GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula edodes Mycelial Metabolome under High-Temperature Stress
Abstract
1. Introduction
2. Results
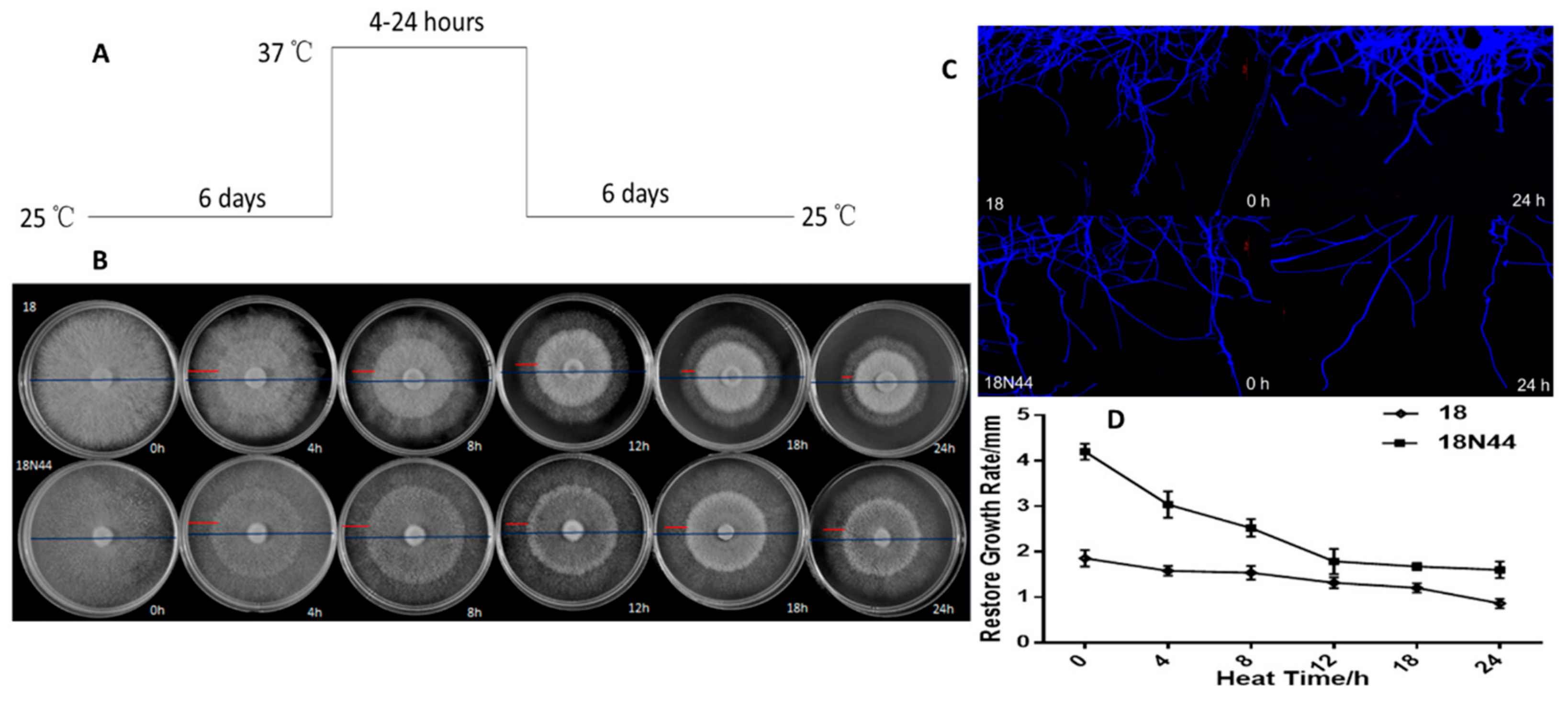
2.1. Physiological Study of Different Strains of L. edodes in Response to High-Temperature Stress
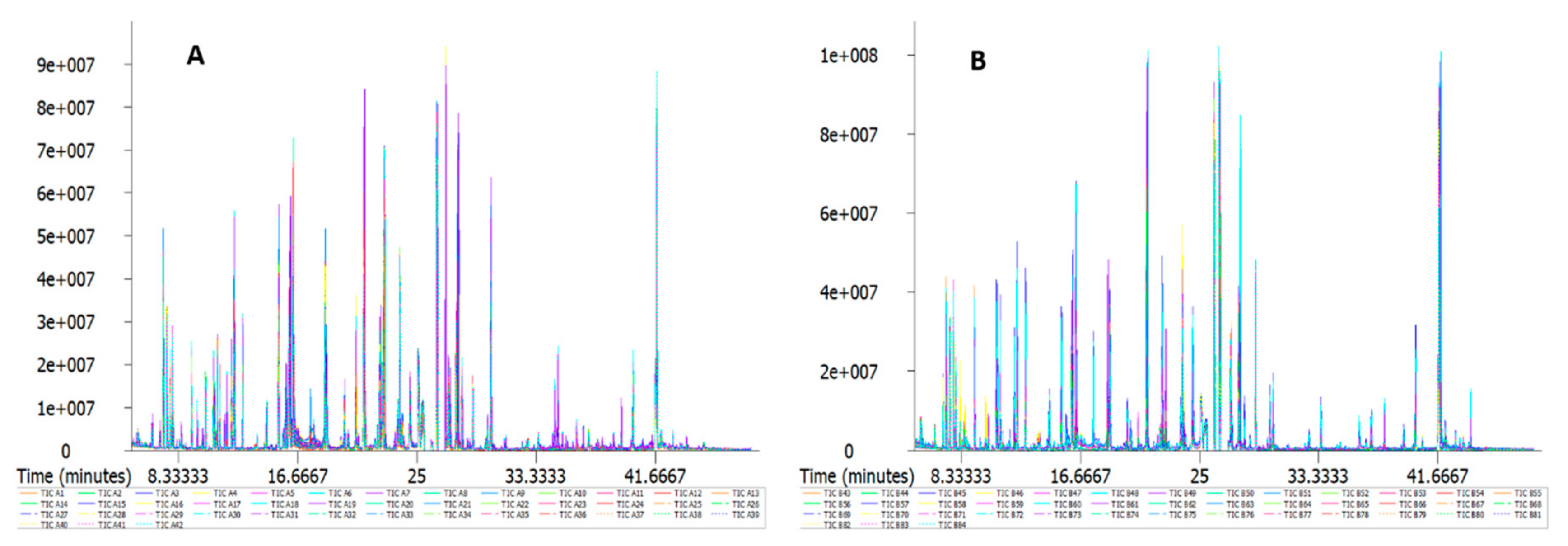
2.2. GC/MS Detection Results and Confirmation by Nontargeted Metabonomics
2.3. Identification of Compounds
2.4. Data Dimension Reduction Processing Result
2.5. Screening of Differential Metabolites and Pathway Enrichment Analysis
2.6. Pathway Analysis of Significantly Differential Metabolites
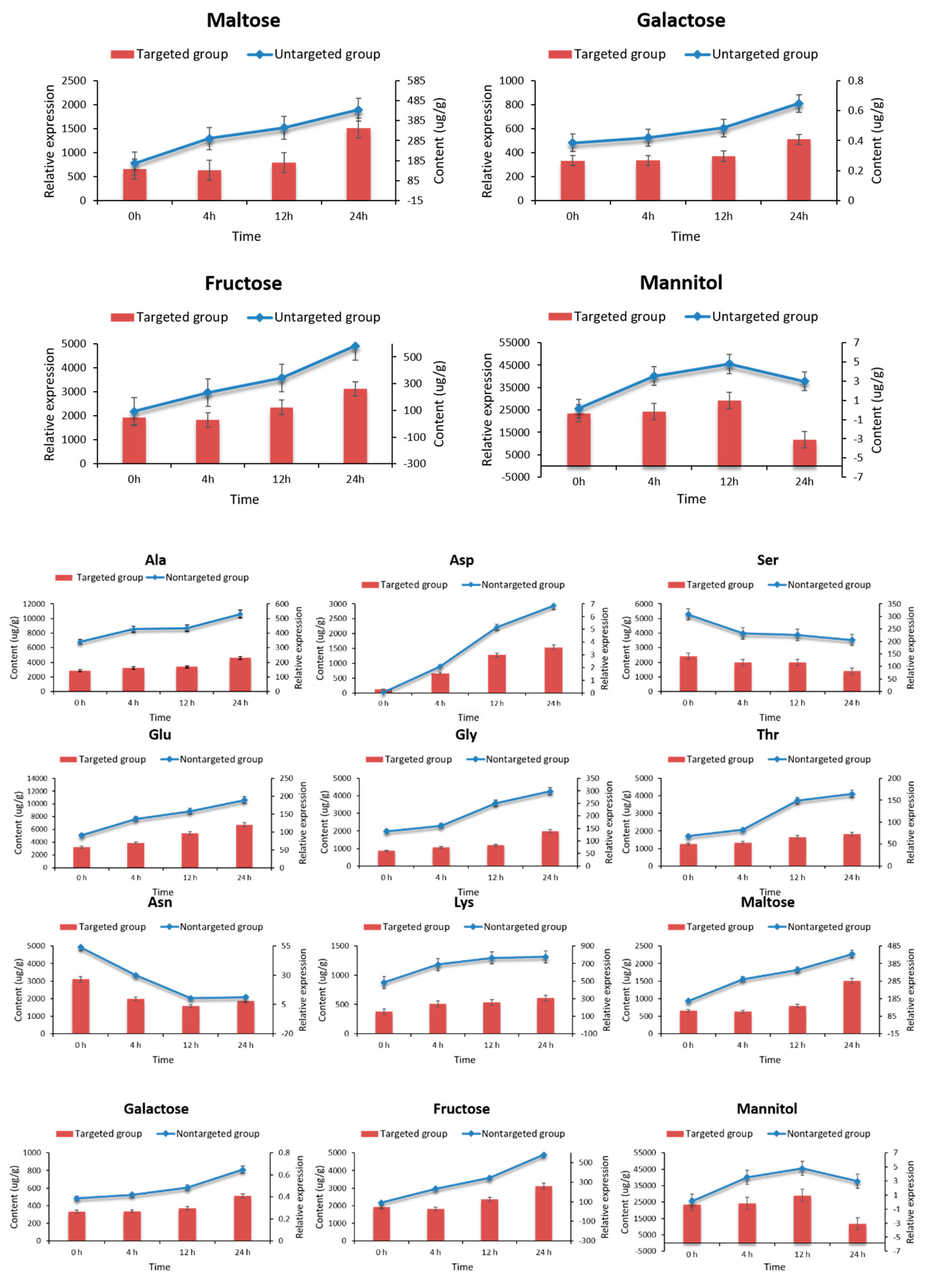
2.7. Targeted Metabolomic Analysis of Key Metabolites
3. Discussion
3.1. Physiological Study on Different Strains of L. edodes in Response to High-Temperature Stress
3.2. Metabonomic Study on Different Strains of L. edodes in Response to High-Temperature Stress
4. Materials and Methods
4.1. Materials and Design
4.1.1. Test Materials
4.1.2. Mycelial Culture and High-Temperature Stress Treatment
4.2. Physiological Determination of Mycelia under High-Temperature Stress
4.2.1. Morphology and Growth Rate of Mycelia
4.2.2. A Method for Determining the Conductivity of Mycelia Damaged by High Temperature
- (1)
- The cultured mycelia were filtered with nonwoven fabric and then rinsed with 200 mL double-distilled water, and the mycelial balls were placed into a triangular bottle again.
- (2)
- The cultured mycelial balls were treated by heat shock for 0 h, 4 h, 8 h, 12 h, 18 h, or 24 h. Then, the treated mycelial balls were put into 30 mL double-distilled water. At room temperature, the conductivity of the solution was determined to be E1.
- (3)
- The centrifuge tube used in the conductivity measurements was sterilized at 121 °C for 20 min and shaken on a shaker at room temperature at 150 rpm for 24 h. The conductivity was measured at E2 at room temperature.
- (4)
- Based on the following formula, the relative conductivity was determined as E% = (E1−E0)/(E2−E0), where E0 refers to the conductivity of double-distilled water [27].
4.2.3. Determination of the Content of Malondialdehyde (MDA) in L. edodes Mycelia under High-Temperature Stress
4.3. Chemicals
4.4. Sample Preparation
4.5. GC/MS Analysis
4.6. Data Preprocessing and Statistical Analysis
4.7. Selection of Differential Metabolites
4.8. Determination of Targeted Metabolites in Key Heat Stress Substances
4.8.1. Preparation of Standard Solution
4.8.2. Sample Treatment Shown in Section 4.4
4.8.3. GC–MS Analysis Method Shown in Section 4.5
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- An, J.; Wang, C.; Liu, G.; Yang, X.; Zhang, X.; Li, J. Analysis of volatile aroma components of fresh and dry Lentinus edodes with gas chromatography-mass spectrometry (GC-MS). Sci. Technol. Food Ind. 2012, 33, 68–71. [Google Scholar]
- Angelcheva, L.; Mishra, Y.; Antti, H.; Kjellsen, T.D.; Funk, C.; Strimbeck, R.G.; Schrã Der, W.P. Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata). New Phytol. 2014, 204, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Baldanzi, G. Role of p38 map kinase in glycine-induced hepatocyte resistance to hypoxic injury. J. Hepatol. 2007, 46, 692–699. [Google Scholar]
- Bao, Y.; Yang, N.; Cang, j.; Feng, M.; lv, Y.; Peng, G.; Tian, Y.; Zhang, D.; Wang, J.; Meng, J. Metabolomic Profiling of Winter Dongnongmai 1Grown at Different Temperatures. J. Triticeae Crop. 2017, 37, 647–655. [Google Scholar]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Degradation and Comparative Metabolomics of Volvariella volvacea Mycelium during Its Subculture. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2017. [Google Scholar]
- Chen, W.; Yang, Y.; Li, W.; Jiang, J.; Yu, H.; Feng, J.; Li, X.; Liu, K. Analysis of Volatile Components in Lentinula edodes by SPME-GC-MS and Establishment of Fingerprint. J. Food Sci. Biotechnol. 2016, 35, 1074–1080. [Google Scholar]
- Chu, C.C.; Chi, T.H. Identification of sulfurous compounds of Shiitake mushroom (Lentinus edodes Sing). J. Agric. Food Chem. 1986, 34, 830–833. [Google Scholar]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef]
- Deng, K.; Zhang, R. Determination of molecular weight of polymer by infrared spectroscopy. J. Hebei Univ. 1996, 35, 62. [Google Scholar]
- Fatma, K.; Joachim, K.; Yul, S.D.; Wei, Z.; Mick, P.; Ron, P.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. Cell Mol. Biol. 2010, 50, 967–981. [Google Scholar]
- Gray, G.R.; Heath, D. A global reorganization of the metabolome in Arabidopsis during cold acclimation is revealed by metabolic fingerprinting. Physiol. Plant. 2010, 124, 236–248. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2010, 132, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.A.; Dana, W.; Susanne, F.; Oliver, F.; Heyer, A.G.; Hincha, D.K. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol. 2006, 142, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hiraide, M.; Kato, A.; Nakashima, T. The smell and odorous components of dried shiitake mushroom, Lentinula edodes V: Changes in lenthionine and lentinic acid contents during the drying process. J. Wood Sci. 2010, 56, 477–482. [Google Scholar] [CrossRef]
- Hiraide, M.; Nakashima, T.; Fujiwara, T. The smell and odorous components of dried shiitake mushroom, Lentinula edodes VI: Increase in odorous compounds of dried shiitake mushroom cultivated on bed logs. J. Wood Sci. 2010, 56, 483–487. [Google Scholar] [CrossRef]
- Hodge, S.; Ward, J.L.; Beale, M.H.; Bennett, M.; Mansfield, J.W.; Powell, G. Aphid-induced accumulation of trehalose in Arabidopsis thaliana is systemic and dependent upon aphid density. Planta 2013, 237, 1057–1064. [Google Scholar] [CrossRef]
- Huang, J.; Wu, N.; Song, J.; Zhang, L.; Jiang, T.; Li, J. Effects of γ-Glutamyl-transpeptidase and Cysteine Sulfoxide Lyase on Endogenous Formaldehyde Production in Shiitake Mushroom. J. Chin. Inst. Food Sci. Technol. 2013, 13, 55–58. [Google Scholar]
- Huang, N. Chinese Edible and Medicinal Bacteriology; Shanghai Scientific and Technical Publishers: Shanghai, China, 2010. [Google Scholar]
- Li, W.; Chen, W.; Yang, Y.; Zhang, J.; Feng, J.; Yu, H. Volatile Flavor Components and Flavor Quality Evaluation of Lentinula edodes Harvested at Different Growth Stages. J. Nucl. Agric. Sci. 2018, 32, 325–334. [Google Scholar]
- Liu, C. Distribution and biological characteristics of Lentinus edodes. For. By Prod. Spec. China 2001, 4, 32–33. [Google Scholar]
- Liu, H.; Yang, H. Physiological effects of glutamic acid and aspartic acid on buckwheat seedlings under high temperature stress. Jiangsu Agric. Sci. 2015, 43, 108–110. [Google Scholar]
- Long, J.; Wang, X.; Gao, H.; Liu, Z.; Liu, C.; Miao, M.; Liu, J. Malonaldehyde acts as a mitochondrial toxin: Inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci. 2006, 79, 1466–1472. [Google Scholar] [CrossRef]
- Lu, L.; Chen, M.; Xing, Z.; Chen, G.; Shao, Y.; Zhao, X. Effect of Perforated Packaging on the Quality, Physiological and Biochemical Indexes of Volvaria volvacea Fruit Bodies during Storage. Acta Edulis Fungi 2013, 20, 56–61. [Google Scholar]
- Keun, H.C.; Ebbels, T.M.; Bollard, M.E.; Beckonert, O.; Antti, H.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Geometric trajectory analysis of metabolic responses to toxicity can define treatment specific profiles. Chem. Res. Toxicol. 2004, 17, 579. [Google Scholar] [CrossRef] [PubMed]
- Ludmila, R.; Hongjian, L.; Joel, S.; Vladimir, S.; Sholpan, D.; Ron, M. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar]
- Mirzaei, M.; Yousefi, M.; Meskinfam, M. Density functional studies of oxygen-terminations versus hydrogen-terminations in carbon and silicon nanotubes. Solid State Sci. 2012, 14, 874–879. [Google Scholar] [CrossRef]
- Ni, J.; Yang, X.; Zhang, H.; Ni, Y.; Wu, H.; Wei, Q. Metabolomics and Its Application to Plant Stress Research. World For. Res. 2014, 27, 11–17. [Google Scholar]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, B.; Aaron, F.; David, B.; MoLler, S.G.; Hillel, F. Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar]
- Peng, Z.; Zhengfu, Z.; Wei, Z.; Min, L.; Ming, C.; Gehong, W. Global transcriptional analysis of Escherichia coli expressing IrrE, a regulator from Deinococcus radiodurans, in response to NaCl shock. Mol. Biosyst. 2015, 11, 1165–1171. [Google Scholar]
- Pineau, B.; Bourge, M.; Marion, J.; Mauve, C.; Gilard, F.; Maneta-Peyret, L.; Moreau, P.; Satiat-Jeunemaitre, B.; Brown, S.C.; De Paepe, R.; et al. The importance of cardiolipin synthase for mitochondrial ultrastructure, respiratory function, plant development, and stress responses in Arabidopsis. Plant Cell 2013, 25, 4195–4208. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.H.; Song, C.Y.; Tan, Q.; Chen, M.; Pan, Y. Use of ITS and ISSR markers to identify cultivated strains for Lentinula edodes. Mycosystema 2006, 25, 94–100. [Google Scholar]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell. Biochem. 2004, 256–257, 319–327. [Google Scholar] [CrossRef]
- Shan, X. Effect of Exogenous Spermidine on Carbon and Nitrogen Metabolism Mechanism in Tomato Seedlings under High Temperature Stress. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2010, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Chen, M. Monospore Cross-Breeding of Xianggu Mushroom (Lentinula edodes) ISSR Analysis of Thermo-Tolerant Hybrid. J. Microbiol. 2015, 3, 43–47. [Google Scholar]
- Wang, L.; Zhao, Y.; Zhang, B.; Chen, M. Breeding thermo-tolerant strains of Lentinula edodes by UV mutagenesis. Sci. Technol. Food Ind. 2014, 35, 171–174. [Google Scholar]
- Want, E.J.; Wilson, I.D.; Helen, G.; Georgios, T.; Plumb, R.S.; John, S.; Elaine, H.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Bueschl, C.; Schoefbeck, D.; Neumann, N.K.N.; Kluger, B.; Schuster, K.; Krska, R.; Adam, G.; Lemmens, M. GC–MS based targeted metabolic profiling identifies changes in the wheat metabolome following deoxynivalenol treatment. Metabolomics 2015, 11, 722–738. [Google Scholar] [CrossRef]
- Wei, J. Analysis of Gene Expression Level and Activity of Key Antioxidant Enzymes from Volvariella volvacea in Response to Cold Stress. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef]
- Wu, G.; Xie, B.; Jiang, Y.; Xiao, K.; Wang, D.; Peng, C.; Su, Y. Effect of Different Packaging Regimes on the Quality of Volvariella volvacea Fruit Bodes. Acta Edulis Fungi 2014, 21, 60–65. [Google Scholar]
- Xie, F.; Xie, B.; Lin, Y.; Yi, H.; Fu, R. Effect of 60Co-γ Ray Irradiation on the Physiological and Biochemical Indexes and the Fresh-keeping of V. volvacea. Acta Edulis Fungi 2005, 12, 43–48. [Google Scholar]
- Xie, H.L.; Pei-Wu, L.I.; Wang, X.P.; Zhang, Q.; Zhang, L.X.; Wang, T.; Zhang, W.; Wang, X.F. Study on Effects of Temperature on Metabolism of Aspergillus Flavus Based on Untargeted Metabolomics. J. Instrum. Anal. 2017, 26, 51–78. [Google Scholar]
- Xin, M. The Gene Expression and Function Study of Hydrophobin and Heat Shock Proteins from Lentinula edodes. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Xin, M.; Zhao, Y.; Huang, J.; Song, C.; Chen, M. Expression and Bioinformatic Analysis of Hydrophobin Protein Gene (hyd1) in Lentinula edodes under High Temperature Stress. Mol. Plant Breed. 2016, 112, 2645–2652. [Google Scholar]
- Xu, Z.; Song, X.; Li, Y.; Yu, C.; Yan, Z.; Ming, G.; Shen, X.; Chen, M. Gene expression related to trehalose metabolism and its effect on Volvariella volvacea under low temperature stress. Sci. Rep. 2018, 8, 11011. [Google Scholar]
- Yang, N.; Wang, C.L.; He, W.P.; Qu, Y.Z.; Li, Y.S. Photosynthetic characteristics and effects of exogenous glycine of Chorispora bungeana under drought stress. Photosynthetica 2016, 54, 459–467. [Google Scholar] [CrossRef]
- Ye, Y.; Zhu, Y.; Pan, L.; Li, L.; Wang, X.; Lin, Y. Gaining insight into the response logic of Saccharomyces cerevisiae to heat shock by combining expression profiles with metabolic pathways. Biochem. Biophys. Res. Commun. 2009, 385, 357–362. [Google Scholar] [CrossRef]
- Yuqing, W. Identification of Mushroom of Germplasm Resources and the Preservation Methods. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fujian, China, 2009. [Google Scholar]
- Zhang, J.; Xie, X.; Dong, Z. An evaluation on the heat tolerance of coolseason turf grasses under field heat stress. Pratacultural Sci. 2007, 24, 105–109. [Google Scholar]
- Zhao, W.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar]
- Zheng, S.; Zhang, G.; Zhang, X.; Cui, L.; Wang, H. Effect of Different Temperature on the Storage of Different Water Content of Edible Mushrooms. Edible Fungi China 2008, 27, 52–53. [Google Scholar]
- Zhou, L.; Li, Y.; Wang, W.; Zhong, S. Research Progress in the Metabolomics for Plants Response to Temperature Stress. J. Shanxi Agric. Sci. 2017, 45, 317–320. [Google Scholar]
- Jia, Z.; Lei, Z.; Yuwei, C.; Xin, L.; Zhen, Z.; Guowang, X. Alteration of leaf metabolism in Bt-transgenic rice (Oryza sativa L.) and its wild type under insecticide stress. J. Proteome Res. 2012, 11, 4351. [Google Scholar]
- Jianrong, L.; Ju, H.; Jie, Y.; Ning, W.; Jun, S.; Lei, Z.; Tianjia, J. Rapid purification and characterization of γ-glutamyl-transpeptidase from shiitake mushroom (Lentinus edodes). J. Food Sci. 2012, 77, C640–C645. [Google Scholar]
- Jing-Yu, L.; Zheng-He, Y.; Fang, L.; Xin-Rui, L.; Bao-Gui, X. Evaluation of the use of SCAR markers for screening genetic diversity of Lentinula edodes strains. Curr. Microbiol. 2012, 64, 317–325. [Google Scholar]
- Kantvilas, G. Ainsworth Bisby’s Dictionary of the Fungi, 9th ed.; Kirk, P.M., Cannon, P.F., David, J.C., Stalpers, J.A., Eds.; CAB International: Wallingford, UK, 2001; p. 655. ISBN 0-85199-377-X. [Google Scholar]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Kong, H.; Dai, W.; Xu, G. Advances of metabolite identification in liquid chromatography-mass spectrometry based metabolomics. Chin. J. Chromatogr. 2014, 32, 1052. [Google Scholar] [CrossRef]
- Li, L.; Ye, Y.; Pan, L.; Zhu, Y.; Zheng, S.; Lin, Y. The induction of trehalose and glycerol in Saccharomyces cerevisiae in response to various stresses. Biochem. Biophys. Res. Commun. 2009, 387, 778–783. [Google Scholar] [CrossRef] [PubMed]




 Strain 18 upregulation,
Strain 18 upregulation,  strain 18 downregulation.
strain 18 downregulation.  Strain 18N44 upregulation,
Strain 18N44 upregulation,  strain 18N44 downregulation. Gray indicates no significantly different metabolic.
strain 18N44 downregulation. Gray indicates no significantly different metabolic.
 Strain 18 upregulation,
Strain 18 upregulation,  strain 18 downregulation.
strain 18 downregulation.  Strain 18N44 upregulation,
Strain 18N44 upregulation,  strain 18N44 downregulation. Gray indicates no significantly different metabolic.
strain 18N44 downregulation. Gray indicates no significantly different metabolic.
| Different Metabolites | VIP | p | Different Metabolites | VIP | p |
|---|---|---|---|---|---|
| Alanine | 1.17812 | 4.88607 × 10−4 | Gallic acid | 1.30530 | 6.52562 × 10−5 |
| Aspartate/aspartic acid | 1.03574 | 3.17468 × 10−7 | Ethanolamine | 1.38919 | 4.47419 × 10−10 |
| Glutamate/L-glutamic acid | 1.35766 | 3.41897 × 10−7 | 2-aminoethanethiol | 1.35147 | 1.89102 × 10−6 |
| Proline | 1.03591 | 2.860688 × 10−3 | Dithioerythritol | 1.38845 | 2.77433 × 10−7 |
| Phenylalanine | 1.07183 | 7.741247 × 10−3 | Phytanic acid | 1.40061 | 1.16715 × 10−7 |
| Tyrosine | 1.33479 | 4.72696 × 10−4 | Adenine | 1.38915 | 8.3489 × 10−7 |
| Serine | 1.46222 | 1.20382 × 10−5 | Adenosine | 1.16933 | 7.23195 × 10−4 |
| L-glutamic acid | 1.02125 | 7.3379 × 10−5 | D-Fructose 1,6-bisphosphate | 1.17656 | 5.699884 × 10−3 |
| Glycine | 1.22217 | 2.05583 × 10−8 | Fructose-6-phosphate | 1.36802 | 1.73526 × 10−4 |
| Threonine | 1.17647 | 2.305898 × 10−3 | Alpha-ketoglutaric acid | 1.38852 | 2.1916 × 10−6 |
| Glutathione | 1.25819 | 1.36582 × 10−12 | 1,3-diaminopropane | 1.40000 | 4.07715 × 10−8 |
| Citrate cycle | 1.38953 | 6.38001 × 10−10 | 1-Methylhydantoin | 1.34342 | 2.69444 × 10−6 |
| Fructose | 1.22170 | 5.66071 × 10−8 | 3,6-Anhydro-D-galactose | 1.44528 | 7.65583 × 10−12 |
| Maltose | 1.24022 | 3.8289 × 10−13 | 5-Methoxytryptamine | 1.42654 | 3.86987 × 10−9 |
| Sophorose | 1.23167 | 4.39968 × 10−7 | Acetol | 1.37941 | 4.87405 × 10−8 |
| Trehalose | 1.07189 | 2.184293 × 10−3 | Allylmalonic acid | 1.30494 | 2.91429 × 10−5 |
| Sorbitol | 1.32470 | 1.68173 × 10−5 | Cycloleucine | 1.36591 | 2.25922 × 10−4 |
| 3-Hydroxypropionic acid | 1.41517 | 5.02333 × 10−9 | Dibenzofuran | 1.31198 | 5.32826 × 10−6 |
| 5-Aminovaleric acid | 1.42903 | 2.50953 × 10−9 | Gly-pro | 1.40607 | 1.92801 × 10−8 |
| Aminomalonic acid | 1.33618 | 3.96316 × 10−6 | N-Acetyl-D-galactosamine | 1.03300 | 4.551565 × 10−3 |
| Dehydroascorbic Acid | 1.42405 | 5.98067 × 10−6 | Octanal | 1.39123 | 4.41212 × 10−7 |
| Oxalic acid | 1.27637 | 1.62664 × 10−9 | Panthenol | 1.25303 | 7.58015 × 10−4 |
| Trehalose-6-phosphate | 1.24712 | 4.56643 × 10−6 | p-benzoquinone | 1.35201 | 2.09886 × 10−6 |
| Mannitol | 1.22484 | 1.58142 × 10−11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Chen, M.; Zhao, Y.; Zha, L.; Yang, H.; Wu, Y. GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula edodes Mycelial Metabolome under High-Temperature Stress. Int. J. Mol. Sci. 2019, 20, 2330. https://doi.org/10.3390/ijms20092330
Zhao X, Chen M, Zhao Y, Zha L, Yang H, Wu Y. GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula edodes Mycelial Metabolome under High-Temperature Stress. International Journal of Molecular Sciences. 2019; 20(9):2330. https://doi.org/10.3390/ijms20092330
Chicago/Turabian StyleZhao, Xu, Mingjie Chen, Yan Zhao, Lei Zha, Huanling Yang, and Yuejin Wu. 2019. "GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula edodes Mycelial Metabolome under High-Temperature Stress" International Journal of Molecular Sciences 20, no. 9: 2330. https://doi.org/10.3390/ijms20092330
APA StyleZhao, X., Chen, M., Zhao, Y., Zha, L., Yang, H., & Wu, Y. (2019). GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula edodes Mycelial Metabolome under High-Temperature Stress. International Journal of Molecular Sciences, 20(9), 2330. https://doi.org/10.3390/ijms20092330






