Circadian Regulation in Tissue Regeneration
Abstract
1. Introduction
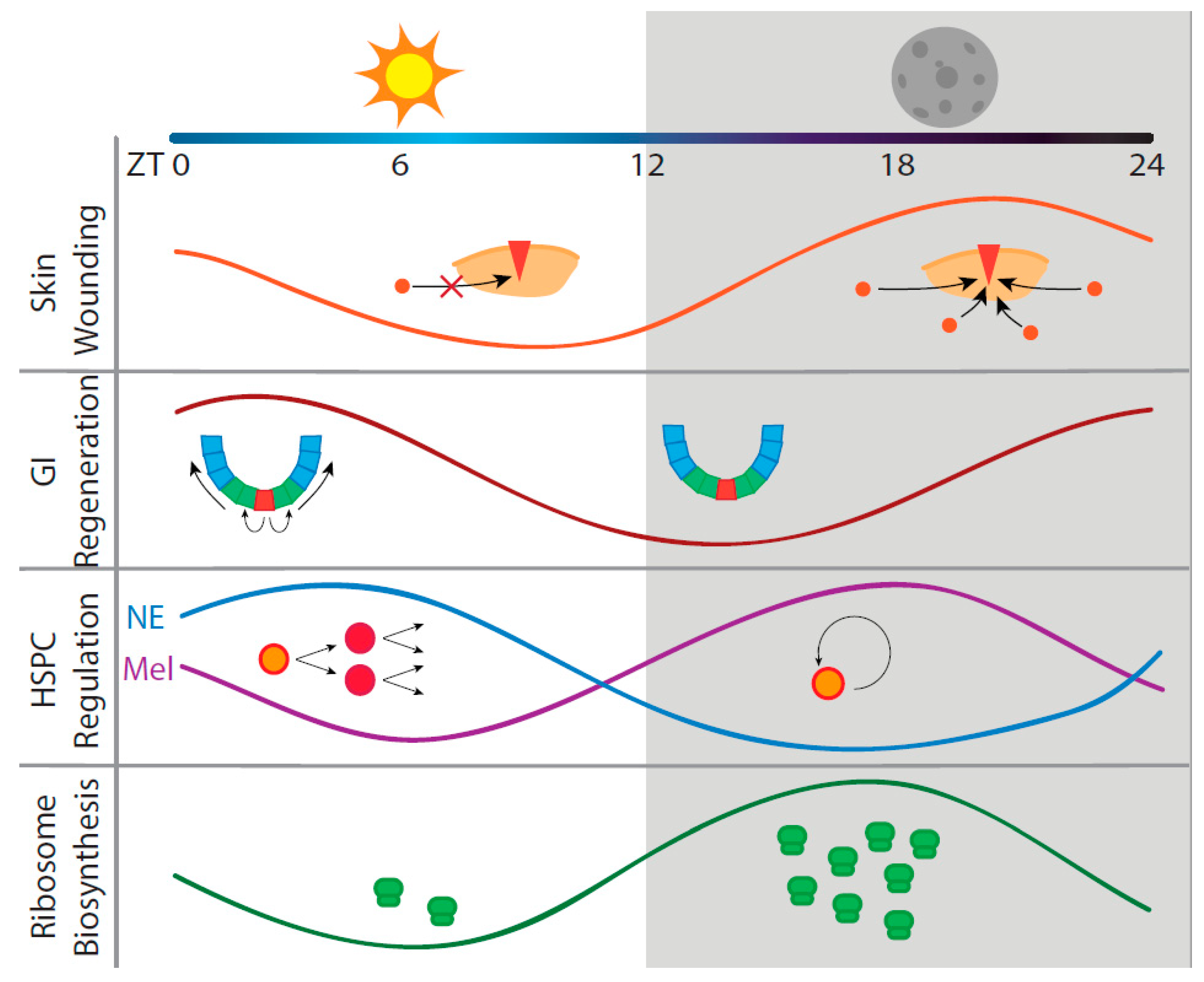
2. Circadian Regeneration in Three Representative Organ Systems
2.1. Skin Regeneration
2.2. Intestinal Regeneration
2.3. Hematopoietic Regeneration
3. Circadian Translation May Be a Factor in Regeneration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCN | Suprachiasmatic nucleus |
| ROR | Retinoic acid receptor-related orphan receptor |
| Rev-ErbA | Reverse c-erb A |
| RRE | Rev-ErbA/ROR response element |
| ROS | Reactive oxygen species |
| UV | Ultraviolet |
| CBC | Crypt-base columnar cell |
| ISC | Intestinal stem cell |
| TA | Transit-amplifying |
| EC | Enterocyte |
| GC | Goblet cell |
| EEC | Enteroendocrine cell |
| PC | Paneth cell |
| GI | Gastrointestinal |
| DSS | Dextran–sodium sulfate |
| CRD | Circadian rhythm disruption |
| RNAi | RNA interference |
| ZT | Zeitgeber time |
| BM | Bone marrow |
| HSC | Hematopoietic stem cell |
| HSPC | Hematopoietic stem and progenitor cell |
| MPP | Multipotent progenitor |
| LT-HSC | Long-term hematopoietic stem cell |
| SP | Side population |
| CAR | CXCL12-abundant reticular |
| NE | Norepinephrine |
| TNF | Tumor necrosis factor |
| MAPK | Mitogen-activated protein kinase |
References
- Refinetti, R. Comparison of light, food, and temperature as environmental synchronizers of the circadian rhythm of activity in mice. J. Physiol. Sci. 2015, 65, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Phenotyping Circadian Rhythms in Mice. Curr. Protoc. Mouse Biol. 2015, 5, 271–281. [Google Scholar] [CrossRef]
- Balsalobre, A.; Damiola, F.; Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998, 93, 929–937. [Google Scholar] [CrossRef]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacner, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, P.L.; Takahashi, J.S. Genetics of circadian rhythms in mammalian model organisms. Adv. Genet. 2011, 74, 175–230. [Google Scholar] [PubMed]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013, 3–27. [Google Scholar]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Ko, C.H.; Lowrey, P.L.; Buhr, E.D.; Song, E.; Chang, S.; Yoo, O.J.; Yamazaki, S.; Lee, C.; Takahashi, J.S. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Zylka, M.J.; Sriram, S.; Shearman, L.P.; Weaver, D.R.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M. mCRY1 and mCRY2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop. Cell 1999, 98, 193–205. [Google Scholar] [CrossRef]
- Griffin, E.A.; Staknis, D.; Weitz, C.J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 1999, 286, 768–771. [Google Scholar] [CrossRef]
- Sato, T.K.; Yamada, R.G.; Ukai, H.; Baggs, J.E.; Miraglia, L.J.; Kobayashi, T.J.; Welsh, D.K.; Kay, S.A.; Ueda, H.R.; Hogenesch, J.B. Feedback repression is required for mammalian circadian clock function. Nat. Genet. 2006, 38, 312–319. [Google Scholar] [CrossRef]
- Busino, L.; Bassermann, F.; Maiolica, A.; Lee, C.; Nolan, P.M.; Godinho, S.I.H.; Draetta, G.F.; Pagano, M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 2007, 316, 900–904. [Google Scholar] [CrossRef]
- Reischl, S.; Vanselow, K.; Westermark, P.O.; Thierfelder, N.; Maier, B.; Herzel, H.; Kramer, A. β-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J. Biol. Rhythms 2007, 22, 375–386. [Google Scholar] [CrossRef]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; Fitzgerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Weger, M.; Diotel, N.; Dorsemans, A.C.; Dickmeis, T.; Weger, B.D. Stem cells and the circadian clock. Dev. Biol. 2017, 431, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, P.; Van Laake, L.W.; Geijsen, N. Circadian clocks: From stem cells to tissue homeostasis and regeneration. EMBO Rep. 2018, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Yamaguchi, S.; Mitsui, S.; Emi, A.; Shimoda, F.; Okamura, H. Control mechanism of the circadian clock for timing of cell division in vivo. Science 2003, 302, 255–259. [Google Scholar] [CrossRef]
- Masri, S.; Cervantes, M.; Sassone-Corsi, P. The circadian clock and cell cycle: Interconnected biological circuits. Curr. Opin. Cell Biol. 2013, 25, 730–734. [Google Scholar] [CrossRef]
- Soták, M.; Sumová, A.; Pácha, J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann. Med. 2014, 46, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, S.; Beaulieu-Laroche, L.; Blum, I.D.; Landgraf, D.; Welsh, D.K.; Storch, K.F.; Labrecque, N.; Cermakian, N. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 2017, 15, 13. [Google Scholar] [CrossRef]
- Gery, S.; Koeffler, H.P. Circadian rhythms and cancer. Cell Cycle 2010, 9, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Okyar, A.; Dulong, S.; Innominato, P.F.; Clairambault, J. Circadian Timing in Cancer Treatments. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 377–421. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Przybek, M. Circadian Genes in Breast Cancer. Adv. Clin. Chem. 2016, 75, 53–70. [Google Scholar]
- Hoyle, N.P.; Seinkmane, E.; Putker, M.; Feeney, K.A.; Krogager, T.P.; Chesham, J.E.; Bray, L.K.; Thomas, J.M.; Dunn, K.; Blaikley, J.; et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci. Transl. Med. 2017, 9, eaal2774. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.; Cooke, A.; Chang, H.; Weaver, D.R.; Breault, D.T.; Karpowicz, P. The Circadian Clock Gene BMAL1 Coordinates Intestinal Regeneration. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Kumari, A.; Kollet, O.; Khatib-Massalha, E.; Subramaniam, M.D.; Ferreira, Z.S.; Avemaria, F.; Rzeszotek, S.; García-García, A.; Xie, S.; et al. Daily Onset of Light and Darkness Differentially Controls Hematopoietic Stem Cell Differentiation and Maintenance. Cell Stem Cell 2018, 23, 572–585.e7. [Google Scholar] [CrossRef]
- Jouffe, C.; Cretenet, G.; Symul, L.; Martin, E.; Atger, F.; Naef, F.; Gachon, F. The Circadian Clock Coordinates Ribosome Biogenesis. PLoS Biol. 2013, 11, e1001455. [Google Scholar] [CrossRef]
- Martin, P. Wound healing - Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, B.K.; Wang, M.; Qin, X.J.; Parks, W.C.; Senior, R.M.; Welgus, H.G. Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. N. Y. Acad. Sci. 1999, 878, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.P.; Gil, S.G.; Carter, W.G. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J. Biol. Chem. 2000, 275, 31896–31907. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.J.; Cramer, L.P. Actin-based cell motility and cell locomotion. Cell 1996, 84, 371–379. [Google Scholar] [CrossRef]
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005, 11, 1351–1354. [Google Scholar] [CrossRef]
- Plikus, M.V.; Van Spyk, E.N.; Pham, K.; Geyfman, M.; Kumar, V.; Takahashi, J.S.; Andersen, B. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythms 2015, 30, 163–182. [Google Scholar] [CrossRef]
- Janich, P.; Toufighi, K.; Solanas, G.; Luis, N.M.; Minkwitz, S.; Serrano, L.; Lehner, B.; Benitah, S.A. Human Epidermal Stem Cell Function Is Regulated by Circadian Oscillations. Cell Stem Cell 2013, 13, 745–753. [Google Scholar] [CrossRef]
- Sporl, F.; Korge, S.; Jurchott, K.; Wunderskirchner, M.; Schellenberg, K.; Heins, S.; Specht, A.; Stoll, C.; Klemz, R.; Maier, B.; et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 10903–10908. [Google Scholar] [CrossRef]
- Janich, P.; Pascual, G.; Merlos-Suárez, A.; Batlle, E.; Ripperger, J.; Albrecht, U.; Cheng, H.Y.M.; Obrietan, K.; Di Croce, L.; Benitah, S.A. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 2011, 480, 209–214. [Google Scholar] [CrossRef]
- Lin, K.K.; Kumar, V.; Geyfman, M.; Chudova, D.; Ihler, A.T.; Smyth, P.; Paus, R.; Takahashi, J.S.; Andersen, B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009, 5, e1000573. [Google Scholar] [CrossRef]
- Plikus, M.V.; Vollmers, C.; de la Cruz, D.; Chaix, A.; Ramos, R.; Panda, S.; Chuong, C.-M. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc. Natl. Acad. Sci. USA 2013, 110, E2106–E2115. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Lee, J.; Tumbar, T. Hairy tale of signaling in hair follicle development and cycling. Semin. Cell Dev. Biol. 2012, 23, 906–916. [Google Scholar] [CrossRef]
- Stevens, C.P.; Leblond, C.E. Rate of renewal of the cells of the intestinal epithelium in the rat. Anat. Rec. 1947, 97, 373. [Google Scholar]
- Dubrovsky, G.; Dunn, J.C.Y. Mechanisms for intestinal regeneration. Curr. Opin. Pediatr. 2018, 30, 424–429. [Google Scholar] [CrossRef]
- van der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2008, 71, 241–260. [Google Scholar] [CrossRef]
- Hall, P.A.; Coates, P.J.; Ansari, B.; Hopwood, D. Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J. Cell Sci. 1994, 107, 3569–3577. [Google Scholar]
- Parasram, K.; Bernardon, N.; Hammoud, M.; Chang, H.; He, L.; Perrimon, N.; Karpowicz, P. Intestinal Stem Cells Exhibit Conditional Circadian Clock Function. Stem Cell Rep. 2018, 11, 1287–1301. [Google Scholar] [CrossRef]
- Pagel, R.; Bär, F.; Schröder, T.; Sünderhauf, A.; Künstner, A.; Ibrahim, S.M.; Autenrieth, S.E.; Kalies, K.; König, P.; Tsang, A.H.; et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. 2017, 31, 4707–4719. [Google Scholar] [CrossRef]
- Karpowicz, P.; Zhang, Y.; Hogenesch, J.B.; Emery, P.; Perrimon, N. The circadian clock gates the intestinal stem cell regenerative state. Cell Rep. 2013, 3, 996–1004. [Google Scholar] [CrossRef]
- Matsu-Ura, T.; Dovzhenok, A.; Aihara, E.; Rood, J.; Le, H.; Ren, Y.; Rosselot, A.E.; Zhang, T.; Lee, C.; Obrietan, K.; et al. Intercellular Coupling of the Cell Cycle and Circadian Clock in Adult Stem Cell Culture. Mol. Cell 2016, 64, 900–912. [Google Scholar] [CrossRef]
- Yu, X.; Rollins, D.; Ruhn, K.A.; Stubblefield, J.J.; Green, C.B.; Kashiwada, M.; Rothman, P.B.; Takahashi, J.S.; Hooper, L. V TH17 cell differentiation is regulated by the circadian clock. Science 2013, 342, 727–730. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Scheving, L.E.; Tsai, T.H.; Scheving, L.A. Chronobiology of the intestinal tract of the mouse. Am. J. Anat. 1983, 168, 433–465. [Google Scholar] [CrossRef]
- Al-Nafussi, A.I.; Wright, N.A. Circadian rhythm in the rate of cellular proliferation and in the size of the functional compartment of mouse jejunal epithelium. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1982, 40, 71–79. [Google Scholar] [CrossRef]
- Potten, C.S.; Al-Barwari, S.E.; Hume, W.J.; Searle, J. Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinet. 1977, 10, 557–568. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Gombert, M. Circadian rhythms in the pathogenesis of gastrointestinal diseases. World J. Gastroenterol. 2018, 24, 4297–4303. [Google Scholar] [CrossRef]
- Nojkov, B.; Rubenstein, J.H.; Chey, W.D.; Hoogerwerf, W.A. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am. J. Gastroenterol. 2010, 105, 842–847. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150. [Google Scholar]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue Damage-Induced Intestinal Stem Cell Division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef]
- Parkar, S.; Kalsbeek, A.; Cheeseman, J. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Calvi, L.M.; Link, D.C. Cellular complexity of the bone marrow hematopoietic stem cell niche. Calcif. Tissue Int. 2014, 94, 112–124. [Google Scholar] [CrossRef]
- Hoffman, C.M.; Calvi, L.M. Minireview: Complexity of Hematopoietic Stem Cell Regulation in the Bone Marrow Microenvironment. Mol. Endocrinol. 2014, 28, 1592–1601. [Google Scholar] [CrossRef]
- Morrison, S.J.; Weissman, I.L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1994, 1, 661–673. [Google Scholar] [CrossRef]
- Seita, J.; Weissman, I.L. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Chow, A.; Merad, M.; Frenette, P.S. Circadian rhythms influence hematopoietic stem cells. Curr. Opin. Hematol. 2009, 16, 235–242. [Google Scholar] [CrossRef]
- Tsinkalovsky, O.; Rosenlund, B.; Laerum, O.D.; Eiken, H.G. Clock gene expression in purified mouse hematopoietic stem cells. Exp. Hematol. 2005, 33, 100–107. [Google Scholar] [CrossRef]
- Tsinkalovsky, O.; Filipski, E.; Rosenlund, B.; Sothern, R.B.; Eiken, H.G.; Wu, M.W.; Claustrat, B.; Bayer, J.; Lévi, F.; Laerum, O.D. Circadian expression of clock genes in purified hematopoietic stem cells is developmentally regulated in mouse bone marrow. Exp. Hematol. 2006, 34, 1248–1260. [Google Scholar] [CrossRef]
- Lucas, D.; Battista, M.; Shi, P.A.; Isola, L.; Frenette, P.S. Mobilized Hematopoietic Stem Cell Yield Depends on Species-Specific Circadian Timing. Cell Stem Cell 2008, 3, 364–366. [Google Scholar] [CrossRef]
- Kollet, O.; Vagima, Y.; D’Uva, G.; Golan, K.; Canaani, J.; Itkin, T.; Gur-Cohen, S.; Kalinkovich, A.; Caglio, G.; Medaglia, C.; et al. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and CXCL12 expression by bone marrow stromal progenitors. Leukemia 2013, 27, 2006–2015. [Google Scholar] [CrossRef]
- Hemmers, S.; Rudensky, A.Y. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep. 2015, 11, 1339–1349. [Google Scholar] [CrossRef]
- Bollinger, T.; Leutz, A.; Leliavski, A.; Skrum, L.; Kovac, J.; Bonacina, L.; Benedict, C.; Lange, T.; Westermann, J.; Oster, H.; et al. Circadian Clocks in Mouse and Human CD4+ T Cells. PLoS ONE 2011, 6, e29801. [Google Scholar] [CrossRef]
- Silver, A.C.; Arjona, A.; Hughes, M.E.; Nitabach, M.N.; Fikrig, E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain. Behav. Immun. 2012, 26, 407–413. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.I.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.-J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive Oxygen Species Regulate Hematopoietic Stem Cell Self-Renewal, Migration and Development, As Well As Their Bone Marrow Microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef]
- So, A.Y.-L.; Bernal, T.U.; Pillsbury, M.L.; Yamamoto, K.R.; Feldman, B.J. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc. Natl. Acad. Sci. USA 2009, 106, 17582–17587. [Google Scholar] [CrossRef] [PubMed]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA 2013, 111, 167–172. [Google Scholar] [CrossRef]
- Kojima, S.; Shingle, D.L.; Green, C.B. Post-transcriptional control of circadian rhythms. J. Cell Sci. 2011, 124, 311–320. [Google Scholar] [CrossRef]
- Lim, C.; Allada, R. Emerging roles for post-transcriptional regulation in circadian clocks. Nat. Neurosci. 2013, 16, 1544–1550. [Google Scholar] [CrossRef]
- Preußner, M.; Heyd, F. Post-transcriptional control of the mammalian circadian clock: Implications for health and disease. Pflugers Arch. 2016, 468, 983–991. [Google Scholar] [CrossRef]
- Shibata, S.; Hamada, T.; Tominaga, K.; Watanabe, S. An in vitro circadian rhythm of protein synthesis in the rat suprachiasmatic nucleus under tissue culture conditions. Brain Res. 1992, 584, 251–256. [Google Scholar] [CrossRef]
- Scammell, T.E.; Schwartz, W.J.; Smith, C.B. No evidence for a circadian rhythm of protein synthesis in the rat suprachiasmatic nuclei. Brain Res. 1989, 494, 155–158. [Google Scholar] [CrossRef]
- Huang, Y.; Ainsley, J.A.; Reijmers, L.G.; Jackson, F.R. Translational Profiling of Clock Cells Reveals Circadianly Synchronized Protein Synthesis. PLoS Biol. 2013, 11, e1001703. [Google Scholar] [CrossRef]
- Janich, P.; Arpat, A.B.; Castelo-Szekely, V.; Lopes, M.; Gatfield, D. Ribosome profiling reveals the rhythmic liver translatome and circadian clock regulation by upstream open reading frames. Genome Res. 2015, 25, 1848–1859. [Google Scholar] [CrossRef]
- Missra, A.; Ernest, B.; Lohoff, T.; Jia, Q.; Satterlee, J.; Ke, K.; von Arnim, A.G. The Circadian Clock Modulates Global Daily Cycles of mRNA Ribosome Loading. Plant Cell 2015, 27, 2582–2599. [Google Scholar] [CrossRef]
- Lipton, J.O.; Yuan, E.D.; Boyle, L.M.; Ebrahimi-Fakhari, D.; Kwiatkowski, E.; Nathan, A.; Güttler, T.; Davis, F.; Asara, J.M.; Sahin, M. The circadian protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell 2015, 161, 1138–1151. [Google Scholar] [CrossRef]
- Marti, A.R.; Patil, S.; Mrdalj, J.; Meerlo, P.; Skrede, S.; Pallesen, S.; Pedersen, T.T.; Bramham, C.R.; Grønli, J. No Escaping the Rat Race: Simulated Night Shift Work Alters the Time-of-Day Variation in BMAL1 Translational Activity in the Prefrontal Cortex. Front. Neural Circuits 2017, 11, 70. [Google Scholar] [CrossRef]
- Caster, S.Z.; Castillo, K.; Sachs, M.S.; Bell-Pedersen, D. Circadian clock regulation of mRNA translation through eukaryotic elongation factor eEF-2. Proc. Natl. Acad. Sci. USA 2016, 113, 9605–9610. [Google Scholar] [CrossRef]
| Cell Type | Model | Circadian Regulation Mechanism | Conclusions | Ref. |
|---|---|---|---|---|
| Mouse fibroblasts | Skin explants, in vivo, synchronized culture | Cell-intrinsic—actin lamellipodia formation | Correlation between high Per2 in night-time and increased mobilization and wound healing | [27] |
| Human keratinocytes | Synchronized keratinocyte culture, in vivo competition in nude mice | Cell-intrinsic differentiation or proliferation response | Transcriptome: high differentiation in early morning, high proliferation in evening | [41] |
| Epidermal biopsies, neonatal keratinocyte culture | Cortisol-induced KLF9 expression | High KLF9 in morning, associated with increased differentiation | [42] | |
| Mouse hair follicle bulge stem cells | In vivo reporter mouse | Cell-intrinsic Bmal1 and Per1/2 regulation | Circadian cycling maintains homeostasis of stem cell population | [43] |
| Mouse hair germ progenitors | In vivo mouse dorsal skin | Cell-intrinsic regulation of cell cycle | Clock genes regulate G1-S phase transition in hair germ | [44] |
| Mouse hair epithelial matrix cells | In vivo mouse dorsal skin–radiation hair loss | Cell-intrinsic regulation of cell cycle | More hair loss in morning during high mitotic activity, clock genes regulate G2-M phase transition | [45] |
| Cell Type | Model | Circadian Regulation Mechanism | Conclusions | Ref. |
|---|---|---|---|---|
| Drosophila crypt cells | In vivo physiological turnover, circadian knockouts | Intercellular niche signaling from ECs to ISCs | ISC rhythmicity influenced by ECs | [52] |
| Mouse crypt cells | In vivo DSS-induced colitis, circadian knockouts | Intercellular signaling | Arrythmicity leads to more severe colitis through loss of crypt cells and G2-M inhibition | [53] |
| Drosophila crypt cells | In vivo RNAi screens in DSS-induced colitis | Intercellular signaling of circadian factors | per transcript peaks ZT12-18, induces peak ISC mitosis at dawn, local signaling of clock components essential for G1-S phase | [54] |
| Mouse Paneth cells in crypt | Enteroid culture | Wnt secretion from PCs | PCs are necessary for pacemaker circadian regulation of ISC cell division | [55] |
| Mouse crypt cells | Radiation-induced GI syndrome in vivo and in enteroids | Circadian mitotic schedule in response to injury | Mitotic activity peak ZT0-4 and nadir ZT12-16 | [28] |
| Mouse Intestinal TH17 cells | In vivo intestine and colon | Balance of TH17 differentiation through competing Rev-Erb and Nfil3 | TH17 cells are pro-inflammatory, Nfil3 and Rev-Erb necessary to balance TH17 population—disruption exacerbates GI diseases | [56] |
| Mouse gut bacterial cells | Gut microbiome | Cyclical fluctuations in microbial population | Food intake timing can influence microbial effect on intestines | [57] |
| Cell Type | Model | Circadian Regulation Mechanism | Conclusions | Ref. |
|---|---|---|---|---|
| Mouse BM SP (LT-HSC enriched) | Isolated BM cells | Cell-intrinsic circadian clock in SP LT-HSCs | LT-HSCs show high Per1 and low Cry1 expression compared to total BM cells, only Per2 is oscillating in SP cells, irregular circadian clock in LT-HSCs | [72,73] |
| Mouse BM and blood cells | Isolated BM and blood cell culture | NE and CXCL12 signaling from BM nerve and CAR cells | Circulating HSPC number peaks at ZT5 and shows nadir at ZT17, NE from nerves downregulates CAR CXCL12 and induces HSC egress | [70] |
| Circulating human and mouse HSCs and HSPCs | Peripheral blood isolation at different time-points | CXCR4 and CXCL12 circadian regulation in the BM | Mice and humans showed opposite egress patterns, human egress peak in the evening | [74] |
| Mouse BM and circulating HSCs | In vivo, cultured BM and circulating HSCs | NE induced TNF bursts at light and dark onset | NE and TNF bursts at light onset induce HSPC differentiation and egress, while TNF and melatonin bursts at dark onset induce HSC self-renewal | [29] |
| Mouse BM and circulating HSPCs | In vivo in WT and Corticosterone deficient mice, cultured BM, and circulating HSPCs | Corticosterone modulation of BM CXCL12 secretion | Corticosterone peaks at dawn and downregulates CXCL12. Rhythmicity essential to balance HSPC egress and self-renewal | [75] |
| Model | Circadian Regulation Mechanism | Conclusion | Ref. |
|---|---|---|---|
| Mouse liver | Ribosomal mRNA association with polysomes | More ribosome subunit synthesis and assembly during nighttime. | [30] |
| Mouse liver | Ribosomal mRNA association with polysomes | Peak polysome association with ribosome transcripts ZT10-16, 150 non-oscillating transcripts had preferential translational timing based on function | [90] |
| Arabidopsis thaliana seedlings | Ribosomal mRNA association with polysomes | Proteins with daytime or nighttime function preferentially associate with ribosomes at that time of day. Ribosomal mRNAs bound to polysome at night | [91] |
| Mouse embryonic fibroblasts | Bmal1 association with translation machinery | S6K1-mediated phosphorylation of Bmal1 promotes its binding to mRNA cap-binding complex and increased translation | [92] |
| Neurospora crassa | Circadian MAPK phosphorylation of elongation factors | MAPK factors rhythmically phosphorylate eEF-2, increasing translation efficiency | [94] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paatela, E.; Munson, D.; Kikyo, N. Circadian Regulation in Tissue Regeneration. Int. J. Mol. Sci. 2019, 20, 2263. https://doi.org/10.3390/ijms20092263
Paatela E, Munson D, Kikyo N. Circadian Regulation in Tissue Regeneration. International Journal of Molecular Sciences. 2019; 20(9):2263. https://doi.org/10.3390/ijms20092263
Chicago/Turabian StylePaatela, Ellen, Dane Munson, and Nobuaki Kikyo. 2019. "Circadian Regulation in Tissue Regeneration" International Journal of Molecular Sciences 20, no. 9: 2263. https://doi.org/10.3390/ijms20092263
APA StylePaatela, E., Munson, D., & Kikyo, N. (2019). Circadian Regulation in Tissue Regeneration. International Journal of Molecular Sciences, 20(9), 2263. https://doi.org/10.3390/ijms20092263




