Experiments with Snails Add to Our Knowledge about the Role of aPKC Subfamily Kinases in Learning
Abstract
1. Introduction
1.1. Atypical PKCs and Their Common Properties
1.2. The Role of C1 Domain in Atypical PKCs Function
1.3. PKMζ Structure, Regulation, and Function
1.4. Mechanisms of PKMζ Formation in Different Species
1.5. Land Snail as a Model Object for Neuroscience Studies
2. Results
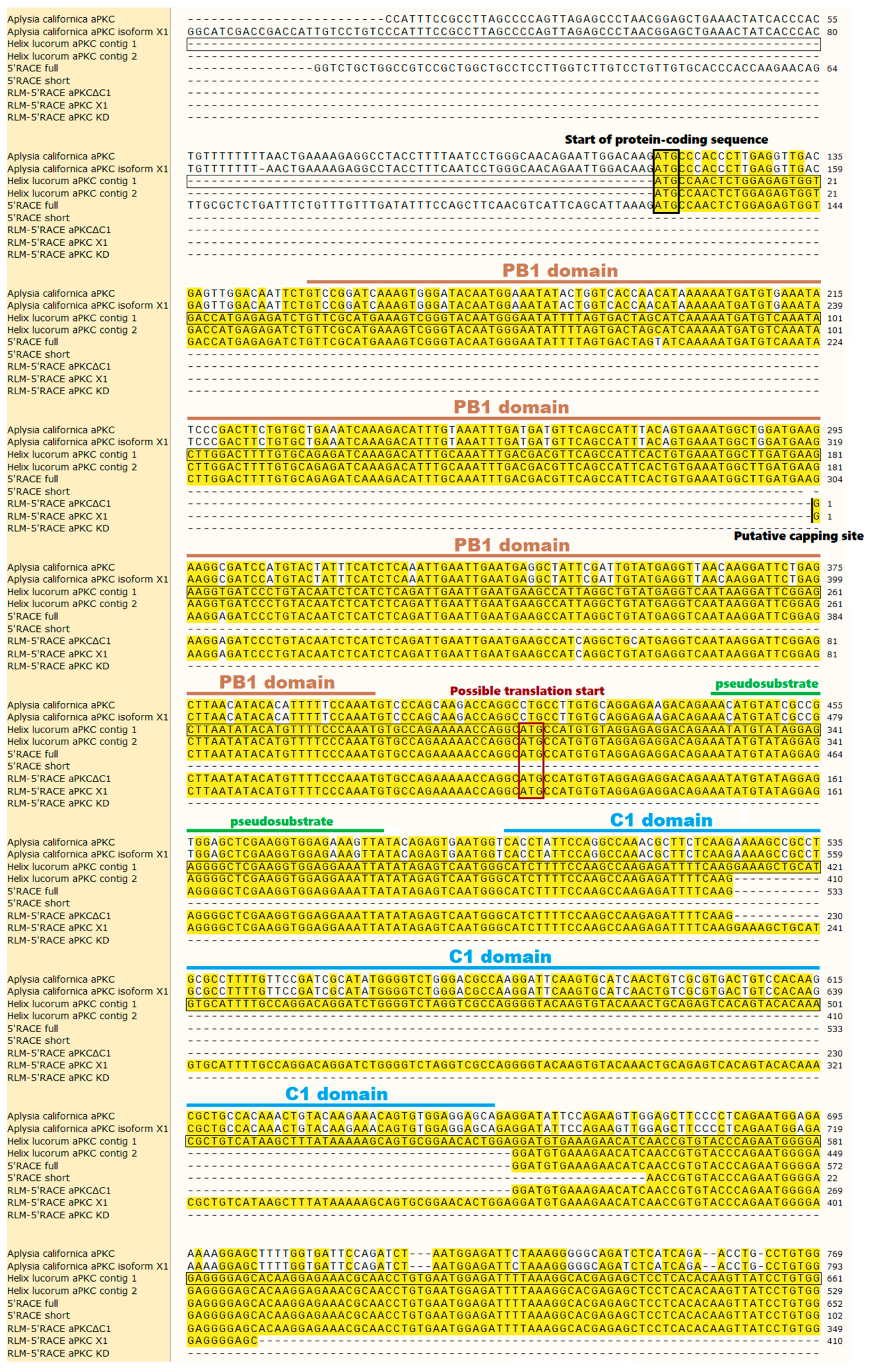
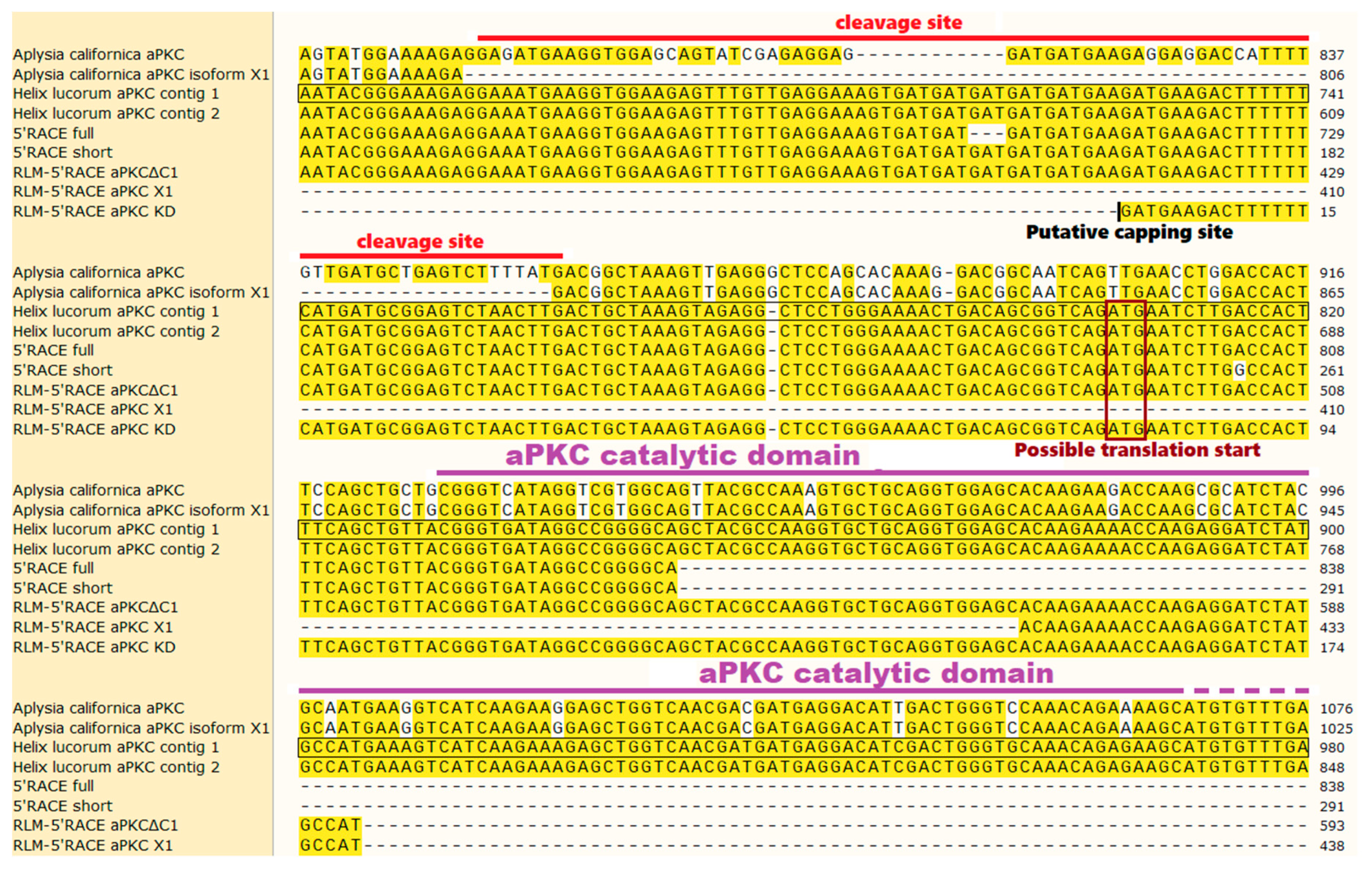
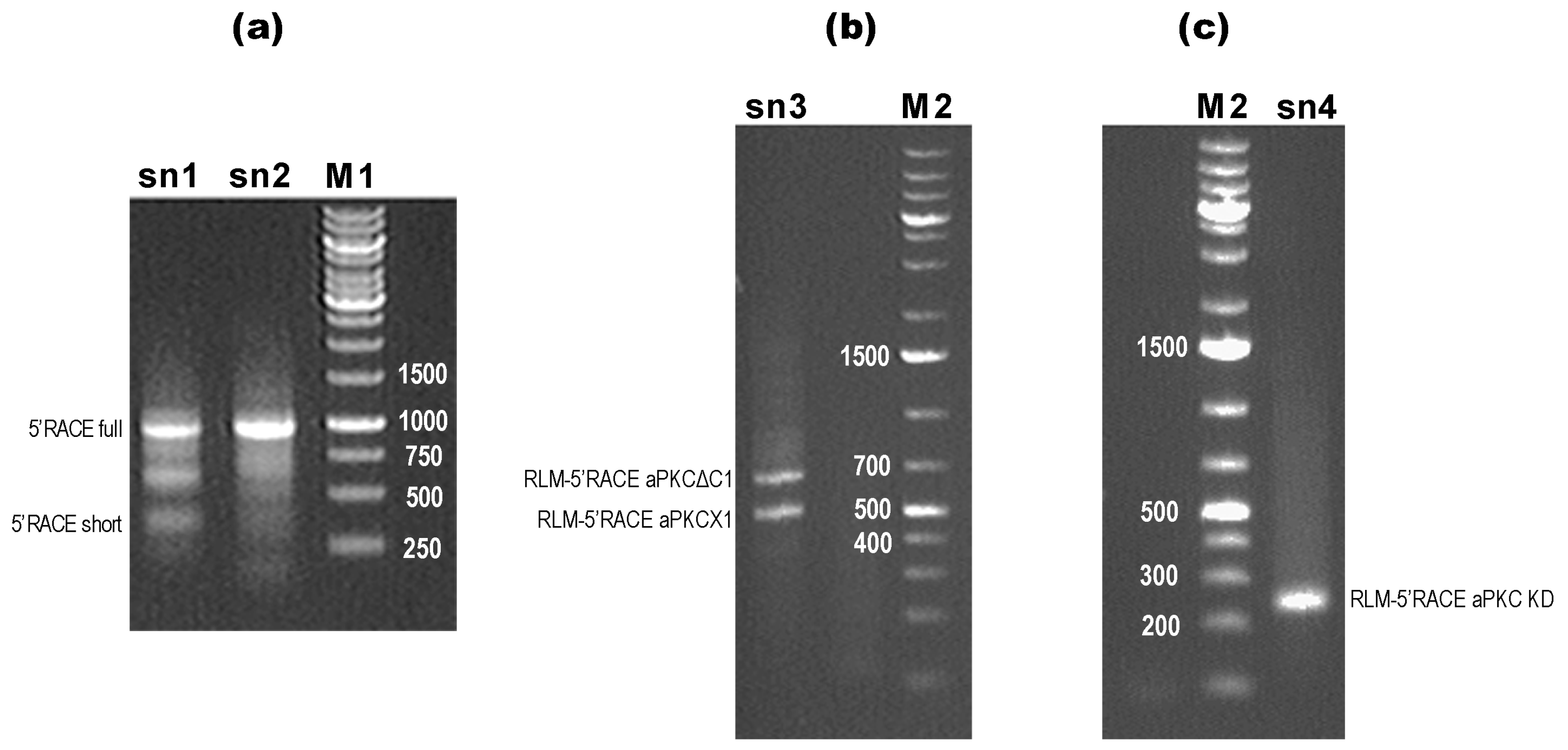
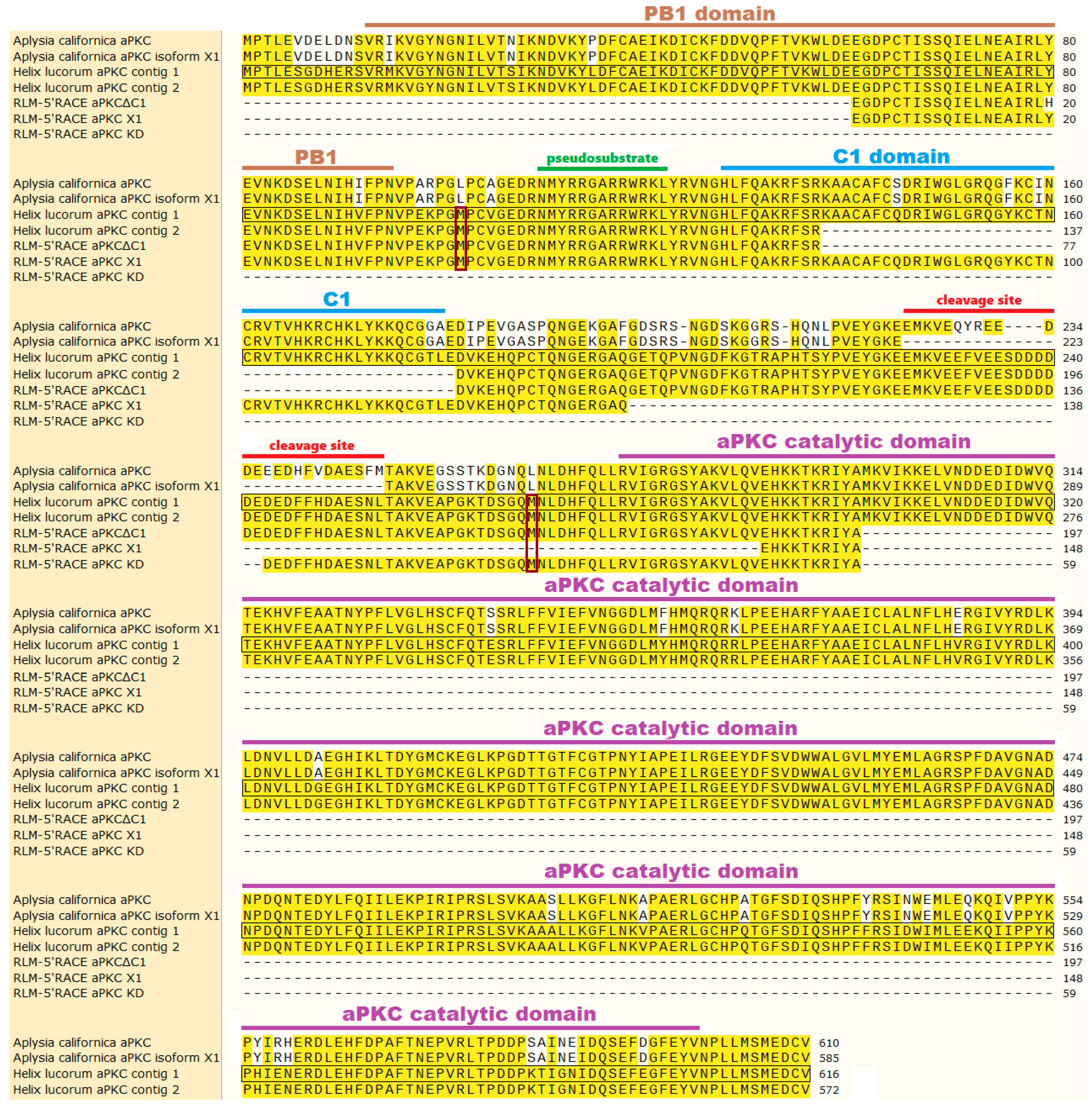
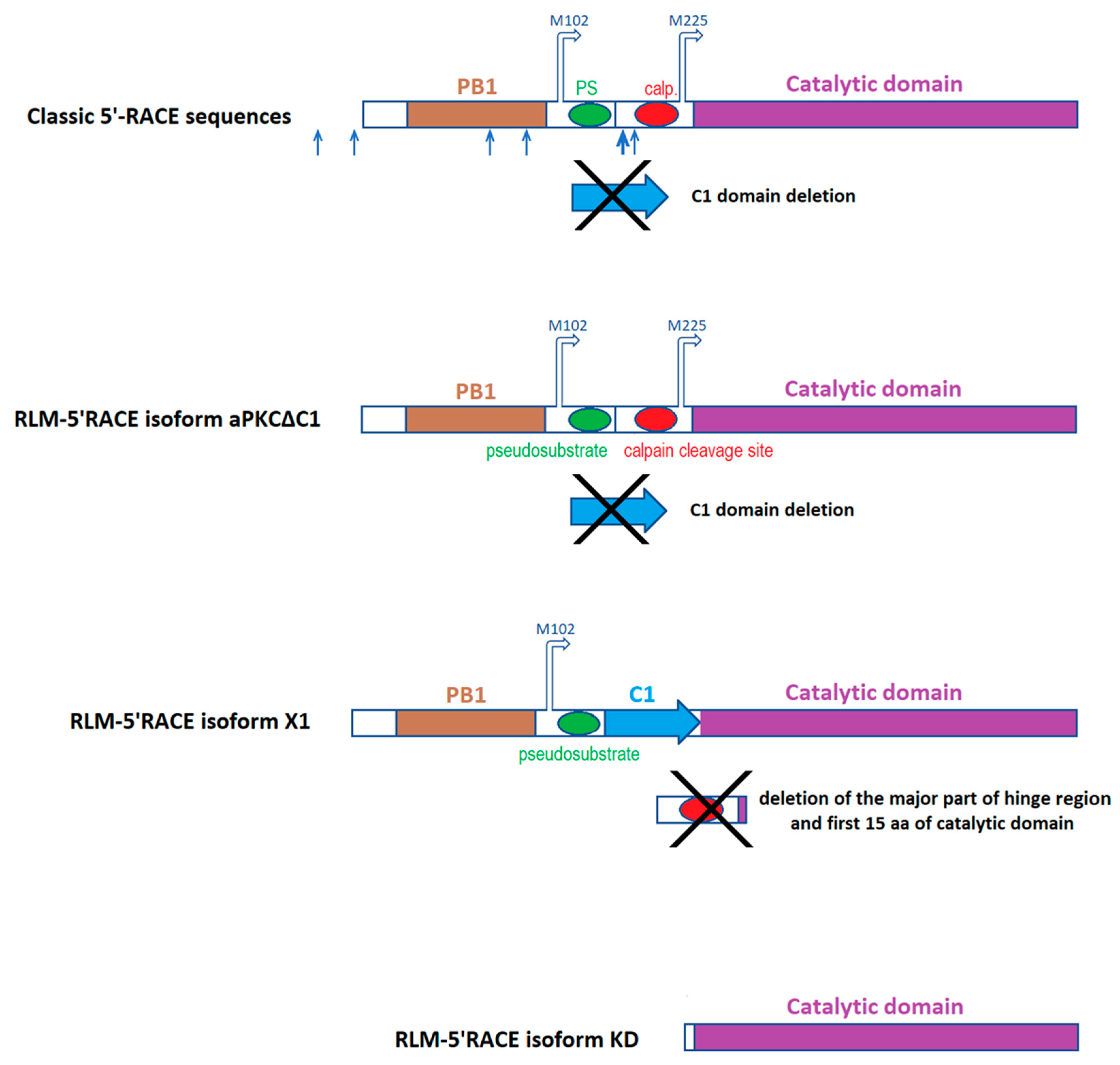
2.1. Classic 5′-RACE Revealed a Full-Size Transcript and a Few Shorter Fragments
2.2. RLM-5’RACE Revealed Two Putative Capping Sites and Two Alternative Splicing Sites
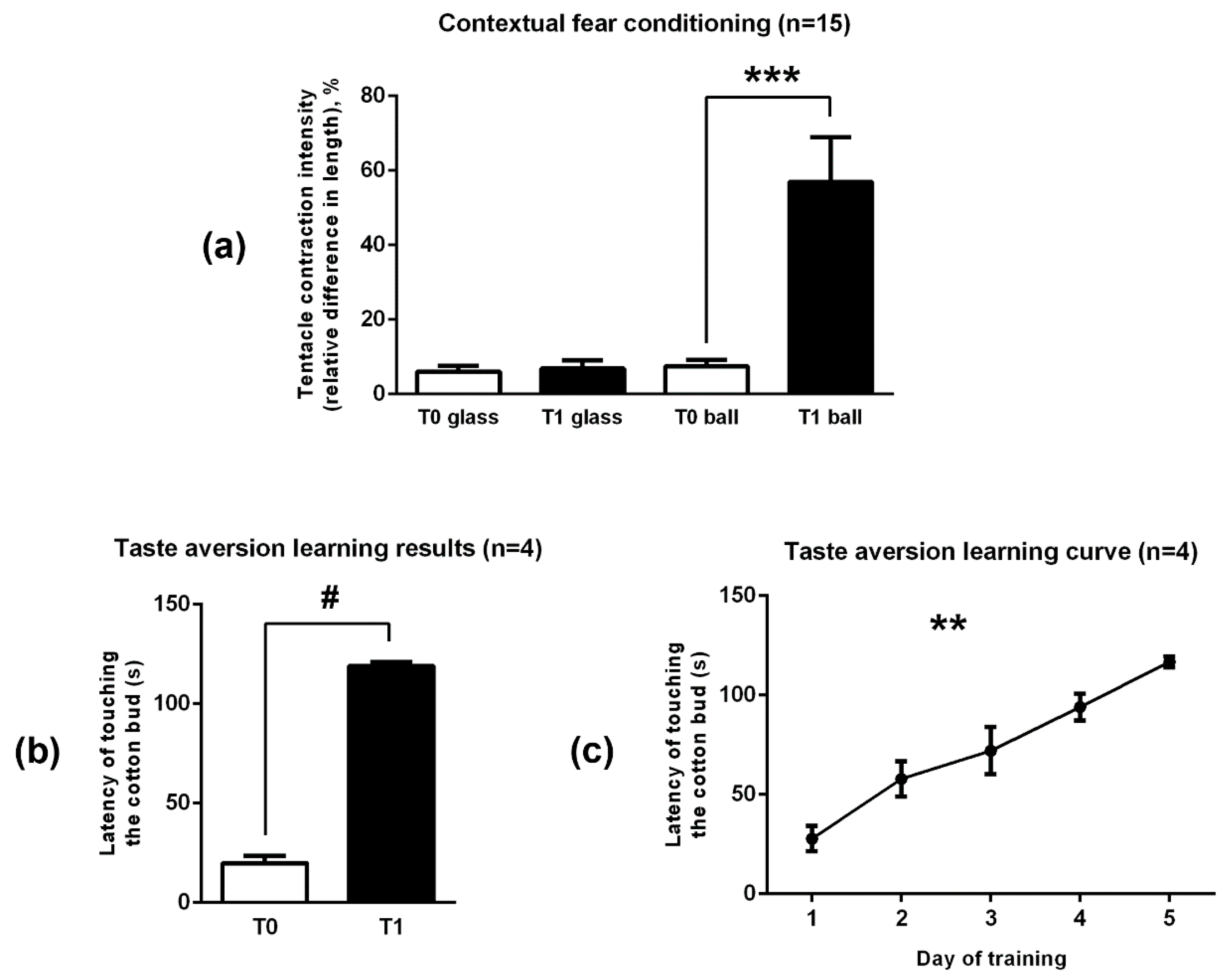
2.3. The Training of Snails Was Successful in Both Experiments
2.3.1. Contextual Fear Conditioning
2.3.2. Taste Aversion Learning
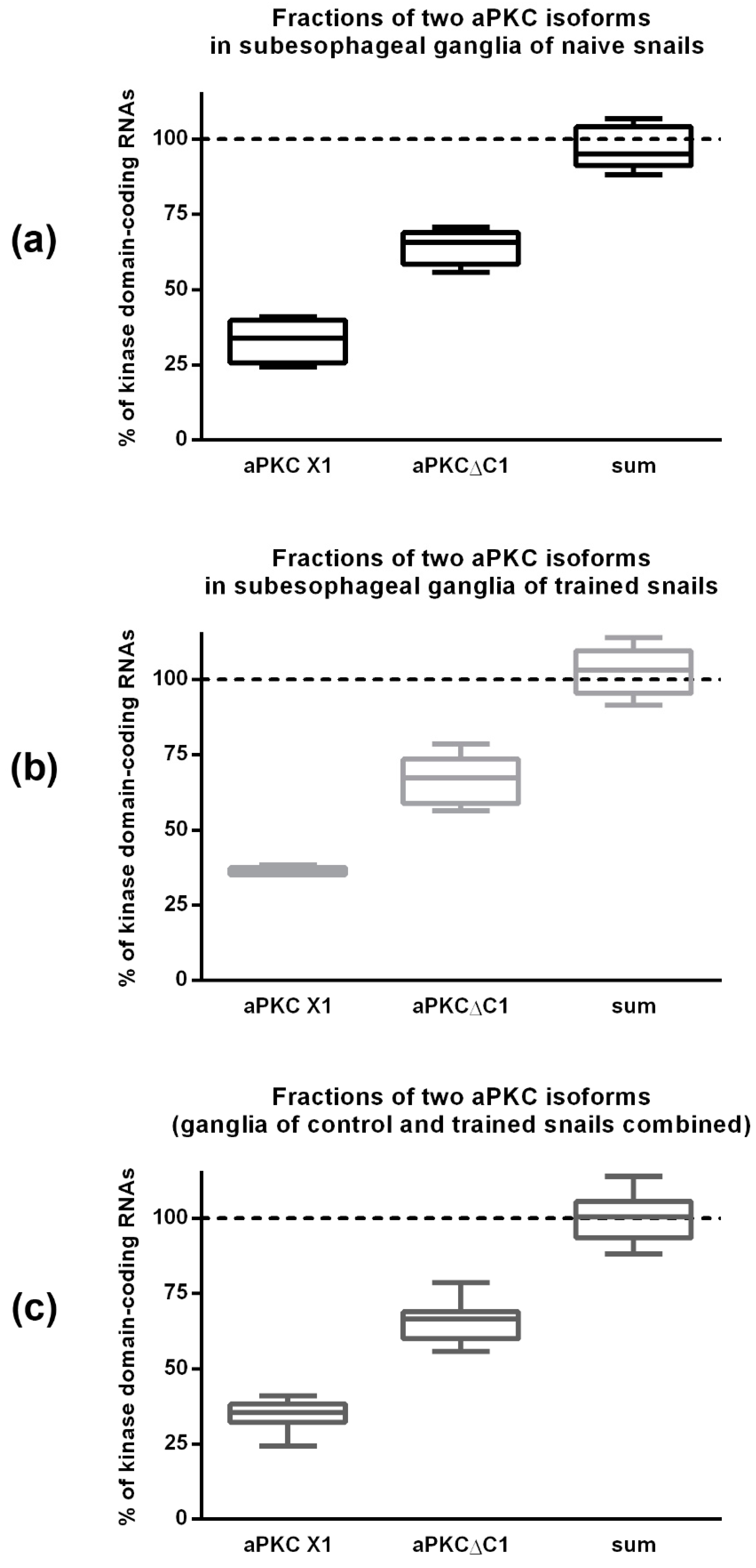
2.4. ddPCR Demonstrated That the Ratio of Two Splice Isoforms in Subesophagial Ganglia is Roughly 2:1 and Does Not Change after Contextual Fear Conditioning
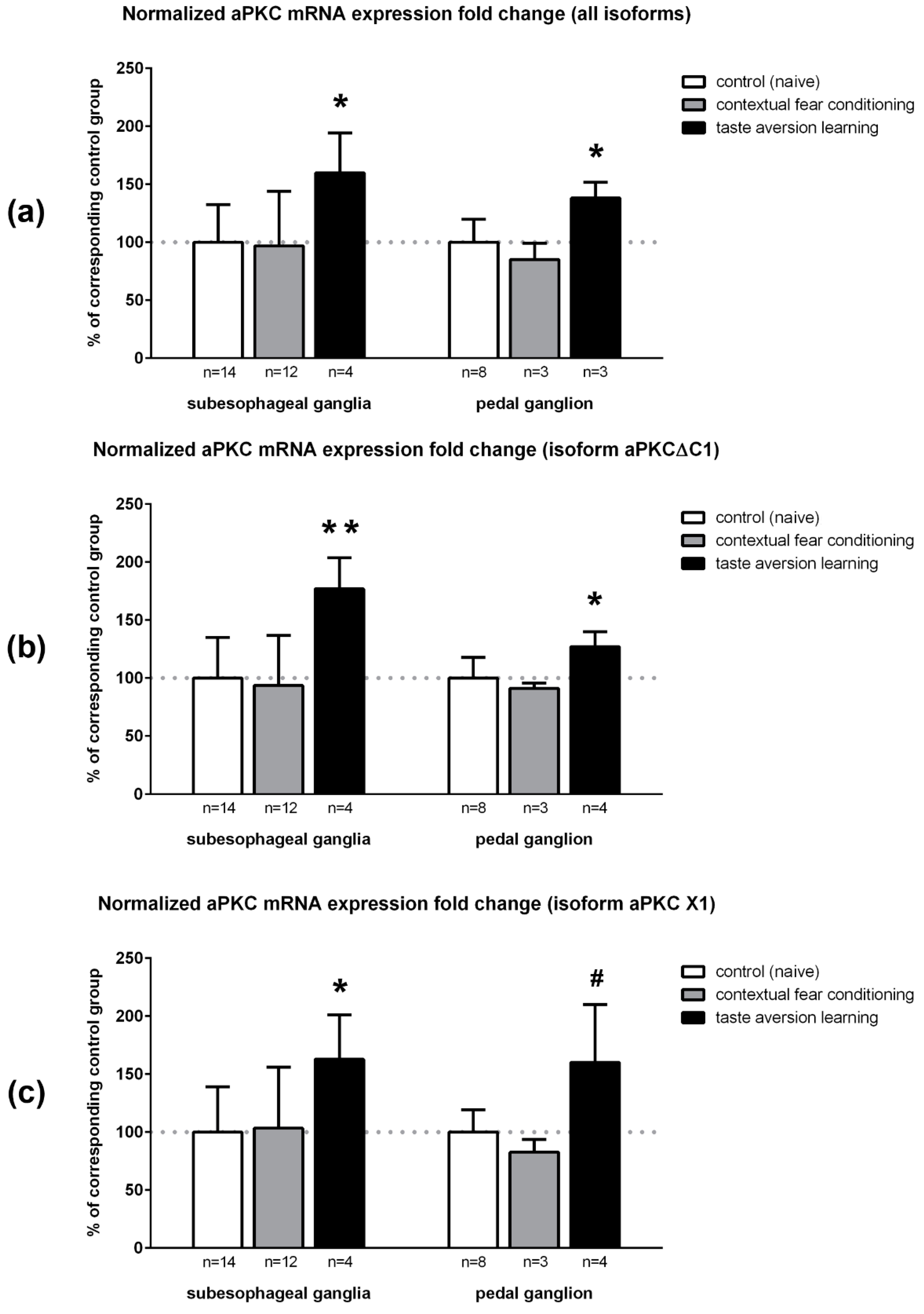
2.5. qPCR Results Demonstrated That aPKC mRNA Expression Is Increased in Snail Ganglia after Taste Aversion Learning, but Not After Contextual Fear Conditioning
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. 5′-End of Snail aPKC mRNA Cloning and Sequencing
4.2.1. Primer Design
4.2.2. RNA Extraction
4.2.3. Classic 5′-RACE cDNA Preparation and Amplification
4.2.4. RLM-5’RACE cDNA Preparation and Amplification
4.2.5. Molecular Cloning and Sequencing
4.2.6. Alignment
4.3. Behavioral Methods
4.3.1. Contextual Fear Conditioning
4.3.2. Taste Aversion Learning
4.4. Quantitative Assessment of Expression of Putative Snail aPKC mRNA Isoforms
4.4.1. RNA Extraction and Reverse Transcription
4.4.2. Primer Design
4.4.3. Droplet Digital PCR
- Initial denaturation: 95 °C, 5 min, ramp rate 2 °C/s
- 40 cycles of amplification:
- Denaturation: 95 °C, 30 s, ramp rate 2 °C/s
- Annealing: 55.9 °C, 30 s, ramp rate 2 °C/s
- Elongation: 72 °C, 30 s
- Signal stabilization:
- 4 °C, 5 min
- 90 °C, 5 min
- Infinite hold, 12 °C
4.4.4. Droplet Digital PCR Analysis and Calculations
4.4.5. Quantitative PCR
- Initial denaturation: 95 °C, 5 min
- 40 cycles of amplification:
- Denaturation: 95 °C, 30 s
- Annealing: 63 °C, 30 s
- Elongation: 72 °C, 30 s, with detection of fluorescence
- Melt curve: 65–95 °C, ramp rate 0.5 °C/5 s, with continuous detection of fluorescence
4.4.6. Quantitative PCR Analysis and Calculations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5,7-DHT | 5,7-Dihydroxytryptamine |
| 5′-RACE | Rapid amplification of cDNA 5′-end |
| 5′-UTR | 5′-Untranslated region |
| AGC | The group of kinases named after the protein kinase A, G, and C families |
| AM | Average mean |
| aPKC | Atypical kinases from PKC family |
| aPKC KD | The isoform of snail aPKC mRNA encoding only kinase domain |
| aPKC X1 | The isoform of snail aPKC mRNA with deletion in the hinge region |
| aPKCΔC1 | The isoform of snail aPKC mRNA with deletion of the sequence encoding C1 domain |
| bp | Base pairs |
| C/EBP | CCAAT/enhancer-binding protein |
| C1 | Protein kinase C conserved region 1 |
| C2 | Protein kinase C conserved region 1 |
| cDNA | Complementary DNA |
| CNS | Central nervous system |
| CRE | cAMP-response element |
| DAG | Diacylglycerol |
| ddPCR | Droplet digital PCR |
| DTT | Dithiothreitol |
| EDTA | Ethylenediaminetetraacetic acid |
| eIF4B | Eucaryotic translation initiation factor 4B |
| eIF4E | Eucaryotic translation initiation factor 4E |
| F | Forward primer |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| gDNA | Genomic DNA |
| GluA2 | Glutamate ionotropic receptor AMPA type subunit 2 |
| H2B | Histone 2B |
| H3 | Histone 3 |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| LB | Lysogeny broth |
| LIP | Lambda-interacting protein |
| MARK2 | Microtubule affinity regulating kinase 2 |
| MEK | Mitogen-activated protein kinase kinase |
| MMLV | Moloney murine leukemia virus |
| MQ | Ultrapure water filtered by Milli-Q purifier |
| mRNA | Messenger RNA |
| NF-κB | Nuclear factor-κB |
| Par-4 | Prostate androgen response-4 |
| PB1 | Phox and Bem1 |
| PC12 | The cell line derived from rat pheochromocytoma |
| PCR | Polymerase chain reaction |
| PEG-4000 | Polyethylene glycol 4000 |
| PICK1 | Protein interacting with C-kinase 1 |
| Pin1 | Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
| PKC | Protein kinase C |
| PKCζ | Protein kinase Cζ |
| PKCζ-reg knockout | Knockout mice in which Prkcz gene was modified by disrupting sequence encoding a regulatory domain |
| PKCι | Protein kinase Cι |
| PKCλ | Protein kinase Cλ |
| PKMζ | Protein kinase Mζ |
| qPCR | Quantitative PCR |
| R | Reverse primer |
| RLM-5’RACE | RNA ligase-mediated rapid amplification of cDNA 5′-end |
| RT | Room temperature |
| RT | Reverse transcription |
| RT-qPCR | Quantitative reverse transcription PCR |
| SD | Standard deviation |
| SMART | Switching mechanism at the 5′-end of the RNA transcript |
| Sp1 | Specificity protein 1 |
| T0 | Testing before learning |
| T1 | Testing after learning |
| TBP | TATA-box binding protein |
| UV | Ultraviolet |
| VAMP2 | Vesicle-associated membrane protein 2 |
| X-Gal | 5-Bromo-4-chloro-3-indolyl-β D-galactopyranoside |
| ZDHHC8 | Zinc finger DHHC-type containing 8 |
Appendix A
References
- Newton, A.C. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001, 101, 2353–2364. [Google Scholar] [PubMed]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef]
- Sossin, W.S. Isoform specificity of protein kinase Cs in synaptic plasticity. Learn. Mem. 2007, 14, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Peach, M.L.; Garfield, S.H.; Wincovitch, S.; Marquez, V.E.; Blumberg, P.M. Effects on ligand interaction and membrane translocation of the positively charged arginine residues situated along the C1 domain binding cleft in the atypical protein kinase C isoforms. J. Biol. Chem. 2006, 281, 33773–33788. [Google Scholar] [CrossRef]
- Sumimoto, H.; Kamakura, S.; Ito, T. Structure and function of the PB1 domain, a protein interaction module conserved in animals, fungi, amoebas, and plants. Sci. STKE. 2007. [Google Scholar] [CrossRef] [PubMed]
- Graybill, C.; Wee, B.; Atwood, S.X.; Prehoda, K.E. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J. Biol. Chem. 2012, 287, 21003–21011. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Xie, L.; Dore, K.; Xie, L.; Del Rio, J.C.; King, C.C.; Martinez-Ariza, G.; Hulme, C.; Malinow, R.; Bourne, P.E.; et al. Zeta inhibitory peptide disrupts electrostatic interactions that maintain atypical protein kinase C in its active conformation on the scaffold p62. J. Biol. Chem. 2015, 290, 21845–21856. [Google Scholar] [CrossRef] [PubMed]
- Perander, M.; Bjorkoy, G.; Johansen, T. Nuclear import and export signals enable rapid nucleocytoplasmic shuttling of the atypical protein kinase C lambda. J. Biol. Chem. 2001, 276, 13015–13024. [Google Scholar] [CrossRef] [PubMed]
- Seidl, S.; Braun, U.B.; Leitges, M. Functional comparison of protein domains within aPKCs involved in nucleocytoplasmic shuttling. Biol Open. 2012, 1, 436–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wooten, M.W.; Vandenplas, M.L.; Seibenhener, M.L.; Geetha, T.; Diaz-Meco, M.T. Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C’s via a src kinase pathway. Mol Cell Biol. 2001, 21, 8414–8427. [Google Scholar] [CrossRef]
- White, W.O.; Seibenhener, M.L.; Wooten, M.W. Phosphorylation of tyrosine 256 facilitates nuclear import of atypical protein kinase C. J. Cell. Biochem. 2002, 85, 42–53. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000, 1, 399–403. [Google Scholar] [CrossRef]
- Hernandez, A.I.; Blace, N.; Crary, J.F.; Serrano, P.A.; Leitges, M.; Libien, J.M.; Weinstein, G.; Tcherapanov, A.; Sacktor, T.C. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J. Biol. Chem. 2003, 278, 40305–40316. [Google Scholar] [CrossRef]
- Bal, N.V.; Susorov, D.; Chesnokova, E.; Kasianov, A.; Mikhailova, T.; Alkalaeva, E.; Balaban, P.M.; Kolosov, P. Upstream open reading frames located in the leader of protein kinase Mζ mRNA regulate its translation. Front. Mol. Neurosci. 2016, 9, 103. [Google Scholar] [CrossRef]
- Chesnokova, E.; Bal, N.; Kolosov, P. Kinases of eIF2a Switch Translation of mRNA Subset during Neuronal Plasticity. Int. J. Mol. Sci. 2017, 18, 2213. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, T.C. Memory maintenance by PKMζ—an evolutionary perspective. Mol. Brain. 2012, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.I.; Oxberry, W.C.; Crary, J.F.; Mirra, S.S.; Sacktor, T.C. Cellular and subcellular localization of PKMζ. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 369, 20130140. [Google Scholar] [CrossRef]
- Pastalkova, E.; Serrano, P.; Pinkhasova, D.; Wallace, E.; Fenton, A.A.; Sacktor, T.C. Storage of spatial information by the maintenance mechanism of LTP. Science 2006, 313, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, T.C. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog. Brain Res. 2008, 169, 27–40. [Google Scholar]
- Sacktor, T.C. How does PKMzeta maintain long-term memory? Nat. Rev. Neurosci. 2011, 12, 9–15. [Google Scholar] [CrossRef]
- Ogasawara, H.; Kawato, M. The protein kinase Mζ network as a bistable switch to store neuronal memory. BMC Syst Biol. 2010, 4, 181. [Google Scholar] [CrossRef]
- Ko, H.G.; Kim, J.I.; Sim, S.E.; Kim, T.; Yoo, J.; Choi, S.L.; Baek, S.H.; Yu, W.J.; Yoon, J.B.; Sacktor, T.C.; et al. The role of nuclear PKMζ in memory maintenance. Neurobiol. Learn. Mem. 2016, 135, 50–56. [Google Scholar] [CrossRef]
- Eom, T.; Muslimov, I.A.; Tsokas, P.; Berardi, V.; Zhong, J.; Sacktor, T.C.; Tiedge, H. Neuronal BC RNAs cooperate with eIF4B to mediate activity-dependent translational control. J. Cell Biol. 2014, 207, 237–252. [Google Scholar] [CrossRef]
- Westmark, P.R.; Westmark, C.J.; Wang, S.; Levenson, J.; O’Riordan, K.J.; Burger, C.; Malter, J.S. Pin1 and PKMzeta sequentially control dendritic protein synthesis. Sci. Signal. 2010, 3. [Google Scholar] [CrossRef]
- Yao, Y.; Kelly, M.T.; Sajikumar, S.; Serrano, P.; Tian, D.; Bergold, P.J.; Frey, J.U.; Sacktor, T.C. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J. Neurosci. 2008, 28, 7820–7827. [Google Scholar] [CrossRef]
- Yoshii, A.; Murata, Y.; Kim, J.; Zhang, C.; Shokat, K.M.; Constantine-Paton, M. TrkB and protein kinase Mζ regulate synaptic localization of PSD-95 in developing cortex. J. Neurosci. 2011, 31, 11894–11904. [Google Scholar] [CrossRef]
- Frutos, S.; Moscat, J.; Diaz-Meco, M.T. Cleavage of zetaPKC but not lambda/iotaPKC by caspase-3 during UV-induced apoptosis. J. Biol. Chem. 1999, 274, 10765–10770. [Google Scholar] [CrossRef]
- Briva, A.; Vadász, I.; Lecuona, E.; Welch, L.C.; Chen, J.; Dada, L.A.; Trejo, H.E.; Dumasius, V.; Azzam, Z.S.; Myrianthefs, P.M.; et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE 2007, 2, e1238. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T.; Wooten, M.W. Signal integration and diversification through the p62 scaffold protein. Trends Biochem. Sci. 2007, 32, 95–100. [Google Scholar] [CrossRef]
- Sontag, E.; Sontag, J.M.; Garcia, A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J. 1997, 16, 5662–5671. [Google Scholar] [CrossRef] [PubMed]
- Sjöqvist, M.; Antfolk, D.; Ferraris, S.; Rraklli, V.; Haga, C.; Antila, C.; Mutvei, A.; Imanishi, S.Y.; Holmberg, J.; Jin, S.; et al. PKCζ regulates Notch receptor routing and activity in a Notch signaling-dependent manner. Cell Res. 2014, 24, 433–450. [Google Scholar] [CrossRef]
- Brady, S.C.; Allan, L.A.; Clarke, P.R. Regulation of caspase 9 through phosphorylation by protein kinase C zeta in response to hyperosmotic stress. Mol. Cell. Biol. 2005, 25, 10543–10555. [Google Scholar] [CrossRef]
- Fritzius, T.; Frey, A.D.; Schweneker, M.; Mayer, D.; Moelling, K. WD-repeat-propeller-FYVE protein, ProF, binds VAMP2 and protein kinase Czeta. FEBS J. 2007, 274, 1552–1566. [Google Scholar] [CrossRef]
- Suzuki, A.; Hirata, M.; Kamimura, K.; Maniwa, R.; Yamanaka, T.; Mizuno, K.; Kishikawa, M.; Hirose, H.; Amano, Y.; Izumi, N.; et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 2004, 14, 1425–1435. [Google Scholar] [CrossRef]
- Tobias, I.S.; Newton, A.C. Protein Scaffolds Control Localized Protein Kinase Cζ Activity. J. Biol. Chem. 2016, 291, 13809–13822. [Google Scholar] [CrossRef] [PubMed]
- Bougie, J.K.; Lim, T.; Farah, C.A.; Manjunath, V.; Nagakura, I.; Ferraro, G.B.; Sossin, W.S. The atypical protein kinase C in Aplysia can form a protein kinase M by cleavage. J. Neurochem. 2009, 109, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.S.; Price, G.; Powell, C.T. Rat protein kinase c zeta gene contains alternative promoters for generation of dual transcripts with 5′-end heterogeneity. DNA Cell. Biol. 2000, 19, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Bougie, J.K.; Cai, D.; Hastings, M.; Farah, C.A.; Chen, S.; Fan, X.; McCamphill, P.K.; Glanzman, D.L.; Sossin, W.S. Serotonin-induced cleavage of the atypical protein kinase C Apl III in Aplysia. J. Neurosci. 2012, 32, 14630–14640. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.D.; Kandel, E.R.; Bailey, C.H. Molecular mechanisms of memory storage in Aplysia. Biol. Bull. 2006, 210, 174–191. [Google Scholar] [CrossRef]
- Pittenger, C.; Kandel, E.R. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003, 358, 757–763. [Google Scholar] [CrossRef]
- Balaban, P. Behavioral neurobiology of learning in terrestrial snails. Prog. Neurobiol. 1993, 41, 1–19. [Google Scholar] [CrossRef]
- Balaban, P.M. Cellular mechanisms of behavioral plasticity in terrestrial snail. Neurosci. Biobehav. Rev. 2002, 26, 597–630. [Google Scholar] [CrossRef]
- Ganger, M.T.; Dietz, G.D.; Ewing, S.J. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinformatics 2017, 18, 534. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C: Structure, function, and regulation. J. Biol. Chem. 1995, 270, 28495–28498. [Google Scholar] [CrossRef]
- Balaban, P.M.; Vehovszky, A.; Maximova, O.A.; Zakharov, I.S. Effect of 5,7-dihydroxytryptamine on the food-aversive conditioning in the snail Helix lucorum L. Brain Res. 1987, 404, 201–210. [Google Scholar] [CrossRef]
- Balaban, P.M.; Vinarskaya, A.K.; Zuzina, A.B.; Ierusalimsky, V.N.; Malyshev, A.Y. Impairment of the serotonergic neurons underlying reinforcement elicits extinction of the repeatedly reactivated context memory. Sci. Rep. 2016, 6, 36933. [Google Scholar] [CrossRef]
- Xu, C.; Li, Q.; Efimova, O.; Jiang, X.; Petrova, M.; Vinarskaya, A.; Kolosov, P.; Aseyev, N.; Koshkareva, K.; Ierusalimsky, V.; et al. Immediate early genes in the nervous system of snail Helix lucorum. eNeuro, 2019; In press. [Google Scholar]
- Matz, M.; Shagin, D.; Bogdanova, E.; Britanova, O.; Lukyanov, S.; Diatchenko, L.; Chenchik, A. Amplification of cDNA ends based on template-switching effect and step-out PCR. Nucleic Acids Res. 1999, 27, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Machleder, E.M.; Chenchik, A.; Li, R.; Siebert, P.D. Reverse transcriptase template switching: A SMART approach for full-length cDNA library construction. Biotechniques 2001, 30, 892–897. [Google Scholar] [CrossRef]
- Matz, M.V.; Alieva, N.O.; Chenchik, A.; Lukyanov, S. Amplification of cDNA ends using PCR suppression effect and step-out PCR. Methods Mol. Biol. 2003, 221, 41–49. [Google Scholar]
- Maruyama, K.; Sugano, S. Oligo-capping: A simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides. Gene 1994, 138, 171–174. [Google Scholar]
- Shaefer, B.C. Revolution in rapid amplification of cDNA ends: New strategies for polymerase chain reaction cloning of full-length cDNA ends. Anal. Biochem. 1995, 227, 255–273. [Google Scholar] [CrossRef]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef]
- Balaban, P.M.; Roshchin, M.V.; Korshunova, T.A. Two-faced nitric oxide is necessary for both erasure and consolidation of memory. Zh. Vyssh. Nerv. Deiat. Im. I.P. Pavlova 2011, 61, 274–280. [Google Scholar]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

| Primer Function | Primer Name | Primer Sequence (5′→3′) |
|---|---|---|
| Reverse primer for the 1st round of nested PCR | R1 | GTTTGCACCCAGTCGATGTCC |
| Reverse primer for the 2nd round of nested PCR | R2 | GACCAGCTCTTTCTTGATGACTTTC |
| Reverse primer for the 3rd round of nested PCR | R3 | TGCAGCACCTTGGCGTAGC |
| Primer Target | Primer Name | Primer Sequence (5′→3′) |
|---|---|---|
| aPKC X1 mRNA isoform | aPKC X1 F | CTGCATGTGCATTTTGCC |
| aPKC X1 R | TTTCACATCCTCCAGTGTTCC | |
| aPKCΔC1 mRNA isoform (primers for qPCR) | aPKCΔC1 F | TATAGGAGAGGGGCTCG |
| aPKCΔC1 R | ATGTTCTTTCACATCCCTTG | |
| aPKCΔC1 mRNA isoform (primers for ddPCR) | dd aPKCΔC1 F | GCCAAGAGATTTTCAAGGGAT |
| dd aPKCΔC1 R | CTCCATTCACAGGTTGCG | |
| Putative kinase domain-coding part of aPKC mRNA | aPKC KD F | TGAGTTTGTGAATGGAGGCG |
| aPKC KD R | AGTCTGTTAGTTTGATGTGTCCC | |
| TATA-box binding protein | TBP F | GGTTGGTAGCTGTGATGTC |
| TBP R | CCATGCGGTAGATAAGTCC | |
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH F | CCCAGAACATCATTCCCTCCTC |
| GAPDH R | CGGAAAGCCATGCCGGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesnokova, E.; Zuzina, A.; Bal, N.; Vinarskaya, A.; Roshchin, M.; Artyuhov, A.; Dashinimaev, E.; Aseyev, N.; Balaban, P.; Kolosov, P. Experiments with Snails Add to Our Knowledge about the Role of aPKC Subfamily Kinases in Learning. Int. J. Mol. Sci. 2019, 20, 2117. https://doi.org/10.3390/ijms20092117
Chesnokova E, Zuzina A, Bal N, Vinarskaya A, Roshchin M, Artyuhov A, Dashinimaev E, Aseyev N, Balaban P, Kolosov P. Experiments with Snails Add to Our Knowledge about the Role of aPKC Subfamily Kinases in Learning. International Journal of Molecular Sciences. 2019; 20(9):2117. https://doi.org/10.3390/ijms20092117
Chicago/Turabian StyleChesnokova, Ekaterina, Alena Zuzina, Natalia Bal, Aliya Vinarskaya, Matvey Roshchin, Alexander Artyuhov, Erdem Dashinimaev, Nikolay Aseyev, Pavel Balaban, and Peter Kolosov. 2019. "Experiments with Snails Add to Our Knowledge about the Role of aPKC Subfamily Kinases in Learning" International Journal of Molecular Sciences 20, no. 9: 2117. https://doi.org/10.3390/ijms20092117
APA StyleChesnokova, E., Zuzina, A., Bal, N., Vinarskaya, A., Roshchin, M., Artyuhov, A., Dashinimaev, E., Aseyev, N., Balaban, P., & Kolosov, P. (2019). Experiments with Snails Add to Our Knowledge about the Role of aPKC Subfamily Kinases in Learning. International Journal of Molecular Sciences, 20(9), 2117. https://doi.org/10.3390/ijms20092117





