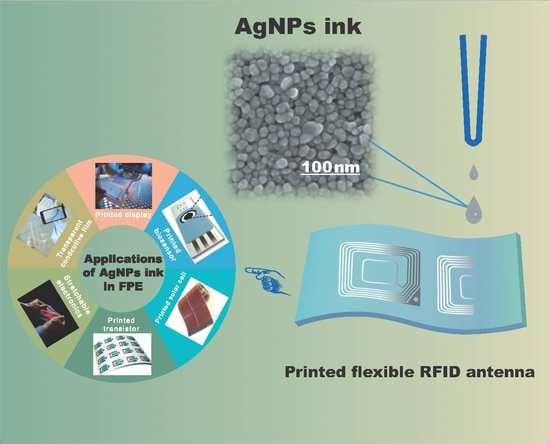
Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics
Abstract
1. Introduction
2. Strategies of Achieving Highly Conductive Ag NPs Based Ink under Moderate Sintering
2.1. Protective Agents
2.2. Ag NPs Sizes and Shapes
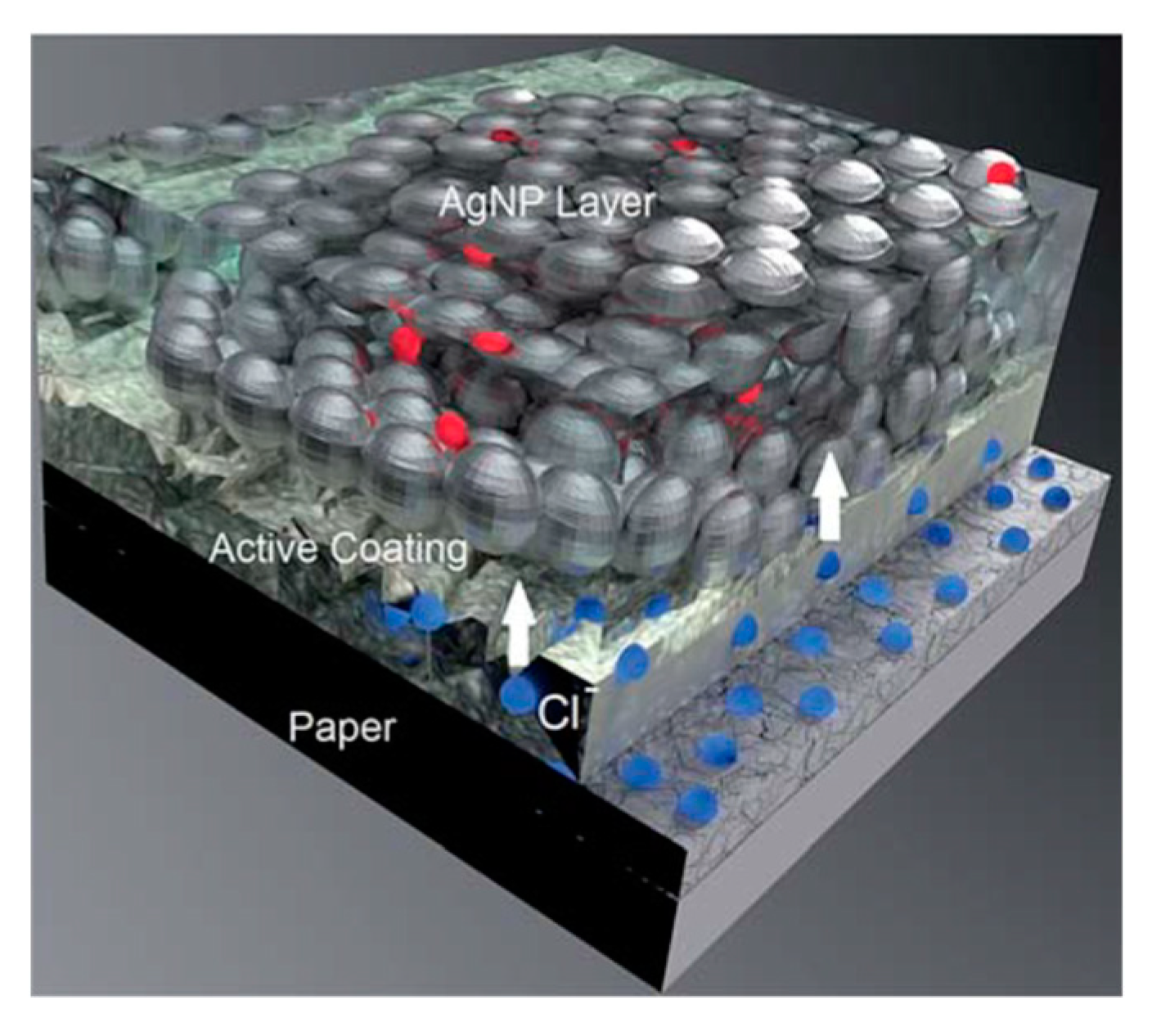
2.3. Substrate Facilitated Sintering
2.4. Photonic Sintering Method
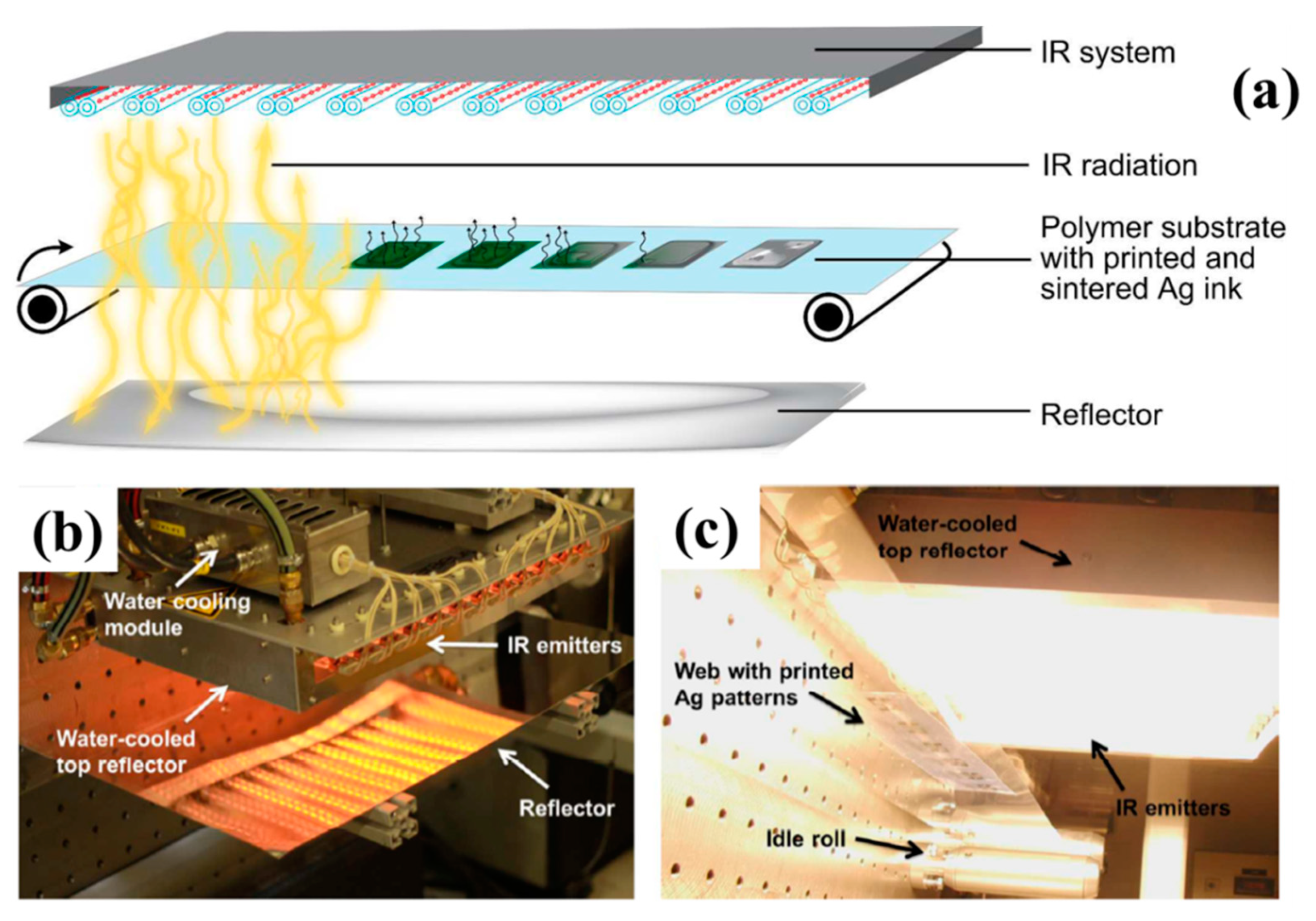
2.4.1. Infra-Red (IR) Sintering
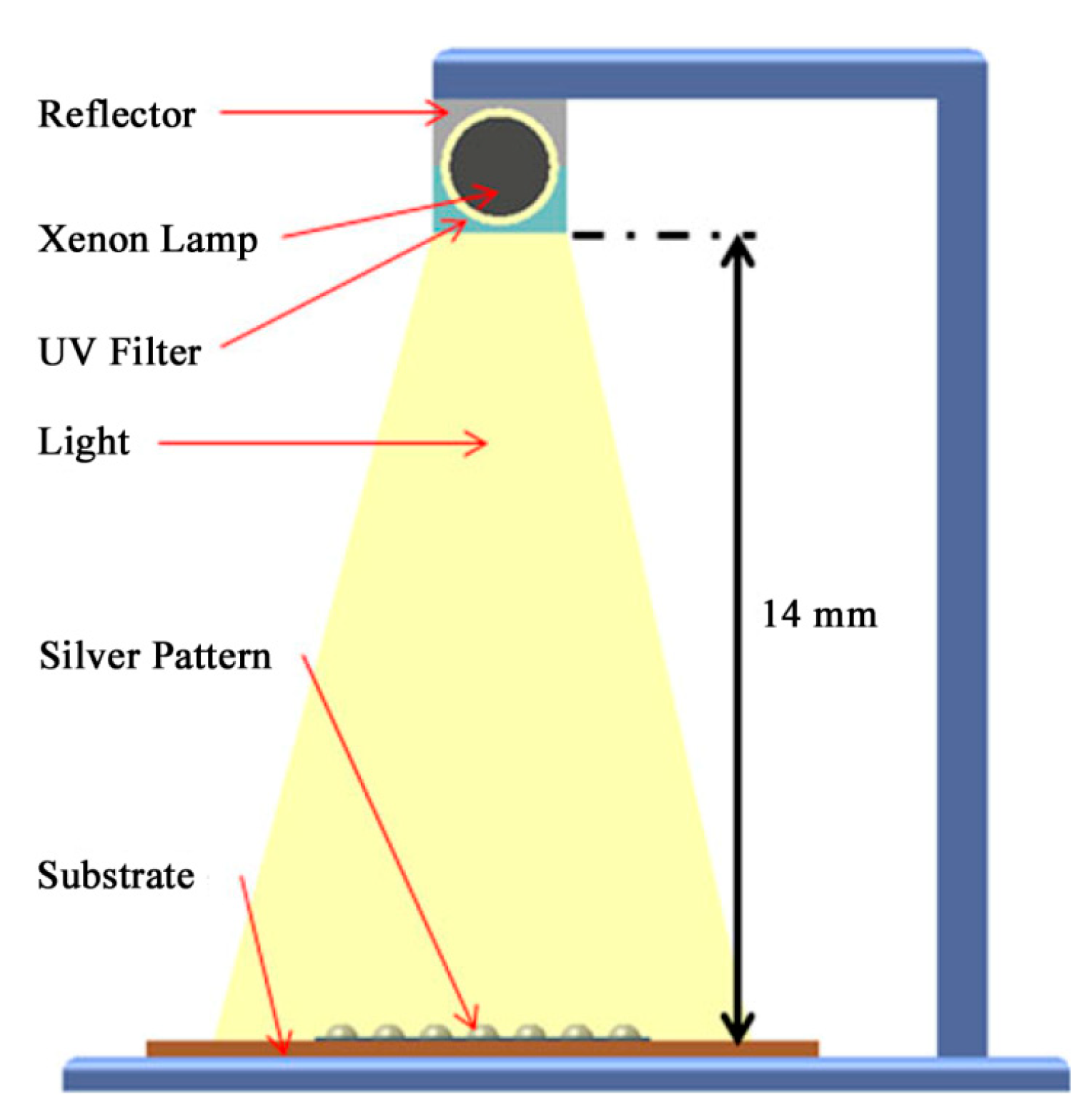
2.4.2. Intense Pulsed Light (IPL) Sintering
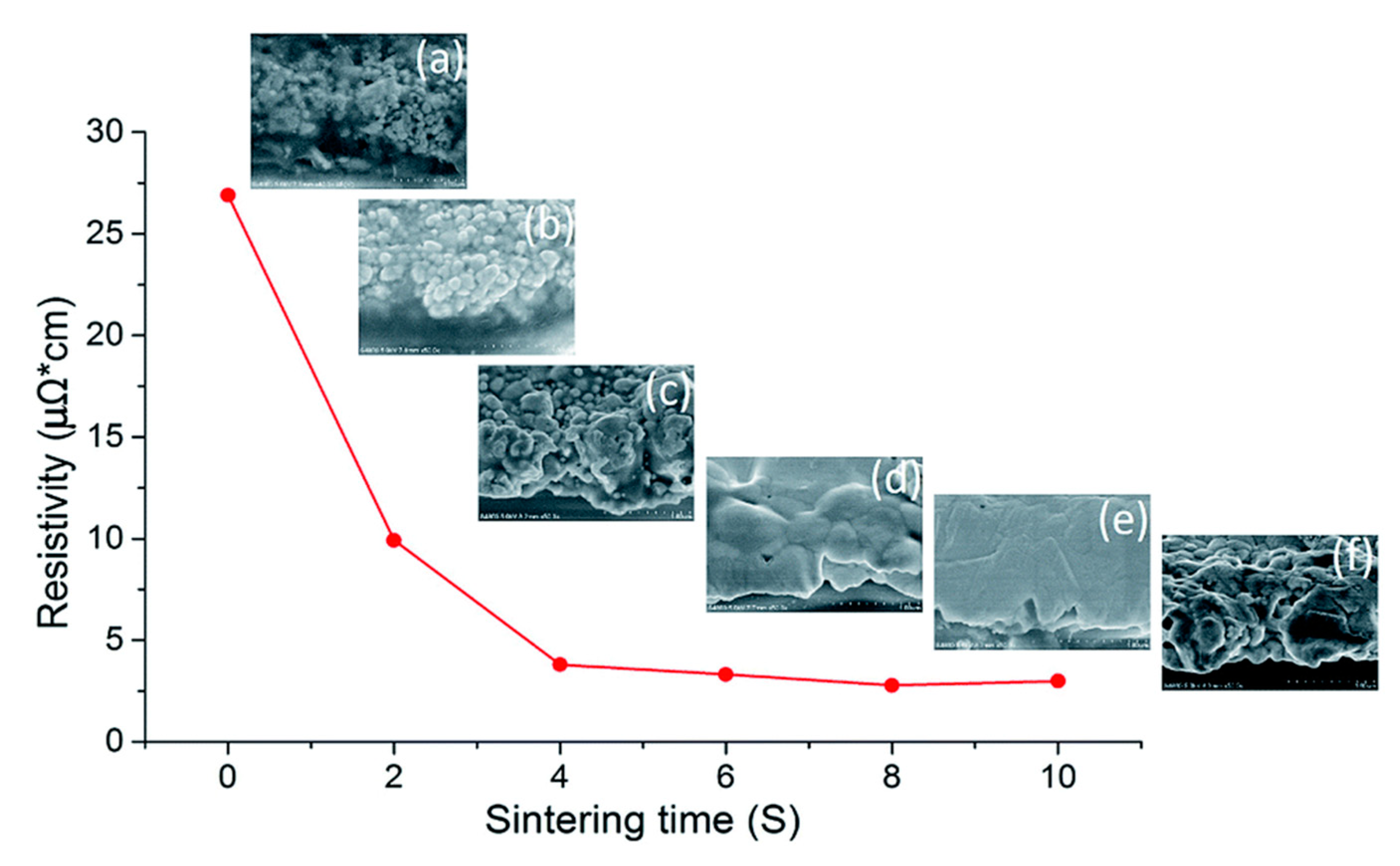
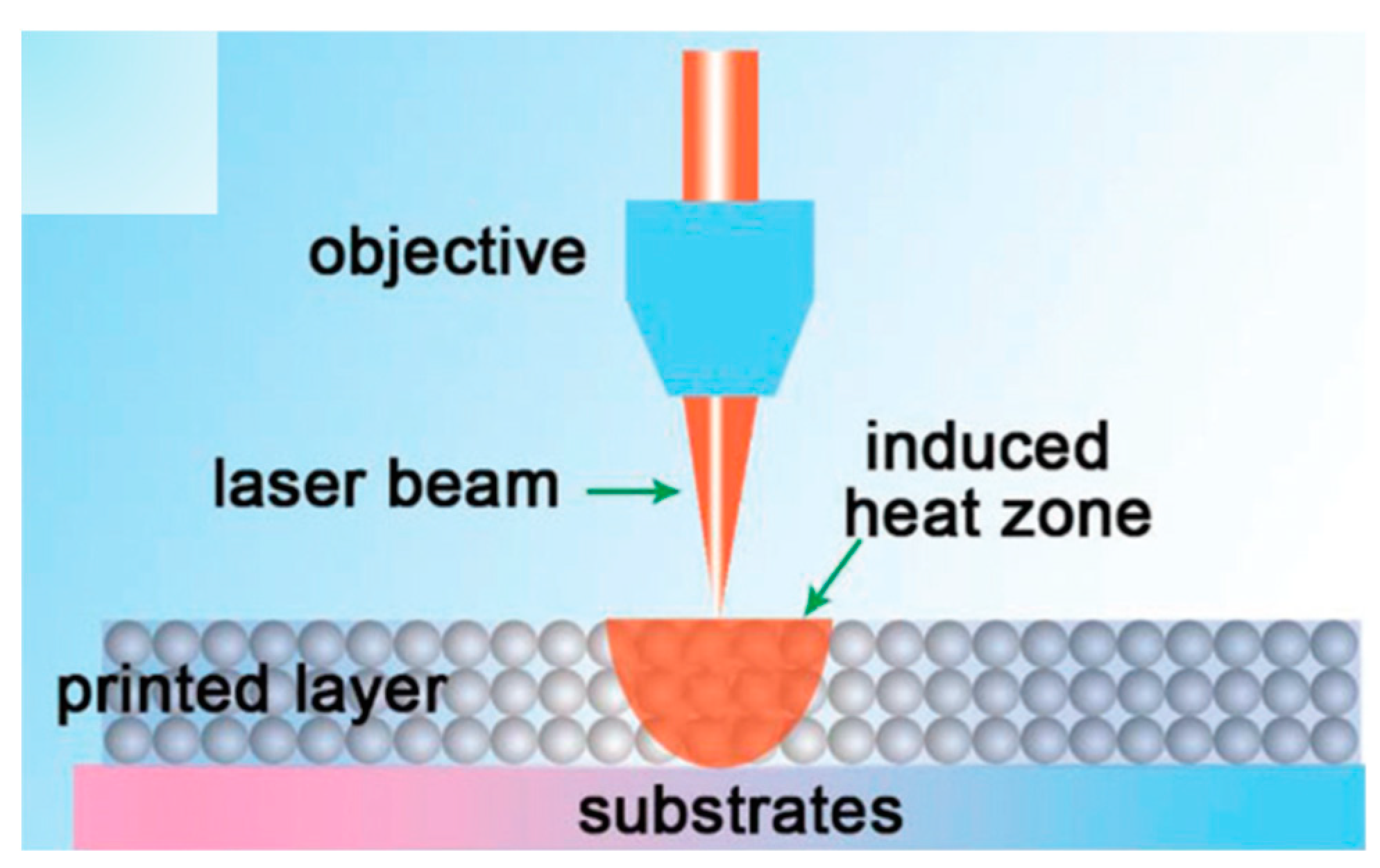
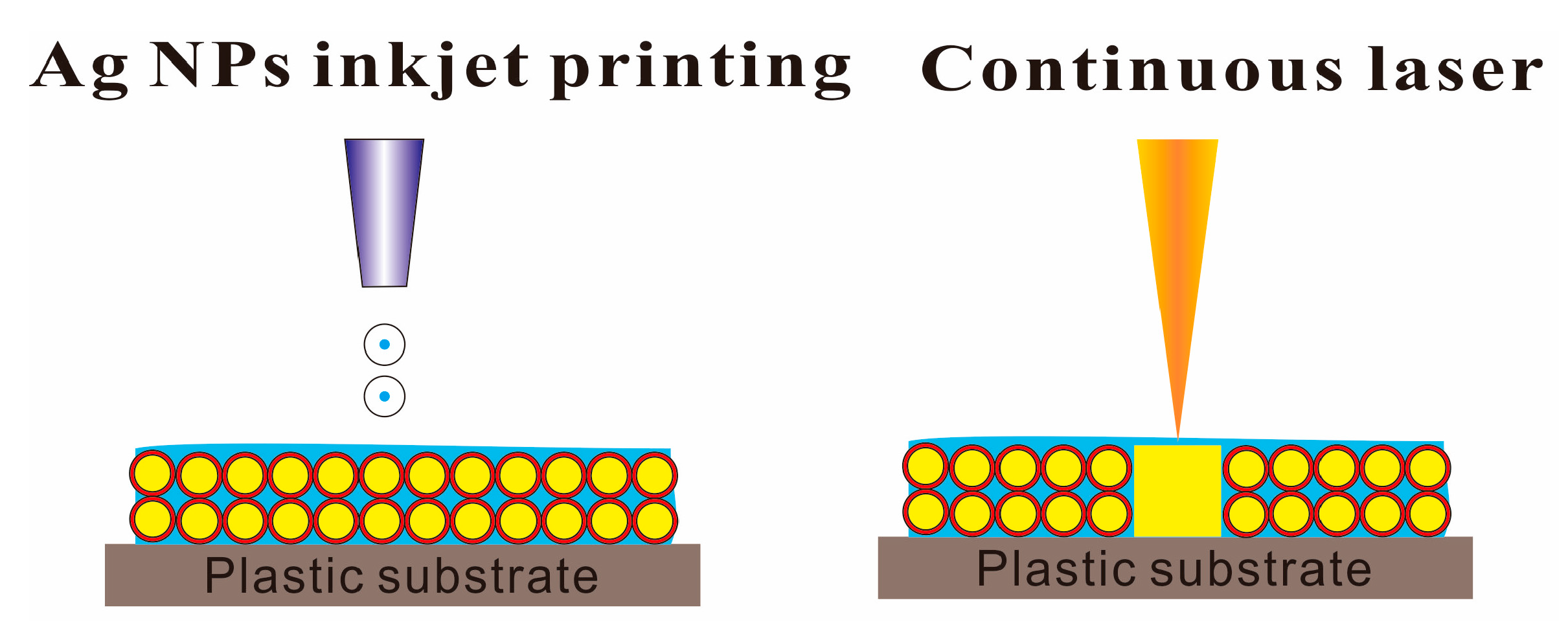
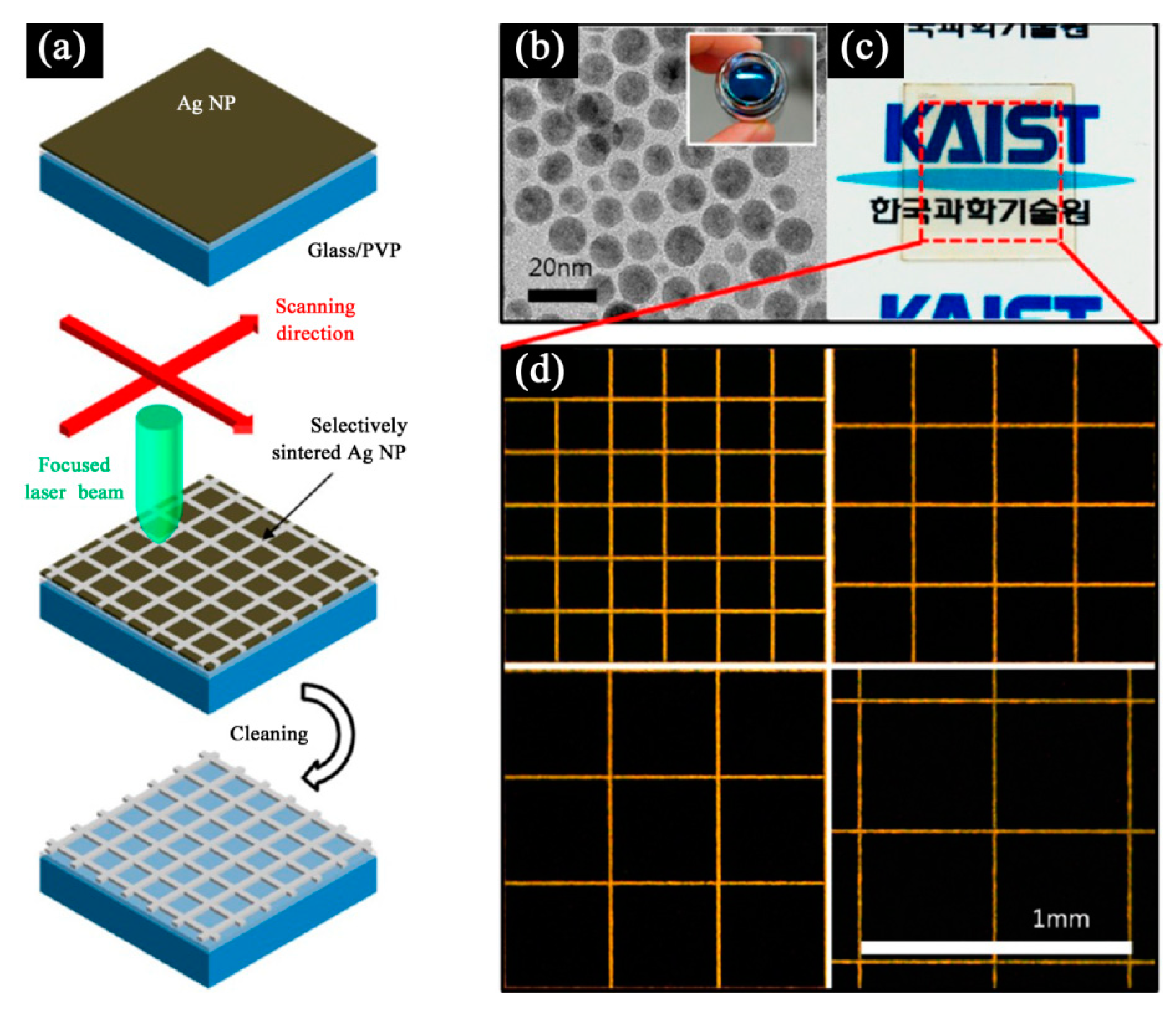
2.4.3. Laser Sintering
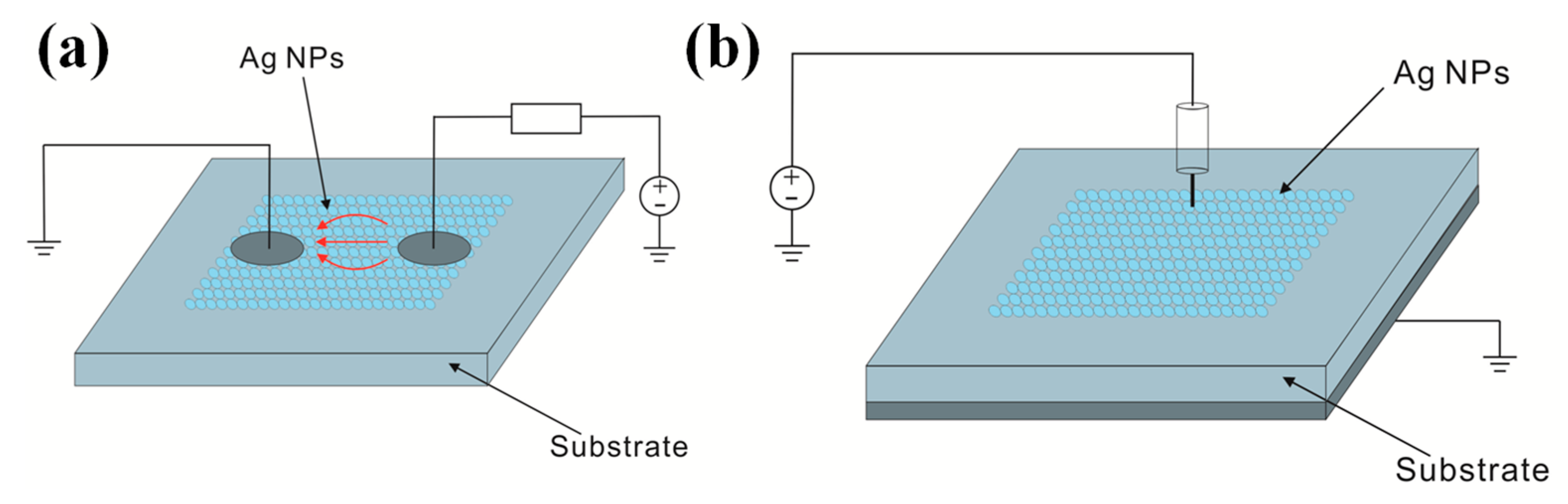
2.5. Other Emerging Sintering Methods
3. Applications of the Ag NPs Based Ink
3.1. Transparent Conductive Films
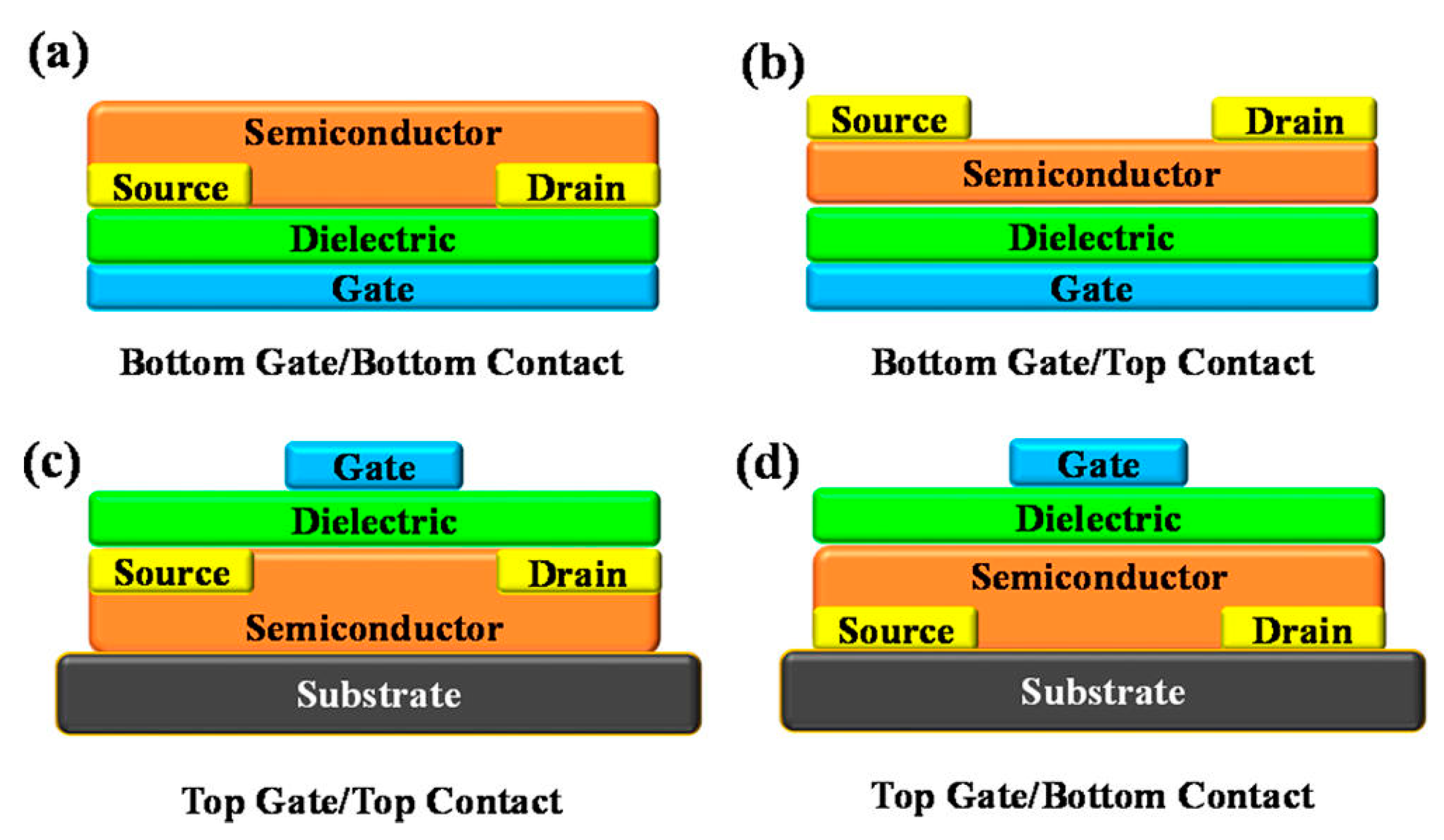
3.2. Thin Film Transistor
3.3. Biosensors
3.4. RFID
3.5. Stretchable Electronics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ag NPs | Silver nanoparticles |
| TCF | Transparent conductive film |
| TFTs | Thin film transistors |
| FPE | Flexible and printed electronics |
| PAA | Poly(acrylic acid) |
| PVP | Poly(vinyl pyrolidone) |
| PVA | Poly(vinyl alcohol) |
| DDA | Dodecylamine |
| DDT | Dodecanethiol |
| AA | Acetic acid |
| OA | Oleylamine |
| CNF | Cellulose nanofibers |
| EM | Electromagnetic |
| UV | Ultra-violet |
| IR | Infra-red |
| IPL | Intense pulsed light |
| MOD | Metal organic compounds |
| NIR | Near-IR |
| R2R | Roll to roll |
| RFID | Radio frequency identification |
| TEM | Transmission electron microscope |
| PET | Polyethylene terephthalate |
| rGO | Reduced graphene oxide |
| PSA | Prostate specific antigen |
| PDMS | Polydimethylsiloxane |
| PI | Polyimide |
| PEN | Polyethylene naphthalate |
| RES | Rapid electrical sintering |
| DC | Direct current |
| AC | Alternating current |
| ITO | Indium tin oxide |
| CNTs | Carbon nanotubes |
| OLEDs | Organic light emitting diodes |
| IGZO | Indium gallium zinc oxide |
| RMS | Root-mean-square |
| PSA | Prostate specific antigen |
| EIs | Electrochemical immunosensors |
| LSPR | Localized surface plasmon response |
| RFID | Radio frequency identification |
| MIBK | Methylisobutylketone |
| Ag NWs | Ag nanowires |
References
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Burdusel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Jiang, F.; Han, Z.; Chen, J. Electrodeposition of silver nanoparticle arrays on transparent conductive oxides. Appl. Surf. Sci. 2016, 369, 178–182. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.R.; Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013, 15, 1814. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Min, B.K.; Hwang, Y.J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. [Google Scholar] [CrossRef]
- Chopra, R.; Sharma, K.; Kumar, M.; Bhalla, V. Pentacenequinone-Stabilized Silver Nanoparticles: A Reusable Catalyst for the Diels-Alder [4 + 2] Cycloaddition Reactions. J. Org. Chem. 2016, 81, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, S.M.; Titus, E.J.; Willets, K.A. SERS Orientational Imaging of Silver Nanoparticle Dimers. J. Phys. Chem. Lett. 2011, 2, 2711–2715. [Google Scholar] [CrossRef]
- Yan, C.C.; Zhang, D.H.; Li, D.D. Spherical metallic nanoparticle arrays for super-resolution imaging. J. Appl. Phys. 2011, 109, 063105. [Google Scholar] [CrossRef]
- Hsiao, H.H.; Yeh, P.C.; Wang, H.H.; Cheng, T.Y.; Chang, H.C.; Wang, Y.L.; Wang, J.K. Enhancing bright-field image of microorganisms by local plasmon of Ag nanoparticle array. Opt. Lett. 2014, 39, 1173–1176. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Mahalakshmi, V.; Raichur, A.M. Silver nanoparticles modified nanocapsules for ultrasonically activated drug delivery. Mater. Sci. Eng. C 2012, 32, 2349–2355. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef]
- Benyettou, F.; Rezgui, R.; Ravaux, F.; Jaber, T.; Blumer, K.; Jouiad, M.; Motte, L.; Olsen, J.C.; Platas-Iglesias, C.; Magzoub, M.; et al. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B 2015, 3, 7237–7245. [Google Scholar] [CrossRef]
- Nantaphol, S.; Chailapakul, O.; Siangproh, W. A novel paper-based device coupled with a silver nanoparticle-modified boron-doped diamond electrode for cholesterol detection. Anal. Chim. Acta 2015, 891, 136–143. [Google Scholar] [CrossRef]
- Kim, W.; Kim, Y.H.; Park, H.K.; Choi, S. Facile Fabrication of a Silver Nanoparticle Immersed, Surface-Enhanced Raman Scattering Imposed Paper Platform through Successive Ionic Layer Absorption and Reaction for On-Site Bioassays. ACS. Appl. Mater. Int. 2015, 7, 27910–27917. [Google Scholar] [CrossRef]
- Ye, Y.D.; Xia, L.; Xu, D.D.; Xing, X.J.; Pang, D.W.; Tang, H.W. DNA-stabilized silver nanoclusters and carbon nanoparticles oxide: A sensitive platform for label-free fluorescence turn-on detection of HIV-DNA sequences. Biosens. Bioelectron. 2016, 85, 837–843. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Smith, L.E.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Substrate independent silver nanoparticle based antibacterial coatings. Biomaterials 2014, 35, 4601–4609. [Google Scholar] [CrossRef]
- Kamyshny, A.; Steinke, J.; Magdassi, S. Metal-based Inkjet Inks for Printed Electronics. Open Appl. Phys. J. 2011, 4, 19–36. [Google Scholar] [CrossRef]
- Karthik, P.S.; Singh, S.P. Conductive silver inks and their applications in printed and flexible electronics. RSC Adv. 2015, 5, 77760–77790. [Google Scholar] [CrossRef]
- Raut, N.C.; Al-Shamery, K. Inkjet printing metals on flexible materials for plastic and paper electronics. J. Mater. Chem. C 2018, 6, 1618–1641. [Google Scholar] [CrossRef]
- Magliulo, M.; Mulla, M.Y.; Singh, M.; Macchia, E.; Tiwari, A.; Torsi, L.; Manoli, K. Printable and flexible electronics: From TFTs to bioelectronic devices. J. Mater. Chem. C 2015, 3, 12347–12363. [Google Scholar] [CrossRef]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Graphene inks for printed flexible electronics: Graphene dispersions, ink formulations, printing techniques and applications. Adv. Colloid Interface Sci. 2018, 261, 41–61. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-Roll fabrication of large area functional organic materials. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.H.; Chen, H.; De Marco, N.; Yang, Y. Recent Progress in Materials and Devices toward Printable and Flexible Sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef] [PubMed]
- Wu, W. Inorganic nanomaterials for printed electronics: A review. Nanoscale 2017, 9, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, S.K.; Divya, M.; Breitung, B.; Kruk, R.; Hahn, H.; Dasgupta, S. Printed Electronics Based on Inorganic Semiconductors: From Processes and Materials to Devices. Adv. Mater. 2018, 30, 1707600. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive Nanomaterials for Printed Electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Perelaer, J.; Smith, P.J.; Mager, D.; Soltman, D.; Volkman, S.K.; Subramanian, V.; Korvink, J.G.; Schubert, U.S. Printed electronics: The challenges involved in printing devices, interconnects, and contacts based on inorganic materials. J. Mater. Chem. 2010, 20, 8446. [Google Scholar] [CrossRef]
- Soukupová, J.; Kvítek, L.; Panáček, A.; Nevěčná, T.; Zbořil, R. Comprehensive study on surfactant role on silver nanoparticles (NPs) prepared via modified Tollens process. Mater. Chem. Phys. 2008, 111, 77–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, B.; Hu, L. PVP Protective Mechanism of Ultrafine Silver Powder Synthesized by Chemical Reduction Processes. J. Solid State Chem. 1996, 121, 105–110. [Google Scholar] [CrossRef]
- Chen, M.; Feng, Y.G.; Wang, X.; Li, T.C.; Zhang, J.Y.; Qian, D.J. Silver nanoparticles capped by oleylamine: Formation, growth, and self-organization. Langmuir 2007, 23, 5296–5304. [Google Scholar] [CrossRef]
- Lovinger, A.J. Development of Electrical Conduction in Silver-filled Epoxy Adhesives. J. Adhesion 1979, 10, 1–15. [Google Scholar] [CrossRef]
- Ruschau, G.R.; Yoshikawa, S.; Newnham, R.E. Resistivities of conductive composites. J. Appl. Phys. 1992, 72, 953–959. [Google Scholar] [CrossRef]
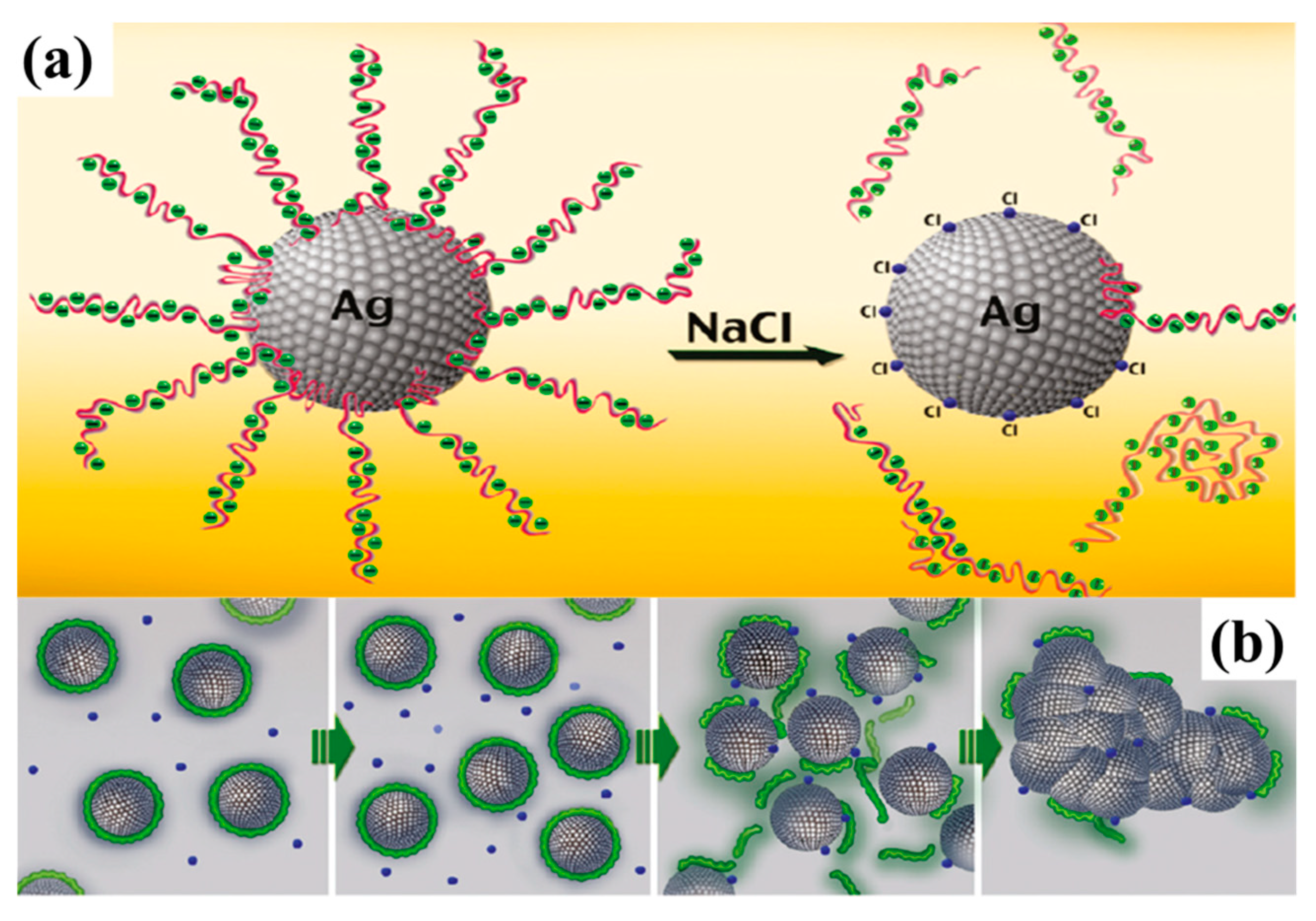
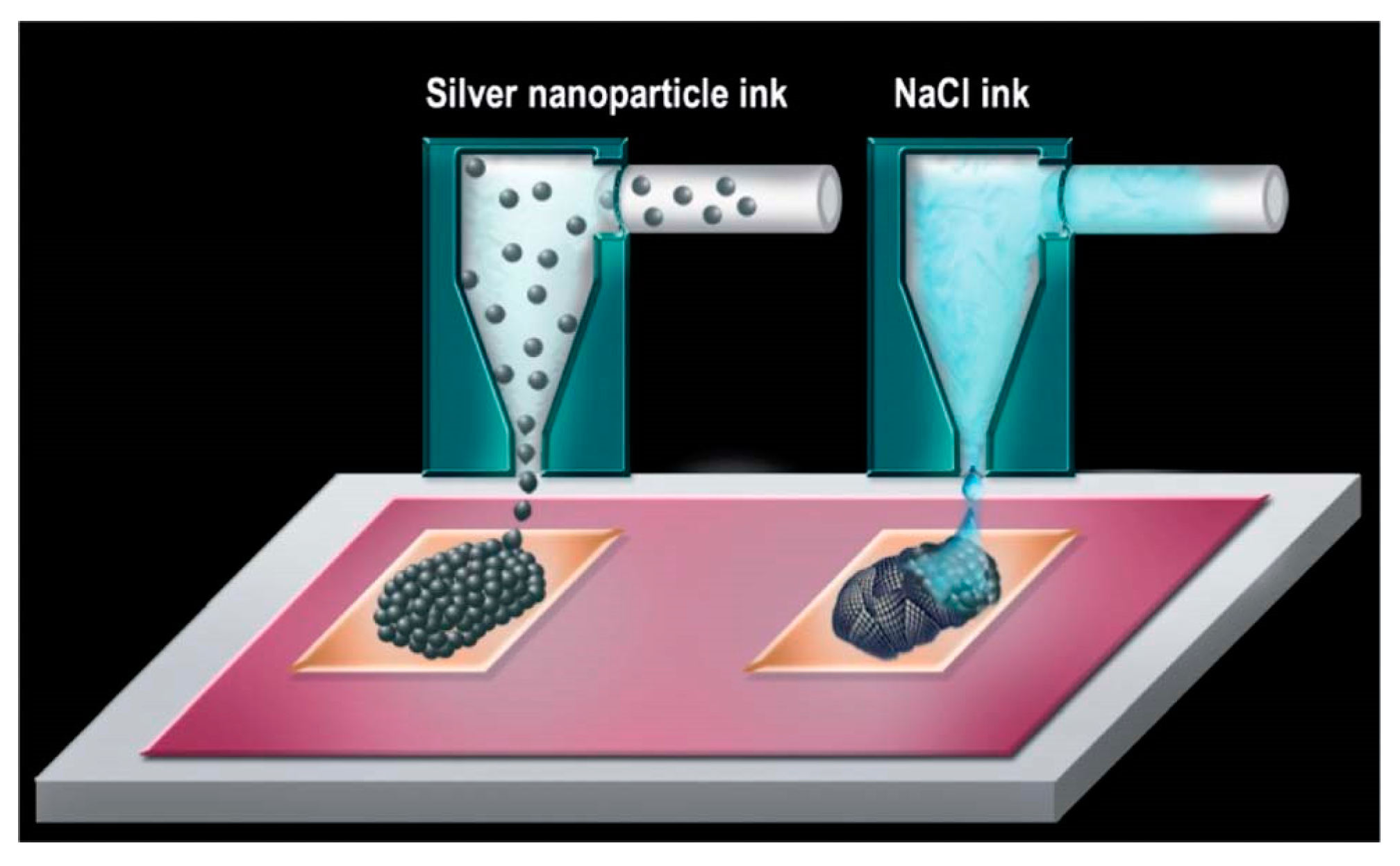
- Shlomo, M.; Michael, G.; Oleg, B.; Alexander, K. Triggering the sintering of silver nanoparticles at room temperature. ACS Nano 2010, 4, 1943–1948. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Mihailescu, C.F.; Anghel, D.F.; Magdassi, S. Conductive Inks with a “Built-In” Mechanism That Enables Sintering at Room Temperature. ACS Nano 2011, 5, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Perelaer, J.; Jani, R.; Grouchko, M.; Kamyshny, A.; Magdassi, S.; Schubert, U.S. Plasma and microwave flash sintering of a tailored silver nanoparticle ink, yielding 60% bulk conductivity on cost-effective polymer foils. Adv. Mater. 2012, 24, 3993–3998. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Wajahat, M.; Jeong, H.; Chang, W.S.; Cho, S.H.; Kim, J.T.; Seol, S.K. Three-dimensional Printing of Silver Microarchitectures Using Newtonian Nanoparticle Inks. ACS Appl. Mater. Interface 2017, 9, 18918–18924. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Ran, J.; Yang, L.; Fang, Y.; Zhai, Q.; Li, L. Flexible transparent conductive films combining flexographic printed silver grids with CNT coating. Nanotechnology 2016, 27, 065202. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, W.; Wang, S.; Tao, Z.; Cheng, L. New insight into the size-controlled synthesis of silver nanoparticles and its superiority in room temperature sintering. CrystEngComm 2014, 16, 4431–4440. [Google Scholar] [CrossRef]
- Khalil, A.M.; Hassan, M.L.; Ward, A.A. Novel nanofibrillated cellulose/polyvinylpyrrolidone/silver nanoparticles films with electrical conductivity properties. Carbohyd. Polym. 2017, 157, 503–511. [Google Scholar] [CrossRef]
- Polavarapu, L.; Manga, K.K.; Cao, H.D.; Loh, K.P.; Xu, Q.-H. Preparation of Conductive Silver Films at Mild Temperatures for Printable Organic Electronics. Chem. Mater. 2011, 23, 3273–3276. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.; Shin, H.; Xia, Y.; Moon, J. Heterogeneous Interfacial Properties of Ink-Jet-Printed Silver Nanoparticulate Electrode and Organic Semiconductor. Adv. Mater. 2008, 20, 3084–3089. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Bai, S.; Liu, Y.; Wang, Q. Fabrication of an ultralight flame-induced high conductivity hybrid sponge based on poly (vinyl alcohol)/silver nitrate composite. Mater. Des. 2018, 139, 96–103. [Google Scholar] [CrossRef]
- Liang, K.-L.; Wang, Y.-C.; Lin, W.-L.; Lin, J.-J. Polymer-assisted self-assembly of silver nanoparticles into interconnected morphology and enhanced surface electric conductivity. RSC Adv. 2014, 4, 15098. [Google Scholar] [CrossRef]
- Tobjörk, D.; Aarnio, H.; Pulkkinen, P.; Bollström, R.; Määttänen, A.; Ihalainen, P.; Mäkelä, T.; Peltonen, J.; Toivakka, M.; Tenhu, H.; et al. IR-sintering of ink-jet printed metal-nanoparticles on paper. Thin Solid Films 2012, 520, 2949–2955. [Google Scholar] [CrossRef]
- Mo, L.; Liu, D.; Li, W.; Li, L.; Wang, L.; Zhou, X. Effects of dodecylamine and dodecanethiol on the conductive properties of nano-Ag films. Appl. Surf. Sci. 2011, 257, 5746–5753. [Google Scholar] [CrossRef]
- Volkman, S.K.; Yin, S.; Bakhishev, T.; Puntambekar, K.; Subramanian, V.; Toney, M.F. Mechanistic Studies on Sintering of Silver Nanoparticles. Chem. Mater. 2011, 23, 4634–4640. [Google Scholar] [CrossRef]
- Yuning, L.; Yiliang, W.; Ong, B.S. Facile synthesis of silver nanoparticles useful for fabrication of high-conductivity elements for printed electronics. J. Am. Chem. Soc. 2005, 127, 3266–3267. [Google Scholar] [CrossRef]
- Polavarapu, L.; Manga, K.K.; Yu, K.; Ang, P.K.; Cao, H.D.; Balapanuru, J.; Loh, K.P.; Xu, Q.H. Alkylamine capped metal nanoparticle “inks” for printable SERS substrates, electronics and broadband photodetectors. Nanoscale 2011, 3, 2268–2274. [Google Scholar] [CrossRef]
- Ankireddy, K.; Vunnam, S.; Kellar, J.; Cross, W. Highly conductive short chain carboxylic acid encapsulated silver nanoparticle based inks for direct write technology applications. J. Mater. Chem. C 2013, 1, 572–579. [Google Scholar] [CrossRef]
- Yiliang, W.; Yuning, L.; Ong, B.S. Printed silver ohmic contacts for high-mobility organic thin-film transistors. J. Am. Chem. Soc. 2006, 128, 4202–4203. [Google Scholar] [CrossRef]
- Layani, M.; Grouchko, M.; Shemesh, S.; Magdassi, S. Conductive patterns on plastic substrates by sequential inkjet printing of silver nanoparticles and electrolyte sintering solutions. J. Mater. Chem. 2012, 22, 14349. [Google Scholar] [CrossRef]
- Jung, I.; Shin, K.; Kim, N.R.; Lee, H.M. Synthesis of low-temperature-processable and highly conductive Ag ink by a simple ligand modification: The role of adsorption energy. J. Mater. Chem. C 2013, 1, 1855. [Google Scholar] [CrossRef]
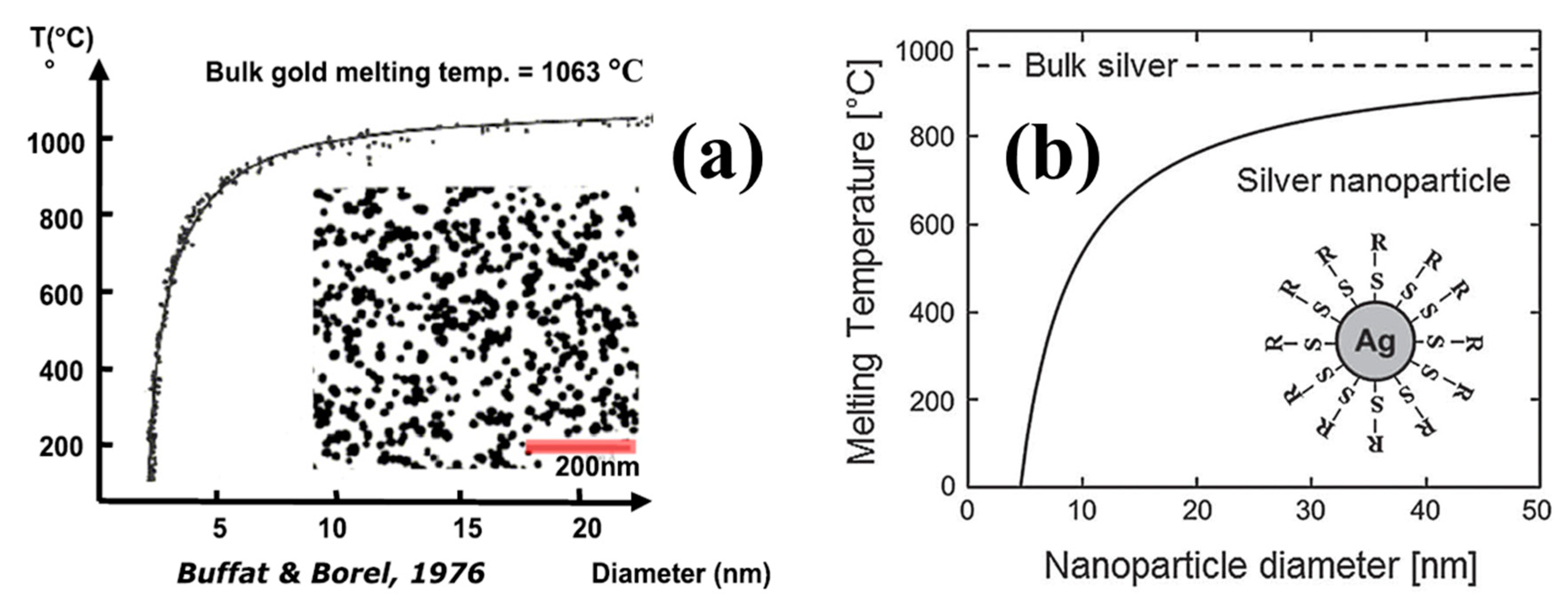
- Buffat, P.; Borel, J.P. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]
- Shyjumon, I.; Gopinadhan, M.; Ivanova, O.; Quaas, M.; Wulff, H.; Helm, C.A.; Hippler, R. Structural deformation, melting point and lattice parameter studies of size selected silver clusters. Eur. Phys. J. D 2005, 37, 409–415. [Google Scholar] [CrossRef]
- Durairaj, R.; Man, L.W. Effect of epoxy and filler concentrations on curing behaviour of isotropic conductive adhesives. J. Therm. Anal. Calorim. 2011, 105, 151–155. [Google Scholar] [CrossRef]
- Balantrapu, K.; McMurran, M.; Goia, D.V. Inkjet printable silver dispersions: Effect of bimodal particle-size distribution on film formation and electrical conductivity. J. Mater. Res. 2010, 25, 821–827. [Google Scholar] [CrossRef]
- Ding, J.; Liu, J.; Tian, Q.; Wu, Z.; Yao, W.; Dai, Z.; Liu, L.; Wu, W. Preparing of Highly Conductive Patterns on Flexible Substrates by Screen Printing of Silver Nanoparticles with Different Size Distribution. Nanoscale Res. Lett. 2016, 11, 412. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Kim, J.S.; Lee, J.G.; Kim, S.B.; Koo, S.M. The effect of silver particle size and organic stabilizers on the conductivity of silver particulate films in thermal sintering processes. Thin Solid Films 2016, 616, 366–374. [Google Scholar] [CrossRef]
- Han, Y.D.; Zhang, S.M.; Jing, H.Y.; Wei, J.; Bu, F.H.; Zhao, L.; Lv, X.Q.; Xu, L.Y. The fabrication of highly conductive and flexible Ag patterning through baking Ag nanosphere−nanoplate hybrid ink at a low temperature of 100 °C. Nanotechnology 2018, 29, 135301. [Google Scholar] [CrossRef]
- Yang, X.; He, W.; Wang, S.; Zhou, G.; Tang, Y.; Yang, J. Effect of the different shapes of silver particles in conductive ink on electrical performance and microstructure of the conductive tracks. J. Mater. Sci. Mater. Electron. 2012, 23, 1980–1986. [Google Scholar] [CrossRef]
- Lee, C.-L.; Chang, K.-C.; Syu, C.-M. Silver nanoplates as inkjet ink particles for metallization at a low baking temperature of 100 °C. Colloids Surf. A Physicochem. Eng. Asp. 2011, 381, 85–91. [Google Scholar] [CrossRef]
- MacDonald, W.A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4. [Google Scholar] [CrossRef]
- Tobjork, D.; Osterbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Park, J.; Lee, C. Characterization of in situ sintering of silver nanoparticles on commercial photo papers in inkjet printing. Flex. Print. Electron. 2018, 3, 025001. [Google Scholar] [CrossRef]
- Öhlund, T.; Schuppert, A.; Andres, B.; Andersson, H.; Forsberg, S.; Schmidt, W.; Nilsson, H.-E.; Andersson, M.; Zhang, R.; Olin, H. Assisted sintering of silver nanoparticle inkjet ink on paper with active coatings. RSC Adv. 2015, 5, 64841–64849. [Google Scholar] [CrossRef]
- Allen, M.; Leppaniemi, J.; Vilkman, M.; Alastalo, A.; Mattila, T. Substrate-facilitated nanoparticle sintering and component interconnection procedure. Nanotechnology 2010, 21, 475204. [Google Scholar] [CrossRef]
- Andersson, H.; Manuilskiy, A.; Lidenmark, C.; Gao, J.; Ohlund, T.; Forsberg, S.; Ortegren, J.; Schmidt, W.; Nilsson, H.E. The influence of paper coating content on room temperature sintering of silver nanoparticle ink. Nanotechnology 2013, 24, 455203. [Google Scholar] [CrossRef] [PubMed]
- Nge, T.T.; Nogi, M.; Suganuma, K. Electrical functionality of inkjet-printed silver nanoparticle conductive tracks on nanostructured paper compared with those on plastic substrates. J. Mater. Chem. C 2013, 1, 5235. [Google Scholar] [CrossRef]
- Wünscher, S.; Abbel, R.; Perelaer, J.; Schubert, U.S. Progress of alternative sintering approaches of inkjet-printed metal inks and their application for manufacturing of flexible electronic devices. J. Mater. Chem. C 2014, 2, 10232–10261. [Google Scholar] [CrossRef]
- Denneulin, A.; Blayo, A.; Neuman, C.; Bras, J. Infra-red assisted sintering of inkjet printed silver tracks on paper substrates. J. Nanoparticle Res. 2011, 13, 3815–3823. [Google Scholar] [CrossRef]
- Cherrington, M.; Claypole, T.C.; Deganello, D.; Mabbett, I.; Watson, T.; Worsley, D. Ultrafast near-infrared sintering of a slot-die coated nano-silver conducting ink. J. Mater. Chem. 2011, 21, 7562. [Google Scholar] [CrossRef]
- Gu, W.; Yuan, W.; Zhong, T.; Wu, X.; Zhou, C.; Lin, J.; Cui, Z. Fast near infrared sintering of silver nanoparticle ink and applications for flexible hybrid circuits. RSC Adv. 2018, 8, 30215–30222. [Google Scholar] [CrossRef]
- Sowade, E.; Kang, H.; Mitra, K.Y.; Weiß, O.J.; Weber, J.; Baumann, R.R. Roll-to-roll infrared (IR) drying and sintering of an inkjet-printed silver nanoparticle ink within 1 second. J. Mater. Chem. C 2015, 3, 11815–11826. [Google Scholar] [CrossRef]
- Kang, J.S.; Ryu, J.; Kim, H.S.; Hahn, H.T. Sintering of Inkjet-Printed Silver Nanoparticles at Room Temperature Using Intense Pulsed Light. J. Electron. Mater. 2011, 40, 2268–2277. [Google Scholar] [CrossRef]
- Chung, W.H.; Hwang, H.J.; Lee, S.H.; Kim, H.S. In situ monitoring of a flash light sintering process using silver nano-ink for producing flexible electronics. Nanotechnology 2013, 24, 035202. [Google Scholar] [CrossRef]
- Abbel, R.; van Lammeren, T.; Hendriks, R.; Ploegmakers, J.; Rubingh, E.J.; Meinders, E.R.; Groen, W.A. Photonic flash sintering of silver nanoparticle inks: A fast and convenient method for the preparation of highly conductive structures on foil. MRS Commun. 2012, 2, 145–150. [Google Scholar] [CrossRef]
- Lee, D.J.; Park, S.H.; Jang, S.; Kim, H.S.; Oh, J.H.; Song, Y.W. Pulsed light sintering characteristics of inkjet-printed nanosilver films on a polymer substrate. J. Micromech. Microeng. 2011, 21, 125023. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Gupta, H.; Gupta, D. Flash Light Sintering of Silver Nanoink for Inkjet-Printed Thin-Film Transistor on Flexible Substrate. IEEE Trans. Nanotechnol. 2017, 16, 375–382. [Google Scholar] [CrossRef]
- Weise, D.; Mitra, K.Y.; Sowade, E.; Baumann, R.R. Intense Pulsed Light Sintering of Inkjet Printed Silver Nanoparticle Ink: Influence of Flashing Parameters and Substrate. Proc. MRS 2015, 1761. [Google Scholar] [CrossRef]
- Balliu, E.; Andersson, H.; Engholm, M.; Ohlund, T.; Nilsson, H.E.; Olin, H. Selective laser sintering of inkjet-printed silver nanoparticle inks on paper substrates to achieve highly conductive patterns. Sci. Rep. 2018, 8, 10408. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, G.; Hong, S.; Kim, M.S.; Kim, D.; Lee, J.; Lee, H.B.; Kwon, J.; Suh, Y.D.; Kang, H.W.; et al. Flexible supercapacitor fabrication by room temperature rapid laser processing of roll-to-roll printed metal nanoparticle ink for wearable electronics application. J. Power Sources 2014, 246, 562–568. [Google Scholar] [CrossRef]
- Bolduc, M.; Trudeau, C.; Beaupre, P.; Cloutier, S.G.; Galarneau, P. Thermal Dynamics Effects using Pulse-Shaping Laser Sintering of Printed Silver Inks. Sci. Rep. 2018, 8, 1418. [Google Scholar] [CrossRef]
- Ko, S.H.; Pan, H.; Grigoropoulos, C.P.; Luscombe, C.K.; Fréchet, J.M.J.; Poulikakos, D. All-inkjet-printed flexible electronics fabrication on a polymer substrate by low-temperature high-resolution selective laser sintering of metal nanoparticles. Nanotechnology 2007, 18, 345202. [Google Scholar] [CrossRef]
- Hong, S.; Yeo, J.; Kim, G.; Kim, D.; Lee, H.; Kwon, J.; Lee, H.; Lee, P.; Ko, S.H. Nonvacuum, Maskless Fabrication of a Flexible Metal Grid Transparent Conductor by Low-Temperature Selective Laser Sintering of Nanoparticle Ink. ACS Nano. 2013, 7, 5024–5031. [Google Scholar] [CrossRef]
- Allen, M.L. Nanoparticle Sintering Methods and Applications for Printed Electronics; Aalto University: Espoo/Helsinki, Finland, 2011. [Google Scholar]
- Allen, M.L.; Aronniemi, M.; Mattila, T.; Alastalo, A.; Ojanpera, K.; Suhonen, M.; Seppa, H. Electrical sintering of nanoparticle structures. Nanotechnology 2008, 19, 175201. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, I.; Hendriks, C.E.; Eckardt, R.; Kranenburg, J.M.; Perelaer, J.; Baumann, R.R.; Schubert, U.S. Argon plasma sintering of inkjet printed silver tracks on polymer substrates. J. Mater. Chem. 2009, 19, 3384. [Google Scholar] [CrossRef]
- Wolf, F.M.; Perelaer, J.; Stumpf, S.; Bollen, D.; Kriebel, F.; Schubert, U.S. Rapid low-pressure plasma sintering of inkjet-printed silver nanoparticles for RFID antennas. J. Mater. Res. 2013, 28, 1254–1261. [Google Scholar] [CrossRef]
- Wünscher, S.; Stumpf, S.; Teichler, A.; Pabst, O.; Perelaer, J.; Beckert, E.; Schubert, U.S. Localized atmospheric plasma sintering of inkjet printed silver nanoparticles. J. Mater. Chem. 2012, 22, 24569. [Google Scholar] [CrossRef]
- Wünscher, S.; Stumpf, S.; Perelaer, J.; Schubert, U.S. Towards single-pass plasma sintering: Temperature influence of atmospheric pressure plasma sintering of silver nanoparticle ink. J. Mater. Chem. C 2014, 2, 1642. [Google Scholar] [CrossRef]
- Ma, S.; Bromberg, V.; Liu, L.; Egitto, F.D.; Chiarot, P.R.; Singler, T.J. Low temperature plasma sintering of silver nanoparticles. Appl. Surf. Sci. 2014, 293, 207–215. [Google Scholar] [CrossRef]
- Perelaer, J.; de Gans, B.J.; Schubert, U.S. Ink-jet Printing and Microwave Sintering of Conductive Silver Tracks. Adv. Mater. 2006, 18, 2101–2104. [Google Scholar] [CrossRef]
- Perelaer, J.; Klokkenburg, M.; Hendriks, C.E.; Schubert, U.S. Microwave flash sintering of inkjet-printed silver tracks on polymer substrates. Adv. Mater. 2009, 21, 4830–4834. [Google Scholar] [CrossRef]
- Perelaer, J.; Abbel, R.; Wunscher, S.; Jani, R.; van Lammeren, T.; Schubert, U.S. Roll-to-roll compatible sintering of inkjet printed features by photonic and microwave exposure: From non-conductive ink to 40% bulk silver conductivity in less than 15 seconds. Adv. Mater. 2012, 24, 2620–2625. [Google Scholar] [CrossRef]
- Layani, M.; Gruchko, M.; Milo, O.; Balberg, I.; Azulay, D.; Magdassi, S. Transparent Conductive Coatings by Printing coffee Ring Arrays Obtained at Room Temperature. ACS Nano 2009, 3, 3537–3542. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Lorang, D.J.; Lewis, J.A. Transparent conductive grids via direct writing of silver nanoparticle inks. Nanoscale 2011, 3, 2700–2702. [Google Scholar] [CrossRef]
- Deganello, D.; Cherry, J.A.; Gethin, D.T.; Claypole, T.C. Patterning of micro-scale conductive networks using reel-to-reel flexographic printing. Thin Solid Films 2010, 518, 6113–6116. [Google Scholar] [CrossRef]
- Kahng, Y.H.; Kim, M.-K.; Lee, J.-H.; Kim, Y.J.; Kim, N.; Park, D.-W.; Lee, K. Highly conductive flexible transparent electrodes fabricated by combining graphene films and inkjet-printed silver grids. Sol. Energy Mater. Sol. C 2014, 124, 86–91. [Google Scholar] [CrossRef]
- Jeong, J.-A.; Kim, J.; Kim, H.-K. Ag grid/ITO hybrid transparent electrodes prepared by inkjet printing. Sol. Energy Mater. Sol. C 2011, 95, 1974–1978. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Gao, Y.; Zhang, P.; Li, C.; Ma, C.; Tu, Y.; Cui, Z.; Chen, L. ITO-free photovoltaic cell utilizing a high-resolution silver grid current collecting layer. Sol. Energy Mater Sol. C 2013, 113, 85–89. [Google Scholar] [CrossRef]
- Mao, L.; Chen, Q.; Li, Y.; Li, Y.; Cai, J.; Su, W.; Bai, S.; Jin, Y.; Ma, C.-Q.; Cui, Z.; et al. Flexible silver grid/PEDOT:PSS hybrid electrodes for large area inverted polymer solar cells. Nano Energy 2014, 10, 259–267. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly Stable Transparent Conductive Silver Grid/PEDOT:PSS Electrodes for Integrated Bifunctional Flexible Electrochromic Supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Fukuda, K.; Someya, T. Recent Progress in the Development of Printed Thin-Film Transistors and Circuits with High-Resolution Printing Technology. Adv. Mater. 2017, 29, 1602736. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Huang, J. Integration of Biomaterials into Sensors Based on Organic Thin-Film Transistors. Macromol. Rapid Commun. 2018, 39, 1800084. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xia, J.; Dong, G.; Tian, B.; Peng, L. Carbon Nanotube Thin Film Transistors for Flat Panel Display Application. Top. Curr. Chem. 2016, 374, 80. [Google Scholar] [CrossRef]
- Tong, S.C.; Sun, J.; Yang, J.L. Printed Thin-Film Transistors: Research from China. ACS Appl. Mater. Interface 2018, 10, 25902–25924. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.R.; Lee, S.S.; Park, H.J.; Jo, Y.; Kim, J.Y.; Lee, H.S.; Kang, Y.C.; Ryu, B.H.; Song, A.; Chung, K.B.; et al. Unraveling the Issue of Ag Migration in Printable Source/Drain Electrodes Compatible with Versatile Solution-Processed Oxide Semiconductors for Printed Thin-Film Transistor Applications. ACS Appl. Mater. Interface 2017, 9, 14058–14066. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Chen, J.; Fang, Z.; Tao, R.; Cai, W.; Yao, R.; Hu, S.; Zhu, Z.; Zhou, Y.; Yang, C.; et al. Direct Inkjet Printing of Silver Source/Drain Electrodes on an Amorphous InGaZnO Layer for Thin-Film Transistors. Materials 2017, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.R.; Jiang, C.; Ma, H.B.; Guo, X.J.; Nathan, A. All ink-jet printed low-voltage organic field-effect transistors on flexible substrate. Org. Electron. 2016, 38, 186–192. [Google Scholar] [CrossRef]
- Castro, H.F.; Sowade, E.; Rocha, J.G.; Alpuim, P.; Lanceros-Mendez, S.; Baumann, R.R. All-Inkjet-Printed Bottom-Gate Thin-Film Transistors Using UV Curable Dielectric for Well-Defined Source-Drain Electrodes. J. Electron. Mater. 2014, 43, 2631–2636. [Google Scholar] [CrossRef]
- Fukuda, K.; Sekine, T.; Kumaki, D.; Tokito, S. Profile Control of Inkjet Printed Silver Electrodes and Their Application to Organic Transistors. ACS Appl. Mater. Interface 2013, 5, 3916–3920. [Google Scholar] [CrossRef]
- Liu, T.T.; Zhao, J.W.; Xu, W.W.; Dou, J.Y.; Zhao, X.L.; Deng, W.; Wei, C.T.; Xu, W.Y.; Guo, W.R.; Su, W.M.; et al. Flexible integrated diode-transistor logic (DTL) driving circuits based on printed carbon nanotube thin film transistors with low operation voltage. Nanoscale 2018, 10, 614–622. [Google Scholar] [CrossRef]
- Tu, L.; Yuan, S.J.; Zhang, H.T.; Wang, P.F.; Cui, X.L.; Wang, J.; Zhan, Y.Q.; Zheng, L.R. Aerosol jet printed silver nanowire transparent electrode for flexible electronic application. J. Appl. Phys. 2018, 123, 174905. [Google Scholar] [CrossRef]
- Cardenas, J.A.; Upshaw, S.; Williams, N.X.; Catenacci, M.J.; Wiley, B.J.; Franklin, A.D. Impact of Morphology on Printed Contact Performance in Carbon Nanotube Thin-Film Transistors. Adv. Funct. Mater. 2019, 29, 1805727. [Google Scholar] [CrossRef]
- Xu, W.W.; Liu, Z.; Zhao, J.W.; Xu, W.Y.; Gu, W.B.; Zhang, X.; Qian, L.; Cui, Z. Flexible logic circuits based on top-gate thin film transistors with printed semiconductor carbon nanotubes and top electrodes. Nanoscale 2014, 6, 14891–14897. [Google Scholar] [CrossRef]
- Shimura, Y.; Nomura, K.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. Specific contact resistances between amorphous oxide semiconductor In-Ga-Zn-O and metallic electrodes. Thin Solid Films 2008, 516, 5899–5902. [Google Scholar] [CrossRef]
- Aoshima, K.; Arai, S.; Fukuhara, K.; Yamada, T.; Hasegawa, T. Surface modification of printed silver electrodes for efficient carrier injection in organic thin-film transistors. Org. Electron. 2017, 41, 137–142. [Google Scholar] [CrossRef]
- Ji, D.Y.; Jiang, L.; Dong, H.L.; Meng, Q.; Zhen, Y.G.; Hu, W.P. Silver mirror reaction for organic electronics: Towards high-performance organic field-effect transistors and circuits. J. Mater. Chem. C 2014, 2, 4142–4146. [Google Scholar] [CrossRef]
- Choi, S.; Larrain, F.A.; Wang, C.Y.; Fuentes-Hernandez, C.; Chou, W.F.; Kippelen, B. Self-forming electrode modification in organic field-effect transistors. J. Mater. Chem. C 2016, 4, 8297–8303. [Google Scholar] [CrossRef]
- Fukuda, K.; Minamiki, T.; Minami, T.; Watanabe, M.; Fukuda, T.; Kumaki, D.; Tokito, S. Printed Organic Transistors with Uniform Electrical Performance and Their Application to Amplifiers in Biosensors. Adv. Electron. Mater. 2015, 1, 1400052. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, Y.H.; Han, J.I. All solution-processed high-resolution bottom-contact transparent metal-oxide thin film transistors. J. Phys. D Appl. Phys. 2009, 42, 125102. [Google Scholar] [CrossRef]
- Hwang, J.K.; Cho, S.; Dang, J.M.; Kwak, E.B.; Song, K.; Moon, J.; Sung, M.M. Direct nanoprinting by liquid-bridge-mediated nanotransfer moulding. Nat. Nanotechnol. 2010, 5, 742–748. [Google Scholar] [CrossRef]
- Ueoka, Y.; Nishibayashi, T.; Ishikawa, Y.; Yamazaki, H.; Osada, Y.; Horita, M.; Uraoka, Y. Analysis of printed silver electrode on amorphous indium gallium zinc oxide. Jpn. J. Appl. Phys. 2014, 53, 125102. [Google Scholar] [CrossRef]
- Ando, E.; Miyazaki, M. Moisture degradation mechanism of silver-based low-emissivity coatings. Thin Solid Films 1999, 351, 308–312. [Google Scholar] [CrossRef]
- Chen, J.Q.; Ning, H.L.; Fang, Z.Q.; Tao, R.Q.; Yang, C.G.; Zhou, Y.C.; Yao, R.H.; Xu, M.; Wang, L.; Peng, J.B. Reduced contact resistance of a-IGZO thin film transistors with inkjet-printed silver electrodes. J. Phys. D Appl. Phys. 2018, 51, 165103. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A Universal Method to Produce Low-Work Function Electrodes for Organic Electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef]
- Gillan, L.; Leppaniemi, J.; Eiroma, K.; Majumdar, H.; Alastalo, A. High performance solution processed oxide thin-film transistors with inkjet printed Ag source-drain electrodes. J. Mater. Chem. C 2018, 6, 3220–3225. [Google Scholar] [CrossRef]
- Secor, E.B.; Smith, J.; Marks, T.J.; Hersam, M.C. High-Performance Inkjet-Printed Indium-Gallium-Zinc-Oxide Transistors Enabled by Embedded, Chemically Stable Graphene Electrodes. ACS Appl. Mater. Interface 2016, 8, 17428–17434. [Google Scholar] [CrossRef]
- Tang, W.; Feng, L.R.; Zhao, J.Q.; Cui, Q.Y.; Chen, S.J.; Guo, X.J. Inkjet printed fine silver electrodes for all-solution-processed low-voltage organic thin film transistors. J. Mater. Chem. C 2014, 2, 1995–2000. [Google Scholar] [CrossRef]
- Sekitani, T.; Noguchi, Y.; Zschieschang, U.; Klauk, H.; Someya, T. Organic transistors manufactured using inkjet technology with subfemtoliter accuracy. Proc. Natl. Acad. Sci. USA 2008, 105, 4976–4980. [Google Scholar] [CrossRef] [PubMed]
- Park, J.U.; Hardy, M.; Kang, S.J.; Barton, K.; Adair, K.; Mukhopadhyay, D.K.; Lee, C.Y.; Strano, M.S.; Alleyne, A.G.; Georgiadis, J.G.; et al. High-resolution electrohydrodynamic jet printing. Nat. Mater. 2007, 6, 782–789. [Google Scholar] [CrossRef]
- Zhang, H.; Ramm, A.; Lim, S.M.; Xie, W.; Ahn, B.Y.; Xu, W.C.; Mahajan, A.; Suszynski, W.J.; Kim, C.; Lewis, J.A.; et al. Wettability Contrast Gravure Printing. Adv. Mater. 2015, 27, 7420–7425. [Google Scholar] [CrossRef]
- Fukuda, K.; Yoshimura, Y.; Okamoto, T.; Takeda, Y.; Kumaki, D.; Katayama, Y.; Tokito, S. Reverse-Offset Printing Optimized for Scalable Organic Thin-Film Transistors with Submicrometer Channel Lengths. Adv. Electron. Mater. 2015, 1, 1500145. [Google Scholar] [CrossRef]
- Ning, H.L.; Tao, R.Q.; Fang, Z.Q.; Cai, W.; Chen, J.Q.; Zhou, Y.C.; Zhu, Z.A.; Zheng, Z.K.; Yao, R.H.; Xu, M.; et al. Direct patterning of silver electrodes with 2.4 mu m channel length by piezoelectric inkjet printing. J. Colloids Interface Sci. 2017, 487, 68–72. [Google Scholar] [CrossRef]
- Tao, R.Q.; Fang, Z.Q.; Zhang, J.H.; Ning, H.L.; Chen, J.Q.; Yang, C.G.; Zhou, Y.C.; Yao, R.H.; Song, Y.S.; Peng, J.B. Capillary force induced air film for self-aligned short channel: Pushing the limits of inkjet printing. Soft Matter 2018, 14, 9402–9410. [Google Scholar] [CrossRef]
- Han, L.; Liu, C.M.; Dong, S.L.; Du, C.X.; Zhang, X.Y.; Li, L.H.; Wei, Y. Enhanced conductivity of rGO/Ag NPs composites for electrochemical immunoassay of prostate-specific antigen. Biosens. Bioelectron. 2017, 87, 466–472. [Google Scholar] [CrossRef]
- Rad, A.S.; Jahanshahi, M.; Ardjmand, M.; Safekordi, A.-A. Hydrogen Peroxide Biosensor Based on Enzymatic Modification of Electrode Using Deposited Silver Nano Layer. Int. J. Electrochem. Sc. 2012, 7, 2623–2632. [Google Scholar]
- Wang, C.; Sun, Y.; Yu, X.; Ma, D.; Zheng, J.; Dou, P.; Cao, Z.; Xu, X. Ag–Pt hollow nanoparticles anchored reduced graphene oxide composites for non-enzymatic glucose biosensor. J. Mater. Sci. Mater. Electron. 2016, 27, 9370–9378. [Google Scholar] [CrossRef]
- Misra, N.; Goel, N.K.; Varshney, L.; Kumar, V. Radiolytically Synthesized Noble Metal Nanoparticles: Sensor Applications. In Reviews in Plasmonics 2015; Geddes, C.D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 51–67. [Google Scholar]
- He, H.; Sydänheimo, L.; Virkki, J.; Ukkonen, L. Experimental Study on Inkjet-Printed Passive UHF RFID Tags on Versatile Paper-Based Substrates. Int. J. Antenn. Propag. 2016, 2016, 9265159. [Google Scholar] [CrossRef]
- Ren, Y.; Sydänheimo, L.; Virkki, J.; Ukkonen, L. Optimisation of manufacturing parameters for inkjet-printed and photonically sintered metallic nanoparticle UHF RFID tags. Electron. Lett. 2014, 50, 1504–1505. [Google Scholar] [CrossRef]
- Salmerón, J.F.; Molina-Lopez, F.; Briand, D.; Ruan, J.J.; Rivadeneyra, A.; Carvajal, M.A.; Capitán-Vallvey, L.F.; de Rooij, N.F.; Palma, A.J. Properties and Printability of Inkjet and Screen-Printed Silver Patterns for RFID Antennas. J. Electron. Mater. 2013, 43, 604–617. [Google Scholar] [CrossRef]
- Jung, M.; Kim, J.; Noh, J.; Lim, N.; Lim, C.; Lee, G.; Kim, J.; Kang, H.; Jung, K.; Leonard, A.D.; et al. All-Printed and Roll-to-Roll-PrinTable 13.56-MHz-Operated 1-bit RF Tag on Plastic Foils. IEEE Trans. Electron. Devices 2010, 57, 571–580. [Google Scholar] [CrossRef]
- Sanchez-Romaguera, V.; Wünscher, S.; Turki, B.M.; Abbel, R.; Barbosa, S.; Tate, D.J.; Oyeka, D.; Batchelor, J.C.; Parker, E.A.; Schubert, U.S.; et al. Inkjet printed paper based frequency selective surfaces and skin mounted RFID tags: The interrelation between silver nanoparticle ink, paper substrate and low temperature sintering technique. J. Mater. Chem. C 2015, 3, 2132–2140. [Google Scholar] [CrossRef]
- Sanchez-Romaguera, V.; Ziai, M.A.; Oyeka, D.; Barbosa, S.; Wheeler, J.S.R.; Batchelor, J.C.; Parker, E.A.; Yeates, S.G. Towards inkjet-printed low cost passive UHF RFID skin mounted tattoo paper tags based on silver nanoparticle inks. J. Mater. Chem. C 2013, 1, 6395. [Google Scholar] [CrossRef][Green Version]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [CrossRef]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.B.; Jeon, S.; Chung, D.Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef]
- Chung, S.; Lee, J.; Song, H.; Kim, S.; Jeong, J.; Hong, Y. Inkjet-printed stretchable silver electrode on wave structured elastomeric substrate. Appl. Phys. Lett. 2011, 98, 153110. [Google Scholar] [CrossRef]
- Albrecht, A.; Bobinger, M.; Salmerón, J.; Becherer, M.; Cheng, G.; Lugli, P.; Rivedeneyra, A. Over-Stretching Tolerant Conductors on Rubber Films by Inkjet-Printing Silver Nanoparticles for Wearables. Polymers 2018, 10, 1413. [Google Scholar] [CrossRef]
- Chun, K.Y.; Oh, Y.; Rho, J.; Ahn, J.H.; Kim, Y.J.; Choi, H.R.; Baik, S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat. Nanotechnol. 2010, 5, 853–857. [Google Scholar] [CrossRef]
- Kim, J.; Wang, Z.; Kim, W.S. Stretchable RFID for Wireless Strain Sensing With Silver Nano Ink. IEEE Sens. J. 2014, 14, 4395–4401. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Kaltenbrunner, M.; Yokota, T.; Jinno, H.; Kuribara, K.; Sekitani, T.; Someya, T. Printable elastic conductors with a high conductivity for electronic textile applications. Nat. Commun. 2015, 6, 7461. [Google Scholar] [CrossRef]
- Mohammed, A.; Pecht, M. A stretchable and screen-printable conductive ink for stretchable electronics. Appl. Phys. Lett. 2016, 109, 184101. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, W.J.; Kwon, B.-S.; Nam, S.-Y.; Choa, S.-H. Highly stretchable and conductive conductors based on Ag flakes and polyester composites. Microelectron. Eng. 2018, 199, 16–23. [Google Scholar] [CrossRef]
- Araki, T.; Nogi, M.; Suganuma, K.; Kogure, M.; Kirihara, O. Printable and Stretchable Conductive Wirings Comprising Silver Flakes and Elastomers. IEEE Electron. Device Lett. 2011, 32, 1424–1426. [Google Scholar] [CrossRef]
- Kumar, A.; Saghlatoon, H.; La, T.-G.; Mahdi Honari, M.; Charaya, H.; Abu Damis, H.; Mirzavand, R.; Mousavi, P.; Chung, H.-J. A highly deformable conducting traces for printed antennas and interconnects: Silver/fluoropolymer composite amalgamated by triethanolamine. Flex. Print. Electron. 2017, 2, 045001. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Tian, Q.; Liu, L.; Yao, W.; Chi, C.; Zeng, P.; Zhang, N.; Wu, W. Highly conductive, flexible and stretchable conductors based on fractal silver nanostructures. J. Mater. Chem. C 2018, 6, 3999–4006. [Google Scholar] [CrossRef]
- Tybrandt, K.; Voros, J. Fast and Efficient Fabrication of Intrinsically Stretchable Multilayer Circuit Boards by Wax Pattern Assisted Filtration. Small 2016, 12, 180–184. [Google Scholar] [CrossRef]
- Liang, J.; Tong, K.; Pei, Q. A Water-Based Silver-Nanowire Screen-Print Ink for the Fabrication of Stretchable Conductors and Wearable Thin-Film Transistors. Adv. Mater. 2016, 28, 5986–5996. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Lee, S.; Seo, J.; Lee, J.; Son, S.; Cho, H.J.; Algadi, H.; Al-Sayari, S.; Kim, D.E.; et al. Ag Nanowire Reinforced Highly Stretchable Conductive Fibers for Wearable Electronics. Adv. Funct. Mater. 2015, 25, 3114–3121. [Google Scholar] [CrossRef]
- Lee, I.; Lee, J.; Ko, S.H.; Kim, T.S. Reinforcing Ag nanoparticle thin films with very long Ag nanowires. Nanotechnology 2013, 24, 415704. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, Y. Highly conductive and stretchable silver nanowire conductors. Adv. Mater. 2012, 24, 5117–5122. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, L.; Guo, Z.; Yang, L.; Zhang, Q.; Fang, Y.; Xin, Z.; Chen, Z.; Hu, K.; Han, L.; Li, L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. https://doi.org/10.3390/ijms20092124
Mo L, Guo Z, Yang L, Zhang Q, Fang Y, Xin Z, Chen Z, Hu K, Han L, Li L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. International Journal of Molecular Sciences. 2019; 20(9):2124. https://doi.org/10.3390/ijms20092124
Chicago/Turabian StyleMo, Lixin, Zhenxin Guo, Li Yang, Qingqing Zhang, Yi Fang, Zhiqing Xin, Zheng Chen, Kun Hu, Lu Han, and Luhai Li. 2019. "Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics" International Journal of Molecular Sciences 20, no. 9: 2124. https://doi.org/10.3390/ijms20092124
APA StyleMo, L., Guo, Z., Yang, L., Zhang, Q., Fang, Y., Xin, Z., Chen, Z., Hu, K., Han, L., & Li, L. (2019). Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. International Journal of Molecular Sciences, 20(9), 2124. https://doi.org/10.3390/ijms20092124