Scholar] [CrossRef]
- Kawagishi, H.; Simada, A.; Shizuki, K.; Ojima, F.; Mori, H.; Okamoto, K.; Sakamoto, H.; Furukawa, S. Erinacine D, a stimulator of NGF-synthesis, from the mycelia of Hericium erinaceum. Heterocycl. Commun. 1996, 2, 51–54. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561–4564. [Google Scholar] [CrossRef]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef]
- Yaoita, Y.; Danbara, K.; Kikuchi, M. Two new aromatic compounds from Hericium erinaceum (Bull.: Fr.) Pers. Chem. Pharm. Bull. 2005, 53, 1202–1203. [Google Scholar] [CrossRef]
- Lee, E.W.; Shizuki, K.; Hosokawa, S.; Suzuki, M.; Suganuma, H.; Inakuma, T.; Li, J.; Ohnishi-Kameyama, M.; Nagata, T.; Furukawa, S.; et al. Two novel diterpenoids, Erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 2000, 64, 2402–2405. [Google Scholar] [CrossRef]
- Kawagishi, H.; Masui, A.; Tokuyama, S.; Nakamura, T. Erinacines J and K from the mycelia of Hericium erinaceum. Tetrahedron 2006, 62, 8463–8466. [Google Scholar] [CrossRef]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a rare sesterterpene from the mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Kenmoku, H.; Shimai, T.; Toyomasu, T.; Kato, N.; Sassa, T. Erinacine Q, a new erinacine from Hericium erinaceum, and its biosynthetic route to erinacine C in the basidiomycete. Biosci. Biotechnol. Biochem. 2002, 66, 571–575. [Google Scholar] [CrossRef]
- Wang, X.L.; Gao, J.; Li, J.; Long, H.P.; Xu, P.S.; Xu, K.P.; Tan, G.S. Three new isobenzofuranone derivatives from the fruiting bodies of Hericium erinaceus. J. Asian Nat. Prod. Res. 2017, 19, 134–139. [Google Scholar] [CrossRef]
- Mesquita, A.R.; Correia-Neves, M.; Roque, S.; Gil Castro, A.; Vieira, P.; Pedrosa, J.; Palha, J.A.; Sousa, N. IL-10 modulates depressive-like behavior. J. Psychiatr. Res. 2008, 43, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Grutz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.A.; Gass, P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef]
- Schweizer, M.C.; Henniger, M.S.H.; Sillaber, I. Chronic mild stress (CMS) in mice: Of anhedonia, ‘anomalous anxiolysis’ and activity. PLoS ONE 2009, 4, e4326. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, P.S.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 163. https://doi.org/10.3390/ijms21010163
Chong PS, Fung M-L, Wong KH, Lim LW. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. International Journal of Molecular Sciences. 2020; 21(1):163. https://doi.org/10.3390/ijms21010163
Chicago/Turabian StyleChong, Pit Shan, Man-Lung Fung, Kah Hui Wong, and Lee Wei Lim. 2020. "Therapeutic Potential of Hericium erinaceus for Depressive Disorder" International Journal of Molecular Sciences 21, no. 1: 163. https://doi.org/10.3390/ijms21010163
APA StyleChong, P. S., Fung, M.-L., Wong, K. H., & Lim, L. W. (2020). Therapeutic Potential of Hericium erinaceus for Depressive Disorder. International Journal of Molecular Sciences, 21(1), 163. https://doi.org/10.3390/ijms21010163